Abstract
The recent emergence of a worldwide epidemic of metabolic disorders, such as obesity and diabetes, demands effective strategy to develop nutraceuticals or pharmaceuticals to halt this trend. Natural products have long been and continue to be an attractive source of nutritional and pharmacological therapeutics. One such natural product is mangiferin (MGF), the predominant constituent of extracts of the mango plant Mangifera indica L. Reports on biological and pharmacological effects of MGF increased exponentially in recent years. MGF has documented antioxidant and anti-inflammatory effects. Recent studies indicate that it modulates multiple biological processes involved in metabolism of carbohydrates and lipids. MGF has been shown to improve metabolic abnormalities and disorders in animal models and humans. This review focuses on the recently reported biological and pharmacological effects of MGF on metabolism and metabolic disorders.
Keywords: Mangiferin, metabolism, inflammation, hyperlipidemia, hyperglycemia
Introduction
The metabolic syndrome has been known as a complex of interwoven risk factors which predispose cardiovascular disease (CVD) and type 2 diabetes mellitus and occur together more likely than alone. According to the most recent Harmonized Definition, metabolic syndrome includes elevated triglyceride (TG) levels and low high-density lipoprotein cholesterol levels, obesity, hyperglycemia and raised blood pressure (1). Obesity and diabetes mellitus remain in high prevalence among all age groups in the USA and worldwide and contribute to the development of metabolic syndrome and a wide range of associated health problems, such as non-alcoholic fatty liver disease (NAFLD) (2–5). Diabetes mellitus and obesity are also major risk factors for development of CVD, which is the leading cause of death worldwide (5).
Metabolic syndrome involves interplay among several organs, including liver, muscles, heart, adipose tissues and pancreas, many types of cells, numerous genes and proteins, and multiple metabolic processes, such as gluconeogenesis, glycolysis, lipogenesis and lipolysis, all of which are regulated by complex networks and influenced by many factors, such as hormones. Insulin resistance (IR) is suggested to be at the core of the syndrome and appears to contribute to multiple processes in the development of metabolic disorders (6).
Treating and preventing metabolic disorders require modulation of a variety of genes, proteins, cellular signaling pathways and biological processes. Current drug development is slow and new drugs are unlikely to appear quickly enough to halt or reverse these long-term trends in the disease. This situation might be due to a focus on a drug discovery strategy dominated by finding an activator or inhibitor specifically targeting one protein or gene, i.e. the higher the specificity, the more desirable it becomes. The approach of high throughput screening of synthetic compounds for modulation of specific targets has not yielded a new wave of pharmacological agents to meet the demands for treating metabolic syndrome, suggesting limitations in this drug discovery approach (7–9). Some of the existing drugs treating obesity and diabetes have a high risk of side effects on the central nervous and cardiovascular systems. For instance, rosiglitazone was suggested to increase the risk of myocardial infarct or death in recent meta-analyses of randomized trials and retrospective case-control studies when compared to placebo or other therapies for type 2 diabetes (10, 11).
Natural compounds, particularly those with histories of medical use, continue to represent an attractive alternative to this approach. Throughout history plants, herbs and fruits have been used as a rich source of medicine. A recent structural comparison between about 10,000 Traditional Chinese Medicine components and about 8,000 modern drugs or candidates identified 908 agent pairs that are structurally similar and 327 agent pairs that are identical in structure (12, 13), indicating an apparent high level of natural products or “natural product-like” representation in modern drugs. Some of these Chinese medicines recently have been proven to ameliorate IR, diabetes and other conditions associated with metabolic syndrome (14–16). These data also suggest that potential medicinal applications are still available for known natural products, and the topic of this review is one such compound, mangiferin (MGF). MGF has interesting potential in treatments of metabolic syndromes via modulation of multiple biological processes.
MGF is wildly distributed in a variety of plants (17). The chief source of MGF is the plant Mangifera indica that produces mango (17, 18). MGF exists in the leaves, heartwood and stem bark of mango plant, the latter being the richest source (18). It is also present in the peels and kernels of mango fruit (18). The first study of MGF was reported in 1960. The interest in MGF has gradually increased before the twenty first century and exponentially increased since then, as indicated by the number of publications (Fig. 1). MGF is a C-glucosyl xanthone and chemically named as C2-β-d-glucopyranosyl-1,3,6,7-tetrahydroxyxanthone (Fig. 2). It features a highly condensed aromatic ring system coupled to a glucose moiety via a C-C bond. As a C-glucoside, mangiferin is poorly absorbed by the gastrointestinal tract. Consequently the bioavailability of MGF is very low (1.2%) (19–21) and the dose required for MGF to exert significant effects is high in vivo experiments (Table 1). The concentrations of MGF in in vitro experiments correlate with in vivo dosage and tend to be high, as well (Table 1). The detailed information on MGF bioavailability and pharmacokinetics of MGF is discussed in other articles of this special issue on MGF. It is also discussed in a recently published review on MGF (22).
Fig. 1.
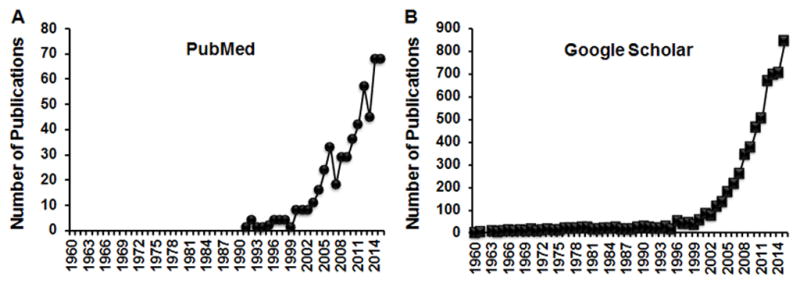
Survey of the studies of MGF in PubMed (A) and Google Scholar (B). The number of publications was obtained by typing “mangiferin” and each year, for instance “1960”, in either PubMed or Google Scholar.
Fig. 2.
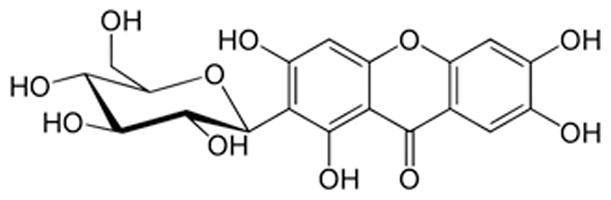
Structure of mangiferin.
Table 1.
Bio- and Pharmacological Effects of Mangiferin Related to Metabolic Disorders.
| In vivo | In vitro | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental model |
HFD | HFD-, fructose- and STZ-induced metabolic syndrome |
Fructose- induced metabolic syndrome |
Overweight patients with hyper- lipidemia |
STZ- induced diabetes |
Genetic model of diabetes |
AGE- induced lipogenesis |
FFA (OA) | |||
| Experimental subject |
Mice (1, 2) |
Rat (3) | Hamster (4) |
Rat (5) | Rat (6, 7) | Human (8) | Rat (9–11) | KK-Ay TSOD mice (12, 13) |
HepG2 cells (14, 15) |
HepG2 cells (3) |
C2C12, L6 myotubes (16, 2) |
| Doses | 400 mg/kg o. |
50–150 mg/kg o. |
50–150 mg/kg o. |
20mg/kg i.p. | 15 mg/kg o. | 150 mg/day | 10–20 mg/kg i.p. 40 mg/kg o. |
90 mg/kg o. | 10 μM | 12.5–100 μM |
2, 200 μM |
| BW | ↓ | ↔ | ↓ | ↔ | ↔ | ↔ | ↔ | ||||
| Plasma glucose | ↓ | ↔ | ↓ | ↔ | ↔ | ↓ | ↓ | ||||
| Plasma insulin | ↓ | ↔ | ↓ | ↔ | ↓ | ||||||
| IR | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||
| Plasma TG | ↓ | ↓ | ↓ | ↓ | ↔ | ↓ | ↓ | ↓ | |||
| Plasma FFA | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||
| Plasma cholesterol | ↓ | ↔ | ↓ | ↔ | ↓ | ↓ | |||||
| Plasma LDL | ↓ | ↔ | ↓ | ↔ | ↓ | ||||||
| Plasma HDL | ↔ | ↑ | ↑ | ↑ | |||||||
| AdipoIR | ↓ | ||||||||||
| β-cell function | ↑ | ||||||||||
| β-hydroxybutyrate | ↑ | ↑ | |||||||||
| Liver TG | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||
| Liver FFA | ↓ | ↓ | |||||||||
| Liver glycogen | ↑ | ||||||||||
| Muscle TG | ↓ | ||||||||||
| Muscle FFA | ↓ | ||||||||||
| CD36 expression | ↑ | ↑ liver muscle | ↓muscular membrane | ↑ | |||||||
| PKB/Akt | ↔ | ||||||||||
| Glut4 | ↑ | ↑ | |||||||||
| Glycolysis enzymes (HK, PK, PDH) | ↑ | ↑ | |||||||||
| Gluconeogenesis | ↓ | ||||||||||
| Cpt-1 | ↑ | ↑ liver | ↔ | ↑ | |||||||
| p-AMPK | ↑ | ↑ | ↑ | ||||||||
| SREBP 1c | ↓ liver | ↔ | ↓ | ||||||||
| Acc | ↓liver | ↓ | ↓ liver | ↔ | ↓(↑ phos) | ||||||
| Scd1 | ↓liver | ↔ | |||||||||
| Dgat2 | ↓ | ↓liver | ↓ | ↓ | |||||||
| PPARα | ↑ liver ↑ muscle |
↔ | |||||||||
| PPARγ | ↔ muscle | ↑ | ↓ | ||||||||
| MAPK/ERK | ↓ | ||||||||||
| IKK | ↓ | ||||||||||
| Adiponectin | ↑ | ↑ serum | ↔ muscle | ||||||||
| TNFα | ↓serum | ↔ muscle | |||||||||
↑ MGF treatment increased indicated parameter; ↓ MGF treatment decreased indicated parameter; ↔ MGF didn’t show effect on indicated parameter.
o., oral administration; i.p., intraperitoneal administration.
Grey-shaded cells - parameter is not applicable; yellow-shaded cells - parameter shows the same trend in most of experiments.
HFD, high fat diet; STZ, streptozotocin; AGE, advanced glycation end products; FFA, free fatty acid; OA, oleic acid; BW, body weight; IR, insulin resistance; TG, triglycerides; LDL, low-density lipoproteins; HDL, high-density lipoproteins; PKB/Akt, protein kinase B/Akt; HK, hexokinase; Glut4, glucose transported type 4; PK, pyruvate kinase; PDH, pyruvate dehydrogenase; Cpt-1, carnitine palmitocyl transferase 1; p-AMPK, phosphorylated AMP-activated protein kinase ; SREBP 1c, sterol regulatory element-binding proteins 1c; Acc, acetyl-CoA carboxylase; Scd1, stearoyl-CoA desaturase 1; Dgat2, diglyceride acyltransferase 2; PPAR, peroxisome proliferator-activated receptors; MAPK/ERK, mitogen-activated protein kinases/extracellular signal-regulated kinases; IKK, IκB kinase; TNFα, tumor necrosis factor α.
P. Apontes, Z. B. Liu, K. Su, O. Benard, D. Y. Youn, X. S. Li, W. Li, R. H. Mirza, C. C. Bastie, L. A. Jelicks, J. E. Pessin, R. H. Muzumdar, A. A. Sauve, Y. L. Chi (2014) Mangiferin Stimulates Carbohydrate Oxidation and Protects Against Metabolic Disorders Induced by High-Fat Diets. Diabetes 63:3626–3636.
J. Lim, Z. Liu, P. Apontes, D. Feng, J. E. Pessin, A. A. Sauve, R. H. Angeletti, Y. Chi (2014) Dual mode action of mangiferin in mouse liver under high fat diet. PloS one 9:e90137.
Y. Niu, S. Li, L. Na, R. Feng, L. Liu, Y. Li, C. Sun (2012) Mangiferin decreases plasma free fatty acids through promoting its catabolism in liver by activation of AMPK. PloS one 7:e30782.
F. Guo, C. Huang, X. Liao, Y. Wang, Y. He, R. Feng, Y. Li, C. Sun (2011) Beneficial effects of mangiferin on hyperlipidemia in high-fat-fed hamsters. Molecular nutrition & food research 55:1809–1818.
S. Saleh, N. El-Maraghy, E. Reda, W. Barakat (2014) Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: role of adiponectin and TNF-alpha. Anais da Academia Brasileira de Ciencias 86:1935–1948.
X. Xing, D. Li, D. Chen, L. Zhou, R. Chonan, J. Yamahara, J. Wang, Y. Li (2014) Mangiferin treatment inhibits hepatic expression of acyl-coenzyme A:diacylglycerol acyltransferase-2 in fructose-fed spontaneously hypertensive rats: a link to amelioration of fatty liver. Toxicology and applied pharmacology 280:207–215.
L. Zhou, Y. Pan, R. Chonan, R. Batey, X. Rong, J. Yamahara, J. Wang, Y. Li (2016) Mitigation of Insulin Resistance by Mangiferin in a Rat Model of Fructose-Induced Metabolic Syndrome Is Associated with Modulation of CD36 Redistribution in the Skeletal Muscle. The Journal of pharmacology and experimental therapeutics 356:74–84.
L. X. Na, Q. Zhang, S. Jiang, S. S. Du, W. Zhang, Y. Li, C. H. Sun, Y. C. Niu (2015) Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: a double-blind randomized controlled trial. Sci Rep-Uk 5.
S. Muruganandan, K. Srinivasan, S. Gupta, P. K. Gupta, J. Lal (2005) Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. Journal of ethnopharmacology 97:497–501.
P. S. Sellamuthu, B. P. Muniappan, S. M. Perumal, M. Kandasamy (2009) Antihyperglycemic Effect of Mangiferin in Streptozotocin Induced Diabetic Rats. J Health Sci 55:206–214.
P. B. Pal, K. Sinha, P. C. Sil (2014) Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFalpha related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PloS one 9:e107220.
T. Miura, H. Ichiki, I. Hashimoto, N. Iwamoto, M. Kato, M. Kubo, E. Ishihara, Y. Komatsu, M. Okada, T. Ishida, K. Tanigawa (2001) Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine : international journal of phytotherapy and phytopharmacology 8:85–87.
T. Miura, N. Iwamoto, M. Kato, H. Ichiki, M. Kubo, Y. Komatsu, H. Sasaki, T. Ishida, K. Tanigawa (2001) Effect of Mangiferin on muscle GLUT4 protein content in TSOD (Tsumura, Suzuki, Obese, Diabetes) mouse, a new type 2 diabetic mice. Biomed Res-Tokyo 22:249–252.
S. K. Mahali, S. K. Manna (2012) Beta-D-glucoside protects against advanced glycation end products (AGEs)-mediated diabetic responses by suppressing ERK and inducing PPAR gamma DNA binding. Biochem Pharmacol 84:1681–1690.
S. K. Mahali, N. Verma, S. K. Manna (2014) Advanced glycation end products induce lipogenesis: regulation by natural xanthone through inhibition of ERK and NF-kappaB. Journal of cellular physiology 229:1972–1980.
M. D. Giron, N. Sevillano, R. Salto, A. Haidour, M. Manzano, M. L. Jimenez, R. Rueda, J. M. Lopez-Pedrosa (2009) Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: role of mangiferin. Clinical nutrition 28:565–574.
MGF bears a catechol moiety, which enables it to form stable MGF-Fe2+/Fe3+ complexes, preventing Fenton-type reactions and corresponding lipid peroxidation reactions (23). Such structural feature enables MGF to scavenge reactive oxygen species (ROS) (24), thus being an effective antioxidant. MGF also helps to maintain the balance among the enzymes, superoxide dismutase, catalase and the glutathione system (25), which have key roles in the cellular defense system against free radical damage. MGF has been shown to affect several biological processes including mitochondrial bioenergetics, glycolysis, lipogenesis, and etc. Fundamental to these biological processes, MGF elicits a spectrum of bioactivities including anti-oxidant and anti-inflammatory effects, and recently discovered anti-hyperlipidemia, anti-hyperglycemia and anti-cancer effects (Fig. 3). The biological and pharmacological effects of MGF are summarized in Table 1.
Fig. 3.
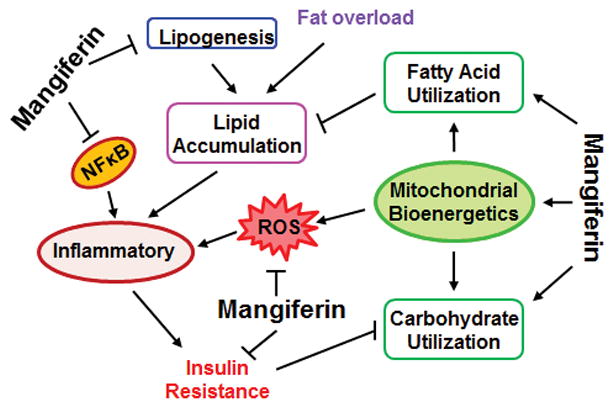
Mangiferin modulated biological processes involved in metabolism and metabolic disorders.
1. MGF Modulates Obesity
Obesity is one of the major risk factors of development of other metabolic disorders including NAFLD and diabetes. Plant extracts rich in MGF prevents obesity in several animal models including high sucrose fed rats (26), high fat diet (HFD) fed C57BL6/J mice (27, 28), spontaneously obese type 2 diabetes mellitus (Tsumura Suzuki obese diabetes, TSOD) mice (29, 30), and obese diabetic KK-Ay mice (31). In overweight and obese humans, mango extracts containing MGF has been shown to reduce body weight, without side effects (32). With pure MGF, Guo et al. showed that MGF reduced HFD induced body weight gain in hamster (33). Recently we showed that MGF prevented HFD induced body weight gain in C57BL6/J mice (34). Additionally, Han et al. showed that a derivative of MGF, which had higher lipophilicities, reduced body weight gain in obese db/db (C57BL/KsJ) mice (35).
The reduction of body weight gain mainly occurs in fat mass (26, 28, 29, 33, 31, 30, 34). In most of these studies MGF was found to have no significant effects on food or water intake (27, 28, 33–35), suggesting that the effects of MGF could be mediated by altered metabolism. Indeed, MGF and MGF rich extracts enhance whole body oxygen consumption and energy expenditure (27, 34). Two main energy sources are carbohydrates and fats. MGF stimulates carbohydrate utilization in skeletal muscle (34). At the cellular level, Shimada et al. showed that MGF containing extracts inhibited differentiation of 3T3-L1 adipocytes (36). At the molecular level, MGF upregulates the enzymes in carbohydrate oxidation in muscle (34) and the enzymes in the pathway of lipid utilization in hepatocytes (33, 37). MGF also suppresses the enzymes in lipogenesis in hepatocytes (33, 37, 38). The ability of stimulating carbohydrate and lipid utilization and simultaneously inhibiting lipid synthesis enables MGF to prevent body weight gain and obesity.
2. MGF Inhibits Hyperlipidemia and Prevents NAFLD
Metabolism of physiological fatty acids and lipids is regulated by two processes, lipogenesis and lipolysis. Lipogenesis is catalyzed by such enzymes as acetyl-CoA carboxylase (Acc), fatty acid synthase (Fas), elongase, stearoyl-CoA desaturase (Scd), glycerol-3-phosphate acyltransferase (Gpat), and diglyceride acyltransferase (Dgat), which are regulated by sterol regulatory element-binding proteins (SREBPs) (39–42). Lipolysis starts with hydrolysis of TG to free fatty acids (FFAs) catalyzed by lipoprotein lipases (LPL). FFAs are then translocated from the circulation into cells by a family of fatty acid transport proteins (FATPs) (43), with one of the most prominent and best characterized members as fatty acid translocase (FAT)/CD36 (44). Intracellular FFAs are converted to fatty acyl CoA, which is subsequently transferred to mitochondria by carnitine palmitocyl transferase 1 (Cpt1). In mitochondria fatty acyl CoA is converted to acetyl-CoA by long chain acyl-CoA dehydrogenase (Lcad), medium chain acyl-CoA dehydrogenase (Mcad), and other enzymes. Most of these enzymes participating lipolysis are target genes of peroxisome proliferator-activated receptors (PPARs) (45).
Certain stress conditions, such as over-nutrition and sedimentary lifestyle, can adversely impact fatty acid and lipid metabolism, leading to accumulation of lipid in plasma, liver and adipose tissues. In several hyperlipidemic animal models, including HFD and/or fructose fed rodents (mice, rats and hamsters), MGF is able to reverse elevated plasma total cholesterol, TG and FFAs, improve balance between LDL and HDL, and reduce atherogenic index (46, 33, 37, 34, 38, 47, 48). In livers of HFD-fed rats, MGF reduced TG and FFAs (49, 50). Xing et al. showed that MGF ameliorated fatty liver in fructose-fed spontaneously hypertensive rats (51). In an unbiased proteomics study (38), using HFD induced obese mouse model, the Chi group showed that MGF reduced plasma TG and prevented lipid accumulation in liver of those mice. More importantly, MGF is able to improve lipid profiles in human (52). In a double-blind randomized control clinical trial, Na and coworkers showed that MGF supplementation improved serum lipid profiles by reducing TG and FFA levels and increasing HDL level and, thus, atherogenis index, in overweight patients with hyperlipidemia (52). MGF also increased LPL activity and L-carnitine, β-hydroxybutyrate and acetoacete levels, suggesting that MGF could promote FFA oxidation (52).
Mechanistically, we and others have provided evidence that MGF inhibits lipogenesis. MGF reduces Acc and Dgat2 in liver of HFD fed rats or hamsters (33, 37). MGF also increases the ratio of phosphorylated-Acc (p-Acc)/Acc by inducing p-AMPK (37). Phosphorylated Acc is the inactive form of Acc. AMPK phosphorylates Acc and inhibits Acc activity. The proteomics study reported by the Chi group revealed that MGF prevented lipid accumulation in liver via down-regulation of acetyl-CoA carboxylase (Acac) and Scd (38). Acac catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA, the rate-determining step in fatty acid synthesis. Scd1 catalyzes the conversion of stearoyl-CoA to oleoyl-CoA, which is a major substrate for TG synthesis. Ingenuity pathway analysis (IPA) of these proteomics data predicted that MGF suppressed SREBPs (38). This data corroborates a report by Guo et al. showing that MGF treatment in hamster resulted in reduced SREBP-1c transcripts in liver (33). Additionally, Mahali et al. demonstrated that MGF could inhibit advanced glycation end productions (AGE)-mediated SREBP-DNA binding activity (33, 53, 54).
While inhibiting lipogenesis, MGF is able to stimulate lipolysis and enhance lipid clearance. Guo et al. showed that MGF increased mRNA of LPL (33). MGF has also been shown to induce CD36 at both transcriptional and translational levels. It induces mRNA of CD36 in liver of hamsters (33) and increases protein level of CD36 in HepG2 (human hepatoblastoma) cells (37). In addition, MGF is able to restore fructose-stimulated sarcolemmal CD36 overexpression and decreased intracellular CD36 distribution in skeletal muscle (48). CD-36 is known to be under genomic control of PPARγ (55), suggesting that MGF might regulate lipid metabolism via modulation of PPARγ, although recent studies differ in their conclusions about MGF and PPARγ interaction (56, 53, 57, 54). For example, a gene reporter assay showed that MGF didn’t have any effect on ciglitazone-induced PPARγ activation (58). The plant extracts, which are rich in MGF, inhibit intracellular TG and fat accumulation and reduce PPARγ2 expression in 3T3-L1 pre-adipocytes (57). In contrast, MGF enhances PPARγ activity in L6-myotubes (56) and increases PPARγ DNA binding ability in various cell lines including HepG2 cells (53, 54). These paradoxical effects of MGF on PPARγ could be due to PPARγ peculiarities in non-adipose tissues and associated with tissue-specificity of MGF action. Besides these effects, MGF promotes fatty acyl CoA translocation from the cytosol to mitochondria, as it induces Cpt1 (33, 37), probably by upregulating PPAR-α (33).
Although more comprehensive studies of the mechanisms of action of MGF are needed to better understand how MGF modulates lipid metabolism, these preliminary reports suggest that MGF suppresses lipogenesis and stimulates lipolysis, thereby preventing lipid accumulation and NAFLD.
3. MGF Reduces Hyperglycemia and Prevents Diabetes
The hallmark of diabetes is hyperglycemia, a result of an overload of carbohydrates, insufficient glucose disposal, and/or the over production of glucose. Insufficient glucose disposal can be caused by insufficient insulin production, insensitivity to insulin signaling, impaired carbohydrate utilization mediated by the enzymes in glycolysis and mitochondrial oxidative processes, such as pyruvate dehydrogenase (PDH). Over production of glucose could be caused by abnormally high levels of abundance or activities of the enzymes in the gluconeogenesis pathway, such as glucose-6-phosphatase (G6P), fructose-1,6-bisphosphatase (FBP) and glucose-6-phosphate dehydrogenase (G6PDH).
MGF has been reported to reduce plasma glucose and insulin levels, and increase insulin sensitivity and glucose tolerance in different genetic mouse models of type 2 diabetes (59, 60), diet induced IR mouse model (34), and streptozotocin (STZ) induced diabetic rats (46, 61, 62). It seems that MGF does so via several unique mechanisms. MGF is an inhibitor of glucosidase (63), which could enable MGF to prevent overloaded carbohydrates from being converted to glucose and absorbed in the intestine. For the existing glucose, MGF appears to stimulate its utilization. The upstream event in the pathway of carbohydrate utilization is insulin signaling. MGF enhances insulin sensitivity and mitigates genetic or environment induced IR (59, 60, 46, 61, 34, 38, 62, 64, 47, 52, 48). In HFD induced IR mouse model, the Chi group showed that MGF reverses HFD caused higher than normal plasma insulin and mitigates HFD induced glucose intolerance (34). MGF also attenuates IR and reduces insulin level in a rat model of fructose-induced metabolic syndrome and in KK-Ay and TSOD diabetic mice (59, 60, 47, 48). In STZ-induced diabetic rats MGF is able to improve insulin sensitivity and correct plasma glucose level, even though without affecting insulin level (46, 61, 62). Also, MGF showed the same effect in STZ-diabetes model enforced with HFD and fructose supplementation (64). In overweight patients with hyperlipidemia, Na et al. reported that MGF supplementation decreased IR index although without improvement of increased plasma insulin and glucose levels (52).
Upon insulin signaling, glucose transporters are translocated from the cytosol to the cell membranes to uptake glucose into cells. While the effects of MGF on GLUT1 is uncertain (56, 35), MGF increases GLUT4 content in the plasma membrane fraction of muscle in mice (60) and in cultured rat myotubes (56) and 3T3 preadipocytes and adipocytes (35), thus increasing glucose uptake (56, 65). MGF caused increase in GLUT4 expression and consequent glucose uptake is probably mediated via activation of AMPK (60, 56). MGF also induces the enzymes in glycogen synthesis (glycogen, glycogen phosphorylase, glycogen synthase) (61) and the enzymes in glycolysis such as hexokinase (HK), pyruvate kinase (PK) and glucose oxidation such as PDH (61, 34). The Chi group provided evidence of molecular mechanisms by which MGF upregulates PDH. MGF acutely activates PDH (34), probably by directly interacting with PDH. It also activates PDH by suppressing PDK4 (34), a negative regulator of PDH.
In addition to stimulating glucose utilization, MGF has also been shown to reduce glucose 6-phosphate (G6P) and fructose bisphosphatase (FBP) and therefore inhibit gluconeogenesis (61). Together, with enhancing insulin sensitivity and glucose utilization, MGF could be an effective anti-hyperglycemia and anti-diabetes agent.
4. MGF Protects Pancreatic β-cells and Mitigates Diabetic Complications
Hyperglycemia and hyperlipidemia are known to reduce viability and insulin secretion of pancreatic β-cells (66). ROS generation, endoplasmic reticulum stress and the following mitochondrial dysfunction have been considered to be important factors mediating pancreatic islets damage and deterioration of type 2 diabetes (67, 68, 66). MGF has well established antioxidant properties (24, 23) and shows its protective action against oxidative damage of various organs in STZ -induced diabetic rats including pancreas, heart, liver and kidney (69–71). Oral administration of MGF improves pancreatic ultramicroscopic architecture and increases β-cell count in STZ diabetic rats (71). These beneficial effects of MGF on pancreas are due to its ability to reduce hydroperoxides and increase reduced glutathione (GSH) (71). In addition, MGF increases nonenzymatic antioxidants in plasma such as vitamin C (71). MGF can also facilitate islet regeneration and β-cell proliferation by regulating cell cycle and essential proteins related to islet regeneration and glucose metabolism (72, 73).
One of the most serious complications of diabetes is diabetic nephropathy (DN) and it is the most common cause of the end stage renal disease. Hyperglycemia-induced overproduction of ROS is the central mechanism of diabetic complications and increased poly pathway flux and formation of AGEs are the two important participators in ROS generation (74). Since MGF is an effective antioxidant, its effects on DN have been studied by several groups using STZ induced diabetic rats (75–77). MGF significantly reverses STZ caused kidney enlargement and structural damage, and kidney dysfunction measured as urinary protein secretion and blood urea nitrogen (75, 76, 62). These effects of MGF are mediated by several mechanisms. Liu et al. showed that MGF reduced AGEs and receptor for advanced glycation end products (RAGE) by upregulating glyoxalase 1 (76). Pal et al. demonstrated that MGF reversed STZ caused reduction in catalase, superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR) and thereby increased the ratio of GSH to GSSG (oxidized glutathione), and reduced malondialdehyde, protein carbonylation and ROS (78, 62). This group also showed that MGF inhibited STZ induced activation of NF-κB pathway (62), which could induce inflammation in kidney. In addition, MGF attenuates sepsis-induced acute kidney injury (79) and renal fibrosis (62, 80) via antioxidant and anti-inflammatory effects. Moreover, MGF inhibits renal urate reabsorption by modulating urate transporters (81).
5. MGF Mitigates Cardiac Vascular Diseases
CVD continues to be the leading cause of death worldwide (5). Abnormal mitochondrial energy metabolism and oxidative stress are critical to CVD. It has been shown that MGF can protect heart from isoproterenol induced structural alteration and functional failure in heart of rats (82), probably due to its ability to enhance mitochondrial oxidative capacity and at the same time to reduce ROS. Being not only an antioxidant but also an anti-inflammatory agent, MGF significantly ameliorates diabetic cardiomyopathy in STZ- and HFD-diabetic rats. Chronic treatment with MGF decreased the levels of myocardial enzymes (CK-MB and LDH) and inflammatory mediators (TNFα and IL-1β) through deactivation of NF-κB nuclear translocation and suppression of RAGE expression (83).
Hyperlipidemia and hyperglycemia are major risk factors contributing to CVD. As MGF is able to mitigate both of these factors, it is conceivable that it can prevent CVD associated with hyperlipidemia and hyperglycemia, although the anti-hyperlipidemia effects of MGF on CVD are likely to be complicated. As mentioned previously, MGF has the potential to not only suppress lipogenesis but also stimulate lipolysis, part of which is fatty acid oxidation. However, increased cardiac fatty acid oxidation plays a role in the development of myocardial dysfunction in diabetes (84, 85). For instance, cardiac overexpression of PPAR-α, which stimulates β-oxidation, induces pathological cardiac changes (86). Interestingly, in the hearts of Zucker diabetic fatty rats (ZDF, a genetic model of type 2 diabetes and obesity), Salacia oblonga extracts containing MGF reduced upregulated PPAR-α and Cpt-1 and Aco mRNAs, while it enhanced hepatic expression of PPAR-α, Cpt-1 and acyl-CoA oxidase (Aco) (87, 85). These putative tissue specific, perhaps paradoxical effects of MGF require further verification. Nevertheless, MGF seems to have beneficial effects in improving CVD.
6. MGF Modulates Cross Talk between Lipid Metabolism and Carbohydrate Metabolism
Cross talk between lipid and carbohydrate metabolisms exists and has been reported (88–91). Hyperglycemia is still considered the principal contributor to the formation of sugar-derived substances AGEs, which accumulate in diabetic persons and contribute to its micro- and macrovascular complications (92, 93). Recent studies revealed that accumulation of AGEs activates lipogenesis in liver and skeletal muscles by interfering with SREBP-1c through downregulation of the SREBP-inhibiting enzyme SIRT-1 and increased glycation of the SREBP-activating protein SCAP (94–96). Mahalli et al. also reported AGE-induced intracellular lipid accumulation in different cell lines, including HepG2, through RAGE-mediated ROS-dependent and -independent ERK and IKK activation, resulting in increased SREBP-DNA binding (53, 54). MGF is able to downregulate these processes and completely suppress AGE-induced lipogenesis, whereas antioxidants, IKK and ERK inhibitors showed only partial protection (53, 54). MGF also stimulates carbohydrate utilization (34), and presumably reduces the sources of AGEs. Enhanced carbohydrate oxidative utilization could potentially raise acetyl-CoA, the end product of carbohydrate oxidation. Acetyl CoA is a substrate of lipogenesis. One would assume that MGF could potentially increase lipogenesis. However, as discussed above, MGF suppresses lipid production. The hypothesized mechanism is that MGF inhibits lipogenesis by suppressing important players in the lipogenesis pathway as supported by the currently available data (33, 37), especially the comprehensive proteomics study reported by the Chi group (38).
While carbohydrate metabolism influences lipid homeostasis, on the other hand, lipid metabolism affects carbohydrate balance. For instance, accumulation of circulating FFAs and lipids could raise glucose level by inducing IR and impairing mitochondrial function, and thereby inhibiting glucose utilization (97, 98). One of the important mediators of cross talk between lipid accumulation and IR is inflammation (Fig. 3). Recent studies established the signaling pathways activated by obesity and involved in development of inflammation and IR in numerous cell types (99–103). One of the theories holds that adipose expansion leads to formation of hypertrophic adipocytes that secreting chemoattractants and leukotriene B4 (LTB4), which in turn causes influx of immune cells to adipose tissue, initiating a cascade of inflammatory events (101). Overload of fat increases lipolysis and augmented lipolysis causes increased plasma levels of saturated FFAs, which can directly activate pro-inflammatory responses in vascular endothelial cells and myeloid-derived cells (102). Excess of intracellular fatty acids activates fatty acid oxidation and leads to excessive generation of peroxidation products in mitochondria (104). Pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, along with peroxidation products, stimulate IKK-β / NFκB and MAPK/ERK/JNK signaling pathways, which contribute to IR due to the ability of IKK-β and JNK serine kinases to phosphorylate serine 307 of IRS1, instead of tyrosine, and hence blunt normal insulin signaling. NF-κB could in turn transcribe its target genes, including those pro-inflammatory cytokines (92).
Using a DNA hybridization array, Leiro et al. found that MGF had significant impacts on the profiles of a large number of NF-κB related cytokine genes that are critical for regulation of inflammation (105). More recent studies reported MGF’s ability to suppress IKK-β / NF-κB and/or MAPK/ ERK/JNK signaling pathways in lymphoid organs (106), leucocytes (107), diabetic kidneys (62) and heart (83), acutely injured liver (78), colon (108) and lungs (109), and to inhibit NF-kB DNA binding and IKK activity in HepG2 cells (110).
MGF’s ability to suppress NF-κB presumably inhibits the production of pro-inflammatory cytokines, and therefore mitigates inflammation caused IR. Indeed, Leiro et al. showed that in primary macrophages from mice, MGF treatment significantly blunted the expression of pro-inflammatory cytokines including IL-1β, IL-6, IL-12, TNFα and other cytokines (105). Tsubaki et al. showed that MGF suppressed the expression of TNFα, IL-6, and IL-1β through inhibiting the activation of NF-κB and ERK1/2 in thymus and spleen of collagen induced arthritis mice (106). MGF treatment reduces serum levels of TNF-alpha, IL-1β and IL-6, and expressions of ERK and JNK in leukocytes after lipopolysaccharide (LPS) stimulation (107). In LPS-stimulated primary hepatocytes, Pan et al. showed that MGF significantly reduced expression of IL-1β and TNFα. In addition, MGF ameliorated LPS/D-galactoseamine-induced acute liver injure in vivo, correcting serum and hepatic inflammatory profiles via blocking hepatic NLRP3 inflammasome activation and activating the Nrf2 pathway (111). In STZ- and HFD-diabetic cardiomyopathy rats, Hou et al. showed that MGF reduced plasma TG (83). In the heart of those rats, MGF reduced not only pro-inflammatory cytokines, but also AGEs (83). Furthermore, MGF improved insulin sensitivity in peripheral tissues (Adipo-IR) by normalizing the fructose-induced increase of NEFA plasma clearance and suppressing fatty acid uptake by muscular tissue (48). Using high-fat/high fructose diet followed by a subdiabetogenic dose of STZ (HFD-Fr-STZ) rat model, Saleh et al. showed that MGF reduced lipids, TNFα, and mitigated IR and hyperglycemia (64). In overweight patients with hyperlipidemia and presenting IR, MGF supplementation decreased IR index (52).
Thus far we have discussed MGF modulation of upstream events in the cross talk between carbohydrate and lipid metabolism. MGF also modulates the downstream events of carbohydrate and lipid metabolisms. The metabolisms of carbohydrates and lipids converge at acetyl-CoA, which is further metabolized by the tricarboxylic acid (TCA) cycle and the subsequent electron transport chain (ETC) to produce energy ATP in mitochondria. Mitochondrial dysfunction is closely associated with over 50 diseases including metabolic syndromes (112–114). Our recent unbiased proteomics study revealed that MGF upregulated proteins participating in mitochondrial bioenergetics (38). These proteins include oxoglutarate dehydrogenase E1 (Dhtkd1), cytochrome c oxidase subunit 6B1 (Cox6b1), fumarate hydratase 1 (Fh1), short-chain specific acyl-CoA dehydrogenase (Acads), enoyl-CoA hydratase and carnitine O-palmitoyltransferase 2 (Cpt2). Others found that MGF raised the levels of state 3, state 4 and ATP in mitochondrial respiration (82). In addition, MGF modulates NAD+/NADH, which are important factors in the TCA cycle and the ETC. MGF and MGF rich extracts reduce NADH (26) and increase NAD+ and therefore increase the ratio of NAD+/NADH (34). These studies indicate that MGF is able to increase mitochondrial bioenergetics in general and thereby stimulate metabolisms of both carbohydrates and fatty acids, setting off the competition between carbohydrate and fatty acid utilization and mitigating both hyperglycemia and hyperlipidemia simultaneously.
The mitochondrial respiratory chain is a major source of ROS within the cell (115–117). Excessive production of ROS and increased oxidative stress are participants in the development and progression of NAFLD, diabetes and its complications, CDV and cancer (118–123). MGF has well established antioxidant properties, for the reasons discussed above, even though it enhances mitochondrial respiration. MGF’s antioxidative properties also account for its anti-inflammatory effects. Given the intimate relationship between the immune and metabolic systems, it is conceivable that anti-inflammatory effects of MGF could be important factors in contributing to its potential ability to mitigate metabolic syndromes.
Conclusions
In summary, the use of synergies of anti-obesity and anti-diabetes drugs with different mechanisms of action is an effective approach for developing new combined pharmaceutical compositions (11). The literature thus far shows that MGF could be an example of one compound exerting multiple beneficial effects. MGF interferes with multiple biological processes critical to the development of metabolic syndrome (Fig. 3). The central effects are probably antioxidant and anti-inflammation, which enable MGF to counteract with IR caused by ROS and inflammation resulted from excessive accumulation of lipids. These are fundamental processes involved in all metabolic disorders. The most studied and the most consistent effects of MGF are reduction of IR and TG and FFAs (Table 1). MGF mitigates IR and consequently promotes glucose uptake. Together with its ability to enhance glycolysis, and perhaps to inhibit gluconeogenesis, MGF can effectively prevent hyperglycemia. MGF stimulates lipolysis and suppresses lipogenesis and thereby reduces lipid accumulation and consequently prevents hyperlipidemia.
What are lacking are in depth mechanistic studies that could pinpoint the molecular targets of MGF. We recently provided evidence that MGF activates PDH (34). Our proteomics study also predicts other molecular targets and signaling pathways affected by MGF (38). While these studies advanced our understanding of the mechanisms of action of MGF, more mechanistic studies are in great need to provide clear pictures of how MGF exerts its beneficial effects. Affecting multiple targets can result in complex effects, some of which could be contradictory to others. This could be the downfall of the strategy of targeting multiple biological markers and events. Nevertheless this strategy has its advantages and is especially applicable to metabolic syndrome as metabolic syndrome includes multiple pathological conditions.
Up-to-date the reported studies of the effects of MGF were mainly conducted in cultured cells and in rodents. There has been only one report on the clinical study of MGF in human (52). More clinical studies in large cohort are necessary to demonstrate that MGF has great potential to be developed into nutritional/pharmacological therapeutics that could prevent and/or reverse metabolic disorders by modulating multiple biological events and processes.
Acknowledgments
This work was supported by the grants that Y.C. received from the American Heart Association (0735066N), from American Diabetes Association (1-11-JF-06), and partially from NIH/NIDDK (DK41296).
Footnotes
Conflict of Interest
Authors have no conflict of interest.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr E. International Diabetes Federation Task Force on, Prevention, L. Hational Heart, I. Blood, A. American Heart, F. World Heart, S. International Atherosclerosis, O. International Association for the Study of. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M G Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Chung A, Backholer K, Wong E, Palermo C, Keating C, Peeters A. Trends in child and adolescent obesity prevalence in economically advanced countries according to socioeconomic position: a systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2015 doi: 10.1111/obr.12360. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB C. American Heart Association Statistics, S. Stroke Statistics. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 6.Lam DW, LeRoith D. In: Endotext. De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. South Dartmouth (MA): 2000. [Google Scholar]
- 7.Grabowski H. Are the economics of pharmaceutical research and development changing?: productivity, patents and political pressures. PharmacoEconomics. 2004;22:15–24. doi: 10.2165/00019053-200422002-00003. [DOI] [PubMed] [Google Scholar]
- 8.Vernon JA. Examining the link between price regulation and pharmaceutical R&D investment. Health Econ. 2005;14:1–16. doi: 10.1002/hec.897. [DOI] [PubMed] [Google Scholar]
- 9.Tobinick EL. The value of drug repositioning in the current pharmaceutical market. Drug news & perspectives. 2009;22:119–125. doi: 10.1358/dnp.2009.22.2.1303818. [DOI] [PubMed] [Google Scholar]
- 10.Bach RG, Brooks MM, Lombardero M, Genuth S, Donner TW, Garber A, Kennedy L, Monrad ES, Pop-Busui R, Kelsey SF, Frye RL Investigators BD. Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2013;128:785–794. doi: 10.1161/CIRCULATIONAHA.112.000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maksimov ML, Svistunov AA, Tarasov VV, Chubarev VN, Avila-Rodriguez M, Barreto GE, Dralova OV, Aliev G. Approaches for the development of drugs for treatment of obesity and metabolic syndrome. Current pharmaceutical design. 2015 doi: 10.2174/1381612822666151209153047. [DOI] [PubMed] [Google Scholar]
- 12.Butler MS. Natural products to drugs: natural product derived compounds in clinical trials. Nat Prod Rep. 2005;22:162–195. doi: 10.1039/b402985m. [DOI] [PubMed] [Google Scholar]
- 13.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 14.Lu JM, Wang YF, Yan HL, Lin P, Gu W, Yu J. Antidiabetic effect of total saponins from Polygonatum kingianum in streptozotocin-induced daibetic rats. Journal of ethnopharmacology. 2015 doi: 10.1016/j.jep.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XT, Yu CJ, Liu JW, Zhang YP, Zhang C, Song CX, Xie JS, Sai JY, Zheng JT, Wang F. Qizhi Jiangtang Jiaonang Improves Insulin Signaling and Reduces Inflammatory Cytokine Secretion and Reactive Oxygen Species Formation in Insulin Resistant HepG2 Cells. Evidence-based complementary and alternative medicine : eCAM. 2015;2015:518639. doi: 10.1155/2015/518639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu T, Sun J, Kagota S, Maruyama K, Wakuda H, Shinozuka K. Panax notoginseng saponins ameliorate impaired arterial vasodilation in SHRSP.Z-Lepr /lzmDmcr rats with metabolic syndrome. Clinical and experimental pharmacology & physiology. 2016 doi: 10.1111/1440-1681.12547. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez GM, Re L, Giuliani A, Nunez-Selles AJ, Davison GP, Leon-Fernandez OS. Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol Res. 2000;42:565–573. doi: 10.1006/phrs.2000.0727. [DOI] [PubMed] [Google Scholar]
- 18.Barreto JC, Trevisan MT, Hull WE, Erben G, de Brito ES, Pfundstein B, Wurtele G, Spiegelhalder B, Owen RW. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.) Journal of agricultural and food chemistry. 2008;56:5599–5610. doi: 10.1021/jf800738r. [DOI] [PubMed] [Google Scholar]
- 19.Han D, Chen C, Zhang C, Zhang Y, Tang X. Determination of mangiferin in rat plasma by liquid-liquid extraction with UPLC-MS/MS. Journal of pharmaceutical and biomedical analysis. 2010;51:260–263. doi: 10.1016/j.jpba.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Hou SY, Wang F, Li YM, Li Y, Wang MQ, Sun DJ, Sun CH. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012;132:289–294. doi: 10.1016/j.foodchem.2011.10.079. [DOI] [PubMed] [Google Scholar]
- 21.Ma H, Chen H, Sun L, Tong L, Zhang T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia. 2014;93:54–61. doi: 10.1016/j.fitote.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Benard O, Chi Y. Medicinal properties of mangiferin, structural features, derivative synthesis, pharmacokinetics and biological activities. Mini Rev Med Chem. 2015;15:582–594. doi: 10.2174/1389557515666150401111410. [DOI] [PubMed] [Google Scholar]
- 23.Andreu GP, Delgado R, Velho JA, Curti C, Vercesi AE. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur J Pharmacol. 2005;513:47–55. doi: 10.1016/j.ejphar.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Leiro JM, Alvarez E, Arranz JA, Siso IG, Orallo F. In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumour necrosis factor-alpha and transforming growth factor-beta genes. Biochem Pharmacol. 2003;65:1361–1371. doi: 10.1016/s0006-2952(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 25.Rajendran P, Ekambaram G, Sakthisekaran D. Cytoprotective effect of mangiferin on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Basic & clinical pharmacology & toxicology. 2008;103:137–142. doi: 10.1111/j.1742-7843.2008.00254.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoshikawa M, Shimoda H, Nishida N, Takada M, Matsuda H. Salacia reticulata and its polyphenolic constituents with lipase inhibitory and lipolytic activities have mild antiobesity effects in rats. J Nutr. 2002;132:1819–1824. doi: 10.1093/jn/132.7.1819. [DOI] [PubMed] [Google Scholar]
- 27.Kishino E, Ito T, Fujita K, Kiuchi Y. A mixture of the Salacia reticulata (Kotala himbutu) aqueous extract and cyclodextrin reduces the accumulation of visceral fat mass in mice and rats with high-fat diet-induced obesity. J Nutr. 2006;136:433–439. doi: 10.1093/jn/136.2.433. [DOI] [PubMed] [Google Scholar]
- 28.Im R, Mano H, Nakatani S, Shimizu J, Wada M. Aqueous Extract of Kotahla Himbutu (Salacia reticulata) Stems Promotes Oxygen Comsumption and Supresses Body Fat Accumulation in Mice. J Health Sci. 2008;54:645–653. [Google Scholar]
- 29.Shimada T, Nagai E, Harasawa Y, Akase T, Aburada T, Iizuka S, Miyamoto K, Aburada M. Metabolic disease prevention and suppression of fat accumulation by Salacia reticulata. J Nat Med-Tokyo. 2010;64:266–274. doi: 10.1007/s11418-010-0401-1. [DOI] [PubMed] [Google Scholar]
- 30.Shimada T, Akase T, Kosugi M, Aburada M. Preventive Effect of Boiogito on Metabolic Disorders in the TSOD Mouse, a Model of Spontaneous Obese Type II Diabetes Mellitus. Evid-Based Compl Alt. 2011 doi: 10.1093/ecam/nep012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikarashi N, Toda T, Okaniwa T, Ito K, Ochiai W, Sugiyama K. Anti-Obesity and Anti-Diabetic Effects of Acacia Polyphenol in Obese Diabetic KKAy Mice Fed High-Fat Diet. Evid-Based Compl Alt. 2011 doi: 10.1093/ecam/nep241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross SM. African mango (IGOB131): a proprietary seed extract of Irvingia gabonensis is found to be effective in reducing body weight and improving metabolic parameters in overweight humans. Holistic nursing practice. 2011;25:215–217. doi: 10.1097/HNP.0b013e318222735a. [DOI] [PubMed] [Google Scholar]
- 33.Guo F, Huang C, Liao X, Wang Y, He Y, Feng R, Li Y, Sun C. Beneficial effects of mangiferin on hyperlipidemia in high-fat-fed hamsters. Molecular nutrition & food research. 2011;55:1809–1818. doi: 10.1002/mnfr.201100392. [DOI] [PubMed] [Google Scholar]
- 34.Apontes P, Liu ZB, Su K, Benard O, Youn DY, Li XS, Li W, Mirza RH, Bastie CC, Jelicks LA, Pessin JE, Muzumdar RH, Sauve AA, Chi YL. Mangiferin Stimulates Carbohydrate Oxidation and Protects Against Metabolic Disorders Induced by High-Fat Diets. Diabetes. 2014;63:3626–3636. doi: 10.2337/db14-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han J, Yi J, Liang FY, Jiang B, Xiao Y, Gao SH, Yang N, Hu HG, Xie WF, Chen WS. X-3, a mangiferin derivative, stimulates AMP-activated protein kinase and reduces hyperglycemia and obesity in db/db mice. Mol Cell Endocrinol. 2015;405:63–73. doi: 10.1016/j.mce.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Shimada T, Nagai E, Harasawa Y, Watanabe M, Negishi K, Akase T, Sai Y, Miyamoto K, Aburada M. Salacia reticulata inhibits differentiation of 3T3-L1 adipocytes. Journal of ethnopharmacology. 2011;136:67–74. doi: 10.1016/j.jep.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Niu Y, Li S, Na L, Feng R, Liu L, Li Y, Sun C. Mangiferin decreases plasma free fatty acids through promoting its catabolism in liver by activation of AMPK. PloS one. 2012;7:e30782. doi: 10.1371/journal.pone.0030782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim J, Liu Z, Apontes P, Feng D, Pessin JE, Sauve AA, Angeletti RH, Chi Y. Dual mode action of mangiferin in mouse liver under high fat diet. PloS one. 2014;9:e90137. doi: 10.1371/journal.pone.0090137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foufelle F, Ferre P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. The Biochemical journal. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. The Journal of clinical investigation. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. The Journal of biological chemistry. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 43.Stahl A. A current review of fatty acid transport proteins (SLC27) Pflugers Archiv : European journal of physiology. 2004;447:722–727. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- 44.Coburn CT, Hajri T, Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. Journal of molecular neuroscience : MN. 2001;16:117–121. doi: 10.1385/JMN:16:2-3:117. discussion 151–117. [DOI] [PubMed] [Google Scholar]
- 45.Rakhshandehroo M, Knoch B, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR research. 2010;2010 doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Lal J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. Journal of ethnopharmacology. 2005;97:497–501. doi: 10.1016/j.jep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Xing X, Li D, Chen D, Zhou L, Chonan R, Yamahara J, Wang J, Li Y. Mangiferin treatment inhibits hepatic expression of acyl-coenzyme A:diacylglycerol acyltransferase-2 in fructose-fed spontaneously hypertensive rats: a link to amelioration of fatty liver. Toxicology and applied pharmacology. 2014;280:207–215. doi: 10.1016/j.taap.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Zhou L, Pan Y, Chonan R, Batey R, Rong X, Yamahara J, Wang J, Li Y. Mitigation of Insulin Resistance by Mangiferin in a Rat Model of Fructose-Induced Metabolic Syndrome Is Associated with Modulation of CD36 Redistribution in the Skeletal Muscle. The Journal of pharmacology and experimental therapeutics. 2016;356:74–84. doi: 10.1124/jpet.115.229005. [DOI] [PubMed] [Google Scholar]
- 49.Guo FC, Huang CH, Liao XL, Wang YM, He Y, Feng RN, Li Y, Sun CH. Beneficial effects of mangiferin on hyperlipidemia in high-fat-fed hamsters. Mol Nutr Food Res. 2011;55:1809–1818. doi: 10.1002/mnfr.201100392. [DOI] [PubMed] [Google Scholar]
- 50.Niu YC, Li ST, Na LX, Feng RN, Liu LY, Li Y, Sun CH. Mangiferin Decreases Plasma Free Fatty Acids through Promoting Its Catabolism in Liver by Activation of AMPK. Plos One. 2012;7 doi: 10.1371/journal.pone.0030782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing X, Li D, Chen D, Zhou L, Chonan R, Yamahara J, Wang J, Li Y. Mangiferin treatment inhibits hepatic expression of acyl-coenzyme A:diacylglycerol acyltransferase-2 in fructose-fed spontaneously hypertensive rats: a link to amelioration of fatty liver. Toxicology and applied pharmacology. 2014 doi: 10.1016/j.taap.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Na LX, Zhang Q, Jiang S, Du SS, Zhang W, Li Y, Sun CH, Niu YC. Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: a double-blind randomized controlled trial. Sci Rep-Uk. 2015;5 doi: 10.1038/srep10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahali SK, Manna SK. Beta-D-glucoside protects against advanced glycation end products (AGEs)-mediated diabetic responses by suppressing ERK and inducing PPAR gamma DNA binding. Biochem Pharmacol. 2012;84:1681–1690. doi: 10.1016/j.bcp.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 54.Mahali SK, Verma N, Manna SK. Advanced glycation end products induce lipogenesis: regulation by natural xanthone through inhibition of ERK and NF-kappaB. Journal of cellular physiology. 2014;229:1972–1980. doi: 10.1002/jcp.24647. [DOI] [PubMed] [Google Scholar]
- 55.Kaul D, Anand PK, Khanna A. Functional genomics of PPAR-gamma in human immunomodulatory cells. Molecular and cellular biochemistry. 2006;290:211–215. doi: 10.1007/s11010-006-9169-8. [DOI] [PubMed] [Google Scholar]
- 56.Giron MD, Sevillano N, Salto R, Haidour A, Manzano M, Jimenez ML, Rueda R, Lopez-Pedrosa JM. Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: role of mangiferin. Clinical nutrition. 2009;28:565–574. doi: 10.1016/j.clnu.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Dudhia Z, Louw J, Muller C, Joubert E, de Beer D, Kinnear C, Pheiffer C. Cyclopia maculata and Cyclopia subternata (honeybush tea) inhibits adipogenesis in 3T3-L1 pre-adipocytes. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2013;20:401–408. doi: 10.1016/j.phymed.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson AS, Monteith GR, Shaw PN, Lin CN, Gidley MJ, Roberts-Thomson SJ. Effects of the mango components mangiferin and quercetin and the putative mangiferin metabolite norathyriol on the transactivation of peroxisome proliferator-activated receptor isoforms. Journal of agricultural and food chemistry. 2008;56:3037–3042. doi: 10.1021/jf800046n. [DOI] [PubMed] [Google Scholar]
- 59.Miura T, Ichiki H, Hashimoto I, Iwamoto N, Kato M, Kubo M, Ishihara E, Komatsu Y, Okada M, Ishida T, Tanigawa K. Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2001;8:85–87. doi: 10.1078/0944-7113-00009. [DOI] [PubMed] [Google Scholar]
- 60.Miura T, Iwamoto N, Kato M, Ichiki H, Kubo M, Komatsu Y, Sasaki H, Ishida T, Tanigawa K. Effect of Mangiferin on muscle GLUT4 protein content in TSOD (Tsumura, Suzuki, Obese, Diabetes) mouse, a new type 2 diabetic mice. Biomed Res-Tokyo. 2001;22:249–252. [Google Scholar]
- 61.Sellamuthu PS, Muniappan BP, Perumal SM, Kandasamy M. Antihyperglycemic Effect of Mangiferin in Streptozotocin Induced Diabetic Rats. J Health Sci. 2009;55:206–214. [Google Scholar]
- 62.Pal PB, Sinha K, Sil PC. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFalpha related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PloS one. 2014;9:e107220. doi: 10.1371/journal.pone.0107220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Peng G, Li Q, Wen S, Huang TH, Roufogalis BD, Yamahara J. Salacia oblonga improves cardiac fibrosis and inhibits postprandial hyperglycemia in obese Zucker rats. Life sciences. 2004;75:1735–1746. doi: 10.1016/j.lfs.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Saleh S, El-Maraghy N, Reda E, Barakat W. Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: role of adiponectin and TNF-alpha. Anais da Academia Brasileira de Ciencias. 2014;86:1935–1948. doi: 10.1590/0001-3765201420140212. [DOI] [PubMed] [Google Scholar]
- 65.Kumar BD, Krishnakumar K, Jaganathan SK, Mandal M. Effect of Mangiferin and Mahanimbine on Glucose Utilization in 3T3-L1 cells. Pharmacogn Mag. 2013;9:72–75. doi: 10.4103/0973-1296.108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M, Lee JS, Oh JE, Nan J, Lee H, Jung HS, Chung SS, Park KS. SIRT3 Overexpression Attenuates Palmitate-Induced Pancreatic beta-Cell Dysfunction. PloS one. 2015;10:e0124744. doi: 10.1371/journal.pone.0124744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan L, Deng X, Chen L, Zhou M. Regulation of insulin secretion and expression of SUR1 gene by chronic exposure to free fatty acids in rat pancreatic beta cells. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2004;24:358–360. 364. doi: 10.1007/BF02861867. [DOI] [PubMed] [Google Scholar]
- 68.Elsner M, Gehrmann W, Lenzen S. Peroxisome-generated hydrogen peroxide as important mediator of lipotoxicity in insulin-producing cells. Diabetes. 2011;60:200–208. doi: 10.2337/db09-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muruganandan S, Gupta S, Kataria M, Lal J, Gupta PK. Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology. 2002;176:165–173. doi: 10.1016/s0300-483x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 70.Sellamuthu PS, Arulselvan P, Kamalraj S, Fakurazi S, Kandasamy M. Protective nature of mangiferin on oxidative stress and antioxidant status in tissues of streptozotocin-induced diabetic rats. ISRN pharmacology. 2013;2013:750109. doi: 10.1155/2013/750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sellamuthu PS, Arulselvan P, Muniappan BP, Fakurazi S, Kandasamy M. Mangiferin from Salacia chinensis prevents oxidative stress and protects pancreatic beta-cells in streptozotocin-induced diabetic rats. Journal of medicinal food. 2013;16:719–727. doi: 10.1089/jmf.2012.2480. [DOI] [PubMed] [Google Scholar]
- 72.Li XJ, Du ZC, Huang Y, Liu BM, Hu WJ, Lu WJ, Deng JG. Synthesis and hypoglycemic activity of esterified-derivatives of mangiferin. Chinese journal of natural medicines. 2013;11:296–301. doi: 10.1016/S1875-5364(13)60032-1. [DOI] [PubMed] [Google Scholar]
- 73.Wang HL, Li CY, Zhang B, Liu YD, Lu BM, Shi Z, An N, Zhao LK, Zhang JJ, Bao JK, Wang Y. Mangiferin Facilitates Islet Regeneration and beta-Cell Proliferation through Upregulation of Cell Cycle and beta-Cell Regeneration Regulators. Int J Mol Sci. 2014;15:9016–9035. doi: 10.3390/ijms15059016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 75.Li XA, Cui XB, Sun XY, Li XD, Zhu Q, Li W. Mangiferin Prevents Diabetic Nephropathy Progression in Streptozotocin-Induced Diabetic Rats. Phytotherapy Research. 2010;24:893–899. doi: 10.1002/ptr.3045. [DOI] [PubMed] [Google Scholar]
- 76.Liu YW, Zhu X, Zhang L, Lu Q, Wang JY, Zhang F, Guo H, Yin JL, Yin XX. Up-regulation of glyoxalase 1 by mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2013;721:355–364. doi: 10.1016/j.ejphar.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 77.Pal PB, Sinha K, Sil PC. Mangiferin Attenuates Diabetic Nephropathy by Inhibiting Oxidative Stress Mediated Signaling Cascade, TNF alpha Related and Mitochondrial Dependent Apoptotic Pathways in Streptozotocin-Induced Diabetic Rats (vol 9, e107220, 2014) PloS one. 2014;9 doi: 10.1371/journal.pone.0107220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pal PB, Sinha K, Sil PC. Mangiferin, a natural xanthone, protects murine liver in Pb(II) induced hepatic damage and cell death via MAP kinase, NF-kappaB and mitochondria dependent pathways. PloS one. 2013;8:e56894. doi: 10.1371/journal.pone.0056894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He LY, Peng XF, Zhu JF, Chen X, Liu H, Tang CY, Dong Z, Liu FY, Peng YM. Mangiferin Attenuate Sepsis-Induced Acute Kidney Injury via Antioxidant and Anti-Inflammatory Effects. Am J Nephrol. 2014;40:441–450. doi: 10.1159/000369220. [DOI] [PubMed] [Google Scholar]
- 80.Zhu X, Cheng YQ, Du L, Li Y, Zhang F, Guo H, Liu YW, Yin XX. Mangiferin attenuates renal fibrosis through down-regulation of osteopontin in diabetic rats. Phytotherapy research : PTR. 2015;29:295–302. doi: 10.1002/ptr.5254. [DOI] [PubMed] [Google Scholar]
- 81.Yang H, Gao LH, Niu YF, Zhou YF, Lin H, Jiang J, Kong XF, Liu X, Li L. Mangiferin Inhibits Renal Urate Reabsorption by Modulating Urate Transporters in Experimental Hyperuricemia. Biol Pharm Bull. 2015;38:1591–1598. doi: 10.1248/bpb.b15-00402. [DOI] [PubMed] [Google Scholar]
- 82.Prabhu S, Jainu M, Sabitha KE, Shyamala Devi CS. Effect of mangiferin on mitochondrial energy production in experimentally induced myocardial infarcted rats. Vascular pharmacology. 2006;44:519–525. doi: 10.1016/j.vph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Hou J, Zheng D, Fung G, Deng H, Chen L, Liang J, Jiang Y, Hu Y. Mangiferin suppressed advanced glycation end products (AGEs) through NF-kappaB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Canadian journal of physiology and pharmacology. 2015:1–9. doi: 10.1139/cjpp-2015-0073. [DOI] [PubMed] [Google Scholar]
- 84.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: Implications for human obesity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang TH, Yang Q, Harada M, Uberai J, Radford J, Li GQ, Yamahara J, Roufogalis BD, Li Y. Salacia oblonga root improves cardiac lipid metabolism in Zucker diabetic fatty rats: modulation of cardiac PPAR-alpha-mediated transcription of fatty acid metabolic genes. Toxicology and applied pharmacology. 2006;210:78–85. doi: 10.1016/j.taap.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 86.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. The Journal of clinical investigation. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang TH, Peng G, Li GQ, Yamahara J, Roufogalis BD, Li Y. Salacia oblonga root improves postprandial hyperlipidemia and hepatic steatosis in Zucker diabetic fatty rats: activation of PPAR-alpha. Toxicology and applied pharmacology. 2006;210:225–235. doi: 10.1016/j.taap.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Goran MI, Walker R, Allayee H. Genetic-related and carbohydrate-related factors affecting liver fat accumulation. Current opinion in clinical nutrition and metabolic care. 2012;15:392–396. doi: 10.1097/MCO.0b013e3283544477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kulavarasalingam K, Bhatnagar D. Interaction between carbohydrates and lipid metabolism. Curr Opin Lipidol. 2014;25:401–402. doi: 10.1097/MOL.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 90.Spriet LL. New Insights into the Interaction of Carbohydrate and Fat Metabolism During Exercise. Sports Med. 2014;44:87–96. doi: 10.1007/s40279-014-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwamoto S, Boonvisut S, Makishima S, Ishizuka Y, Watanabe K, Nakayama K. The role of TRIB1 in lipid metabolism; from genetics to pathways. Biochem Soc T. 2015;43:1063–1068. doi: 10.1042/BST20150094. [DOI] [PubMed] [Google Scholar]
- 92.Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochimica et biophysica acta. 2012;1820:663–671. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 93.Yamagishi S, Matsui T. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. Nutrition. 2016;32:157–165. doi: 10.1016/j.nut.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Mastrocola R, Collino M, Rogazzo M, Medana C, Nigro D, Boccuzzi G, Aragno M. Advanced glycation end products promote hepatosteatosis by interfering with SCAP-SREBP pathway in fructose-drinking mice. American journal of physiology Gastrointestinal and liver physiology. 2013;305:G398–407. doi: 10.1152/ajpgi.00450.2012. [DOI] [PubMed] [Google Scholar]
- 95.Mastrocola R, Collino M, Nigro D, Chiazza F, D’Antona G, Aragno M, Minetto MA. Accumulation of advanced glycation end-products and activation of the SCAP/SREBP Lipogenetic pathway occur in diet-induced obese mouse skeletal muscle. PloS one. 2015;10:e0119587. doi: 10.1371/journal.pone.0119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mastrocola R, Nigro D, Chiazza F, Medana C, Dal Bello F, Boccuzzi G, Collino M, Aragno M. Fructose-derived advanced glycation end-products drive lipogenesis and skeletal muscle reprogramming via SREBP-1c dysregulation in mice. Free radical biology & medicine. 2015;91:224–235. doi: 10.1016/j.freeradbiomed.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 97.Machann J, Haring H, Schick F, Stumvoll M. Intramyocellular lipids and insulin resistance. Diabetes, obesity & metabolism. 2004;6:239–248. doi: 10.1111/j.1462-8902.2004.00339.x. [DOI] [PubMed] [Google Scholar]
- 98.Martins AR, Nachbar RT, Gorjao R, Vinolo MA, Festuccia WT, Lambertucci RH, Cury-Boaventura MF, Silveira LR, Curi R, Hirabara SM. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis. 2012;11 doi: 10.1186/1476-511X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. The Journal of clinical investigation. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS letters. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 102.McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Frontiers in endocrinology. 2013;4:52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pal M, Febbraio MA, Lancaster GI. The roles of c-Jun NH2 -terminal kinases (JNKs) in obesity and insulin resistance. The Journal of physiology. 2016;594:267–279. doi: 10.1113/JP271457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. New Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leiro J, Arranz JA, Yanez M, Ubeira FM, Sanmartin ML, Orallo F. Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. International immunopharmacology. 2004;4:763–778. doi: 10.1016/j.intimp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Tsubaki M, Takeda T, Kino T, Itoh T, Imano M, Tanabe G, Muraoka O, Satou T, Nishida S. Mangiferin suppresses CIA by suppressing the expression of TNF-alpha, IL-6, IL-1 beta, and RANKL through inhibiting the activation of NF-kappa B and ERK1/2. Am J Transl Res. 2015;7:1371–1381. [PMC free article] [PubMed] [Google Scholar]
- 107.Wei Z, Yan L, Deng J, Deng J. Effects of mangiferin on MAPK signaling pathway in chronic inflammation. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2011;36:1798–1802. [PubMed] [Google Scholar]
- 108.Dou W, Zhang J, Ren G, Ding L, Sun A, Deng C, Wu X, Wei X, Mani S, Wang Z. Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-kappaB and MAPK signaling inactivation. International immunopharmacology. 2014;23:170–178. doi: 10.1016/j.intimp.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Nie Y, Li Y, Hou Y, Zhao W, Deng J, Wang PG, Bai G. Identification of Target Proteins of Mangiferin in Mice with Acute Lung Injury Using Functionalized Magnetic Microspheres Based on Click Chemistry. Journal of agricultural and food chemistry. 2015;63:10013–10021. doi: 10.1021/acs.jafc.5b04439. [DOI] [PubMed] [Google Scholar]
- 110.Sahoo BK, Zaidi AH, Gupta P, Mokhamatam RB, Raviprakash N, Mahali SK, Manna SK. A natural xanthone increases catalase activity but decreases NF-kappa B and lipid peroxidation in U-937 and HepG2 cell lines. Eur J Pharmacol. 2015;764:520–528. doi: 10.1016/j.ejphar.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 111.Pan CW, Pan ZZ, Hu JJ, Chen WL, Zhou GY, Lin W, Jin LX, Xu CL. Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. European journal of pharmacology. 2016;770:85–91. doi: 10.1016/j.ejphar.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 112.DiMauro S, Schon EA. Mechanisms of disease: Mitochondrial respiratory-chain diseases. New Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 113.Lowell BB, Shulmanz GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 114.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiological reviews. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 116.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Murphy MP. How mitochondria produce reactive oxygen species. Biochemical Journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 119.Ceriello A. Oxidative stress and glycemic regulation. Metabolism: clinical and experimental. 2000;49:27–29. doi: 10.1016/s0026-0495(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 120.Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease - Roles, mechanisms, and therapeutic implications. Pharmacol Therapeut. 2001;89:187–206. doi: 10.1016/s0163-7258(00)00114-5. [DOI] [PubMed] [Google Scholar]
- 121.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. Journal of biochemical and molecular toxicology. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 122.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rajendran P, Rengarajan T, Nandakumar N, Divya H, Nishigaki I. Mangiferin in cancer chemoprevention and treatment: pharmacokinetics and molecular targets. Journal of receptor and signal transduction research. 2014:1–9. doi: 10.3109/10799893.2014.931431. [DOI] [PubMed] [Google Scholar]


