Abstract
Background
The clinical significance of incidental thyroid abnormalities discovered in 18F-FDG PET/CT (FDG PET/CT) studies remains controversial. The objective of this large retrospective study was to a) determine the prevalence of focal FDG thyroid uptake on whole body FDG PET/CT studies performed for non-thyroid cancers and b) to test whether intense focal FDG thyroid uptake is associated with malignancy.
Methods
A total of 11,921 FDG PET/CT studies in 6,216 patients performed at our institution between 01/2012 and 12/2014 were analyzed. We retrospectively reviewed the medical records of these patients. Eight hundred forty five/6,216 patients (13.6%) had a thyroid incidentaloma based on the clinical 18FDG PET/CT report. One hundred sixty/845 (18.9%) of these underwent ultrasound and 98 of these (61.3%) had a fine needle aspiration (FNA). Twenty-six of these 98 (26.5%) patients underwent thyroidectomy. Thyroid lesion and background SUV's for each patient were measured upon review of the 18FDG PET/CT study. We measured SUVmax, thyroid to background (), thyroid to blood pool () and thyroid to liver () ratios in benign and malignant lesions. Receiver operating curves (ROC) were calculated to determine optimal cut off values between malignant and benign lesions
Results
Twenty-one of the 98 patients who underwent FNA biopsy or thyroidectomy had malignant disease (21.4%). Malignant lesions had significantly higher thyroid lesion SUVmax, , , and than benign nodules. The ROC derived cut off ratio of >2.0 differentiated benign from malignant lesions best with a specificity and sensitivity of 0.76 and 0.88, respectively.
Conclusions
The incidence of malignancy in biopsied focal hypermetabolic thyroid lesions is 21.4%. Lesions on FDG PET/CT studies, exhibiting a ratio , warrant further work up with ultrasound and FNA to exclude malignancy.
Keywords: Thyroid, Incidental findings, PET, FDG
Introduction
18F-FDG PET/CT (FDG PET/CT) imaging is used for initial and subsequent treatment strategy decisions across a variety of cancers (1-3). Whole body imaging is associated with a relatively high number of incidental findings (4, 5) including hypermetabolic thyroid nodules. Thyroid incidentalomas, defined as thyroid lesions identified during an imaging study such as a computed tomography (CT), magnetic resonance imaging (MRI) or PET/CT studies for evaluating non-thyroid disease (6, 7) occur with frequencies of 1-4% (8) (Table 1). FDG uptake in the thyroid can be either focal or diffuse. Whereas diffuse uptake has been widely ascribed to benign conditions such as thyroiditis or Grave's disease (9, 10), focal uptake has been associated with both benign and malignant processes (9). The reported incidence of malignancies in focal hypermetabolic thyroid incidentalomas range from 14-81% (8) (Table 1). Whether semi-quantitative indices of FDG thyroid uptake can predict malignancy remains controversial (8) (Table 1). Thus, the clinical relevance of incidentally detected focal thyroidal FDG uptake remains unclear
Table 1.
Malignancy rate of FDG PET/CT Thyroid Incidentalomas
| First Author | Year | Number of patients | Thyroid Abnormalities* | FNA Biopsy Patients | Number of malignant findings** | SUV significance present (Y, N, NP)*** |
|---|---|---|---|---|---|---|
| Chen | 2009 | 2,549 | 99 (3.8%) | 11 (11.1%) | 7 (63.6%) | N |
| Ho | 2011 | 5,877 | 220 (3.7%) | 55 (25.0%) | 8 (14.5%) | Y |
| Yaylali | 2014 | 2,000 | 57 (2.9%) | 20 (35.1%) | 7 (35.0%) | N |
| Chun | 2015 | 2,584 | 52 (2.0%) | 36 (69.2%) | 15 (41.7%) | Y |
| Stangierski | 2014 | 5,520 | 122 (2.2%) | 82 (67.2%) | 19 (23.1%) | Y |
| Brindle | 2014 | 7,221 | 156 (2.1%) | 30 (19.2%) | 7 (23.3%) | N |
| Lee | 2014 | 2,368 | 64 (2.7%) | 27 (42.2%) | 11 (40.7%) | N |
| Kim | 2013 | 22,674 | 433 (2.1%) | 280 (58.0%) | 68 (24.3%) | NP |
| Bertagna | 2013 | 49,519 | 729 (1.5%) | 211 (28.9%) | 72 (28.9%) | Y |
| Yang | 2012 | 15,948 | 395 (2.5%) | 53 (13.4%) | 43 (81.1%) | Y |
| Bonabi | 2012 | 3,062 | 73 (2.4%) | 58 (79%) | 14 (23.8%) | N |
Thyroid abnormalities were defined by focal or diffuse FDG uptake
Percentage is based on the number of FNA biopsies, not total number of patients
Y = Yes, N= No, NP = Not Performed
The aim of this study was to determine the prevalence of malignancy in patients with incidentally detected focally increased thyroid FDG uptake. Additionally, we attempted to correlate the intensity of thyroid FDG uptake with the histopathological findings. Finally, we performed a ROC curve analysis to identify an optimal threshold for various SUV parameters to separate malignant from benign lesions.
Materials and Methods
Patient Population
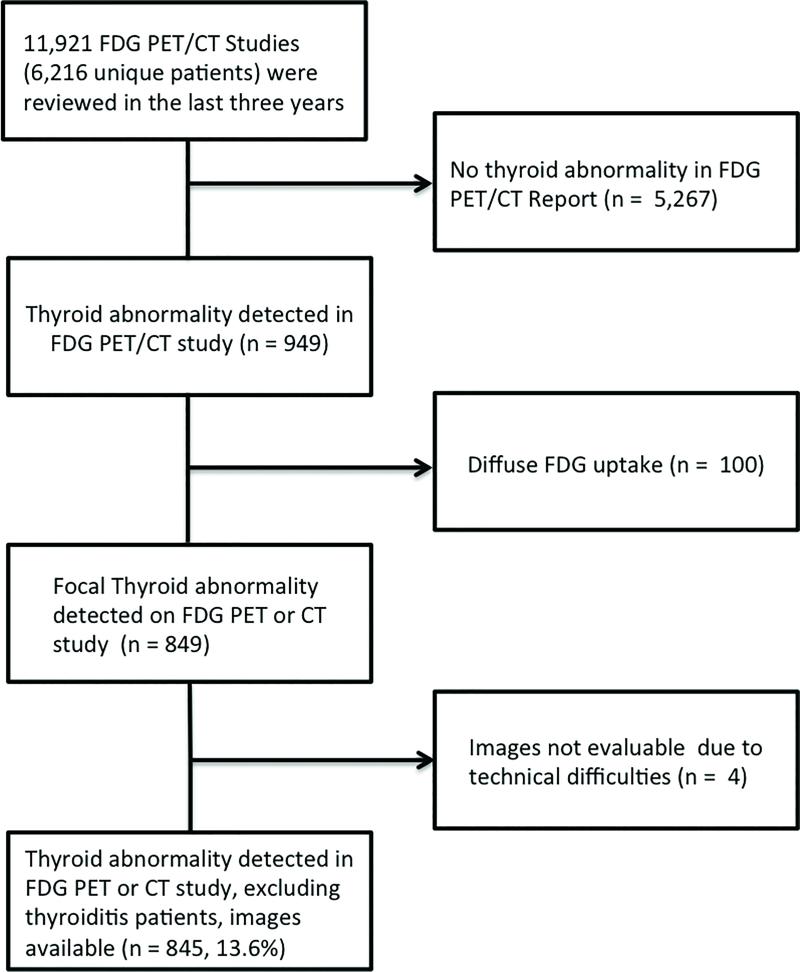
The clinical reports of 11,921 FDG PET/CT studies acquired in 6,216 patients between 01/2012 and 12/2014 were reviewed (Figure 1). Incidental abnormalities defined as an unexpected abnormality in the thyroid based on the CT and/or PET findings (Figure 2) were recorded. Nine hundred forty nine patients meeting these criteria were identified. Patients who exhibited diffusely increased FDG uptake throughout the thyroid gland were excluded (n = 100). An additional 4 patients were excluded because the images were no longer available on the hospital information system. Thus, 845 patients with a thyroid incidentaloma were analysed (Figure 1). Using this information, the prevalence of an incidental thyroid abnormality was calculated. Six hundred two/845 (71.2%) thyroid lesions showed a nodule on CT, but were FDG negative, while 243/845 (28.8%) exhibited a nodule on CT and were FDG positive.
Figure 1.
Flow diagram for the patient population selection
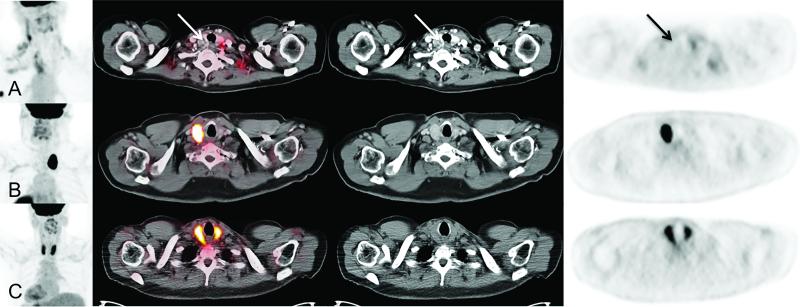
Figure 2.
Examples of thyroid incidentalomas. Row (A) represents a left thyroid lobe lesion seen on CT, but exhibits no FDG uptake (Arrow). Row (B) exhibits an intensely hypermetabolic right thyroid lobe lesion. Row (C) demonstrates diffuse intense FDG uptake throughout the thyroid gland.
Patient Characteristics
The medical records for each patient were reviewed. When available, demographics, ultrasound (US), and pathological reports were collected. Patients were grouped into two cohorts. In Group A, an US was recommended in the PET/CT report to further characterize the thyroid incidentaloma (n =275). In Group B, no such recommendation was made (n = 570). Supplemental Figure 1 represents the flow chart for data analysis.
For interpretation of the FNA all available cytologic reports were independently reviewed by a pathologist (M.F.P.D.). Patients were stratified based on the cytologic and histopathological reports into two groups: patients whose report (positive) suggested further intervention (such as rebiopsy/thyroidectomy), and patients who needed no further follow up (negative). If the patient underwent thyroidectomy (independently whether suggested or not according to the cytologic report), the pathology report was also extracted
Image Acquisition
Emission scans started at 60 ± 10 minutes tracer uptake time and routine PET and CT acquisition and reconstruction algorithms were used (8). All PET/CT studies were conducted using the Siemens mCT or Biograph 64. The image acquisition protocol was reported before (25). In brief, after a minimum fasting period of 4 hours serum glucose levels were below 180 mg/dL in all patients. Patients received 0.21 mCi/kg of 18F-FDG-PET intravenously. PET emission scans were acquired with a weight-based protocol and during shallow breathing as reported previously (27). PET images were reconstructed with an iterative algorithm (OSEM; 2 iterations, 8 subsets). A standard CT protocol was used as previously reported (26). The CT images were reconstructed using filtered back projection at 3.4 mm axial intervals to match the slice separation of the PET data. A standard CT-based algorithm was used for attenuation correction (26).
Image analysis
Several semiquantitative values were explored for their ability to separate malignant from benign lesions. SUVmean/max of the thyroid incidentaloma, as well as the reference tissue SUV (thyroid background, bloodpool, and liver FDG uptake) were measured and recorded. The SUVmax was defined as the metabolically most active pixel in the thyroid incidentaloma, whereas the SUVmean was defined as the average of pixels defined in a 1 cm diameter circular ROI around the hottest pixel. Additionally, we classified the degree of metabolic activity from the PET/CT images into six categories as shown in Supplemental Table 1: no uptake (< blood pool SUVmean or normal thyroid background SUVmean, respectively), mild (= blood pool SUVmean), mild to moderate (> blood pool SUVmean & < liver SUVmean), moderate (= liver SUVmean), moderate to intense (> liver SUVmean & < liver SUVmean × 1.5), and intense (> liver SUV × 1.5). Additionally, the following ratios were defined: , , and . The purpose of these measurements was to determine if a ratio would better predict pathology findings than absolute SUVs. The degree of FDG uptake (SUV measurements) was compared to cytology and histopathology by using an ROC analysis approach.
Statistical analysis
All statistical tests were performed using using MATLAB and Statistics Toolbox Release 2014b, The MathWorks, Inc., Natick, Massachusetts, United States. P-values < 0.05 were considered statistically significant. Quantitative values were expressed as mean ± standard deviation or median and range as appropriate.
Results
Thyroid incidentalomas and follow-up
Eight hundred forty five/6,216 (13.5%) patients presented with a thyroid incidentaloma on the FDG PET/CT images. Six hundred two/845 (71.2%) and 243/845 (28.7%) patients had 18F-FDG negative and positive thyroid lesions, respectively. The demographic data and intervention status are shown in Table 2.
Table 2.
Patient Demographics (n = 845)
| Male | 275 (275/845,32.5%) |
| Female | 570 (570/845, 67.5%) |
| Mean Age | 68 (range: 21-97, 71 deceased) |
| US Patients | 160 (160/845, 18.9%) |
| FNA Biopsy | 98 (98/160, 61.3%) |
| Thyroidectomy | 26 (26/98, 26.5%) |
One hundred sixty/845 (18.9%) patients underwent ultrasound and 98 of these patients (61.3%) subsequently had a FNA biopsy. Based on the review of the pathologist, sixty-six of these patients (67.3%) patients were determined to have benign findings, which according to the pathologist required no further work up; however, 4 of these 66 (6%) patients underwent a thyroidectomy and 2/4 (50%) of these were positive for thyroid malignancy (Supplemental Figure 1). In 32/98 (32.7%) patients, the cytology report recommended further interventions (Table 3). Twenty-two/32 (68.8%) of these patients underwent thyroidectomy, while no intervention was documented in the remaining ten at our institution; they may have undergone thyroidectomy at another institution (Supplemental Figure 1). Seventeen of the 22 patients (77%) who underwent thyroidectomy had thyroid cancer (Supplemental Table 2). Thyroidectomy was performed in the remaining five patients for a variety of pathologies such as atypical follicular cells or Hurthle cell adenoma (Supplemental Table 2).
Table 3.
Classification of FNA Biopsy Histopathologies (n = 98)
| Description | Classification | Frequency |
|---|---|---|
| Adenomatoid nodule (AN) | Negative | 1 |
| Atypia of undetermined significance (AUS) | Positive | 6 |
| Atypical cells with benign colloid nodule (Atypical cells with BCN) | Positive | 1 |
| Atypical Follicular Cells | Positive | 1 |
| Benign Colloid Nodule (BCN) | Negative | 44 |
| Benign Colloid Nodule with Hurthle cell changes (BCN with HC) | Negative | 4 |
| Benign Follicular Nodule (BFN) | Negative | 6 |
| Benign Follicular Nodule with Hurthle cell changes (BFN with HC) | Negative | 2 |
| Benign Hyperplastic Colloid Nodule (BHCN) | Negative | 2 |
| Benign Hyperplastic Nodule with Hurthle cell changes (BHCN with HC) | Negative | 2 |
| Benign Colloid Nodule with extensive Hurthle cell changes (BCN with extensive HC) | Negative | 1 |
| Follicular cell neoplasm | Positive | 2 |
| Follicular lesion of undetermined significance (FLUS) | Positive | 5 |
| Follicular lesion with Hurthle cell features (FL with HC) | Positive | 1 |
| Hurthle cell neoplasm (HC neoplasm) | Positive | 2 |
| Indeterminate(Ind) | Positive | 1 |
| Neoplasm | Positive | 1 |
| PTC | Positive | 12 |
| Polymorphous lymphoid population (PLP) | Negative | 1 |
| Scant follicular cells | Negative | 1 |
| Thyroiditis | Negative | 2 |
FDG PET/CT findings
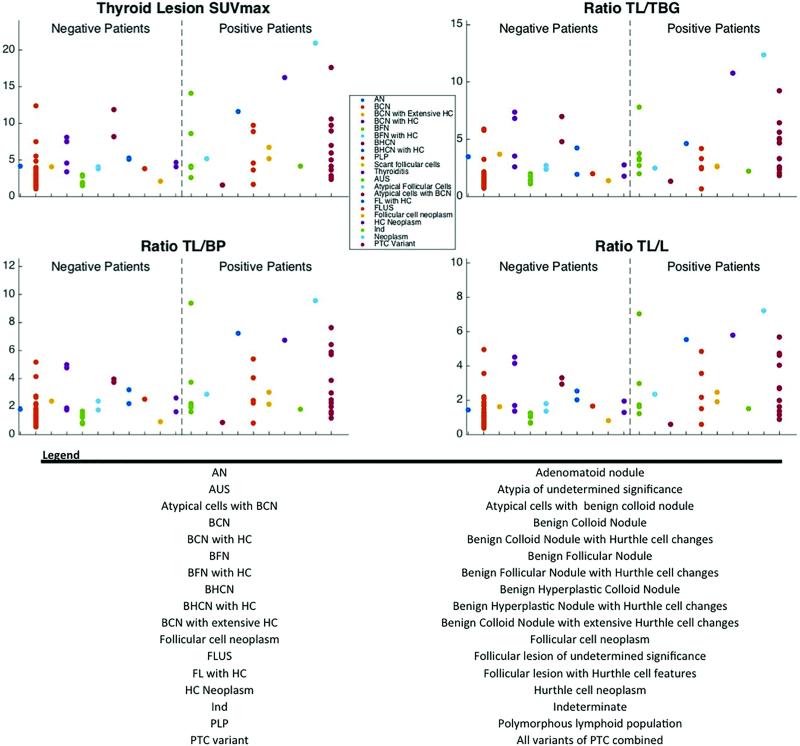
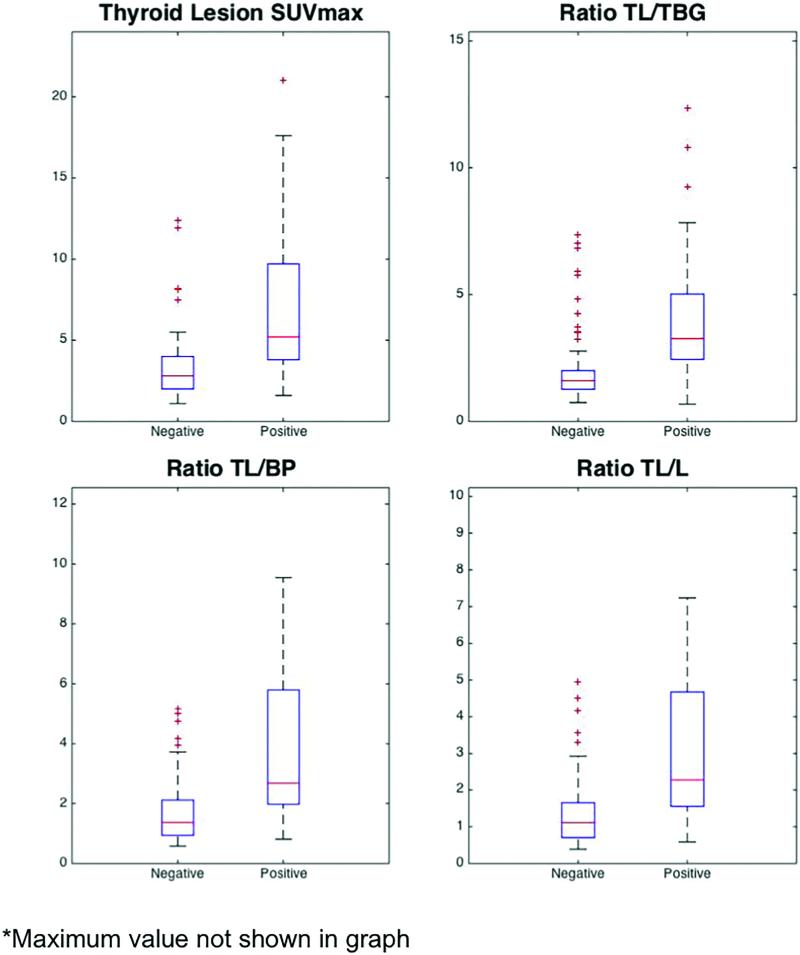
Figure 3 depicts FDG SUV parameters for all thyroid lesions requiring further intervention by report (positive cytology) versus those that did not (negative cytology). The average SUVmax, ratio , ratio , and ratio of positive lesions was significantly higher than for negative lesions (Figure 4, Table 4). However, there was considerable overlap in the SUVmax and SUVratios between positive and negative thyroid lesions (Figure 4).
Figure 3.
The Thyroid lesion SUVmax, ratio TL/TBG, ratio TL/BP, and ratio TL/L across the different histopathologies from the biopsy patients. The patient are separated into two groups, negative, and positive, based on the binary scale method. Please note: One value for HC neoplasm is not shown in graph. TL/TBG = Thyroid lesion/thyroid background, TL/BP = Thyroid lesion/Bloodpool, TL/L = Thyroid lesion/liver
Figure 4.
The Thyroid lesion SUVmax, ratio TL/TBG, ratio TL/BP, and ratio TL/L. TL/TBG = Thyroid lesion/thyroid background, TL/BP = Thyroid lesion/Bloodpool, TL/L = Thyroid lesion/liver
Table 4.
ROC analysis of Thyroid Incidentalomas
| Negative (n = 66) Average Value | Positive (n = 32) Average Value | p-value | Cut Off Value | Sensitivity | Specificity | PPV | NPV | AUC | |
|---|---|---|---|---|---|---|---|---|---|
| Thyroid lesion SUVmax | 3.4 ± 2.3 | 7.9 ± 6.6 | <0.001 | 3.9 | 0.75 | 0.74 | 0.59 | 0.86 | 0.78 |
| Ratio | 2.1 ± 1.5 | 4.7 ± 4.6 | <0.001 | 2.0 | 0.88 | 0.76 | 0.64 | 0.93 | 0.82 |
| Ratio | 1.7 ± 1.1 | 4.1 ± 3.2 | <0.001 | 1.9 | 0.78 | 0.74 | 0.60 | 0.88 | 0.78 |
| Ratio | 1.4 ± 1.0 | 3.2 ± 2.8 | <0.001 | 1.5 | 0.81 | 0.71 | 0.58 | 0.89 | 0.78 |
The ROC analysis is depicted in Table 4. Of note, the ratio cut-off value of 2.0 exhibited the largest AUC (0.82) with a sensitivity and specificity for malignancy of 0.88 and 0.76, respectively.
Malignancy Rate Determination
The thyroid lesions were stratified by the degree of FDG uptake as described above to determine the relationship between metabolic activity of the thyroid lesion and malignancy rate. Here, we defined malignancy rate as the number of pathology proven patients with thyroid cancer (FNA biopsy or thyroidectomy) patients divided by the total number of patients who underwent biopsy.
Thirty one/602 (5.1%) patients with a FDG negative thyroid lesion underwent FNA biopsy. Zero of these 31 patients (0.0%) were positive for thyroid cancer.
Sixty-seven/243 (27.6%) patients with a hypermetabolic thyroid lesion underwent FNA biopsy. Twenty-one/67 (31.3%) patients were positive for malignancy. Table 5 demonstrates the association between the degree of FDG uptake and the malignancy rate. Overall, more than 40% of the thyroid lesions exhibiting intense metabolic activity were malignant, while the lesions with mild or moderate FDG activity were predominantly benign.
Table 5.
Patient Population (n = 845) stratified by FDG uptake
| Patient Number | US | US Result | Biopsy | Malignant* | |
|---|---|---|---|---|---|
| No Uptake | 602 | 75 | 34 | 31 | 0 |
| Mild | 60 | 7 | 6 | 6 | 1 |
| Mild to Moderate | 3 | 0 | 0 | 0 | 0 |
| Moderate | 45 | 15 | 12 | 12 | 2 |
| Moderate to Intense | 46 | 16 | 13 | 13 | 3 |
| Intense | 89 | 47 | 39 | 36 | 17 |
A “malignancy” was defined as a lesion with tissue proven malignant disease
Discussion
The current study demonstrates that thyroid incidentalomas with increased FDG uptake warrant aggressive follow up, especially if FDG uptake is intense. More than 40% of intensely hypermetabolic lesions were malignant. The prevalence of thyroid lesions with focal FDG uptake was close to 4%, which lies at the upper range of previous reports (8). The corresponding malignancy rate was 21/98 (21.4%) which is consistent with previous studies (8). Our key findings are the following: i) As the metabolic activity of the thyroid lesions increases, the malignancy rate increases; ii) thyroid incidentalomas with intense uptake or a should be followed up by an US and FNA biopsy; iii) incidentalomas only seen on CT but not on PET carry a low risk of malignancy. We measured the SUVmax of thyroid lesions and defined specific ratios to test whether malignant lesions can be discriminated from benign lesions. Several studies have shown that the thyroid lesion SUVmax was higher in malignant than in benign lesions (11-15) (Table 1); however, others state the opposite (8, 16-19) (Table 1). While there was some overlap, we identified a statistically significant difference in the thyroid lesion SUVmax, as well as the various target to background ratios among benign and malignant lesions (Figure 4). We performed ROC analysis to determine an optimal cut-off value. Here, we found that the AUC, specificities, and sensitivities for lesion SUVmax, ratio , ratio , and ratio , were significantly higher than the literature values (14, 18, 20). The ratio performed best and yielded a sensitivity and specificity of 0.88 and 0.74, respectively. The AUC was 0.82 (Table 4). Others have attempted but failed to derive SUVmax thresholds that discriminate benign from malignant lesions (14, 17, 18, 20). Many groups solely focused on differentiating benign and malignant thyroid lesions based on SUVmax. To our knowledge, no group has attempted to differentiate lesions based on target to background ratios such as . When we apply the cut off to our data (biopsied patients, n = 98), we find that 44/98 (45%) patients had a ratio . 21/44 (47%) patients were positive for malignancy and 23/44 (53%) patients were false negative. Fifty-four/98 (55%) of the remaining patients had a ratio . Two/55 (3.6%) patients were positive for malignancy, while the remaining 52/54 (96.3%) patients were benign. From this information, we conclude that using a ratio threshold is more accurate predictor of malignant thyroid lesions than a SUVmax threshold. This result is likely due to the fact that the thyroid gland has normal variable physiological uptake that can vary greatly between individuals. Therefore, it is not only important to consider the SUVmax of the thyroid lesion, but also the normal thyroid FDG uptake when determining whether a thyroid lesion is malignant because the ratio is a better predictor.
Currently, no guidelines on the best management of hypermetabolic thyroid incidentalomas exist. According to the American Thyroid Association (ATA), “diagnostic thyroid US should be performed in all patients with a suspected thyroid nodule, nodular goiter, or radiographic abnormality; e.g, a nodule found incidentally on computed tomography (CT) or magnetic resonance imaging (MRI) or thyroid uptake on 18FDG-PET scan” (21). This recommendation seems to be overzealous and leads to numerous unnecessary US studies. The current data suggest that a ratio of 2.0 allows for fairly reliable separation between positive and negative thyroid with a specificity and sensitivity of 0.76 and 0.88, respectively. We thus recommend work-up with US and FNA biopsy in patients with an intensely hypermetabolic (ratio ) thyroid nodule. However, given the relatively low malignancy level of thyroid cancers a watch/wait strategy could be adopted in patients with of <2.0.
It has been reported that well-differentiated PTC thyroid lesions have a low glycolytic phenotype while they avidly take up radioiodine. Once cancers dedifferentiate FDG uptake increases while radioiodine trapping decreases. This has been described as the “flip flop” phenomenon (22-24). However, the SUVmax of primary well-differentiated thyroid papillary carcinomas in the current series was 6.8 ± 5.6 (n = 10). Thus, the flip-flop phenomenon, may only inadequately describe the metabolic phenotype of well-differentiated PTC.
A number of limitations have to be kept in mind prior to translating these results into the clinical routine. First, this study is a retrospective study and to acquire more accurate data, a prospective study must be performed. Secondly, not all of the thyroid incidentalomas were biopsied. Lastly, A selection bias needs to be considered. Patients with intensely hypermetabolic thyroid lesions are more likely to undergo further work up (Table 5), and therefore the thyroid lesions that express milder or even no FDG uptake are not fully evaluated.
Conclusions
The prevalence of thyroid incidentalomas with focal FDG uptake is low (3.9%). However, patients with hypermetabolic thyroid incidentalomas have >20% risk for malignancy. We have calculated an optimal cut off ratio of 2.0 with a specificity and sensitivity of 0.76 and 0.88, respectively, to distinguish between malignant and benign lesions. When the ratio was applied to our data, the rate of malignancy increased to >40%. Such lesions warrant further work-up by US and FNA, whereas a thyroid abnormality with a lower target to background ratio should be watched carefully.
Supplementary Material
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: The authors declare that they have no conflict of interest.
Research involving human participants and/or animals: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Czernin J, Allen-Auerbach M, Nathanson D, Herrmann K. PET/CT in Oncology: Current Status and Perspectives. Current radiology reports. 2013;1:177–190. doi: 10.1007/s40134-013-0016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC, Avril N, Einhorn LH, Suh WW, Samson D, Delbeke D, Gorman M, Shields AF. Recommendations on the use of 18F-FDG PET in oncology. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann K, Benz MR, Krause BJ, Pomykala KL, Buck AK, Czernin J. (18)F-FDG-PET/CT in evaluating response to therapy in solid tumors: where we are and where we can go. The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine. 2011;55:620–632. [PubMed] [Google Scholar]
- 4.Cook GJ, Fogelman I, Maisey MN. Normal physiological and benign pathological variants of 18-fluoro-2-deoxyglucose positron-emission tomography scanning: potential for error in interpretation. Seminars in nuclear medicine. 1996;26:308–314. doi: 10.1016/s0001-2998(96)80006-7. [DOI] [PubMed] [Google Scholar]
- 5.Ozkol V, Alper E, Aydin N, Ozkol HF, Topal NB, Akpinar AT. The clinical value of incidental 18F-fluorodeoxyglucose-avid foci detected on positron emission tomography/computed tomography. Nuclear medicine communications. 2010;31:128–136. doi: 10.1097/MNM.0b013e328332b30e. [DOI] [PubMed] [Google Scholar]
- 6.Iyer NG, Shaha AR, Silver CE, Devaney KO, Rinaldo A, Pellitteri PK, Ferlito A. Thyroid incidentalomas: to treat or not to treat. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2010;267:1019–1026. doi: 10.1007/s00405-010-1207-1. [DOI] [PubMed] [Google Scholar]
- 7.Burguera B, Gharib H. Thyroid incidentalomas. Prevalence, diagnosis, significance, and management. Endocrinology and metabolism clinics of North America. 2000;29:187–203. doi: 10.1016/s0889-8529(05)70123-7. [DOI] [PubMed] [Google Scholar]
- 8.Bertagna F, Treglia G, Piccardo A, Giubbini R. Diagnostic and clinical significance of F-18- FDG-PET/CT thyroid incidentalomas. The Journal of clinical endocrinology and metabolism. 2012;97:3866–3875. doi: 10.1210/jc.2012-2390. [DOI] [PubMed] [Google Scholar]
- 9.Are C, Hsu JF, Schoder H, Shah JP, Larson SM, Shaha AR. FDG-PET detected thyroid incidentalomas: need for further investigation? Annals of surgical oncology. 2007;14:239–247. doi: 10.1245/s10434-006-9181-y. [DOI] [PubMed] [Google Scholar]
- 10.Salvatori M, Melis L, Castaldi P, Maussier ML, Rufini V, Perotti G, Rubello D. Clinical significance of focal and diffuse thyroid diseases identified by (18)F-fluorodeoxyglucose positron emission tomography. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2007;61:488–493. doi: 10.1016/j.biopha.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Bonabi S, Schmidt F, Broglie MA, Haile SR, Stoeckli SJ. Thyroid incidentalomas in FDG PET/CT: prevalence and clinical impact. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2012;269:2555–2560. doi: 10.1007/s00405-012-1941-7. [DOI] [PubMed] [Google Scholar]
- 12.Brindle R, Mullan D, Yap BK, Gandhi A. Thyroid incidentalomas discovered on positron emission tomography CT scanning - Malignancy rate and significance of standardised uptake values. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2014;40:1528–1532. doi: 10.1016/j.ejso.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Parsons M, Torigian DA, Zhuang H, Alavi A. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nuclear medicine communications. 2009;30:240–244. doi: 10.1097/MNM.0b013e328324b431. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Park T, Park S, Pahk K, Rhee S, Cho J, Jeong E, Kim S, Choe JG. The Clinical Role of Dual-Time-Point (18)F-FDG PET/CT in Differential Diagnosis of the Thyroid Incidentaloma. Nuclear medicine and molecular imaging. 2014;48:121–129. doi: 10.1007/s13139-013-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaylali O, Kirac FS, Yuksel D, Marangoz E. Evaluation of focal thyroid lesions incidentally detected in fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography images. Indian journal of cancer. 2014;51:236–240. doi: 10.4103/0019-509X.146737. [DOI] [PubMed] [Google Scholar]
- 16.Chun AR, Jo HM, Lee SH, Chun HW, Park JM, Kim KJ, Jung CH, Mok JO, Kang SK, Kim CH, Kim BY. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose- positron emission tomography. Endocrinology and metabolism. 2015;30:71–77. doi: 10.3803/EnM.2015.30.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho TY, Liou MJ, Lin KJ, Yen TC. Prevalence and significance of thyroid uptake detected by (1)(8)F-FDG PET. Endocrine. 2011;40:297–302. doi: 10.1007/s12020-011-9470-5. [DOI] [PubMed] [Google Scholar]
- 18.Stangierski A, Wolinski K, Czepczynski R, Czarnywojtek A, Lodyga M, Wyszomirska A, Janicka-Jedynska M, Baczyk M, Ruchala M. The usefulness of standardized uptake value in differentiation between benign and malignant thyroid lesions detected incidentally in 18F-FDG PET/CT examination. PloS one. 2014;9:e109612. doi: 10.1371/journal.pone.0109612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Shi W, Zhu B, Hu S, Zhang Y, Wang M, Zhang J, Yao Z, Zhang Y. Prevalence and risk of cancer of thyroid incidentaloma identified by fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Journal of otolaryngology - head & neck surgery = Le Journal d'oto-rhino-laryngologie et de chirurgie cervico-faciale. 2012;41:327–333. [PubMed] [Google Scholar]
- 20.Kim H, Kim SJ, Kim IJ, Kim K. Thyroid incidentalomas on FDG PET/CT in patients with non-thyroid cancer - a large retrospective monocentric study. Onkologie. 2013;36:260–264. doi: 10.1159/000350305. [DOI] [PubMed] [Google Scholar]
- 21.American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 22.Feine U, Lietzenmayer R, Hanke JP, Wohrle H, Muller-Schauenburg W. [18FDG whole-body PET in differentiated thyroid carcinoma. Flipflop in uptake patterns of 18FDG and 131I]. Nuklearmedizin Nuclear medicine. 1995;34:127–134. [PubMed] [Google Scholar]
- 23.Grabellus F, Nagarajah J, Bockisch A, Schmid KW, Sheu SY. Glucose transporter 1 expression, tumor proliferation, and iodine/glucose uptake in thyroid cancer with emphasis on poorly differentiated thyroid carcinoma. Clinical nuclear medicine. 2012;37:121–127. doi: 10.1097/RLU.0b013e3182393599. [DOI] [PubMed] [Google Scholar]
- 24.Hong CM, Ahn BC, Jeong SY, Lee SW, Lee J. Distant metastatic lesions in patients with differentiated thyroid carcinoma. Clinical implications of radioiodine and FDG uptake. Nuklearmedizin Nuclear medicine. 2013;52:121–129. doi: 10.3413/Nukmed-0541-12-11. [DOI] [PubMed] [Google Scholar]
- 25.Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–66. [PubMed] [Google Scholar]
- 26.Kinahan PE, Townsend DW, Beyer T, Sashin D. Attenuation correction for a combined 3D PET/CT scanner. Med Phys. 1998;25:2046–53. doi: 10.1118/1.598392. [DOI] [PubMed] [Google Scholar]
- 27.Halpern BS, Dahlbom M, Quon A, et al. Impact of patient weight and emission scan duration on PET/CT image quality and lesion detectability. J Nucl Med. 2004;45:797–801. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.