Abstract
Children with ADHD demonstrate increased frequent “lapses” in performance on tasks in which the stimulus presentation rate is externally controlled, leading to increased variability in response times. It is less clear whether these lapses are also evident during performance on self-paced tasks, e.g., rapid automatized naming (RAN), or whether RAN inter-item pause time variability uniquely predicts reading performance. A total of 80 children aged 9 to 14 years—45 children with attention-deficit/hyperactivity disorder (ADHD) and 35 typically developing (TD) children—completed RAN and reading fluency measures. RAN responses were digitally recorded for analyses. Inter-stimulus pause time distributions (excluding between-row pauses) were analyzed using traditional (mean, standard deviation [SD], coefficient of variation [CV]) and ex-Gaussian (mu, sigma, tau) methods. Children with ADHD were found to be significantly slower than TD children (p < .05) on RAN letter naming mean response time as well as on oral and silent reading fluency. RAN response time distributions were also significantly more variable (SD, tau) in children with ADHD. Hierarchical regression revealed that the exponential component (tau) of the letter-naming response time distribution uniquely predicted reading fluency in children with ADHD (p < .001, ΔR2 = .16), even after controlling for IQ, basic reading, ADHD symptom severity and age. The findings suggest that children with ADHD (without word-level reading difficulties) manifest slowed performance on tasks of reading fluency; however, this “slowing” may be due in part to lapses from ongoing performance that can be assessed directly using ex-Gaussian methods that capture excessively long response times.
Keywords: Reading, dyslexia, processing speed, executive function, childhood, development
Attention-deficit/hyperactivity disorder (ADHD) and reading disability (RD) represent the two most common neurodevelopmental disorders of childhood (Sexton, Gelhorn, Bell, & Classi, 2012). Furthermore, these two disorders co-occur at a rate of 30–40%, which is higher than would be expected by chance (Couto et al., 2009). Willcutt et al. (2010) have identified a multiple-deficit model to describe the co-occurrence of ADHD and RD in which each disorder has multiple predictors—some unique and some shared. The reading model includes the two unique predictors (phonological awareness and naming speed) and one shared predictor (processing speed), while the ADHD model includes one unique predictor (response inhibition) and one shared predictor (processing speed). Given these observations, processing speed may represent a behavioral “polyphenotype” (i.e., a phenotype constituting core deficits of more than one disorder) whose neuropsychological makeup could account for co-occurrence between the conditions (Grigorenko, 2012). Moreover, while processing speed may be separable from the core phonological deficit in RD, it can nevertheless influence the efficiency of reading (e.g., reading fluency) among those individuals with ADHD with intact single word reading skills, i.e., those without “classic” phonological dyslexia (Shanahan et al., 2006).
Rapid Automatized Naming (RAN) and Processing Speed
The term “processing “ contains components of perception, response preparation and speed motor execution (Jacobson, Ryan, Denckla, Mostofsky, & Mahone, 2013). Development of competence in reading requires not only intact word recognition but also adequate fluency (Perfetti, Marron, & Foltz, 1996), which is dependent on processing speed. However, in the context of text reading, processing speed refers to the speed with which a passage is read with reasonable accuracy (Jacobson et al., 2011), and thus includes other language competencies (e.g., access to phonological codes necessary for developing sound–symbol relationships). Additionally, during fluent passage reading, frontal networks activate to support the executive functions (i.e., controlled attention, working memory) required for the rapid selection and retrieval of orthographic symbols (Leonard et al., 2011).
The automaticity necessary for competent reading fluency is typically assessed via performance on rapid automatized naming (RAN) tasks (Norton & Wolf, 2012; Savage, 2004), with RAN contributing as much as 17% of the unique variance in reading fluency, even after controlling for basic word recognition (Arnell, Joanisse, Klein, Busseri, & Tannock, 2009). While rapid-naming deficits have been primarily observed in RD, recent investigations have identified slowed naming among children with ADHD who do not have basic reading difficulties (Rucklidge & Tannock, 2002; Wodka, Simmonds, Mahone, & Mostofsky, 2009). One hypothesis is that the “slowing” on RAN may reflect momentary inefficiency of the working memory phonological loop (Brooks, Berninger, & Abbott, 2011). Alternatively, weaknesses in executive (response) control or a failure to maintain earlier levels of automaticity may underlie this “slowing”, especially among children with ADHD (Jacobson et al., 2011).
When RAN performance is decomposed, two sources of variance have been hypothesized to contribute to the total time score: (1) time to name each item (articulation time), and (2) the duration of pauses between items (pause time; Neuhaus, Foorman, Francis, & Carlson, 2001). Among children with RD, slowed RAN performance stems primarily from increased inter-item pause times rather than from difficulties at the level of post-lexical access motor production expressed as articulation rates (Araújo et al., 2011). It is not clear whether the slowed performance on RAN (and subsequent slowing in reading fluency) in children with ADHD is due to slow access of lexicon and sound–symbol relationships or to inefficiency in response control, resulting in variability manifest in occasional (but not consistent) longer response times that contribute to a greater mean response time value. Li et al. (2009) showed that, among children with ADHD, inconsistency (variability) of RAN pause times rather than slowed responding was predictive of reading comprehension, suggesting that examination of the total time score alone may not capture the factors influencing impediments to competent reading. Clarifying the reasons behind the variability in performance among children with ADHD is important, as it points to potentially different reasons for poor RAN performance in children ADHD (without RD) and children with RD (and their respective problems with reading fluency) which could potentially lead to different types of intervention.
The Complex Relationship between Processing Speed and Variability
Intra-subject variability in response time (on externally-paced tasks) is thought to reflect within-person fluctuations in performance and is strongly linked to ADHD both at the phenotypic and genetic levels (Kuntsi & Klein, 2012). Several theories have been put forth to account for the observed intra-individual variability in ADHD, including arousal regulation, temporal processing, and anomalies in the “default-mode” network (Kuntsi & Klein, 2012). Among children with comorbid ADHD and RD, reaction time variability appears to influence reading fluency through its effects on word decoding (Tamm et al., 2014); however, the associations between variability and reading in children with ADHD (without RD) are less well understood.
Children with ADHD manifest increased variability in response time across a variety of externally-paced tasks, including choice reaction time, go/no-go, flanker, attention network, and n-back (Epstein et al., 2011; Ryan, Martin, Denckla, Mostofsky, & Mahone, 2010). This increased variability is often interpreted as being due to inefficiency in response preparation and control (Vaurio, Simmonds, & Mostofsky, 2009). Many of these investigations measured intra-subject variability using the coefficient of variation (CV), which is calculated as the standard deviation (SD)/mean of the entire distribution of response times. Assessment of the response-time distribution is complicated, however, since these distributions are typically positively skewed. Among children with ADHD, the skewing of the response-time distribution appears to be exacerbated due to the presence of more frequent “very slow” responses (interpreted as lapses of attention or lapses “off task”), which appear in the extreme tail of the distribution (Hervey et al., 2006). The frequent “lapses”, observed via intermittent long response times in these distributions, make traditional statistical analyses, based on the “Gaussian” distribution, problematic. Specifically, Gaussian analyses examining the mean and SD are reliant on a relatively normal distribution. In the case of response time distributions, both the mean and the SD are increased by the presence of occasional longer response times, which artificially increase both metrics and obscure the actual modal pattern of responding. In these situations, ex-Gaussian analyses may provide a more accurate accounting of the exponential component of the distribution (Whelan, 2008), and better separation of the reaction time (mu), variability (sigma), and atypically slow responses (tau), compared to traditional measures (Ghemlin et al., 2014).
A series of recent investigations noted that the increased response-time variability observed on computerized reaction-time tasks among children with ADHD is the result of this pattern of highly skewed responding, i.e., more frequent lapses in task performance, as manifested by the exponential component of the distribution, i.e., tau (Jacobson et al., 2013; Lee et al., 2015; Vaurio et al., 2009). A recent meta-analysis of 319 studies of reaction-time distributions concluded that children with ADHD show robustly increased variability of reaction time compared to typically developing (TD) children, which appears to often be due to a subset of atypically slow responses (captured analytically by the ex-Gaussian variable tau). When these abnormally long response times are accounted for, children with ADHD do not appear to have more consistently slow response times (Koffer et al., 2013). There is also increasing evidence that this response inconsistency captured by analysis of the ex-Gaussian distribution may represent a potential endophenotype for ADHD, as differences are also observed among unaffected siblings (Lin, Hwang-Gu, & Gau, 2015).
The majority of these studies assessed response time using paradigms (e.g., go/no-go, choice reaction time, simple reaction time) in which stimulus presentation rate was externally controlled (i.e., controlled within the task design rather than by the individual). In contrast, passage reading is typically self-paced, and there is emerging literature that has identified impairments in self-paced decoding skills among children with ADHD without RD (Stubenrauch et al., 2014). Thus, it remains unclear whether the ADHD-related increases in variability observed on externally-paced tasks would be observed on self-paced tasks (e.g., RAN, passage reading) as well, and, if so, how intermittent performance lapses (rather than consistent slowing) contribute to the efficiency of reading fluency.
The present study sought to clarify whether the patterns of variability in ADHD described in previous studies using externally-paced tasks would also be seen on self-paced tasks relevant to academic success. We used ex-Gaussian analyses to (1) examine inter-item pause time variability during rapid naming tasks in children with ADHD (without RD) and (2) determine whether variability on this self-paced naming task predicted performance on measures of reading fluency. It was hypothesized that children with ADHD would manifest increased variability in the pause time distribution due to periodic “lapses in performance” (tau) and that the degree of slowing of these lapses would predict reading fluency.
Methods
Participants
Children aged 9 to 14 years were recruited from outpatient clinics within a large developmental disabilities assessment and treatment center, as well as from local area pediatricians, local chapters of Children and Adults with ADHD (CHADD), schools, social/service organizations (e.g., Boy/Girl Scouts), and community advertisements (e.g., postings at libraries). The sample included 80 children, of whom 45 met study criteria for a diagnosis of ADHD and 35 were TD controls. Participants were screened for comorbidities commonly observed in ADHD (for details, see below). All participants and their parents signed a consent form that met Institutional Review Board standards.
Demographic information, school, and developmental histories were obtained through telephone screenings with parents of participants. Children in both the ADHD and control groups were excluded if they were identified as having a history of speech/language disorder or basic word reading or decoding difficulties, either through parental report or based on a school assessment completed within the past year. Further exclusion criteria included visual impairment or hearing loss, history of other neurological disorder, psychotropic medication use other than stimulants, or comorbid psychiatric diagnoses other than oppositional defiant disorder (ODD) or a specific phobia (which were allowed in both the ADHD and TD groups). Children included in the study were required to have estimated IQ scores of 80 or higher on the Verbal Comprehension Index (VCI) of the Wechsler Intelligence Scale for Children –Fourth Edition (WISC-IV; Wechsler et al., 2004). The VCI was selected as the inclusion measure rather than the Full Scale IQ (FSIQ) so that children whose FSIQ scores were reduced as a function of slowed performance on the Processing Speed Index were not excluded. Additionally, the VCI was chosen over the Perceptual Reasoning Index (PRI) because this is a study of a task that is highly relevant to reading speed, and because the VCI subtests are entirely untimed. Additionally, children with ADHD who were taking stimulant medication (n = 29) were voluntarily removed from the medication by the caregiver on the day prior to testing and the day testing itself.
Screening Measures
Following initial telephone screening, participants were screened for psychiatric diagnoses using a structured parent interview. The Diagnostic Interview for Children and Adolescents– Fourth Edition (DICA-IV; Reich, Welner, & Herjanic, 1997) was used. Additionally, parents and teachers of participants completed behavior rating scales, including the Conners’ Parent Rating Scale – Revised:Long (CPRS-R(L); Conners, 1997), the Conners’ Teacher Rating Scale – Revised: Long (CTRS-R(L); Conners, 1997), and the ADHD Rating Scale-IV (DuPaul, Power, Anastopoulos, & Reid, 1998). Controls with t-scores greater than 60 on either the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV; American Psychiatric Association, 2000) Inattentive or Hyperactive/Impulsive scales of the CPRS-R(L) or CTRS-R(L), or item ratings ≥2 for four or more symptoms of inattention or hyperactivity/impulsivity from the ADHD Rating Scale-IV were also excluded. The CPRS-R(L)/CTRS-R(L) and ADHD Rating Scale-IV were used to confirm ADHD diagnosis and group assignment using the following criteria: (1) positive DSM-IV ADHD diagnosis on DICA-IV and (2) t-scores ≥65 on the DSM-IV Hyperactive/Impulsive or Inattentive scales of the CPRS-R(L)/CTRS-R(L), or (3) 6 out of 9 of the DSM-IV symptoms met (i.e., item rating of 2 or 3) on the Hyperactive/Impulsive or Inattention scales of the ADHD Rating Scale-IV, Home or School version. Positive rating scale responses alone were insufficient for assignment to the ADHD group; children were required to meet ADHD diagnostic criteria on the DICA-IV, which included assessment of the pervasiveness criterion. Children with DSM-IV diagnoses other than ODD or specific phobias were excluded from both groups. Children with ODD and/or specific phobias were eligible for inclusion in the TD group, though no occurrences existed in the TD group for this sample. Additional exclusionary criteria for both groups included history of mental health services for behavior or emotional problems (other than for ADHD-related behaviors in the ADHD group), history of academic problems (other than ADHD) requiring school-based intervention services, or history of defined primary reading or language-based learning disability. Screening also included subtests of the Clinical Evaluation of Language Fundamentals – Fourth Edition (CELF-4; Semel, Wiig, & Secord, 2003); children scoring < −1.5 SDs on either the Receptive or Expressive Language composites, or < −1.0 SDs on both composites, were excluded. Additionally, children scoring less than a standard score of 85 on the Basic Reading Composite of the Woodcock–Johnson Tests of Achievement (WJ-III; Woodcock, McGrew, & Mather, 2001) were also excluded.
Performance-based assessments were completed over two days, less than one month apart. Assessments included measures of intellectual functioning, language, reading, attention, and rapid naming. On the first day, children were administered three measures of reading fluency (described below). Measures completed on the second day included the WISC-IV (Wechsler et al., 2004) and the RAN tests. Registered psychology associates, supervised by licensed psychologists, administered all study measures. Prior to participating in the study, these clinicians were trained in accurate administration, child behavior-management strategies, and motivational techniques to encourage effort. Each individual administering the RAN was requested to note any deviations from the protocol, as well as any concerns with the validity of the administration based on the obvious withdrawal of effort. No such occurrences are noted for any of the participants. It is of note that the average time to administer the RAN Letters trial was less than 30 seconds, and as such, participant cooperation throughout this task was routinely high.
Study Measures
Diagnostic Interview for Children–Fourth Edition (DICA-IV)
Parents of children deemed eligible via telephone screen were administered the DICA-IV (Reich et al., 1997), which is based on the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition, Text Revision (DSM-IV-TR; American Psychiatric Association, 2000). The DICA-IV is a semi-structured interview that is designed for determining selected current and retrospective psychiatric diagnoses. The following modules were included in the psychiatric assessment for the sample: ADHD (past and present), Conduct Disorder, Oppositional Defiant Disorder, Major Depressive Disorder (past and present), Dysthymia, Separation Anxiety Disorder, Panic Disorder, Generalized Anxiety Disorder, Specific Phobia, and Obsessive Compulsive Disorder.
Conners’ Parent Rating Scale – Revised: Long (CPRS-R(L))
The CPRS-R(L) (Conners, 1997) is a parent-report rating scale describing the child’s behavior primarily within the symptom domains characteristic of ADHD (e.g., inattentive and hyperactive-impulsive symptoms). Items are rated according to the frequency of occurrence on a four-point scale ranging from 0 (never) to 3 (almost always). The CPRS-R(L) DSM-IV symptom scales were used for screening participants (as described above) and as one component of the procedure used for diagnostic group assignment. The CPRS-R(L) N score, or the total ADHD symptoms score, was used in regression analyses as the measure of ADHD symptom severity.
ADHD Rating Scale-IV
The ADHD Rating Scale-IV (Dupaul et al., 1998) is an 18-item scale (consisting of 9 inattention items and 9 hyperactivity/impulsivity items) directly corresponding to DSM-IV-TR diagnostic criteria for ADHD, completed by parents describing the child’s behavior over the past 6 months. Responses are coded on a four-step Likert scale from not at all to very much.
Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV)
The WISC-IV (Wechsler et al., 2004) VCI served as the measure of participants’ verbally-based intellectual ability. All children included in the sample had VCI scores above 80. The VCI was used in regression analyses as the estimate for IQ.
Rapid Automatized Naming (RAN)
RAN (Wolf & Denckla, 2005) is a measure of automaticity that assesses a person’s ability to perceive a visual symbol (e.g., a letter) and then quickly and accurately retrieve it through speech. During this task, the child is presented a card with five letters (“a”, “d”, “o”, “p”, “s”), each randomly presented twice in a single row, for a total of 10 items in each row. The child is then asked to name 5 rows of these letters as quickly as possible, resulting in a total of 50 stimuli and 49 pauses, of which 45 are between-stimuli pauses and 4 are between-row pauses. Tasks such as RAN Letters indicate the efficiency with which a child integrates his or her visual and language processes. Naming speed tests, such as the RAN Letters trial, provide an excellent means of differentiating between good and poor readers (Cutting & Denckla, 2001; Semrud-Clikeman, Guy, Griffin, & Hynd, 2000). Furthermore, RAN Letters provides a high degree of test–retest reliability (two-week interval, r = 0.90; Wolf & Denckla, 2005). Therefore, the RAN Letters trial was selected as a primary measure of automaticity of retrieval for the present study.
Word Recognition and Decoding Measures
WJ-III Basic Reading
The Basic Reading Composite of the WJ-III (Woodcock et al., 2001) was used to characterize the word-recognition skills of the sample and to serve as a covariate in regression analyses. The Basic Reading standard score represents a composite of the Letter-Word Identification and Word Attack subtests. The median split-half (Spearman Brown corrected) reliability of the Basic Reading composite is 0.95.
Reading Fluency Measures
Gray Oral Reading Test – Fourth Edition (GORT-IV)
The GORT-IV (Wiederholt & Bryant, 2000) requires children to read text passages of increasing difficulty aloud with the instruction to read for comprehension. The Fluency score represents both the child’s speed of reading (rate) and accuracy (number of deviations from print) for each passage. Scaled scores were calculated for Rate and Accuracy, based upon age norms, and combined to produce the Fluency score. Test–retest reliability for the GORT-IV Fluency score is reported to be 0.93 for the age range under investigation (Wiederholt & Bryant, 2000).
WJ-III Reading Fluency
The WJ-III (Woodcock et al., 2001) Reading Fluency subtest is a timed measure of silent contextual reading fluency, requiring the child to read simple sentences silently, determine whether or not the sentence is true, and circle the appropriate corresponding letter (“T” or “F”). The total score represents the number of correct responses within a three-minute time limit, converted to an age-normed standard score. Test–retest reliability for the Reading Fluency measure is reported to be 0.94 in the age range studied (Woodcock et al., 2001).
Test of Word Reading Efficiency (TOWRE)
The TOWRE (Torgesen, Wagner, & Rashotte, 1999) is an assessment of the child’s single word reading and single pseudoword decoding isolated (non-contextual) word fluency. The child is asked to read as many individual words (Sight Word Efficiency) or non-words (Phonetic Decoding Efficiency) of increasing length and phonetic difficulty as possible in 45 seconds. Scaled scores for Sight Word Efficiency and Phonetic Decoding Efficiency represent the number of correctly read words within the time limit, relative to age norms. The TOWRE Total score is a composite of performance on both the Sight Word Efficiency and Phonetic Decoding Efficiency tasks. Test–retest reliability for the TOWRE Total score is reported to be 0.93 (Torgesen et al., 1999).
Study Procedures
Each child’s oral responses to the RAN stimuli were digitally recorded using Audacity© software. Articulation times and inter-articulation pause times were calculated for each response based on procedures adapted from Li et al. (2009). Articulation onset was marked at the point where the acoustical energy of the appropriate response exceeded the mean noise level; offset was measured at the point where acoustical energy dropped below the mean noise level. Articulation time for each individual response (from onset to offset of acoustical energy) was measured in ms. Pause time was measured as the time between two articulations (i.e., the difference, in ms, between the subsequent articulation onset and the previous articulation offset). Raters of pause and articulation times were trained to an inter-rater reliability criterion of 0.90 for the study. The CV was calculated as the SD/mean of the pause time distribution. For the current investigation, a slightly different analysis method was employed than that of Li et al. (2009) such that inter-stimulus pause time distributions (excluding the four between-row pauses) were analyzed using Gaussian (i.e., mean, SD, CV) and ex-Gaussian (mu, sigma, tau) methodology. Ex-Gaussian variables were extracted using MATLAB and the EGFit toolkit (Lacouture & Cousineau, 2008).
Data Analyses
The three reading fluency scores (GORT-IV, TOWRE, and WJ-III) were converted into age-based standard scores, which were subsequently combined into a single global reading fluency composite score for the purposes of regression analyses. The composite fluency score was calculated as the mean of all three standard score measures for each participant. Group differences on demographic and reading variables were examined using a one-way analysis of covariance (ANCOVA), controlling for VCI. Inter-item RAN pause time distributions were examined on the RAN Letters trial using both Gaussian (mean, SD, CV) and ex-Gaussian (mu, sigma, tau) methodology, as described above. Group differences in these inter-item pause time variables were also examined using ANCOVAs (covarying for VCI). Hierarchical regression analyses were then used to examine the predictions of the reading fluency composite scores from each of the six RAN inter-item pause variables, controlling for the WISC-IV VCI, WJ-III Basic Reading composite, and ADHD symptom severity (CPRS-R(L) DSM-IV Total score).
Results
Participants
Detailed demographic information and reading performance for sample participants is shown in Table 1. The sample consisted of 45 children with ADHD and 35 TD children in a comparison group, aged 9 to 14 years (mean = 11.26 ± 1.54). The racial composition of the sample was as follows: Caucasian 69%, African-American 19%, multi-racial 6%, Asian 5%, and Pacific Islander 1%. One participant reported Hispanic ethnicity. The ADHD group was comprised of 26 boys and 19 girls, and the TD group was comprised of 14 boys and 21 girls. Only 1 participant in the sample (a boy in the ADHD group) had comorbid diagnoses of oppositional defiant disorder (ODD) and a specific phobia. There were no significant differences in sex distribution between the ADHD and TD groups, χ2(1) = 2.489, p = .176. Additionally, there were no significant differences between the groups in age, F(1, 78) = 0.062, p = .804, η2p = .001, or socioeconomic status (SES), as determined by calculation of the Hollingshead Index, F(1, 78) = 0.004, p = .947, η2p < .001. However, consistent with a recently published meta-analysis examining the effects of ADHD-related symptomatology on test-taking behavior (Jepsen, Fagerlund, & Mortensen, 2009), the TD children had a significantly higher IQ (as measured by the WISC-IV VCI) than the (unmedicated) children with ADHD, F(1, 78) = 8.48, p = .005, η2p = .098. As such, the VCI was used as a covariate in all subsequent analyses, and age was used as a covariate for group comparisons for mean pause time (MPT), SD, CV, mu, sigma, and tau.
Table 1.
Demographic Information and Reading Performance.
| ADHD (n = 45)
|
TD (n = 35)
|
|||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | p | η2p | |
| Age | 11.22 | 1.64 | 11.31 | 1.41 | .804 | .001 |
| WISC-IV VCI | 102.51 | 13.03 | 111.14 | 13.31 | .005 | .098 |
| SES (Hollingshead) | 40.13 | 21.15 | 39.79 | 23.58 | .947 | <.001 |
| WJ-III BR SSa | 103.93 | 10.30 | 112.76 | 9.80 | .006 | .098 |
| GORT-IV Fluency SSa | 102.44 | 17.76 | 118.57 | 14.58 | .001 | .125 |
| WJ-III RF SSa | 97.60 | 20.21 | 108.94 | 26.23 | .043 | .053 |
| TOWRE Total SSa | 103.49 | 13.44 | 111.26 | 23.51 | .003 | .112 |
| RAN Letters SSa | 98.47 | 13.79 | 110.49 | 11.81 | <.001 | .179 |
| CPRS t-score | 69.90 | 18.44 | 45.63 | 10.68 | <.001 | .375 |
Note. ADHD = attention-deficit/hyperactivity disorder; BR = Basic Reading; CPRS = Conners’ Parent Rating Scale –Revised:Long (ADHD Total – Scale N[]); GORT-IV = Gray Oral Reading Test – Fourth Edition; RAN = rapid automatized naming; RF = Reading Fluency; SES = socioeconomic status; SS = standard score; TD = typically developing; TOWRE = Test of Word Reading Efficiency; VCI = Verbal Comprehension Index; WISC-IV = Wechsler Intelligence Scale for Children – Fourth Edition; WJ-III = Woodcock–Johnson Tests of Achievement – Third Edition.
Comparisons are calculated after covarying for the WISC-IV VCI.
RAN Performance
The results of the traditional Gaussian and ex-Gaussian analyses of the RAN Letters trial are listed in Table 2. After controlling for VCI, the children with ADHD showed significantly reduced (slower) performance on the RAN Letters trial (standard score) compared to the TD children, F(1, 75) = 16.34, p < .001, η2p = .18. Using traditional Gaussian analyses (i.e., examining the mean and SD of inter-item pause times) and controlling for VCI and age, the ADHD group had significantly greater overall MPTs (p = .002) and SDs (p = .005) compared to the TD group, but not CV (p = .448).
Table 2.
Group Differences on the RAN Letters Pause Time Variables.
| ADHD (n = 45)
|
TD (n= 35)
|
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | η2p | |
| MPT | 0.160 | 0.093 | 0.101 | 0.056 | .002 | .115 |
| mu | 0.020 | 0.041 | 0.019 | 0.029 | .920 | <.001 |
| SD | 0.220 | 0.148 | 0.130 | 0.097 | .005 | .101 |
| CV | 1.379 | 0.451 | 1.281 | 0.528 | .448 | .008 |
| sigma | 0.010 | 0.022 | 0.009 | 0.012 | .656 | .003 |
| tau | 0.142 | 0.096 | 0.081 | 0.048 | .002 | .118 |
Note. ADHD = attention-deficit/hyperactivity disorder; CV = coefficient of variation (SD/mean); MPT = mean pause time (ms); SD = standard deviation; TD = typically developing. All group comparisons are made after covarying for the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV) Verbal Comprehension Index (VCI) and age.
Group comparisons were made for the ex-Gaussian measures (mu, sigma, tau) after covarying for VCI and age. Analyses of inter-item pause times using ex-Gaussian methods showed a different pattern of performance. There were no significant group differences observed in the mean (mu; p = .920) or the SD (sigma; p = .656) of the normal component of the distribution. Conversely, the exponential component of the distribution (tau) was significantly greater in the ADHD group compared to the TD group (p = .002), suggesting that significant group differences observed in overall MPT were driven by periodic longer pauses represented in the tail of the distribution (tau). These group comparisons were also made using transformed pause time variables (SD transformation), and the pattern of results was exactly the same (i.e., significant group differences for MPT, SD, and tau).
Basic Reading
After controlling for VCI, the children with ADHD showed significantly lower mean scores for basic reading skills (WJ-III Basic Reading) compared to the TD children, F(1, 73) = 7.92, p = .002, η2p = 0.098.
Reading Fluency
After controlling for VCI, the children with ADHD showed significantly lower mean scores than the TD children on silent reading fluency (WJ-III Reading Fluency), F(1, 76) = 4.25, p = .043, η2p = 0.053, contextual oral reading fluency (GORT-IV Fluency), F (1, 76) = 10.98, p = .001, η2p= 0.125, and non-contextual oral fluency (TOWRE), F(1, 76) = 9.58, p = .003, η2p= 0.112.
A series of hierarchical linear regression analyses were employed to examine the contribution of the Gaussian (MPT, SD, CV) and ex-Gaussian (mu, sigma, tau) measures of pause-time distribution to reading fluency after controlling for verbal reasoning (WISC-IV VCI), basic reading (WJ-III Basic Reading composite), ADHD symptom severity (CPRS-R DSM-IV Total) and age (see Table 3). MPT and SD were significant predictors of performance on the reading fluency composite (both values of p < .001), while CV was not a significant predictor of reading fluency (p = .067). Examining the ex-Gaussian measures, only tau was observed to be a significant predictor of reading fluency (p < .001), explaining approximately 8% of the unique variance in reading fluency over and above that accounted for by verbal reasoning, basic word-recognition skills, ADHD symptom severity and age (see Figure 1).
Table 3.
Hierarchical Regression Predicting the Reading Fluency Composite Scores.
| RAN Variable | β | ΔR2 | p |
|---|---|---|---|
| Mean Pause Time (MPT) | −.302 | .073 | <.001 |
| Standard Deviation (SD) | −.323 | .091 | <.001 |
| CV | −.140 | .019 | .067 |
| mu | .025 | .001 | .741 |
| sigma | .064 | .004 | .406 |
| tau | −.322 | .081 | <.001 |
Note. In each of the six regression analyses, the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV) Verbal Comprehension Index (VCI), Woodcock–Johnson Tests of Achievement – Third Edition (WJ-III) Basic Reading Standard Score, ADHD Symptom Severity, i.e., Conners’ Parent Rating Scale –Revised:Long (CPRS-R(L)) Scale N: DSM-IV Total Scale, and age were entered on the first step, followed by the RAN variable on the second step. In each analysis, the first step accounted for 59% of the variance in the Reading Fluency Composite. CV = coefficient of variation (SD/mean).
Figure 1.
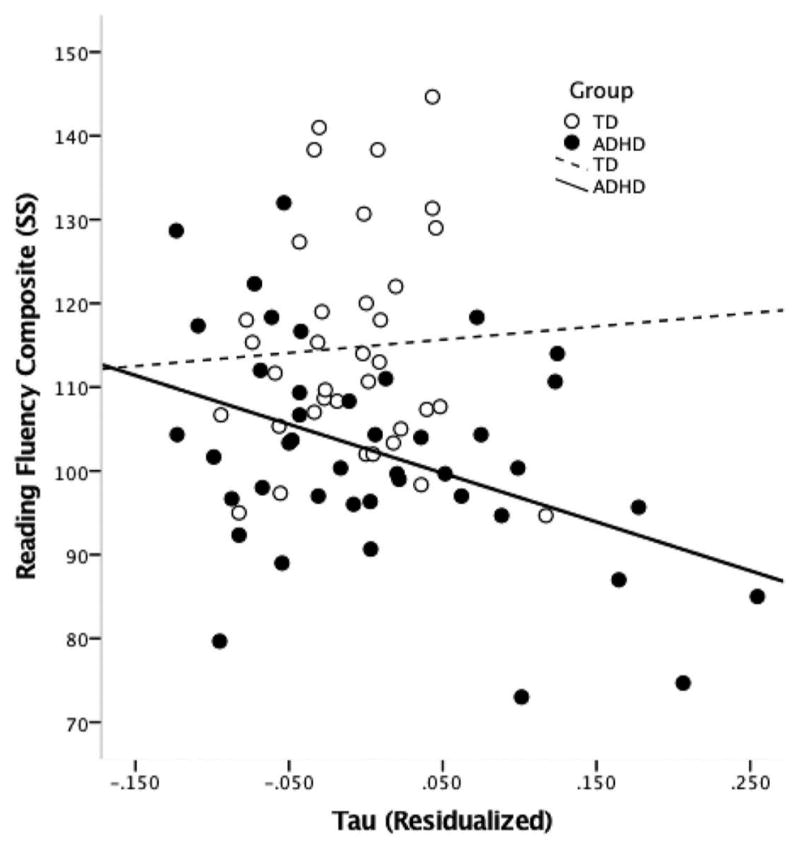
Association between tau (residualized to control for VCI, ADHD symptoms, basic reading, and age) and reading fluency composite in ADHD (R2 = .16) and TD groups (R2 = .01).
Additional exploratory regression analyses were used within each group to determine whether patterns of association between tau and reading fluency differ between children with ADHD and TD children. Indeed, the significant association between tau and reading fluency was driven primarily by the correlation in the ADHD group, wherein tau was significantly associated with reading fluency, ΔR2 = .162, p < .001. In contrast, within the TD group, tau was not significantly associated with reading fluency, ΔR2 = .015, p = .318.
Discussion
The present study represents one of the first investigations to demonstrate that the lapses that are characteristic of the performance of children with ADHD on externally controlled reaction-time tasks are also evident on self-paced rapid naming tasks. These findings indicate that, among carefully-screened children with ADHD and TD children, the children with ADHD were substantially more variable than the TD children with regard to inter-item pauses on a rapid letter-naming task. Even after controlling for basic word-recognition skills, verbal ability, symptom severity and age, variability in inter-item pause time was a significant predictor of performance on reading fluency tasks, especially among children with ADHD, for whom tau accounted for 16% of unique variance in reading fluency.
An examination of the inter-item pause times via both Gaussian and ex-Gaussian analyses revealed longer MPTs and greater variability (SD, tau) in children with ADHD. Specifically, the MPT for the TD children was 0.10 ms (± 0.06 ms) and the MPT for the ADHD group was 0.16 ms (± 0.06 ms). Using a standard of an MPT of greater than +1 SD for the TD group mean (i.e., > 0.16 ms) to indicate a “longer than average” pause time, 14 of the TD children (40%) had longer than average pause times, while 30 of the children with ADHD (67%) had longer than average pause times. In “real-life” terms, the longer pauses on self-generated rapid naming tasks are represented in portions of ms—largely undetectable to the examiner or the child—but are nevertheless related to the relative cognitive inefficiency that adversely affects reading fluency in children with ADHD.
Moreover, the increased variability captured by tau suggests that group differences in the MPT and SD are likely driven by these brief, intermittent “lapses” in performance or periodic longer response latencies (tau) rather than by consistently slowed performance across the course of the naming task. Supporting this interpretation, and consistent with the findings of the Koffer et al. (2013) meta-analysis, neither mu nor sigma was elevated, suggesting that the children with ADHD were not actually slower to respond (i.e., slower to name letters) or more variable within the normal component of the response distribution. As such, the results indicate that the increased naming-speed times observed in children with ADHD appear to be driven by occasional lapses in consistent performance during word retrieval and not by overall slowing per se. Unlike children with RD, for whom slow RAN performance is considered to be (at least partly) a function of slower access to phonological codes (Hulme & Snowling, 1992), we believe that the slowing on RAN tests in children with ADHD is more likely due to inefficiency in top-down response control, reflected in the occasional very long response times captured by the tau variable in the ex-Gaussian analyses. These findings highlight the importance of using ex-Gaussian analytical methods to examine patterns of inconsistent responding on self-paced tasks, as well as on externally-controlled computer-administered reaction-time tasks (go/no-go, simple reaction time, choice reaction time, flanker).
These findings further imply that the isolated (relatively) longer response times (i.e., inter-item pauses) apparent in the performance of children with ADHD on both externally-controlled and self-paced tasks may reflect performance lapses that are believed to be associated with inefficient response control and/or poor allocation of controlled effort. This association parallels that seen when considering the lapses observed in externally-controlled tasks, which have been linked to anomalous development and the use of both prefrontal and premotor brain regions responsible for response preparation and execution in ADHD, as these areas have been implicated in timing and automaticity of responding (Mahone et al., 2011). Other frontal-subcortical circuits (i.e., oculomotor-caudate) have also been implicated in children with ADHD, based on observations of increased response variability on reflexive saccade tasks (Mahone, Mostofsky, Lasker, Zee, & Denckla, 2009). Additional research will be required to better clarify the nature of these interrelated pathways and their role in the shared functional impairments underlying ADHD and reading.
Regardless, it is becoming clear from an increasing number of studies examining response control (e.g., Jacobson et al., 2013; Stubenrauch et al., 2014) that the presence of these performance lapses observed in children with ADHD places them at increased risk for failure on both core reading-related tasks and on more complex tasks requiring longer periods of controlled and sustained performance within the classroom. That is, controlled attention and processing speed underlie basic decoding and reading fluency skills, and these skills in turn support the more difficult tasks of reading comprehension and writing. Core weaknesses in allocation of attention and response control put children with ADHD at risk of experiencing difficulties at school, particularly on tasks requiring periods of sustained effort, rapid responding, and working memory. While controlled variation of stimulus presentation rate (i.e., “jittering”) on externally controlled tasks appears to reduce the presence of performance lapses in children with ADHD (Lee et al., 2015), it remains a challenge to identify non-pharmacological interventions designed to produce similar normalization of performance for self-paced tasks (i.e., passage reading).
While the present findings are specific to rapid letter naming (and perhaps more directly applicable to reading), a series of prior investigations has also identified rapid color-naming deficits among children with ADHD, wherein color-naming deficits were more pronounced than deficits in letter naming (Ghelani, Sidhu, Jain, & Tannock, 2004). Moreover, treatment with methylphenidate has been shown to improve (but not normalize) color-naming deficits among children with ADHD (Bedard, Ickowicz, & Tannock, 2002; Tannock, Martinussen, & Frijters, 2000). One hypothesis for the relative impairment in color naming is that letter naming continues to be “practiced” in school, and thus becomes part of continually updated stimulus–response repertoires (or even habits). Conversely, color naming drops out of academic daily practice and remains a more novel challenge to efficient response preparation (Li et al., 2009). Given these considerations, the present findings of ADHD-specific performance lapses in letter naming are even more striking and suggest a unique cognitive mechanism that can potentially interfere with the development of reading competence.
The strengths of the current study include the use of a carefully-screened sample of children with and without ADHD, as well as the separation of their responses on a rapid naming task into pause and articulation times, enabling careful examination of performance at the individual item level. This conservative approach to participant screening also represents a limitation of the study. As a consequence of the comorbidities excluded (especially language disorders and word-reading difficulties), the sample may not fully represent the full spectrum of youth with ADHD in the community, many of whom have complex patterns of comorbidities. Nevertheless, these data represent an important extension of earlier work examining the reaction-time distribution in ADHD (Li et al., 2009) using a new cohort. At the same time, given the relatively small size of the current sample and the generally above-average cognitive ability levels within the TD group, these results need to be replicated in a larger sample.
Acknowledgments
A portion of this study was presented at the Fortieth Annual North American Meeting of the International Neuropsychological Society in Waikoloa, Hawaii on February 7, 2013.
Funding
This work was supported by the National Institutes of Health [P50 HD 52121, U54 HD 079123] and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program [UL1 RR025005].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
E. Mark Mahone http://orcid.org/0000-0002-5022-1499
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. Text Revision. [Google Scholar]
- Araújo S, Inácio F, Francisco A, Faísca L, Petersson KM, Reis A. Component processes subserving rapid automatized naming in dyslexic and non-dyslexic readers. Dyslexia. 2011;17:242–255. doi: 10.1002/dys.433. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Joanisse MF, Klein RM, Busseri MA, Tannock R. Decomposing the relation between rapid automatized naming (RAN) and reading ability. Canadian Journal of Experimental Psychology. 2009;63:173–184. doi: 10.1037/a0015721. [DOI] [PubMed] [Google Scholar]
- Bedard A, Ickowicz A, Tannock R. Methylphenidate improves Stroop naming speed, but not response interference, in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2002;12:301–309. doi: 10.1089/104454602762599844. [DOI] [PubMed] [Google Scholar]
- Brooks AD, Berninger VW, Abbott RD. Letter naming and letter writing reversals in children with dyslexia: Momentary inefficiency in the phonological and orthographic loops of working memory. Developmental Neuropsychology. 2011;36:847–868. doi: 10.1080/87565641.2011.606401. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales –Revised. North Tonawanda, New York: Multi-Health Systems; 1997. [Google Scholar]
- Couto JM, Gomez L, Wigg K, Ickowicz A, Pathare T, Malone M, … Barr CL. Association of attention-deficit/hyperactivity disorder with a candidate region for reading disabilities on chromosome 6p. Biological Psychiatry. 2009;66:368–375. doi: 10.1016/j.biopsych.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting LE, Denckla MB. The relationship of rapid automatized naming and word reading in normally developing readers. Reading and Writing: An Interdisciplinary Journal. 2001;14:673–705. doi: 10.1023/A:1012047622541. [DOI] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. New York, NY: The Guilford Press; 1998. [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Antonini TN, … Altaye M. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelani K, Sidhu R, Jain U, Tannock R. Reading comprehension and reading related abilities in adolescents with reading disabilities and attention-deficit/hyperactivity disorder. Dyslexia. 2004;10:364–384. doi: 10.1002/dys.285. [DOI] [PubMed] [Google Scholar]
- Ghemlin D, Fuermaier ABM, Walther S, Debelak R, Rentrop M, Westermann C, Weisbrod M. Intraindividual variability in inhibitory function in adults with ADHD –Anex-Gaussian approach. PLoS One. 2014 doi: 10.1371/journal.pone.0112298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko EL. Commentary: Translating quantitative genetics into molecular genetics: Decoupling reading disorder and ADHD – reflections on Greven et al. and Rosenberg et al. (2012) Journal of Child Psychology and Psychiatry. 2012;53:252–253. doi: 10.1111/j.1469-7610.2011.02524.x. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Arnold LE, Conners CK, … Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychology. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Hulme C, Snowling M. Deficits in output phonology: An explanation of reading failure? Cognitive Neuropsychology. 1992;9:47–72. doi: 10.1080/02643299208252052. [DOI] [Google Scholar]
- Jacobson LA, Ryan M, Denckla MB, Mostofsky SH, Mahone EM. Performance Lapses in Children with Attention-Deficit/Hyperactivity Disorder Contribute to Poor Reading Fluency. Archives of Clinical Neuropsychology. 2013;28:672–683. doi: 10.1093/arclin/act048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LA, Ryan M, Martin RB, Ewen J, Mostofsky SH, Denckla MB, Mahone EM. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychology. 2011;17:209–224. doi: 10.1080/09297049.2010.532204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen JRM, Fagerlund B, Mortensen EL. Do attention deficits influence IQ assessment in children and adolescents with ADHD? Journal of Attention Disorders. 2009;12:551–562. doi: 10.1177/1087054708322996. [DOI] [PubMed] [Google Scholar]
- Koffer MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, Kolomeyer EG. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Review. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Klein C. Intraindividual variability in ADHD and its implications for research of causal links. Current Topics in Behavioral Neurosciences. 2012;9:67–91. doi: 10.1007/7854_2011_145. [DOI] [PubMed] [Google Scholar]
- Lacouture Y, Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology. 2008;4:35–45. [Google Scholar]
- Lee RWY, Jacobson LA, Pritchard AE, Ryan MS, Yu Q, Denckla MB, … Mahone EM. Jitter reduces response-time variability in ADHD: An ex-Gaussian analysis. Journal of Attention Disorders. 2015;19:794–804. doi: 10.1177/1087054712464269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Low P, Jonczak EE, Schmutz KM, Siegel LS, Beaulieu C. Brain anatomy, processing speed, and reading in school-age children. Developmental Neuropsychology. 2011;36:828–846. doi: 10.1080/87565641.2011.606398. [DOI] [PubMed] [Google Scholar]
- Li JJ, Cutting LE, Ryan M, Zilioli M, Denckla MB, Mahone EM. Response variability in rapid automatized naming predicts reading comprehension. Journal of Clinical and Experimental Neuropsychology. 2009;31:877–888. doi: 10.1080/13803390802646973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Hwang-Gu SL, Gau SSF. Intra-individual reaction time variability based on ex-Gaussian distribution as a potential endophenotype for attention-deficit/hyper-activity disorder. Acta Psychiatrica Scandinavica. 2015;132:39–50. doi: 10.1111/acps.12393. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Mostofsky SH, Lasker AG, Zee D, Denckla MB. Oculomotor anomalies in attention-deficit/hyperactivity disorder: Evidence for deficits in response preparation and inhibition. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:749–756. doi: 10.1097/CHI.0b013e3181a565f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Ranta ME, Crocetti D, O’Brien JW, Kaufmann WE, Denckla MB, Mostofsky SH. Comprehensive examination of frontal regions in boys and girls with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2011;17:1047–1057. doi: 10.1017/S1355617711001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus G, Foorman BR, Francis DJ, Carlson CD. Measures of information processing in rapid automatized naming (RAN) and their relation to reading. Journal of Experimental Child Psychology. 2001;78:359–373. doi: 10.1006/jecp.2000.2576. [DOI] [PubMed] [Google Scholar]
- Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: Implications for understanding and treatment of reading disabilities. Annual Review of Psychology. 2012;63:427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Marron MA, Foltz PW. Sources of comprehension failure: Theoretical perspectives and case studies. In: Cornoldi C, Oakhill J, editors. Reading comprehension difficulties: Processes and intervention. Mahwah, NJ: Lawrence Erlbaum; 1996. pp. 137–165. [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. North Towanda Falls, NY: Multi-Health Systems; 1997. [Google Scholar]
- Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: Effects of reading difficulties and gender. Journal of Child Psychology and Psychiatry. 2002;43:988–1003. doi: 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Ryan M, Martin R, Denckla MB, Mostofsky SH, Mahone EM. Interstimulus jitter facilitates response control in children with ADHD. Journal of the International Neuropsychological Society. 2010;16:388–393. doi: 10.1017/S1355617709991305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage R. Motor skills, automaticity, and developmental dyslexia: A review of the research literature. Reading and Writing: An Interdisciplinary Journal. 2004;17:301–324. doi: 10.1023/B:READ.0000017688.67137.80. [DOI] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 4. Toronto, Canada: The Psychological Corporation/A Harcout Assessment Company; 2003. ((CELF-4)) [Google Scholar]
- Semrud-Clikeman M, Guy K, Griffin JD, Hynd GW. Rapid naming deficits in children and adolescents with reading disabilities and attention deficit hyperactivity disorder. Brain and Language. 2000;74:70–83. doi: 10.1006/brln.2000.2337. [DOI] [PubMed] [Google Scholar]
- Sexton CC, Gelhorn HL, Bell JA, Classi PM. The co-occurrence of reading disorder and ADHD: Epidemiology, treatment, psychosocial impact, and economic burden. Journal of Learning Disabilities. 2012;45:538–564. doi: 10.1177/0022219411407772. [DOI] [PubMed] [Google Scholar]
- Shanahan MA, Pennington BF, Yerys BE, Scott A, Boada R, Willcutt RG, … DeFries JC. Processing speed deficits in attention deficit/hyperactivity disorder and reading disability. Journal of Abnormal Child Psychology. 2006;34:585–602. doi: 10.1007/s10802-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Stubenrauch C, Freund J, Alecu De Flers S, Scharke W, Braun M, Jacobs AM, Konrad K. Nonword reading and Stroop interference: differentiates attention-de What dificit/hyperactivity disorder and reading disability? Journal of Clinical and Experimental Neuropsychology. 2014;36:244–260. doi: 10.1080/13803395.2013.878690. [DOI] [PubMed] [Google Scholar]
- Tamm L, Epstein JN, Denton CA, Vaughn AJ, Peugh J, Willcutt EG. Reaction time variability associated with reading skills in poor readers with ADHD. Journal of the International Neuropsychological Society. 2014;20:292–301. doi: 10.1017/S1355617713001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock R, Martinussen R, Frijters J. Naming speed performance and stimulant effects indicate effortful, semantic processing deficits in attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:237–252. doi: 10.1023/A:1005192220001. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency. Austin, TX: PRO-ED; 1999. [Google Scholar]
- Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler DL, Kaplan E, Fein D, Kramer JH, Morris R, Delis DC. Wechsler Intelligence Scale for Children –Fourth Edition: Integrated, technical and interpretive manual. San Antonio, TX: Harcourt Assessment; 2004. [Google Scholar]
- Whelan R. Effective analysis of reaction time data. The Psychological Record. 2008;58:475–482. [Google Scholar]
- Wiederholt L, Bryant B. Examiner’s manual: Gray oral reading test. 4. Austin, TX: PRO-ED; 2000. [Google Scholar]
- Willcutt EG, Pennington BF, Duncan L, Smith SD, Keenan JM, Wadsworth S, … Olson RK. Understanding the complex etiologies of developmental disorders: Behavioral and molecular genetic approaches. Journal of Developmental & Behavioral Pediatrics. 2010;31:533–544. doi: 10.1097/DBP.0b013e3181ef42a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodka EL, Simmonds DJ, Mahone EM, Mostofsky SH. Moderate variability in stimulus presentation improves motor response control. Journal of Clinical and Experimental Neuropsychology. 2009;31:483–488. doi: 10.1080/13803390802272036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Denckla MB. Examiner’s manual: The rapid automatized naming and rapid alternating stimulus tests. Austin, TX: PRO-ED; 2005. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson –III. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]


