Abstract
Background
Factors associated with the prognosis of patients with small cell lung cancer (SCLC) is relatively unknown, than of those with non-small cell lung cancer. This study was undertaken to identify the prognostic factors of SCLC.
Methods
The medical records of 333 patients diagnosed with SCLC at tertiary hospital from January 1, 2008, to December 31, 2012 were retrospectively reviewed. Patients were categorized by age (≤65 years vs. >65 years) and by extent of disease (limited disease [LD] vs extensive disease [ED]). Overall survival and progression free survival rates were determined. Factors associated with prognosis were calculated using Cox's proportional hazard regression model.
Results
Most baseline characteristics were similar in the LD and ED groups. Eastern Cooperative Oncology Group (ECOG) performance status (PS), first chemotherapy regimen, and prophylactic cranial irradiation (PCI) differed significantly in patients with LD and ED. Mean ECOG PS was significantly lower (p<0.001), first-line chemotherapy with etoposide-cisplatin was more frequent than with etoposide-carboplatin (p<0.001), and PCI was performed more frequently (p=0.019) in LD-SCLC than in ED-SCLC. Prognosis in the LD group was better in younger (≤65 years) than in older (>65 years) patients, but prognosis in the ED group was unrelated to age.
Conclusion
This study showed that overall survival (OS) was significantly improved in younger than in older patients with LD-SCLC. Univariate and multivariate analyses showed that age, PCI and the sum of cycles were significant predictors of OS in patients with LD-SCLC. However, prognosis in the ED group was unrelated to age.
Keywords: Prognosis, Small Cell lung Carcinoma, Age Groups
Introduction
Lung cancer is one of the most common forms of cancer worldwide. Patients with this disease have a bad prognosis. The main risk factor of lung cancer is smoking. It can be divided into two major types, non-small cell lung cancer and small cell lung cancer (SCLC)1.
The prevalence of SCLC increases with age. At diagnosis, patients with SCLC can be subdivided into those with limited disease (LD) and extensive disease (ED). Multimorbid conditions have been associated with a slightly increased hazard of death in patients with LD-SCLC, independent of treatment2. By contrast, the prognosis in patients with ED-SCLC is associated with treatment, not with age2.
This study was designed to determine the prognostic factors affecting survival in patients with SCLC, who were diagnosed with this disease at Asan Medical Center from January 1, 2008, to December 31, 2012.
Materials and Methods
1. Patient selection
This study included 333 patients who were newly diagnosed with SCLC at tertiary hospital from January 1, 2008, to December 31, 2012.
2. Study design
Patient records were compiled and analyzed retrospectively using the ABLE3,4 (Asan Biomedical Research Environment) system. Factors analyzed included type of lung cancer, weight, height, body mass index, smoking history (pack-years [PY]), comorbidities (diabetes mellitus, heart failure, chronic renal failure, liver cirrhosis, cerebrovascular disease, and pulmonary tuberculosis), Eastern Cooperative Oncology Group (ECOG) performance status, stage at diagnosis, first chemotherapy regimen (etoposide-cisplatin [EP] and etoposide-carboplatin [EC]), the sum of cycles, concurrent chemoradiotherapy (CCRT) and cause of death.
Patients were divided into two groups by age (≤65 years vs. >65 years) and extent of disease (LD vs. ED).
Eligible SCLC patients were ambulatory. They were newly diagnosed of SCLC at tertiary hospital during this period. Patients had never been treated SCLC before. They had 0–2 in ECOG performance status. Patients aged ≤18 years were excluded. Primary end points is overall survival (OS). Secondary end points is progression-free survival (PFS).
3. Statistical analysis
Clinical factors in patients aged ≤65 years vs. >65 years and those with LD and ED were compared by Mann-Whitney U tests for continuous variables and chi-square tests or Fisher exact tests for categorical variables. OS and PFS were estimated by the Kaplan-Meier method, with results compared by log-rank tests. Risk factors for survival were determined by multivariable analysis using Cox's proportional hazard regression model. Statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA) and R ver. 3.2.0 (Microsoft (R), Seattle, WA, USA). All p-values were two-sided statistical (α=0.05), with p-values <0.05 considered statistically significant.
Results
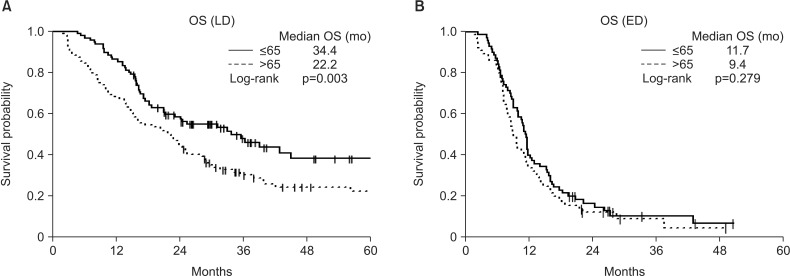
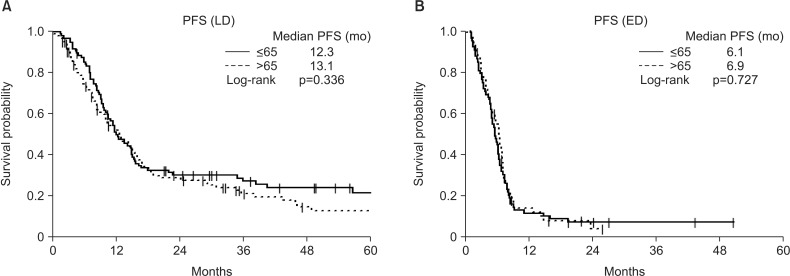
The baseline characteristics of the 333 included patients are shown in Table 1. By age, 165 of these patients were ≤65 years old and 168 were >65 years old. Of the 333 patients with SCLC, 198 had LD, including 95 aged ≤65 and 103 aged >65 years, whereas 135 patients had ED, including 70 aged ≤65 and 65 aged >65 years. A comparison of the two age groups showed that younger patients were significantly more likely to receive EP than EC as first-line chemotherapy and were significantly more likely to undergo prophylactic cranial irradiation (PCI). The results showed that first-line chemotherapy with EP more frequent than with EC, ≥4 which is the sum of cycles and PCI performed more frequently in aged ≤65 than aged >65 years in LD-SCLC and ED-SCLC (Tables 2, 3). We divided the cause of death as disease progression and others. The death by disease progression was 137 (80.1%) and others was 34 (19.9%) (Table 1). And each percent of disease progression and others was not different in LD patients (Table 2). But death by disease progression were much more than others regardless of age in ED patients (Table 3). A comparison of survival outcomes (Figure 1A) showed that OS was significantly greater in younger than in older patients with LD-SCLC but did not differ in younger and older patients with ED (Figure 1B). PFS (Figure 2A, B) analysis showed that PFS was similar in younger and older patients with LD and ED.
Table 1. Baseline characteristics.
| Variable | Total | LD (n=198) | ED (n=135) | p-value |
|---|---|---|---|---|
| Age, yr | 0.488 | |||
| ≤65 | 165 (49.5) | 95 (48.0) | 70 (51.9) | |
| >65 | 168 (50.5) | 103 (52.0) | 65 (48.1) | |
| BMI, kg/m2 | 0.767 | |||
| ≤23 | 162 (48.6) | 95 (48.0) | 67 (49.6) | |
| >23 | 171 (51.4) | 103 (52.0) | 68 (50.4) | |
| ECOG PS | <0.001 | |||
| 0, 1 | 239 (71.8) | 176 (88.9) | 63 (46.7) | |
| 2 | 94 (28.2) | 22 (11.1) | 72 (53.3) | |
| Smoking | 0.643 | |||
| Current or ex-smoker | 285 (86) | 168 (85) | 117 (87) | |
| Non-smoker | 48 (14) | 18 (13) | ||
| Smoking (PY) | 40.0 (30.0–50.0) | 40.0 (28.0–50.0) | 42.5 (30.0–50.0) | 0000.152 |
| Comorbidity | ||||
| DM | 55 (16.5) | 31 (15.7) | 24 (17.8) | 0000.609 |
| HF | 1 (0.3) | 1 (0.5) | 0 (0) | >0.999* |
| CRF | 0 (0) | 0 (0) | 0 (0) | |
| LC | 2 (0.6) | 1 (0.5) | 1 (0.7) | >0.999* |
| CVA | 8 (2.4) | 7 (3.5) | 1 (0.7) | 0.149* |
| Inactive TB | 32 (9.6) | 16 (8.1) | 16 (11.9) | 0.252 |
| First CTx regimen | <0.001 | |||
| EP | 257 (77.2) | 179 (90.4) | 78 (57.8) | |
| EC | 76 (22.8) | 19 (9.6) | 57 (42.2) | |
| The sum of cycles | 0.099 | |||
| 2–3 | 48 (14.4) | 22 (11.1) | 26 (19.3) | |
| ≥4 | 285 (85.6) | 176 (88.9) | 109 (80.7) | |
| Thoracic RT (CCRT) | 0.053 | |||
| Yes | 209 (62.8) | 177 (89.4) | 32 (23.7) | |
| PCI | 0.019 | |||
| Yes | 113 (33.9) | 77 (38.9) | 36 (26.7) | |
| Progression of disease | 0.022 | |||
| Progression | 56 (16.8) | 41 (20.7) | 15 (11.1) | |
| Non-progression | 277 (83.2) | 157 (79.3) | 120 (88.9) | |
| Cause of death | <0.001 | |||
| Progression | 137 (80.1) | 28 (52.8) | 109 (92.4) | |
| Else | 34 (9.9) | 25 (47.2) | 9 (7.6) |
Values are presented as number (%).
*Using Fisher exact test.
LD: limited disease; ED: extensive disease; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; PY: pack years; DM: diabetes mellitus; HF: heart failure: CRF: chronic renal failure; LC: liver cirrhosis; CVA: cerebrovascular disease; TB: tuberculosis; CTx: chemotherapy; EP: etoposide-cisplatin; EC: etoposide-carboplatin; RT: radiotherapy; CCRT: concurrent chemoradiotherapy; PCI: prophylactic cranial irradiation.
Table 2. Baseline characteristics (limited disease small cell lung cancer).
| Variable | Total | ≤65 Years (n=95) | >65 Years (n=103) | p-value |
|---|---|---|---|---|
| BMI, kg/m2 | 0.006 | |||
| ≤23 | 95 (48.0) | 36 (37.9) | 59 (57.3) | |
| >23 | 103 (52.0) | 59 (62.1) | 44 (42.7) | |
| ECOG PS | 0.360 | |||
| 0, 1 | 177 (89.3) | 83 (87.4) | 94 (91.3) | |
| 2 | 21 (10.7) | 12 (12.6) | 9 (8.7) | |
| Smoking | 0.178 | |||
| Current or ex-smoker | 168 (84.8) | 84 (88.4) | 84 (81.6) | |
| Non-smoker | 30 (15.2) | 11 (11.6) | 19 (18.4) | |
| Smoking (PY) | 40.0 (30.0–53.0) | 40.0 (23.0–47.5) | 45.0 (30.0–60.0) | 0.003 |
| Comorbidity | ||||
| DM | 31 (15.7) | 16 (16.8) | 15 (14.6) | 0.659 |
| HF | 1 (0.5) | 0 (0) | 1 (1.0) | >0.999* |
| CRF | 0 (0) | 0 (0) | 0 (0) | |
| LC | 1 (0.5) | 1 (1.1) | 0 (0) | 0.480* |
| CVA | 7 (3.5) | 2 (2.1) | 5 (4.9) | 0.447* |
| Inactive TB | 16 (8.1) | 8 (8.4) | 8 (7.8) | 0.866 |
| First CTx regimen | 0.003 | |||
| EP | 179 (90.4) | 92 (96.8) | 87 (84.5) | |
| EC | 19 (9.6) | 3 (3.2) | 16 (15.5) | |
| The sum of cycles | 0.303 | |||
| 2–3 | 22 (11.1) | 7 (7.4) | 15 (14.6) | |
| ≥4 | 176 (88.9) | 88 (92.6) | 88 (85.4) | |
| Thoracic RT (CCRT) | 0.338 | |||
| Yes | 177 (89.4) | 87 (91.6) | 90 (87.4) | |
| PCI | 0.002 | |||
| Yes | 77 (38.9) | 48 (50.5) | 29 (28.2) | |
| Progression of disease | 0.641 | |||
| Progression | 41 (20.7) | 21 (22.1) | 20 (19.4) | |
| Non-progression | 157 (79.3) | 74 (77.9) | 83 (80.6) | |
| Cause of death | 0.859 | |||
| Progression | 28 (52.8) | 15 (51.7) | 13 (54.2) | |
| Else | 25 (47.2) | 14 (48.3) | 11 (45.8) |
Values are presented as number (%).
*Using Fisher exact test.
BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; PY: pack years; DM: diabetes mellitus; HF: heart failure: CRF: chronic renal failure; LC: liver cirrhosis; CVA: cerebrovascular disease; TB: tuberculosis; CTx: chemotherapy; EP: etoposide-cisplatin; EC: etoposide-carboplatin; RT: radiotherapy; CCRT: concurrent chemoradiotherapy; PCI: prophylactic cranial irradiation.
Table 3. Baseline characteristics (extensive disease small cell lung cancer).
| Variable | Total | ≤65 Years (n=70) | >65 Years (n=65) | p-value |
|---|---|---|---|---|
| BMI, kg/m2 | 0.549 | |||
| ≤23 | 67 (49.6) | 33 (47.1) | 34 (52.3) | |
| >23 | 68 (50.4) | 37 (52.9) | 31 (47.7) | |
| ECOG PS | 0.645 | |||
| 0, 1 | 63 (46.7) | 34 (48.6) | 29 (44.6) | |
| 2 | 72 (53.3) | 36 (51.4) | 36 (55.4) | |
| Smoking | 0.499 | |||
| Current or ex-smoker | 117 (86.7) | 62 (88.6) | 55 (84.6) | |
| Non-smoker | 18 (13.3) | 8 (11.4) | 10 (15.4) | |
| Smoking (PY) | 40.0 (25.0–50.0) | 30.0 (20.0–45.0) | 40.0 (30.0–50.0) | 0.008 |
| Comorbidity | ||||
| DM | 24 (17.8) | 11 (15.7) | 13 (20.0) | 0.515 |
| HF | 0 (0) | 0 (0) | 0 (0) | |
| CRF | 0 (0) | 0 (0) | 0 (0) | |
| LC | 1 (0.7) | 0 (0) | 1 (1.5) | 0.482* |
| CVA | 1 (0.7) | 0 (0) | 1 (1.5) | 0.482* |
| Inactive TB | 16 (11.9) | 7 (10) | 9 (13.9) | 0.490 |
| First CTx regimen | <0.001 | |||
| EP | 78 (57.8) | 53 (75.7) | 25 (38.5) | |
| EC | 57 (42.2) | 17 (24.3) | 40 (61.5) | |
| The sum of cycles | 0.039 | |||
| 2–3 | 26 (19.3) | 11 (15.7) | 15 (23.1) | |
| ≥4 | 109 (80.7) | 59 (84.3) | 50 (76.9) | |
| Thoracic RT (CCRT) | 0.338 | |||
| Yes | 32 (23.7) | 21 (30.0) | 11 (16.9) | |
| PCI | 0.091 | |||
| Yes | 36 (26.7) | 23 (32.9) | 13 (20.0) | |
| Progression of disease | 0.128 | |||
| Progression | 15 (11.1) | 5 (7.1) | 10 (15.4) | |
| Non-progression | 120 (88.9) | 65 (92.9) | 55 (84.6) | |
| Cause of death | 0.016* | |||
| Progression | 59 (98.3) | 50 (86.2) | ||
| Else | 9 (7.6) | 1 (1.7) | 8 (13.8) |
*Using Fisher exact test.
BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; PY: pack years; DM: diabetes mellitus; HF: heart failure: CRF: chronic renal failure; LC: liver cirrhosis; CVA: cerebrovascular disease; TB: tuberculosis; CTx: chemotherapy; EP: etoposide-cisplatin; EC: etoposide-carboplatin; RT: radiotherapy; CCRT: concurrent chemoradiotherapy; PCI: prophylactic cranial irradiation.
Figure 1. (A, B) Subgroup analysis showed that overall survival (OS) was significantly longer in younger than in older patients with limited disease (LD) (p=0.003), but did not differ with age in extensive disease (ED) (p=0.279).
Figure 2. (A, B) Subgroup analysis showed that progression-free survival (PFS) was similar in all patients with limited disease (LD) (p=0.336) and extensive disease (ED) (p=0.727).
Univariate analysis showed that older age, increased number of smoking (PY), fewer the sum of cycles (EC/EP) and absence of PCI were associated with significantly shorter OS. Multivariate analysis showed that older age and fewer than four of cycles were statistically independent predictors of shorter OS, significantly (Table 4). Univariate analysis showed that fewer the sum of cycles (EC/EP) and absence of CCRT were associated with significantly shorter PFS. Multivariate analysis showed that fewer the sum of cycles (EC/EP), absence of CCRT and absence of PCI were statistically independent predictors of shorter PFS, significantly (Table 5).
Table 4. Univariate and multivariate analyses of factors associated with OS in patients with limited disease.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% Hazard ratio | p-value | Hazard ratio | 95% Hazard ratio | p-value | |
| Age (≤65 yr) | 0.590 | 0.413–0.841 | 0.003 | 0.503 | 0.344–0.735 | <0.001 |
| BMI (>23) | 0.782 | 0.553–1.107 | 0.166 | - | - | - |
| ECOG PS (2) | 1.026 | 0.566–1.860 | 0.933 | - | - | - |
| Smoking (current or Ex) | 0.685 | 0.439–1.070 | 0.096 | - | - | - |
| PY | 1.008 | 1.000–1.015 | 0.051 | - | - | - |
| DM (+) | 1.419 | 0.903–2.232 | 0.130 | - | - | - |
| HF (+) | 1 | 0.574–30.319 | 0.158 | - | - | - |
| LC (+) | 1.435 | 0.200–10.269 | 0.719 | - | - | - |
| CVA (+) | 0.553 | 0.176–1.740 | 0.311 | - | - | - |
| Inactive TB (+) | 0.880 | 0.461–1.680 | 0.699 | - | - | - |
| The sums of cycles | ||||||
| 3 | 0.855 | 0.338–2.166 | 0.741 | 1.633 | 0.626–4.26 | 0.316 |
| ≥4 | 0.257 | 0.129–0.512 | <0.001 | 0.242 | 0.12–0.487 | <0.001 |
| CCRT (no) | 1.450 | 0.758–2.773 | 0.261 | - | - | - |
| PCI (yes) | 0.561 | 0.388–0.810 | 0.002 | 0.604 | 0.409–0.891 | 0.011 |
| First CTx regimen (EP) | 0.844 | 0.475–1.499 | 0.563 | - | - | - |
OS: overall survival; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; Ex: stop smoking now; PY: pack years; DM: diabetes mellitus; HF: heart failure; LC: liver cirrhosis; CVA: cerebrovascular disease; TB: tuberculosis; CCRT: concurrent chemoradiotherapy; PCI: prophylactic cranial irradiation; CTx: chemotherapy; EP: etoposide-carboplatin.
Table 5. Univariate and multivariate analyses of factors associated with PFS in patients with LD.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% Hazard ratio | p-value | Hazard ratio | 95% Hazard ratio | p-value | |
| Age (≤65 yr) | 0.856 | 0.624–1.175 | 0.337 | - | - | - |
| BMI (>23) | 0.951 | 0.693–1.304 | 0.754 | - | - | - |
| ECOG PS (2) | 0.816 | 0.452–1.473 | 0.500 | - | - | - |
| Smoking (current or Ex) | 0.565 | 0.373–0.855 | 0.007 | 0.612 | 0.395–0.948 | 0.028 |
| PY | 1.003 | 0.996–1.011 | 0.339 | - | - | - |
| DM (+) | 1.084 | 0.693–1.695 | 0.725 | - | - | - |
| HF (+) | 0.000 | 0.000 | 0.982 | - | - | - |
| LC (+) | 1.607 | 0.224–11.542 | 0.637 | - | - | - |
| CVA (+) | 0.544 | 0.201–1.471 | 0.230 | - | - | - |
| Inactive TB (+) | 0.987 | 0.558–1.747 | 0.965 | - | - | - |
| The sums of cycles | - | |||||
| 3 | 0.227 | 0.075–0.684 | 0.008 | 0.827 | 0.269–2.54 | 0.74 |
| ≥4 | 0.161 | 0.072–0.360 | <0.001 | 0.15 | <0.001 | |
| CCRT (yes) | 2.531 | 1.237–5.179 | 0.011 | 4.605 | 1.913–11.084 | 0.001 |
| PCI (yes) | 0.749 | 0.543–1.034 | 0.079 | 0.683 | 0.486–0.96 | 0.028 |
| First CTx regimen (EP) | 1.208 | 0.669–2.178 | 0.531 | - | - | - |
progression-free survival; LD: limited disease; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; Ex: stop smoking now; PY: pack years; DM: diabetes mellitus; HF: heart failure; LC: liver cirrhosis; CVA: cerebrovascular disease; TB: tuberculosis; CCRT: concurrent chemoradiotherapy; PCI: prophylactic cranial irradiation; CTx: chemotherapy; EP: etoposide-carboplatin.
Discussion
This study found that patients with LD-SCLC had a better prognosis when aged ≤65 than >65 years. However, another studies5,6,7 found that age was not significantly prognostic of survival in patients with LD-SCLC. This discrepancy may have been due to differences in patient populations, including ethnicity and comorbidity. By contrast, age was not associated with prognosis in patients with ED-SCLC.
Older patients are associated with decreased performance status and increased comorbidity. Therefore, survival rates were lower with advancing age in LD-SCLC8. Also, other study2 announced that treatment led to a slightly increase of risk of death in patients with comorbidities in LD-SCLC. In ED-SCLC patients, OS was not different between two age groups. At diagnosis, the extension of disease were much larger regardless of age. Therefore, the prognosis have no difference by age in ED-SCLC patients.
Moreover, thoracic radiotherapy, CCRT, and platinum-based chemotherapy have been found to significantly improve survival9. Our results showed that younger age, PCI and increased the sum of cycles were associated with better prognosis, whereas CCRT and the first chemotherapy regimen were not. Although patients aged ≤65 years were treated more aggressively and had better prognosis than patients >65 years10, chemotherapy should not be withheld from older patients based solely on age. The survival of patients who receive chemotherapy is significantly longer than that of untreated patients, despite requiring frequent dose reductions for toxicity. Survival benefits are due to the effects of treatment and not to a selection bias in patients chosen for therapy11.
This study had several limitations. It is a retrospective design and the performance at a single center. And we had better study about treatment plan considering biological age, performance status and patient's attitude.
This study showed that OS was significantly improved in younger than in older patients with LD-SCLC. But, age was not associated with prognosis in patients with ED-SCLC. Clinician will make a decision about treatment considering biological age. It is multiple concept including comorbidity index and tolerability for treatment.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Martini B. Lung cancer: epidemiology, prognosis and therapy. Med Monatsschr Pharm. 2006;29:217–221. [PubMed] [Google Scholar]
- 2.Aarts MJ, Aerts JG, van den Borne BE, Biesma B, Lemmens VE, Kloover JS. Comorbidity in patients with small-cell lung cancer: trends and prognostic impact. Clin Lung Cancer. 2015;16:282–291. doi: 10.1016/j.cllc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Shin SY, Lyu Y, Shin Y, Choi HJ, Park J, Kim WS, et al. Lessons learned from development of de-identification system forbiomedical research in a Korean tertiary hospital. Healthc Inform Res. 2013;19:102–109. doi: 10.4258/hir.2013.19.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin SY, Park YR, Shin Y, Choi HJ, Park J, Lyu Y, et al. A Deidentification method for bilingual clinical texts of various note types. J Korean Med Sci. 2015;30:7–15. doi: 10.3346/jkms.2015.30.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siu LL, Shepherd FA, Murray N, Feld R, Pater J, Zee B. Influence of age on the treatment of limited-stage small-cell lung cancer. J Clin Oncol. 1996;14:821–828. doi: 10.1200/JCO.1996.14.3.821. [DOI] [PubMed] [Google Scholar]
- 6.Gridelli C, Casaluce F, Sgambato A, Monaco F, Guida C. Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that's the question. Transl Lung Cancer Res. 2016;5:150–154. doi: 10.21037/tlcr.2016.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi A, Maione P, Colantuoni G, Guerriero C, Ferrara C, Del Gaizo F, et al. Treatment of small cell lung cancer in the elderly. Oncologist. 2005;10:399–411. doi: 10.1634/theoncologist.10-6-399. [DOI] [PubMed] [Google Scholar]
- 8.Ludbrook JJ, Truong PT, MacNeil MV, Lesperance M, Webber A, Joe H, et al. Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? a community-based population analysis. Int J Radiat Oncol Biol Phys. 2003;55:1321–1330. doi: 10.1016/s0360-3016(02)04576-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Jiang R, Garces YI, Jatoi A, Stoddard SM, Sun Z, et al. Prognostic factors for limited-stage small cell lung cancer: a study of 284 patients. Lung Cancer. 2010;67:221–226. doi: 10.1016/j.lungcan.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radzikowska E, Roszkowski K, Glaz P. Lung cancer in patients under 50 years old. Lung Cancer. 2001;33:203–211. doi: 10.1016/s0169-5002(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd FA, Amdemichael E, Evans WK, Chalvardjian P, Hogg-Johnson S, Coates R, et al. Treatment of small cell lung cancer in the elderly. J Am Geriatr Soc. 1994;42:64–70. doi: 10.1111/j.1532-5415.1994.tb06075.x. [DOI] [PubMed] [Google Scholar]