Abstract
Oxidative stress drives cell death in a number of diseases including ischemic stroke and neurodegenerative diseases. A better understanding of how cells recover from oxidative stress is likely to lead to better treatments for stroke and other diseases. The recent evidence obtained in several models ties the process of lysosomal exocytosis to the clearance of protein aggregates and toxic metals. The mechanisms that regulate lysosomal exocytosis, under normal or pathological conditions, are only beginning to emerge. Here we provide evidence for the biphasic effect of oxidative stress on lysosomal exocytosis. Lysosomal exocytosis was measured using the extracellular levels of the lysosomal enzyme beta-hexosaminidase (β-hex). Low levels or oxidative stress stimulated lysosomal exocytosis, but inhibited it at high levels. Deletion of the lysosomal ion channel TRPML1 eliminated the stimulatory effect of low levels of oxidative stress. The inhibitory effects of oxidative stress appear to target the component of lysosomal exocytosis that is driven by extracellular Ca2+. We propose that while moderate oxidative stress promotes cellular repair by stimulating lysosomal exocytosis, at high levels oxidative stress has a dual pathological effect: it directly causes cell damage and impairs damage repair by inhibiting lysosomal exocytosis. Harnessing these adaptive mechanisms may point to pharmacological interventions for diseases involving oxidative proteotoxicity or metal toxicity.
Keywords: Lysosomes, exocytosis, calcium, TRPML1, oxidative stress
Graphical Abstract
1. Introduction
Production of reactive oxygen species (ROS) normally accompanies mitochondrial and cytoplasmic enzymatic activities [1, 2]. It can be accelerated by mitochondrial damage and by toxic compounds that promote ROS production [2]. The cellular defense system comprising catalases and superoxide dismutases effectively fights ROS at near-physiological conditions [3–8]. However, a significant imbalance towards the ROS accumulation leads to oxidative stress and to organellar and cytoplasmic damage.
Oxidative stress causes lipid peroxidation [9–11], and the ensuing loss of membrane integrity damages membrane organelles. The injured mitochondria and lysosomes accelerate the damage by stimulating ROS production and by leaking the digestive enzymes and pro-apoptotic factors [12–18]. The repair of oxidative damage necessitates a rapid elimination of the damaged organelles by autophagy, which depends upon proper lysosomal function [19–24]. Oxidative stress damages the cytoplasmic proteins as well, leading to protein aggregation. Elimination of such aggregates depends on autophagy, which, in turn, depends on lysosomes as well.
The endocytic/autophagic/lysosomal roles in cell health are not limited to degradation, but also involve the capture and rapid evacuation of organelles and cytoplasmic compartments. Indeed, lysosomal and autophagic exocytosis has recently emerged as an important cytoprotective mechanism [25–35]. Lysosomal exocytosis was observed more than 50 years ago as a mechanism to limit focal cellular injuries by sequestration followed by digestion or extrusion [36]. Although early studies focused upon its role in a plasma-membrane repair [37], exocytosis has recently re-emerged as an important component of the cellular clearance pathway. Upregulation of lysosomal exocytosis is proposed to underlie the therapeutic effect of TFEB overexpression in several disease models [25, 31, 33].
The lysosomal divalent cation channel TRPML1 was proposed to drive lysosomal exocytosis because of suppression of lysosomal exocytosis observed in mucolipidosis type IV models [32, 38]. The proposed underlying mechanisms involves releasing the calcium ions stored in lysosomes [32], which actuates the conformational change in SNARE that drive the fusion of the lysosomes with the plasma membrane. A role of TRPML1 in lysosomal traffic and positioning [39] is likely to contribute to the exocytosis process as well. Finally, TRPML1 has been proposed to facilitate the expulsion of the copper-filled lysosomes [28], a process that is crucial for limiting oxidative stress [26, 40]. The recent evidence of TRPML1 activation by oxidative stress [41] raises the possibility that lysosomal, exocytosis responds to oxidative stress as well. Such a response would modulate the cellular clearance pathways in a manner integrating signals from oxidative stress and cellular energy sending pathway due to the regulation of TRPML1 expression via mTORC1 and TFEB/TFE3.
As the details of the lysosomal exocytosis mechanisms are emerging, the complexity of the machinery that drives it is becoming apparent. This provides opportunities for pharmacological interventions into many diseases, as many additional targets of pathologies can now be recognized. We have recently shown that lysosomal exocytosis limits the oxidative damage caused by transition metal exposure [29, 40]. A surprising finding that emerged in the course of our previous studies is that while short-term exposure to transition metals stimulated lysosomal exocytosis, long-term exposure had an inhibitory effect [26, 40]. Since the long-term metal exposure is associated with oxidative stress [42, 43], here we investigate the role of oxidative stress in modulating lysosomal exocytosis and provide novel insights into its mechanisms.
2. Methods
Cell Culture and treatments
HeLa cells were maintained in DMEM (Dulbecco’s modified Eagle’s medium; Lonza) supplemented with 10% FBS (Atlanta Biologicals) at 37ºC in the presence of 5% CO2. tBHP was from Invitrogen (Carlsbag, CA) and ML-SA1 was from Sigma-Aldrich (St. Louis, MO). Primary cortical neuron cultures were prepared as previously described [44], from E15–16 C57BL/6 (Charles River) mouse embryos. The neurons were plated on poly-L-lysine-coated four-well chambered slides (Nunc Laboratory-Tek; Fisher Scientific, Agawam, MA, USA) at 3105 cells/cm2 in serum-free Neurobasal media (Gibco, Thermo Fisher) and supplemented with 0.75mM L-glutamine (BioWhittaker, Walkersville, MD, USA) and B27 supplement (Gibco).
siRNA transfection
ON-TARGET plus siRNA probes were custom synthesized by Dharmacon (Lafayette, CO). The first siRNA probe was custom designed to target nucleotides 959–977 with the sequence 5′-CCCACATCCAGGAGTGTAA-3′ in human MCOLN1, as previously described (TRPML1 959 siRNA) [45, 46]. Non-targeting control siRNA purchased from Sigma was used as a negative control. For knockdown of endogenous TRPML1, cells were plated in 12 well plates and grown to ~70% confluency. As described by manufacturer’s protocol, cells were transfected with either control or TRPML1-specific siRNA using RNAiMAX (Invitrogen). To confirm TRPML1 knockdown, SYBR-green based qPCR was performed as described before [29, 40, 47].
β-Hex activity assay
The assay was performed as before [26, 29, 40]. In brief, HeLa cells on 12-well plates were washed once with regular buffer and 250 μl of buffer was added to each well. The buffer contained, in mM: 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, pH adjusted to 7.4. The buffer was collected at the indicated times and incubated with 300 μl of 3 mM 4-nitrophenyl N-acetyl-β-D-glucosaminide (N9376, Sigma-Aldrich) for 30 minutes at 37ºC in 0.1 M citrate buffer (0.1 M sodium citrate, 0.1 M citric acid, pH 4.5). Reactions were stopped by adding 650 μl borate buffer (100 mM boric acid, 75 mM NaCl, 25 mM sodium borate, pH 9.8) and the absorbance was measured in a spectrophotometer at 405 nm. To determine total cellular β-hex content, cells were lysed with 250 μl of 1% Triton X-100 in PBS and after a 10,000 x g spin for 5 minutes at 4oC, 25 μl of the cell extracts were used for the enzyme activity reaction. Enzyme activity was determined as the amount of 4-nitrophenol produced. Absorbance was calibrated with different amounts of 4-nitrophenol (N7660, Sigma-Aldrich, St Louis, MO) in 0.1 M citrate buffer.
Statistical significance was calculated using a one-tailed, unpaired t-test with p<0.05 considered significant. Data are presented as mean ± S.E.M.
3. Results
We have previously used β-hex assays in HeLa cells to study the role of lysosomal exocytosis on transition metal clearance and the effects of transition metals on lysosomal exocytosis [26, 29, 40]. β-hex is a lysosomal enzyme, the delivery of which to the extracellular medium is commonly used as a measure of lysosomal exocytosis. In the previous studies we performed a detailed analysis and verification of this process using LAMP1 exposure and siRNA for the components of the protein complex responsible for the lysosomal fusion with the plasma membrane [26].
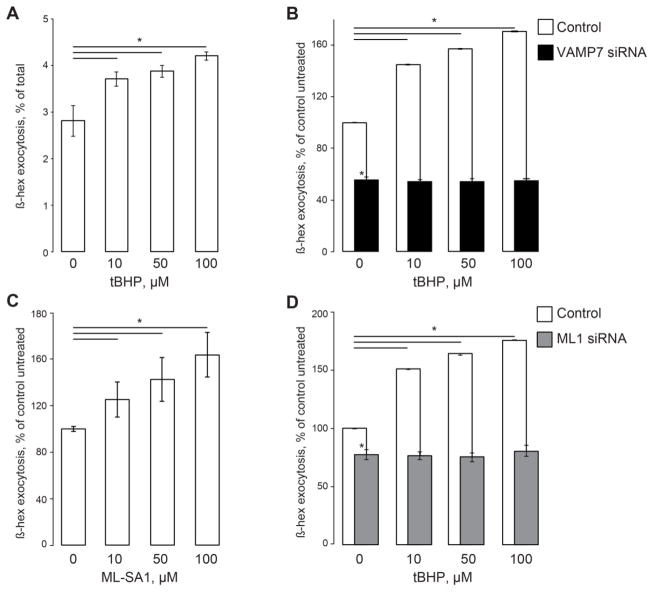
Tert-Butyl hydroperoxide (tBHP), a stable form of hydrogen peroxide is commonly used to induce oxidative stress [48–50]. In our hands, tBHP changed the delivery of β-hex activity to the extracellular medium bathing HeLa cells in a concentration-dependent manner. Fig 1A shows that cells treated with 10 to 100 μM tBHP for 1 hr increased the rate of the lysosomal exocytosis. In this set of experiments, untreated cells released 2.81±0.32% of their total β-hex context (measured by lysing cells with CHAPS) into the medium during the 1 hr exposure. Cells treated with 50 μM tBHP released 3.87±0.13% of total enzyme, while cells treated with 100 μM tBHP released 4.20±0.09% of the total β-hex (3 individual samples per condition per experiment, 6 experiments, p<0.05 for 10, 50 and 100 μM tBHP). An independent series of experiments Fig 1B shows that the tBHP-induced increase was eliminated by siRNA-mediated knockdown of VAMP7, which was implicated in the lysosomal exocytosis [51, 52] (3 measurements per condition per experiment, 6 experiments, p<0.05 for 10, 50 and 100 μM tBHP, p<0.05 for all VAMP7-siRNA points). TRPML1 has been implicated in lysosomal exocytosis [32, 38]. Accordingly, TRPML1 activator ML-SA1 [32] stimulated lysosomal exocytosis (Fig 1C, 3 measurements per condition per experiment, 3 experiments, p<0.05 for 10, 50 and 100 μM ML-SA1). siRNA-mediated knockdown of TRPML1 eliminated the tBHP-dependent increase in the lysosomal exocytosis rate as well (Fig 1D, 3 measurements per condition per experiment, 6 experiments, p<0.05 for 10, 50 and 100 μM tBHP). TRPML1 mRNA levels following the knockdown are shown in Supplementary Fig S1. These data suggest that a) TRPML1 activation by oxidative stress contributes to the increase in β-hex exocytosis in response to oxidative stress, and b) such an increase occurs according to the common paradigm of lysosomal exocytosis: Ca2+ release through TRPML1 actuating or facilitating VAMP7-dependent fusion events.
Fig 1. Moderate ROS levels activate lysosomal exocytosis.
A. β-hex exocytosis in cells treated with low and moderate tBHP concentrations. β-hex activity in the extracellular medium expressed as the percentage of total cellular β-hex content at different tBHP concentrations. B. β-hex exocytosis normalized to its levels in untreated cells, which were taken as 100%. VAMP7 knockdown using siRNA suppresses the basal and the tBHP-stimulated β-hex exocytosis. C. β-hex exocytosis expressed as in panel B as a function of ML-SA1 concentration. D. β-hex exocytosis analyzed as in panel B in mock- and TRPML1 siRNA-transfected cells. β-hex assays in HeLa cells performed as in our previous publications [26, 29, 40]. β-hex activity in the culture medium was analyzed one hour after the fresh medium was introduced. The activity was normalized to the total β-hex content, which was analyzed by lysing cells; total β-hex content in cell lysates of untreated cells was taken for 100% (shown in Supplementary Fig S2). tBHP and ML-SA1 were added immediately after the recording had begun. siRNA transfections were performed 24 hours before the experiment. Data represent 3 experiments, 3 independent biological replicates each; * denotes p<0.05 relative to untreated control.
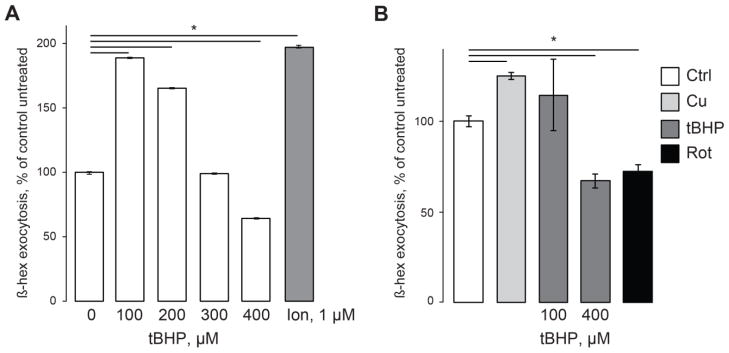
Interestingly, the trend of lysosomal exocytosis activation by oxidative stress reversed when tBHP concentration was raised over 200 μM. Fig 2A shows that while in cells treated with 100 μM tBHP, β-hex exocytosis averaged 180% of its levels in untreated cells (n=9, p<0.05), increasing tBHP concentration to 300 μM dropped the exocytosis to about 100% of control cells. At 400 μM tBHP β-hex exocytosis averaged 60% of its levels in control cells (Fig 2A, n=9, p<0.05). The total β-hex cellular activity measured after the lysing of the cells using Triton X-100 did not show the same degree of suppression (Supplementary Fig S2).
Fig 2. Inhibition of lysosomal exocytosis by high levels of oxidative stress.
β-hex assays performed in HeLa cells (A) and mouse primary cortical neuron cultures (B). All treatments are as in Fig 1. As in our recent publication [26], 100 μM Cu stimulated lysosomal exocytosis. Rotenone (Rot, 20 μM) was applied 12 hours before the experiment. Data represent 3 experiments, 2 independent biological replicates each; * denotes p<0.05 relative to untreated control.
The lysosomal exocytosis decline in response to oxidative stress was detected in the mouse primary cortical neuron cultures as well. In this set of experiments, β-hex exocytosis rate in cells treated with 400 μM tBHP averaged 67% of its levels in untreated cells (n=6, p<0.05) (Fig 2B). We conclude that at high levels oxidative stress suppresses lysosomal exocytosis in both transformed and primary cells. As an additional treatment we used the mitochondrial uncoupler rotenone [53, 54], known to increase the mitochondrial electron-leak causing oxidative stress. Fig 2B shows that rotenone suppressed levels of lysosomal exocytosis as well (72% of control levels, n=9, p<0.05). These tBHP and rotenone concentrations are routinely used to study the effects of oxidative stress in neuronal cultures in vivo and in vitro [19, 49, 50, 55].
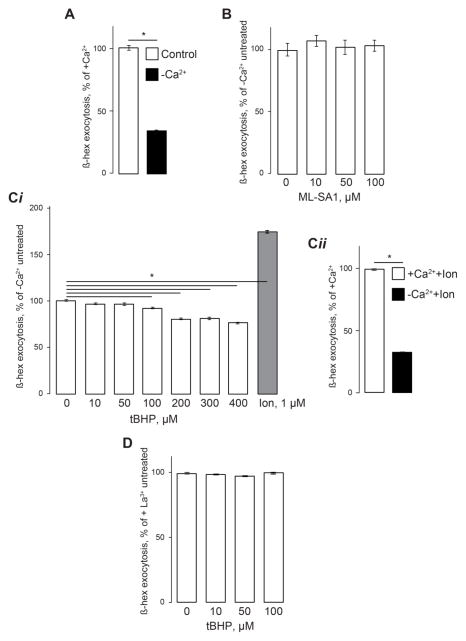
In the next set of experiments, we sought to determine the source of lysosomal exocytosis inhibition by oxidative stress. Lysosomal exocytosis probably involves multiple steps including vesicle positioning, delivery, docking and fusion. The previous evidence and data in Fig 1C,D show that TRPML1 is critical for this process. However, whether it controls all faucets of lysosomal exocytosis is unclear. Indeed, we have recently shown that Ca2+ influx from the extracellular medium has an important role in lysosomal exocytosis [26] Accordingly, the removal of Ca2+ from the extracellular buffer inhibited the basal rate of lysosomal exocytosis (Fig 3A). When measured in Ca2+-free buffer, basal lysosomal exocytosis averaged 34% or its levels in regular buffer containing 1 mM Ca2+ (3 experiments, 3 individual samples in each experiment, p<0.06). In these experiments, Ca2+ was removed at the onset of the experiment. In our hands, ML-SA1 did not induce β-hex exocytosis in Ca2+-free buffer (Fig 3B). The removal of extracellular Ca2+ suppressed the lysosomal exocytosis activation by tBHP as well (Fig 3Ci). Under these conditions, ionomycin induced some β-hex exocytosis, although its magnitudes were significantly suppressed compared with the ionomycin effect in normal Ca2+ buffer (Fig 3Cii) The nonspecific plasma-membrane Ca2+ channel blocker La3+ (100 μM) also suppressed the β-hex exocytosis activation by tBHP (Fig 3D).
Fig 3. Involvement of extracellular Ca2+ in lysosomal exocytosis.
β-hex assays performed in HeLa cells as in Fig 1 and 2. A. Ca2+ removal from the extracellular medium suppresses lysosomal exocytosis. Data are expressed as percentage of lysosomal exocytosis in cells exposed to normal 1 mM Ca2+. B. ML-SA1 (100 μM) added immediately after beginning of the experiment as in Fig 1C is not effective in stimulating β-hex exocytosis in the absence of extracellular Ca2+. The data are expressed as percentage of β-hex exocytosis relative to untreated cells exposed to Ca2+-free solution. C. (i) Cells exposed to Ca2+-free medium immediately after the beginning of experiment and treated as in Fig 1C and 2A do not show an increase in β-hex exocytosis in response to tBHP. (ii) Comparison of the ionomycin effect on lysosomal exocytosis in Ca2+-containing and Ca2+-free buffers. Exocytosis is expressed as in panel B. D. tBHP is ineffective in inducing lysosomal exocytosis in the presence of 100 μM LaCl3. The experiments were performed in a normal Ca2+ buffer to which La3+ was added, and the data are expressed as percentage of β-hex exocytosis relative to untreated cells in La3+ buffer. Data represent 3 experiments, 3 independent biological replicates each; * denotes p<0.05 relative to untreated control.
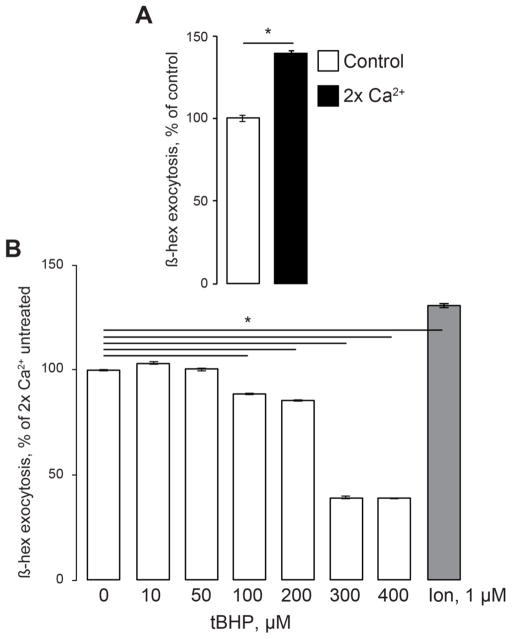
Increasing the extracellular Ca2+ levels to 2 mM raised the basal β-hex exocytosis rates by about 40% (Fig 4A; 3 experiments, 3 individual samples in each experiment, p<0.06). The magnitude of β-hex signal under the high-Ca2+ conditions was comparable with that induced by ionomycin or tBHP under normal conditions. Under the high-Ca2+ conditions, the tBHP concentrations that activated β-hex exocytosis in normal Ca2+ produced little or no increase in β-hex exocytosis (Fig 4B; 3 experiments, 3 individual samples in each experiment, p<0.06). However, the tBHP concentrations that inhibited β-hex exocytosis in normal Ca2+, produced marked decrease in β-hex exocytosis. Based on this, we propose that extracellular Ca2+ influx is important for the lysosomal exocytosis. While the exocytosis activation by low levels of ROS depends on TRPML1, lysosomal exocytosis inhibition by oxidative stress involves inhibiting of plasma membrane Ca2+ influx.
Fig 4. Extracellular Ca2+ influx and lysosomal exocytosis.
β-hex assays performed in HeLa cells that were bathed in 2 x Ca2+ media (2 mM Ca2+) starting immediately after beginning of the experiment. A. Raising extracellular Ca2+ increases lysosomal exocytosis. Data are expressed as percentage of lysosomal exocytosis in cells exposed to normal 1 mM Ca2+. B. High levels of tBHP cause significant suppression of lysosomal exocytosis in 2x Ca2+ buffer. Cells were treated and the data are expressed essentially the same as in Fig 3C. Data represent 3 experiments, 3 independent biological replicates each; * denotes p<0.05 relative to untreated control.
4. Discussion
Oxidative stress drives pathological processes in many diseases including ischemic stroke, reperfusion injury, heart failure, Alzheimer’s disease, ALS, genetic and toxic forms of Parkinson’s disease, autism and Asperger’s syndrome [20, 53, 56–65]. A better understanding of the mechanisms of oxidative damage and the recovery from oxidative stress is crucial for better treatments for these diseases. An important component of the cellular defense against oxidative stress is the recovery from oxidative damage. The removal of damaged organelles from the cytoplasm is a function of the autophagic/lysosomal pathway [19, 24]. Beyond the degradation of damaged organelles, the expulsion of damaged cellular components via exocytosis has recently emerged as an important component of the cellular clearance mechanism [21, 31, 66–71]. Therefore, the lysosomal exocytosis pathway is a potentially important pharmacological target for modulating the effects of oxidative stress.
The recent evidence of TRPML1 activation by ROS [41] raised the possibility that TRPML1-dependent processes are regulated by ROS as well. The initial evidence for the role of TRPML1 in lysosomal exocytosis comes from resting unstimulated human skin fibroblasts. TRPML-deficient fibroblasts from mucolipidosis type IV patients were found to have lower exocytosis rates than age-matched heterozygous controls [38]. TRPML1 has been implicated in the exocytosis of Cu-filled lysosomes [28]. Finally, inhibition of lysosomal exocytosis and the resulting membrane deficits were used to explain phagocytosis suppression in TRPML1-deficient cells [32]. Our data on the stimulation of the lysosomal exocytosis by low and moderate levels of the pro-oxidant tBHP in a manner requiring TRPML1 agrees with this evidence.
Our data show that oxidative stress (high levels of ROS) suppresses the process of lysosomal exocytosis, which would be predicted to impair the expulsion of toxic compounds from cells. Therefore, we propose that ROS have a biphasic effect on the lysosomal clearance: consistent with their role as a second messenger moderate levels of ROS stimulate the lysosomal exocytosis, but pathological levels of ROS inhibit the lysosomal exocytosis. Therefore, the impact of oxidative stress pathology on cells is two-pronged: beyond direct damage, it impairs the ability of the cell to limit and repair damage by extrusion of damaged materials. Indeed, an accumulation of damaged mitochondria and protein aggregates, which in turn can further amplify oxidative stress [24], is observed in multiple human diseases. An impact of oxidative stress on the cellular clearance pathway has been studied in many models; its specific effects are being disputed. An inhibitory effect of oxidative stress on the cellular clearance is also supported by data emerging from studies of ALS [72] and Alzheimer’s disease [66, 73].
Several candidate mechanisms for the observed effects can be discussed. Ca2+ release through TRPML1 has been implicated in lysosomal exocytosis [32]. Lysosomal exocytosis probably involves the stages of vesicle delivery to the plasma membrane and their fusion. TRPML1 has been implicated in vesicle positioning and motility [39] and membrane fusion [32]. Impairments in either of these processes would impact lysosomal exocytosis. Both processes appear to require Ca2+. Our previously published data [26] and the results presented here show that extracellular Ca2+ is necessary for the lysosomal exocytosis as well. These data suggest a contribution of the extracellular Ca2+ influx into cellular clearance and repair processes driven by lysosomal exocytosis. Oxidative stress was shown to inhibit the store-operated Ca2+ entry component Orai [74–76], and thus the inhibition of the store-operated Ca2+ entry by oxidative stress may be responsible for some of the loss of lysosomal exocytosis under oxidative stress. Since cell signaling involving G protein-coupled receptors drives the store-operated Ca2+ entry, this may serve as evidence connecting G protein-coupled receptor signaling and the cellular clearance via lysosomal exocytosis.
We show that increasing extracellular Ca2+ stimulates lysosomal exocytosis to the extent that renders oxidative stress ineffective (Fig 4). We think that the under these conditions, the increased Ca2+ influx triggers the processes normally actuated by TRPML1-dependent Ca2+ release, such as lysosomal positioning or fusion. Since the loss of lysosomal exocytosis was proposed to contribute to mucolipidosis type IV pathology [32, 38], it would be interesting to answer whether increased Ca2+ influx reverses some aspects of this pathology.
5. Conclusions
Oxidative stress drives pathological processes in many diseases including ischemic stroke, heart attack and neurodegenerative diseases. Lysosomal exocytosis has emerged as an important cellular clearance pathway. We show that oxidative stress suppresses lysosomal exocytosis. Therefore, beyond direct damage, oxidative stress suppresses cellular repair by inhibiting the expulsion of toxic compounds.
Supplementary Material
Highlights.
Oxidative stress regulated lysosomal exocytosis in a biphasic manner.
Stimulation of lysosomal exocytosis by low levels of oxidative stress requires TRPML1.
Inhibition of lysosome exocytosis by high levels of oxidative stress involves calcium influx.
Acknowledgments
These studies were supported by NIH grants HD058577, ES01678, NS096755 and NS094860, and Mucolipidosis type IV foundation grant to KK, and NIH grants AG026389 and NS065789 to CTC.
Footnotes
Co-author Approval Statement
- Publication of this manuscript has been approved by all authors.
Conflict of Interest Statement
The authors declare no conflicting interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox biology. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drose S, Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Rodriguez A, Egea-Guerrero JJ, Murillo-Cabezas F, Carrillo-Vico A. Oxidative stress in traumatic brain injury. Curr Med Chem. 2014;21:1201–1211. doi: 10.2174/0929867321666131217153310. [DOI] [PubMed] [Google Scholar]
- 4.Milton NG. Inhibition of catalase activity with 3-amino-triazole enhances the cytotoxicity of the Alzheimer’s amyloid-beta peptide. Neurotoxicology. 2001;22:767–774. doi: 10.1016/s0161-813x(01)00064-x. [DOI] [PubMed] [Google Scholar]
- 5.Caruano-Yzermans AL, Bartnikas TB, Gitlin JD. Mechanisms of the copper-dependent turnover of the copper chaperone for superoxide dismutase. J Biol Chem. 2006;281:13581–13587. doi: 10.1074/jbc.M601580200. [DOI] [PubMed] [Google Scholar]
- 6.Callio J, Oury TD, Chu CT. Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J Biol Chem. 2005;280:18536–18542. doi: 10.1074/jbc.M413224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- 8.Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 9.Gasier HG, Fothergill DM. Oxidative stress, antioxidant defenses and nitric oxide production following hyperoxic exposures, Undersea & hyperbaric medicine : journal of the Undersea and Hyperbaric Medical Society. Inc. 2013;40:125–134. [PubMed] [Google Scholar]
- 10.Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G, Radak Z, Calabrese EJ, Cuzzocrea S. Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal. 2013;19:836–853. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 11.Mazzio EA, Reams RR, Soliman KF. The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro. Brain Res. 2004;1004:29–44. doi: 10.1016/j.brainres.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Schafer MK, Pfeiffer A, Jaeckel M, Pouya A, Dolga AM, Methner A. Regulators of mitochondrial Ca(2+) homeostasis in cerebral ischemia. Cell Tissue Res. 2014;357:395–405. doi: 10.1007/s00441-014-1807-y. [DOI] [PubMed] [Google Scholar]
- 13.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Park MH, Kim HJ, Koh JY. Metallothionein-3 regulates lysosomal function in cultured astrocytes under both normal and oxidative conditions. Glia. 2010;58:1186–1196. doi: 10.1002/glia.20998. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Koh JY. Roles of zinc and metallothionein-3 in oxidative stress-induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytes. Molecular brain. 2010;3:30. doi: 10.1186/1756-6606-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang JJ, Kim HN, Kim J, Cho DH, Kim MJ, Kim YS, Kim Y, Park SJ, Koh JY. Zinc(II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals. 2010;23:997–1013. doi: 10.1007/s10534-010-9346-9. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JJ, Lee SJ, Kim TY, Cho JH, Koh JY. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:3114–3122. doi: 10.1523/JNEUROSCI.0199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiskum G, Starkov A, Polster BM, Chinopoulos C. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:111–119. doi: 10.1111/j.1749-6632.2003.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 19.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osellame LD, Duchen MR. Quality control gone wrong: mitochondria, lysosomal storage disorders and neurodegeneration. Br J Pharmacol. 2014;171:1958–1972. doi: 10.1111/bph.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giordano S, Darley-Usmar V, Zhang J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox biology. 2014;2:82–90. doi: 10.1016/j.redox.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raben N, Wong A, Ralston E, Myerowitz R. Autophagy and mitochondria in Pompe disease: nothing is so new as what has long been forgotten. American journal of medical genetics. Part C Seminars in medical genetics. 2012;160C:13–21. doi: 10.1002/ajmg.c.31317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korolchuk VI, Rubinsztein DC. Regulation of autophagy by lysosomal positioning. Autophagy. 2011;7:927–928. doi: 10.4161/auto.7.8.15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherra SJ, 3rd, Chu CT. Autophagy in neuroprotection and neurodegeneration: A question of balance. Future Neurol. 2008;3:309–323. doi: 10.2217/14796708.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Settembre C, Medina DL. TFEB and the CLEAR network. Methods Cell Biol. 2015;126:45–62. doi: 10.1016/bs.mcb.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Peña KA, Coblenz J, Kiselyov K. Brief exposure to copper activates lysosomal exocytosis. Cell Calcium. 2015;57:257–262. doi: 10.1016/j.ceca.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samie MA, Xu H. Lysosomal exocytosis and lipid storage disorders. J Lipid Res. 2014;55:995–1009. doi: 10.1194/jlr.R046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polishchuk EV, Concilli M, Iacobacci S, Chesi G, Pastore N, Piccolo P, Paladino S, Baldantoni D, van ISC, Chan J, Chang CJ, Amoresano A, Pane F, Pucci P, Tarallo A, Parenti G, Brunetti-Pierri N, Settembre C, Ballabio A, Polishchuk RS. Wilson Disease Protein ATP7B Utilizes Lysosomal Exocytosis to Maintain Copper Homeostasis. Dev Cell. 2014 doi: 10.1016/j.devcel.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kukic I, Kelleher SL, Kiselyov K. Zn2+ efflux through lysosomal exocytosis prevents Zn2+-induced toxicity. J Cell Sci. 2014;127:3094–3103. doi: 10.1242/jcs.145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X, Zhang X, Gao Q, Ali Samie M, Azar M, Tsang WL, Dong L, Sahoo N, Li X, Zhuo Y, Garrity AG, Wang X, Ferrer M, Dowling J, Xu L, Han R, Xu H. The intracellular Ca(2)(+) channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med. 2014;20:1187–1192. doi: 10.1038/nm.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spampanato C, Feeney E, Li L, Cardone M, Lim JA, Annunziata F, Zare H, Polishchuk R, Puertollano R, Parenti G, Ballabio A, Raben N. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol Med. 2013;5:691–706. doi: 10.1002/emmm.201202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samie M, Wang X, Zhang X, Goschka A, Li X, Cheng X, Gregg E, Azar M, Zhuo Y, Garrity AG, Gao Q, Slaugenhaupt S, Pickel J, Zolov SN, Weisman LS, Lenk GM, Titus S, Bryant-Genevier M, Southall N, Juan M, Ferrer M, Xu H. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev Cell. 2013;26:511–524. doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feeney EJ, Spampanato C, Puertollano R, Ballabio A, Parenti G, Raben N. What else is in store for autophagy? Exocytosis of autolysosomes as a mechanism of TFEB-mediated cellular clearance in Pompe disease. Autophagy. 2013;9:1117–1118. doi: 10.4161/auto.24920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Li X, Xu H. Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1202194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011;21:421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hruban Z, Spargo B, Swift H, Wissler RW, Kleinfeld RG. Focal cytoplasmic degradation. Am J Pathol. 1963;42:657–683. [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 38.Laplante JM, Sun M, Falardeau J, Dai D, Brown EM, Slaugenhaupt SA, Vassilev PM. Lysosomal exocytosis is impaired in mucolipidosis type IV. Mol Genet Metab. 2006;89:339–348. doi: 10.1016/j.ymgme.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W, Gao Q, Cheng X, Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016;18:404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peña KA, Kiselyov K. Transition metals activate TFEB in overpexpressing cells. Biochem J. 2015;470:65–76. doi: 10.1042/BJ20140645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, Delling M, Marugan J, Ferrer M, Xu H. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun. 2016;7:12109. doi: 10.1038/ncomms12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 43.Granzotto A, Sensi SL. Intracellular zinc is a critical intermediate in the excitotoxic cascade. Neurobiol Dis. 2015;81:25–37. doi: 10.1016/j.nbd.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Dagda RK, Gusdon AM, Pien I, Strack S, Green S, Li C, Van Houten B, Cherra SJ, 3rd, Chu CT. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson’s disease. Cell Death Differ. 2011;18:1914–1923. doi: 10.1038/cdd.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colletti GA, Miedel MT, Quinn J, Andharia N, Weisz OA, Kiselyov K. Loss of Lysosomal Ion Channel Transient Receptor Potential Channel Mucolipin-1 (TRPML1) Leads to Cathepsin B-dependent Apoptosis. J Biol Chem. 2012;287:8082–8091. doi: 10.1074/jbc.M111.285536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miedel MT, Rbaibi Y, Guerriero CJ, Colletti G, Weixel KM, Weisz OA, Kiselyov K. Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. J Exp Med. 2008;205:1477–1490. doi: 10.1084/jem.20072194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kukic I, Lee JK, Coblentz J, Kelleher SL, Kiselyov K. Zinc-dependent lysosomal enlargement in TRPML1-deficient cells involves MTF-1 transcription factor and ZnT4 (Slc30a4) transporter. Biochem J. 2013;451:155–163. doi: 10.1042/BJ20121506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coblentz J, St Croix C, Kiselyov K. Loss of TRPML1 promotes production of reactive oxygen species: is oxidative damage a factor in mucolipidosis type IV? Biochem J. 2014;457:361–368. doi: 10.1042/BJ20130647. [DOI] [PubMed] [Google Scholar]
- 49.Rabin DM, Rabin RL, Blenkinsop TA, Temple S, Stern JH. Chronic oxidative stress upregulates Drusen-related protein expression in adult human RPE stem cell-derived RPE cells: a novel culture model for dry AMD. Aging. 2013;5:51–66. doi: 10.18632/aging.100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291:F840–855. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arantes RM, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26:4630–4637. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem. 2004;279:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 53.Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson’s disease. Antioxid Redox Signal. 2005;7:685–693. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- 55.Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, Wood NW, Willems PH, Smeitink JA, Cookson MR, Bandmann O. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hegde ML, Hegde PM, Rao KS, Mitra S. Oxidative genome damage and its repair in neurodegenerative diseases: function of transition metals as a double-edged sword. J Alzheimers Dis. 2011;24(Suppl 2):183–198. doi: 10.3233/JAD-2011-110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nunez MT, Garrick MD, Raisman-Vozari R, Hirsch EC. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci U S A. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherer TB, Greenamyre JT. Oxidative damage in Parkinson’s disease. Antioxid Redox Signal. 2005;7:627–629. doi: 10.1089/ars.2005.7.627. [DOI] [PubMed] [Google Scholar]
- 60.Schrag M, Mueller C, Oyoyo U, Smith MA, Kirsch WM. Iron, zinc and copper in the Alzheimer’s disease brain: a quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol. 2011;94:296–306. doi: 10.1016/j.pneurobio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 62.Parellada M, Moreno C, Mac-Dowell K, Leza JC, Giraldez M, Bailon C, Castro C, Miranda-Azpiazu P, Fraguas D, Arango C. Plasma antioxidant capacity is reduced in Asperger syndrome. J Psychiatr Res. 2012;46:394–401. doi: 10.1016/j.jpsychires.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Gu F, Chauhan V, Chauhan A. Glutathione redox imbalance in brain disorders. Current opinion in clinical nutrition and metabolic care. 2015;18:89–95. doi: 10.1097/MCO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 64.De Filippis B, Valenti D, de Bari L, De Rasmo D, Musto M, Fabbri A, Ricceri L, Fiorentini C, Laviola G, Vacca RA. Mitochondrial free radical overproduction due to respiratory chain impairment in the brain of a mouse model of Rett syndrome: protective effect of CNF1. Free Radic Biol Med. 2015;83:167–177. doi: 10.1016/j.freeradbiomed.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol. 2014;5:150. doi: 10.3389/fphys.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polito VA, Li H, Martini-Stoica H, Wang B, Yang L, Xu Y, Swartzlander DB, Palmieri M, di Ronza A, Lee VM, Sardiello M, Ballabio A, Zheng H. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med. 2014;6:1142–1160. doi: 10.15252/emmm.201303671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martina JA, Diab HI, Li H, Puertollano R. Novel roles for the MiTF/TFE family of transcription factors in organelle biogenesis, nutrient sensing, and energy homeostasis. Cell Mol Life Sci. 2014;71:2483–2497. doi: 10.1007/s00018-014-1565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pastore N, Blomenkamp K, Annunziata F, Piccolo P, Mithbaokar P, Maria Sepe R, Vetrini F, Palmer D, Ng P, Polishchuk E, Iacobacci S, Polishchuk R, Teckman J, Ballabio A, Brunetti-Pierri N. Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency. EMBO Mol Med. 2013;5:397–412. doi: 10.1002/emmm.201202046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pastore N, Ballabio A, Brunetti-Pierri N. Autophagy master regulator TFEB induces clearance of toxic SERPINA1/alpha-1-antitrypsin polymers. Autophagy. 2013;9:1094–1096. doi: 10.4161/auto.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1817–1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.La Spada AR. PPARGC1A/PGC-1alpha, TFEB and enhanced proteostasis in Huntington disease: Defining regulatory linkages between energy production and protein-organelle quality control. Autophagy. 2012;8:1845–1847. doi: 10.4161/auto.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie Y, Zhou B, Lin MY, Wang S, Foust KD, Sheng ZH. Endolysosomal Deficits Augment Mitochondria Pathology in Spinal Motor Neurons of Asymptomatic fALS Mice. Neuron. 2015;87:355–370. doi: 10.1016/j.neuron.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barnett A, Brewer GJ. Autophagy in aging and Alzheimer’s disease: pathologic or protective? J Alzheimers Dis. 2011;25:385–394. doi: 10.3233/JAD-2011-101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nunes P, Demaurex N. Redox regulation of store-operated Ca2+ entry. Antioxid Redox Signal. 2014;21:915–932. doi: 10.1089/ars.2013.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bogeski I, Kilch T, Niemeyer BA. ROS and SOCE: recent advances and controversies in the regulation of STIM and Orai. J Physiol. 2012;590:4193–4200. doi: 10.1113/jphysiol.2012.230565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, Mori Y, Hoth M, Niemeyer BA. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Science signaling. 2010;3:ra24. doi: 10.1126/scisignal.2000672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.