Abstract
Tumor metastasis is the main reason for the poor prognosis of lung cancer patients. The GABAA receptor subunit GABRA3 is reportedly upregulated in lung cancer. Herein, we show that high GABRA3 protein expression in lung adenocarcinoma correlated positively with disease stage, lymphatic metastasis status and poor patient survival. In addition, GABRA3 induced MMP-2 and MMP-9 expression through activation of the JNK/AP-1 signaling pathway, which enhanced lymphatic metastasis by lung adenocarcinoma both in vitro and in vivo. These results indicate that GABRA3 promotes lymph node metastasis and may thus be an effective therapeutic target for anticancer treatment.
Keywords: non-small cell lung cancer, GABRA3, MMP, JNK/AP-1, lymphatic metastasis
INTRODUCTION
Lung cancer is a common malignancy with a high mortality rate that results in approximately a quarter million deaths per year in the United States alone [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all cases of lung cancer, more than 40% of which is lung adenocarcinoma (LUAD) [2]. Despite improvements in diagnostics and treatment strategies, the prognosis of metastatic lung adenocarcinoma remains poor, with the average 5-year survival rate of approximately 15% [3]. There is thus need to better understand the mechanisms of tumor metastasis in NSCLC and for development of a pharmacological intervention.
Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter in the mammalian brain that specifically interacts with two major classes of receptors: GABA A receptor (α1–6, β1–3, γ1–3, δ, ε, θ, π, ρ1–3) and GABA B receptor (GABBR1 and GABBR2) [4–6]. Fava et al. [7] reported that GABA treatment decreased the proliferation and metastatic potential of cholangiocarcinoma, and Joseph et al. [8] reported that GABA reduced migration of colon cancer. By contrast, Azuma et al. [9] showed that GABA promotes metastasis and invasion by prostate cancer cells through upregulation of metalloproteinase expression. One explanation for these conflicting results may be that different GABA receptor subunits mediate different responses via distinct intracellular signaling pathways, leading to beneficial or deleterious effects.
We previously investigated GABA receptor expression profiles in samples of NSCLC and non-cancerous lung tissues and found that gene expression of GABRA3, GABRE and GABBR2 was significantly higher in primary NSCLC tissues [10]. Moreover, expression of GABRA3 mRNA was associated with a poor prognosis in patients with NSCLC. Consistent with that observation, Liu et al. found that GABRA3 gene is overexpressed in NSCLC [11]. This suggests that dominant expression of the GABRA3 subunit may result in cancer progression. To test that idea, we assessed the effect GABRA3 on the development of lymphatic metastasis in NSCLC.
RESULTS
Upregulation of GABRA3 levels correlates with progression of LUAD
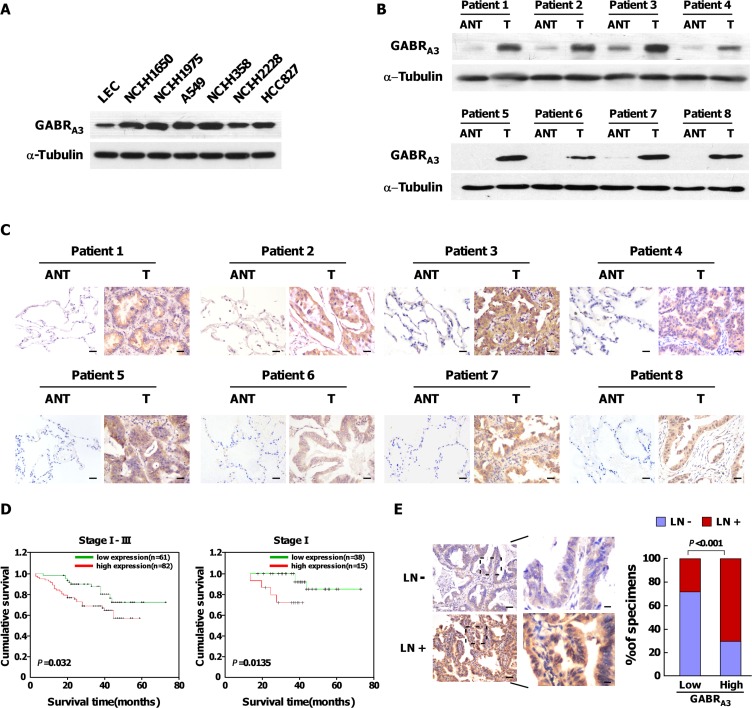
We analyzed the GABA receptor expression profile in 31 pairs of LUAD and their corresponding adjacent non-tumorous lung tissues using RNAseqV2 data sets for LUAD on the TCGA website (https://tcga-data.nci.nih.gov/tcga/). The results showed that the mRNA expression of GABRA3 (t = 3.477, P = 0.002), GABRA5 (t = 2.121, P = 0.042), GABRD (t = 3.259, P = 0.003), GABRG2 (t = 2.318, P = 0.027), and GABRQ (t = 2.219, P = 0.034) was higher in LUAD tissues (Supplementary Figures 1 and 2). In addition, expression of GABRA3 mRNA in lung squamous cell carcinoma was higher than in matched adjacent non-tumor tissues (t = 4.219, P = 0.0007; Supplementary Figure 3A). Using real-time quantitative PCR, we confirmed that GABRA3 gene expression was upregulated in LUAD cell lines and fresh clinical LUAD tissues as compared to paired non-cancerous tissues (Supplementary Figure 3B and 3C). Correspondingly, levels of GABRA3 protein were markedly higher in LUAD cell lines than in normal human lung epithelial cells (Figure 1A), and higher in clinical LUAD tissues than in matched adjacent non-tumor tissues (Figure 1B and 1C). These results demonstrate that GABRA3 is overexpressed in human LUAD, at both the protein and mRNA levels.
Figure 1. Upregulation of GABRA3 correlates with poor prognosis in LUAD.
(A) Western blots showing expression of GABRA3 protein in lung epithelial cells and LUAD cells. a-Tubulin was used as a loading control. (B–C)Comparison of GABRA3 protein expression between primary LUAD tissues (T) and matched adjacent non-tumor tissues (ANT) using Western blot analysis (B) and IHC (C). Scale bars, 50 μm. (D). Kaplan-Meier survival curves for LUAD patients showing low and high levels of GABRA3 expression. (E). Correlation of GABRA3 expression patients with lymphatic metastasis and those without metastasis. Left panel: micrographs of two representative cases. Scale bars, 50 mm; insets, 10 mm. Right panel: Chi-square analysis of the relation between low or high GABRA3 expression and lymphatic metastasis.
We also used immunohistochemistry to assess GABRA3 protein expression in 143 paraffin-embedded, archived LUAD tissue samples, including 54 cases of grade I (37.7%), 29 cases of grade II (20.3%), and 60 cases of grade III (42%; Supplementary Table 1). Statistical analyses revealed that levels of GABRA3 protein correlated significantly with TNM clinical stage (P < 0.001), lymph node metastasis status (P < 0.001) and patient survival (P = 0.042; Supplementary Table 2). Additionally, Kaplan-Meier survival analysis and log-rank test showed that GABRA3 overexpression correlated with shorter overall survival in stages I-III (P = 0.032) or stage I (P = 0.0135; Figure 1D), but not in stage II or III patients. Immunohistochemical detection of GABRA3 was adversely associated with survival in univariate analysis (P = 0.037; Supplementary Table 3), but was not statistically significant in the multivariate analysis (P = 0.554; Supplementary Table 3).
GABRA3 expression correlates with lymphatic metastasis and promotes the invasiveness of LUAD cells in vitro
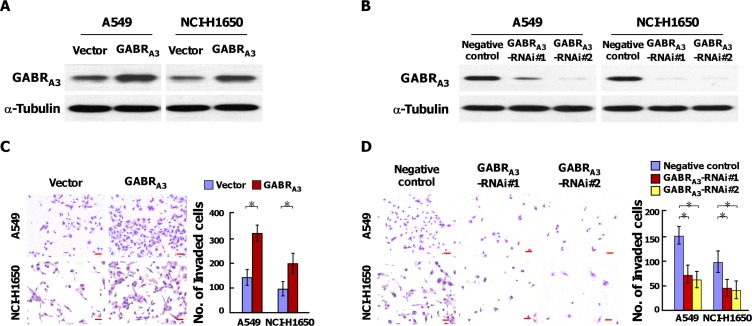
As shown in Figure 1E and Supplementary Table 2, levels of GABRA3 expression were higher in patients with lymphatic metastasis than without it. This suggests GABRA3 may contribute to lymphatic metastasis of LUAD. To test that idea, we established stable GABRA3-overexpressing and GABRA3 knockdown cells in the A549 and NCI-H1650 lines, respectively (Figure 2A and 2B). Subsequent Transwell matrix penetration assays showed that cells overexpressing GABRA3 were more invasive than control cells, whereas silencing GABRA3 diminished invasive activity (Figure 2C and 2D). Likewise, knocking down GABRA3 expression in our previously established primary Am1010 cells, derived from muscle metastases of a human lung adenocarcinoma (Supplementary Figure 5A) [12], inhibited invasive activity in Transwell assays (Supplementary Figure 5B). These results suggest that GABRA3 promotes the invasiveness of LUAD cells.
Figure 2. GABRA3 promotes the invasiveness of LUAD cells.
(A–B) Western blot analysis of the effect of overexpressing (A) or silencing (B) GABRA3 in the A549 and NCI-H1650 cell lines. a-Tubulin was used as a loading control. (C–D) Representative micrographs (left panel) and quantification (right panel) of invaded cells in a Transwell matrix penetration assay. Scale bars, 50 mm. Error bars depict the mean ± SD from three independent experiments, *P < 0.05.
In vitro MTT assay, it demonstrated that the proliferation rate of GABRA3-transduced cells, GABRA3-silenced cells or control cells has no significant difference (Supplementary Figure 4A and 4B). But, GABRA3-transduced cells were more resistant to cisplatin than vector cells, and GABRA3-silenced cells were more sensitive to cisplatin than vector cells in vitro (Supplementary Figure 4C and 4D).
GABRA3 increases lymph node metastasis in vivo
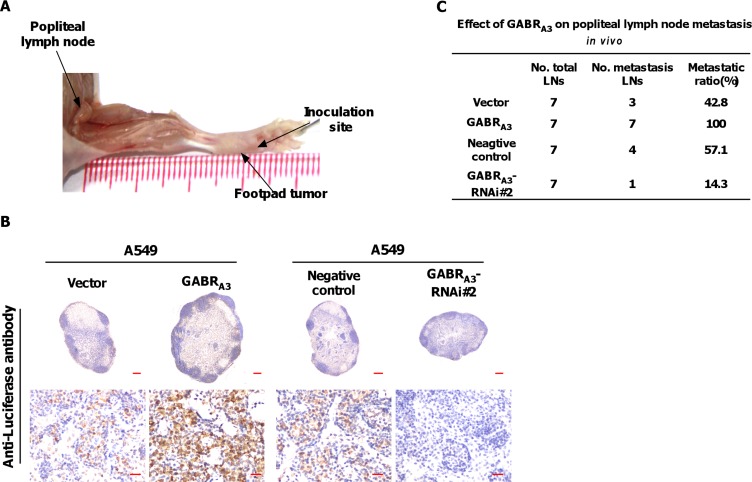
We inoculated firefly luciferase-expressing, GABRA3-overexpressing, GABRA3-silenced or control cells into the footpads of nude mice (n = 7/group; Figure 3A). When the popliteal lymph nodes were enucleated and fixed 5 weeks later, we found more luciferase-positive tumor cells within lymph nodes from mice injected with GABRA3-overexpressing cells than with vector-control cells (Figure 3B). Moreover, lymph nodes from mice injected with GABRA3-silenced cells had fewer luciferase-positive tumor cells (Figure 3B). Strikingly, the ratios of metastatic to total nodes were significantly higher in the GABRA3-overexpressing group (100% [7/7]) than the vector-control (42.8% [3/7]) or negative control groups (57.1% [4/7]). And only a single metastatic lymph node was detected in the GABRA3-silenced groups (Figure 3C). These results indicate that GABRA3 promotes lymph node metastasis of LUAD cells in vivo.
Figure 3. GABRA3 promotes lymph node metastasis in vivo .
(A) Representative micrographs of the popliteal lymph node metastasis model. The indicated cells stably expressing firefly luciferase were inoculated into the footpads of mice. (B) Representative micrographs of popliteal lymph nodes immunostained with anti-luciferase antibody. Scale bars: upper panel, 200 mm; lower panel, 20 mm. (C) Ratios of metastatic to total lymph notes in the indicated cells. *P < 0.05.
GABRA3 upregulates MMP-2 and MMP-9 expression in LUAD
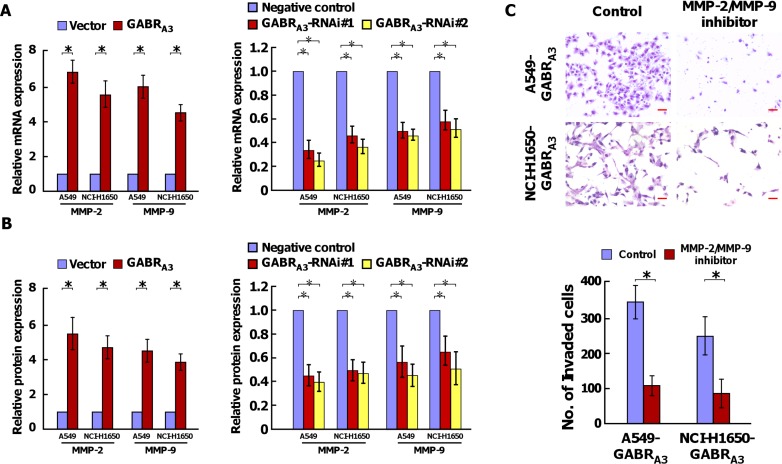
Real-time quantitative PCR analysis showed that levels of MMP-2 and MMP-9 mRNA were increased in GABRA3-overexpressing cells and decreased in GABRA3-silenced cells (Figure 4A and Supplementary Figure 5C). In addition, ELISAs showed that overexpressing GABRA3 also led to increased expression of MMP-2 and MMP-9 proteins, while silencing GABRA3 reduced expression of the two enzymes (Figure 4B and Supplementary Figure 5D). Moreover, inhibition of MMP-2 and MMP-9 enzyme activity using MMP-2/MMP-9 Inhibitor V (200 mM) reduced the invasive ability of GABRA3-overexpressing LUAD cells (Figure 4C).
Figure 4. GABRA3 induces MMP-2 and MMP-9 expression in LUAD cells.
(A) Real-time PCR analysis of MMP-2 and MMP-9 mRNA expression in the indicated cells. Transcript levels were normalized to GAPDH expression. (B) Levels of MMP-2 and MMP-9 protein in supernatants from the indicated cell cultures assessed using ELISAs. (C) Representative micrographs (upper panel) and quantification (lower panel) of invaded cells with or without treatment with MMP-2/MMP-9 inhibitor (200 nM). Scale bars, 50 mm. Error bars depict the mean ± SD of three independent experiments, *P < 0.05.
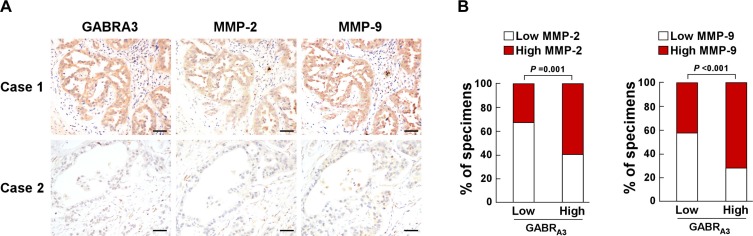
Immunohistochemical analysis of paraffin-embedded clinical LUAD specimens revealed that MMP-2 and MMP-9 expression was strong in samples with high GABRA3 expression, but low in samples with low GABRA3 level (Figure 5A). Chi-square analysis showed that GABRA3 expression correlated significantly with MMP-2 (P = 0.001) and MMP-9 expression (P < 0.001; Figure 5B). These results indicate that upregulation of MMP-2 and MMP-9 expression plays a central role in GABRA3-mediated LUAD invasiveness.
Figure 5. Clinical relevance between GABRA3 and MMP-2/MMP-9 expression in human LUAD.
(A) Relation between GABRA3 and MMP-2/MMP-9 expression. Shown are representative micrographs of two cases. Scale bars, 50 mm. (B) Percentages of LUAD specimens showing low- or high- GABRA3 expression in relation to MMP-2/MMP-9 expression.
JNK/AP-1 signaling pathway strongly contributes to GABRA3 induced MMP-2, MMP-9 expression and invasiveness
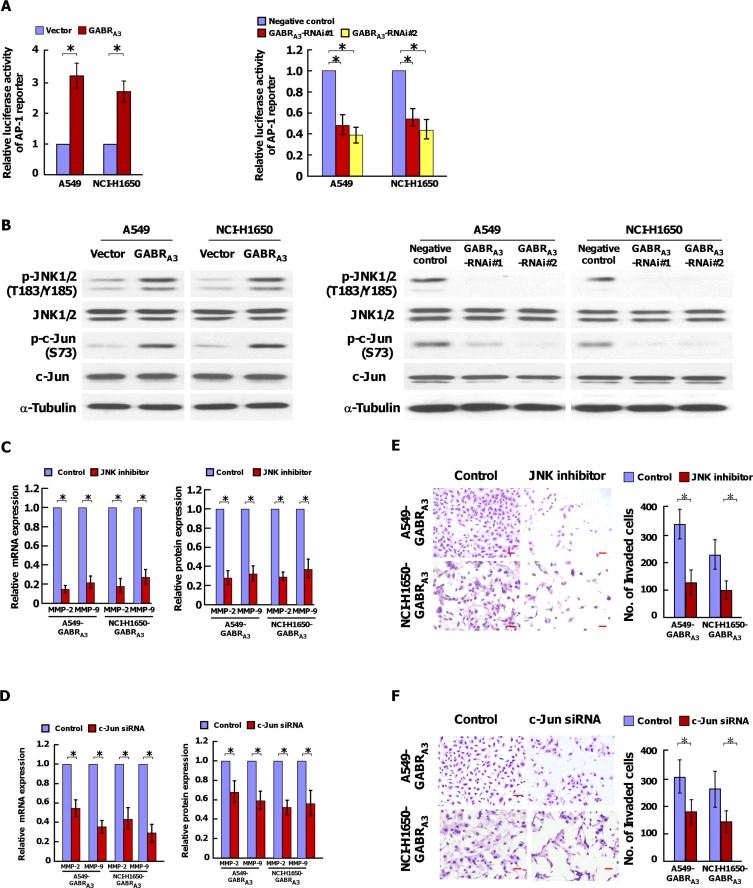
Using dual-luciferase assays, we found that the AP-1-driven luciferase reporter activity was stimulated in GABRA3-overexpessing cells, but inhibited in GABRA3 silencing cells (Figure 6A). Consistently, the transcriptional levels of cyclin D, cyclin E, p16INK4, p21CIP, Bcl-2, Bcl-xl, VEGF, downstream genes of AP-1 signaling pathway, were unregulated in GABRA3-transduced cells, and decreased in GABRA3-silenced cells (Supplementary Figure 6). And, overexpression of GABRA3 increased, and silencing GABRA3 decreased phosphorylation of JNK1/2 and c-Jun (Figure 6B). Inhibition of the JNK/AP-1 signaling pathway using a JNK inhibitor or silencing c-Jun using targeted siRNA reduced GABRA3-driven MMP-2 and MMP-9 expression (Figure 6C and 6D). Furthermore, the stimulatory effect of GABRA3 on LUAD invasiveness was reversed by a JNK enzyme inhibitor or c-Jun siRNA, which indicates the importance of the JNK/AP-1 signaling pathway for activation of GABRA3-induced LUAD invasion.
Figure 6. GABRA3 activates the JNK/AP-1 signaling pathway.
(A) Transcriptional activities of an AP-1 luciferase reporter plasmid in the indicated cells. (B) Western blot analysis of p-JNK1/2(T183/Y185), total JNK1/2, p-c-Jun (S73), and total c-Jun expression in the indicated cells. a-Tubulin was used as a loading control. (C and D) MMP-2 and MMP-9 mRNA and protein expression in the indicated cells with or without treatment with a JNK inhibitor (150 nM) (C) or c-Jun siRNA (D). E and F. Representative micrographs (left panel) and quantification (right panel) of invaded cells with or without treatment with a JNK inhibitor (E) or c-Jun siRNA (F). Scale bars, 50 mm. Error bars depict the mean ± SD of three independent experiments, *P < 0.05.
DISCUSSION
Lymph-node metastasis, a crucial step in tumor progression, is a risk factor for disease recurrence and poor prognosis in lung cancer [13, 14]. However, the molecular mechanism underlying lymph node metastasis remains poorly understood. In this study, we report that overexpression of GABRA3 in LUAD cells was significantly associated with lymphatic metastasis. Through selective modulation of a JNK/AP-1/MMPs pathway, GABRA3 promoted lymphatic metastasis in LUAD cells in vitro and in vivo.
GABRA3 is a subunit of the GABA A receptor [15]. Liu et al. demonstrated that GABRA3 is overexpressed in hepatocellular carcinoma and that GABA promoted hepatocellular carcinoma cell proliferation through overexpression of GABRA3 [16]. Liu et al. also reported that GABRA3 is overexpressed in NSCLC tissues, and that the level of GABRA3 expression was associated with the NSCLC grade [11]. Consistent with this observation, we previously showed that higher levels of GABRA3 mRNA are associated with disease progression in NSCLC patients [10]. In the present study, our univariate analysis showed that overexpression of GABRA3 protein was significantly associated with lymphatic metastasis status (P < 0.001) and patient survival (P = 0.032) in LUAD. In a multivariate analysis, however, GABRA3 expression was not a significant independent prognostic factor (P = 0.554). This may be due to insufficient sample size, or it could reflect the relation between GABRA3 expression and tumor stage. GABRA3 expression and tumor stage, including lymph node involvement, were highly correlated. As such, they would not be significant independent factors when entered into the Cox model at the same time.
MMP-2 and MMP-9 are members of a family of zinc-dependent enzymes that digest and degrade components of the extracellular matrix [17]. This facilitates multiple steps in cancer metastasis, including detachment, invasion, intravasation and extravasation, as well as angiogenesis and lymphangiogenesis [18–23]. Moreover, expression of MMP-2 and MMP-9 is upregulated in many cancer types, including lung cancer, breast cancer, and glioma, and is considered an important prognostic factor [24–26]. Upregulation of MMPs is induced by several oncogenic pathways, the including JNK/AP-1 signaling pathway [27–32]. In the present study, overexpression of GABRA3 increased MMP-2 and MMP-9 expression, while silencing GABRA3 reduced it. Inhibiting MMP-2/MMP-9 activity abrogated the invasiveness of GABRA3-overexpressing cells. In addition, overexpressing GABRA3 markedly increased, while silencing GABRA3 decreased, the expression of phospho-JNK1/2 (T183/Y185) and phospho-c-Jun (S73) in LUAD cells. What's more the expression of MMP-2/MMP-9 and the invasiveness of GABRA3-overexpressing cells was inhibited by the JNK inhibitor SP600125 or by c-Jun siRNA. Thus GABRA3-induced lymphatic metastasis in LUAD appears to be via activation of the JNK/AP-1/MMPs axis.
Identifying a key molecule involved in lymphatic metastasis could provide new therapeutic targets for cancer treatment. Our research on GABRA3 uncovered the novel molecular mechanism underlying the lymphatic metastasis in LUAD, and may lead to the development of a new therapeutic strategy for the treatment of LUAD.
MATERIALS AND METHODS
Cell lines
The A549, NCI-H1650, NCI-H1975, NCI-H358, NCI-H1395 and HCC827 lung adenocarcinoma cell lines were purchased from Shanghai Institutes of Biological Sciences (Shanghai, China) and cultured under the manufacturers suggested conditions. Normal human lung epithelial cells were collected for lung samples as described previously [33].
Patient information and tissue specimens
A total of 143 paraffin-embedded lung adenocarcinoma specimens that had been histopathologically and clinically diagnosed at the first Affiliated Hospital of Guangzhou Medical University were collected for this study. Follow-up information and cause of death were obtained from a review of telephone follow-ups conducted every 3 months. Twelve (8.39%) patients were lost to follow-up. Clinical information on the patients after complete surgical resection is summarized in Supplementary Table 1. Fresh lung adenocarcinoma tissues and the adjacent normal tissues were obtained from patients who underwent surgical resection with no other anticancer therapies before surgery in the Thoracic Surgery Department of The First Affiliated Hospital of Guangzhou Medical University. Normal lung tissues were obtained from individuals who underwent surgical resection of pulmonary bullae and were confirmed to be free of any prior pathologically detectable conditions. The use of these clinical materials for research purposes was approved by the patients and the Institutional Research Ethics Committee.
Plasmids, virus production and infection of target cells
The human GABRA3 coding sequence was amplified using PCR and cloned into the pMSCV vector. To silence endogenous GABRA3, two short hairpin RNA (shRNA; synthesized by Invitrogen) oligonucleotides (GCTGAAGTGGTTTATTCTTGG and GCTCTTTGCCATATTCAATCT) were separately cloned into pSuper-retro-puro vector. A non-targeting shRNA, TTCTCCGAACGTGTCACGT, was used as a negative control. HEK293T cell lines stably expressing GABRA3 and GABRA3 shRNA were generated through retroviral infection and selected using 0.5 μg/ml puromycin for 10 days. GABRA3 levels in the stable cells were detected by Western blotting.
Real-time quantitative PCR
The relative mRNA levels of selected genes were calculated as 2−[(Ct of gene) – (Ct of GAPDH)] normalized the level of GAPDH mRNA. The sequences of the primers were as follows: for GABRA3, 5′-TCGGTCTCTCCAAGTTTG TGC-3′ and 5′-TTCCGTTGTCCACCAATCTGA-3′; for MMP2, 5′-AAGAAGTAGC TGTGACCGCC-3′ and 5′-TTGCTGGAGACAAATTCTGG-3′; for MMP9, 5′-TTGG TCCACCTGGTTCAACT-3′ and 5′-ACGACGTCTTCCA GTACCGA-3′; for GAPDH, 5′-AATCCCATCACCATCT TCCA-3′ and 5′-CCTGCTTCACCACCTTCTTG-3′.
Western blotting (WB)
Western blotting was performed as previously described [34]. The primary antibodies used were anti-GABRA3 (Sigma, Saint Louis, MO), anti-p-JNK1/2, anti-JNK1/2, anti-p-c-Jun and anti-c-Jun (Abcam, Cambridge, MA). The blotted membranes were then stripped and reprobed with an anti-α-tubulin antibody (Sigma, Saint Louis, MO) as a loading control.
Chemical reagents
MMP-2/MMP-9 inhibitor V was purchased from Calbiochem (San Diego, CA; Cat. No. 444274). JNK inhibitor SP600125 was purchased from R&D Systems (Minneapolis, MN; Cat. No. 1496).
Immunohistochemistry (IHC)
IHC analysis was performed with 143 paraffin-embedded, archived samples of clinical LUAD tissue as previously described [34]. The intensity of immunostaining was scored separately by two independent pathologists. A staining index (SI) was calculated as the staining intensity score × the proportion of positive tumor cells. Cut-off values for high and low expression of proteins of interest were chosen on the basis of a measure of heterogeneity.
Popliteal lymph node metastasis model
Cells of interest labeled with firefly luciferase (3 × 106) were inoculated into the footpads of male BALB/c-nu mice (18–20 g), which were then randomly divided into groups (n = 7/group) at day 0. All mice were sacrificed after 5 weeks, and all popliteal lymph nodes were enucleated and fixed in formalin. To detect the cancer cells within the popliteal lymph nodes, serial 4.0-μm sections of paraffin-embedded samples were immunohistochemically analyzed using an anti-luciferase antibody (Abcam, Cambridge, MA).
ELISA
The concentrations of MMP-2 and MMP-9 in medium conditioned by cells of interest were determined using commercially available MMP-2 and MMP-9 ELISA Kits (Abcam, Cambridge, MA) according to the manufacturer's instructions.
Luciferase assay
AP-1 luciferase reporter plasmids plus pRL-TK renilla plasmid (Promega, Madison, WI) were transfected into cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Twenty-four hours after transfection, the luciferase and renilla signals were measured using a Dual Luciferase Reporter Assay Kit (Promega, Madison, WI).
Cell invasion assay
The indicated cells (2 × 104) in serum-free DMEM were plated on the top surface of polycarbonate membranes (coated with Matrigel) in Transwell chambers (Costar, Corning Inc., NY). After incubation for 22 h at 37°C, invaded cells on the lower membrane surface were fixed, stained, photographed and counted under a microscope (10 random fields per well, 100× magnification).
Statistical analysis
All statistical analyses were performed using SPSS 13.0 statistical software. Paired-samples t-tests were used to assess differences in gene expression between LUAD and adjacent non-tumorous lung tissues. The relationship between GABRA3 expression and the clinicopathological characteristics was assessed using the chi-square test. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. P < 0.05 was considered statistically significant in all experiments.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
ACKNOWLEDGMENTS AND FUNDING
This project was supported by the National Natural Science Foundation of China (Grant No. 81301998); the Pearl River Nova Program of Guangzhou (Grant No. 2014J2200011); the Natural Science Foundation for Distinguished Young Scholars of Guangdong Province (Grant No. 2014A030306013); the Science and Technology Planning Project of Guangdong Province, China (Grants No. 2007B031515017 and 2008A030201024); the Science and Technology Planning Project of Guangzhou, China (Grants No. 2007Z1-E0111 and 2007Z3-E0261), and the Ministry of Health, China (Grant No. W2011FAI01).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Authors' Contributions
Liping Liu and Chenglin Yang carried out most of the experimental work. Jianfei Shen and Liyan Huang conducted animal experiments. Weixuan Lin and Hailing Tang conducted Real-time PCR analysis and luciferase reporter assay. Wenhua Liang and Wenlong Shao conducted the data analysis. Haibo Zhang and Jianxing He directed the project and edited the paper. All the authors reviewed the manuscript.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P, International Staging C, Participating I. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 5.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 6.Kerr DI, Ong J. GABAB receptors. Pharmacol Ther. 1995;67:187–246. doi: 10.1016/0163-7258(95)00016-a. [DOI] [PubMed] [Google Scholar]
- 7.Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T, Taffetani S, Marzioni M, Summers R, et al. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–11446. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- 8.Joseph J, Niggemann B, Zaenker KS, Entschladen F. The neurotransmitter gamma-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 2002;62:6467–6469. [PubMed] [Google Scholar]
- 9.Azuma H, Inamoto T, Sakamoto T, Kiyama S, Ubai T, Shinohara Y, Maemura K, Tsuji M, Segawa N, Masuda H, Takahara K, Katsuoka Y, Watanabe M. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res. 2003;63:8090–8096. [PubMed] [Google Scholar]
- 10.Zhang X, Zhang R, Zheng Y, Shen J, Xiao D, Li J, Shi X, Huang L, Tang H, Liu J, He J, Zhang H. Expression of gamma-aminobutyric acid receptors on neoplastic growth and prediction of prognosis in non-small cell lung cancer. J Transl Med. 2013;11:102. doi: 10.1186/1479-5876-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Guo F, Dai M, Wang D, Tong Y, Huang J, Hu J, Li G. Gammaaminobutyric acid A receptor alpha 3 subunit is overexpressed in lung cancer. Pathol Oncol Res. 2009;15:351–358. doi: 10.1007/s12253-008-9128-7. [DOI] [PubMed] [Google Scholar]
- 12.Li HL, Xie SM, Zhang L, Cai CJ, Wang W, Huang J, Wang DY, Wen DP, Deng QH, Zhong NS, He JX. Establishment and characterization of a new drug surviving cell line Am1010, derived directly from muscle metastases of a human lung adenocarcinoma patient with multi-drug-resistance to cisplatin, taxol, and gefitinib. Acta Pharmacol Sin. 2010;31:601–608. doi: 10.1038/aps.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 14.Wright G, Manser RL, Byrnes G, Hart D, Campbell DA. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax. 2006;61:597–603. doi: 10.1136/thx.2005.051995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABA(A) receptor genes in the human genome. J Biol Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Li YH, Guo FJ, Wang JJ, Sun RL, Hu JY, Li GC. Gamma-aminobutyric acid promotes human hepatocellular carcinoma growth through overexpressed gamma-aminobutyric acid A receptor alpha 3 subunit. World J Gastroenterol. 2008;14:7175–7182. doi: 10.3748/wjg.14.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandler S, Miller KM, Clements JM, Lury J, Corkill D, Anthony DC, Adams SE, Gearing AJ. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–161. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 18.Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci. 2006;11:479–491. doi: 10.2741/1811. [DOI] [PubMed] [Google Scholar]
- 19.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 21.Fink K, Boratynski J. [The role of metalloproteinases in modification of extracellular matrix in invasive tumor growth, metastasis and angiogenesis] Postepy Hig Med Dosw (Online) 2012;66:609–628. doi: 10.5604/17322693.1009705. [DOI] [PubMed] [Google Scholar]
- 22.Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK, Kim JS, Oh SC. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011;71:7061–7070. doi: 10.1158/0008-5472.CAN-11-1338. [DOI] [PubMed] [Google Scholar]
- 23.Bruyere F, Melen-Lamalle L, Blacher S, Roland G, Thiry M, Moons L, Frankenne F, Carmeliet P, Alitalo K, Libert C, Sleeman JP, Foidart JM, Noel A. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods. 2008;5:431–437. doi: 10.1038/nmeth.1205. [DOI] [PubMed] [Google Scholar]
- 24.Iniesta P, Moran A, De Juan C, Gomez A, Hernando F, Garcia-Aranda C, Frias C, Diaz-Lopez A, Rodriguez-Jimenez FJ, Balibrea JL, Benito M. Biological and clinical significance of MMP-2, MMP-9, TIMP-1 and TIMP-2 in non-small cell lung cancer. Oncol Rep. 2007;17:217–223. [PubMed] [Google Scholar]
- 25.Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakada M, Okada Y, Yamashita J. The role of matrix metalloproteinases in glioma invasion. Front Biosci. 2003;8:e261–269. doi: 10.2741/1016. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 28.Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 29.Ozanne BW, McGarry L, Spence HJ, Johnston I, Winnie J, Meagher L, Stapleton G. Transcriptional regulation of cell invasion: AP-1 regulation of a multigenic invasion programme. Eur J Cancer. 2000;36:1640–1648. doi: 10.1016/s0959-8049(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 30.Lin CM, Chen YH, Ma HP, Wang BW, Chiu JH, Chua SK, Ong JR, Shyu KG. Silibinin inhibits the invasion of IL-6-stimulated colon cancer cells via selective JNK/AP-1/MMP-2 modulation in vitro. J Agric Food Chem. 2012;60:12451–12457. doi: 10.1021/jf300964f. [DOI] [PubMed] [Google Scholar]
- 31.Hong IK, Kim YM, Jeoung DI, Kim KC, Lee H. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp Mol Med. 2005;37:230–239. doi: 10.1038/emm.2005.31. [DOI] [PubMed] [Google Scholar]
- 32.Shin M, Yan C, Boyd D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim Biophys Acta. 2002;1589:311–316. doi: 10.1016/s0167-4889(02)00195-7. [DOI] [PubMed] [Google Scholar]
- 33.Cai J, Fang L, Huang Y, Li R, Yuan J, Yang Y, Zhu X, Chen B, Wu J, Li M. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res. 2013;73:5402–5415. doi: 10.1158/0008-5472.CAN-13-0297. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Zhang N, Song LB, Liao WT, Jiang LL, Gong LY, Wu J, Yuan J, Zhang HZ, Zeng MS, Li M. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14:3319–3326. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.