Abstract
Follicular dendritic-cell sarcoma (FDCS) is a rare and recalcitrant disease. In the present study, a patient-derived orthotopic xenograft (PDOX) mouse model of FDCS was established in the biceps muscle of nude mice. The FDCS PDOX was resistant to both doxorubicin (DOX) and NVP-BEZ235, dactolisib (BEZ) an experimental agent which is a dual pan-phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor. However, in contrast to DOX and BEZ, the FDCS PDOX was sensitive to the tumor-targeting bacterial strain, Salmonella typhimurium A1-R (S. typhimurium A1-R). The combination of S. typhimurium A1-R and either DOX or BEZ did not increase the antitumor efficacy of S. typhimurium A1-R, indicating that DOX and BEZ were not active in this PDOX model. The efficacy of S. typhimurium A1-R in this recalcitrant FDCS gives strong impetus to move bacterial therapy to clinical trials for this disease. The findings of the present study are of particular importance since it demonstrates that S. typhimurium A1-R is effective in a PDOX model of FDCS established from a patient who failed DOX therapy.
Keywords: Salmonella typhimurium A1-R, tumor-targeting, GFP, sarcoma, soft-tissue
INTRODUCTION
Follicular dendritic-cell sarcoma (FDCS) is a highly recalcitrant disease. FDCS is rare and arises from follicular dendritic cells [1]. CHOP chemotherapy, which contains cyclophosphamide (CTX), doxorubicin (DOX), vincristine and prednisone, is most frequently used with FDCS with transient, partial responses observed in some patients [2]. Complete responses to CHOP are infrequent [1]. The 5-year overall survival (OS) for localized FDCS is 55% and for metastatic disease is 38% [3]. Therefore, novel approaches to FDCS are needed [1, 2].
The tumor-targeting Salmonella typhimurium A1-R (S. typhimurium A1-R) strain was developed by our laboratory [4]. S. typhimurium A1-R is auxotrophic for Leu–Arg, which prevents it from mounting a continuous infection in normal tissues. S. typhimurium A1-R was able to inhibit or eradicate primary and metastatic tumors as monotherapy in nude mouse models of major cancers [5], including prostate [6, 7], breast [8–10], lung [11, 12], pancreatic [13–17], ovarian [18, 19] stomach [20], and cervical cancer [21], as well as sarcoma cell lines [22–25] and glioma [26, 27], all of which are highly aggressive tumor models.
Previously, we developed a patient-derived nude-mouse model of soft tissue sarcoma resistant to gemcitabine. However, S. typhimurium A1-R significantly inhibited tumor growth compared to the untreated mice. These results suggest tumor-targeting S. typhimurium A1-R is a promising treatment for chemo-resistant soft tissue sarcoma [28].
Recently, a patient with high-grade undifferentiated pleomorphic soft tissue sarcoma from a striated muscle was grown in the right biceps femoris muscle of mice to establish a patient-derived orthotopic xenograft (PDOX) model. This sarcoma PDOX was sensitive to DOX and S. typhimurium A1-R followed by DOX, could eradicate this tumor [25].
The present study evaluates S. typhimurium A1-R efficacy on a DOX-resistant FDCS PDOX model established from a patient who failed DOX therapy.
RESULTS AND DISCUSSION
The treatment schedule for the FDCS PDOX is shown in Figure 1. Three weeks after orthotopic implantation, tumors reached 5 mm in diameter and continued to grow rapidly (Figure 1A).
Figure 1. PDOX model of follicular dendritic-cell sarcoma (FDCS) and treatment protocol.
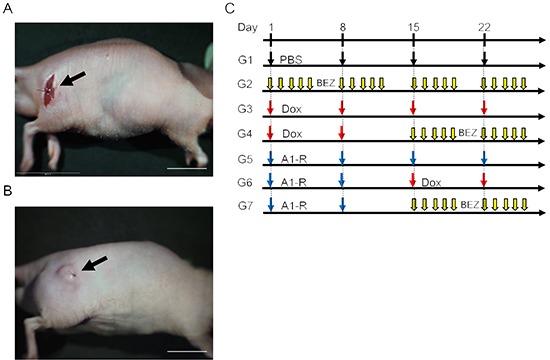
A. During the sarcoma transplant procedure in the muscle. B. Three weeks after implantation. C. Treatment protocol.
After intraperitoneal (i.p.) administration of S. typhimurium A1-R for four weeks, and two subsequent weeks without treatment, the green fluorescent protein (GFP)-expressing bacteria could be visualized by fluorescence imaging in the resected tumor. S. typhimurium A1-R was imaged directly as well as by mincing of the tumor and subsequent colony outgrowth from the minced tissue on agar medium (Figure 2).
Figure 2. Imaging tumor-targeting Salmonella typhimurium A1-R in the FDCS PDOX.
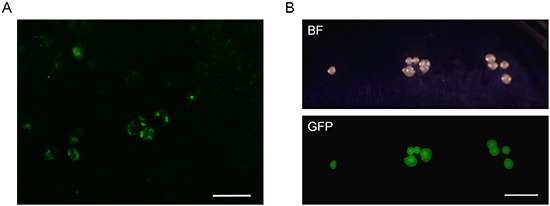
A. FDCS was resected from a PDOX model after 4 weeks treatment of S. typhimurium A1-R and a subsequent two weeks without treatment. FV1000 confocal microscopy. Scale bar = 100 μm. B. Colonies of S. typhimurium A1-R isolated from the tumor of the bacterially-treated FDCS PDOX after 4 weeks treatment and a subsequent 2 weeks without treatment.
The FDCS PDOX was resistant to doxorubicin (DOX) (p = 0.11 at day-22 of treatment, Group 3) (Figure 3). The FDCS PDOX was also resistant to NVP-BEZ235 (dactolisib) (BEZ), which is a dual pan-phosphoinositide 3-kinase-mammalian target of rapamycin mTOR inhibitor [29] (p = 0.48 at day-18 of treatment, Group 2). In a Phase II trial, investigators reported a durable partial response in a patient with metastatic FDCS treated with ridaforolimus, an mTOR inhibitor [30]. The FDCS PDOX was also resistant to the combination of DOX and BEZ (p = 0.14 at day-22, Group 4).
Figure 3. Efficacy of chemotherapy and S. typhimurium A1-R in the FDCS PDOX A, B.
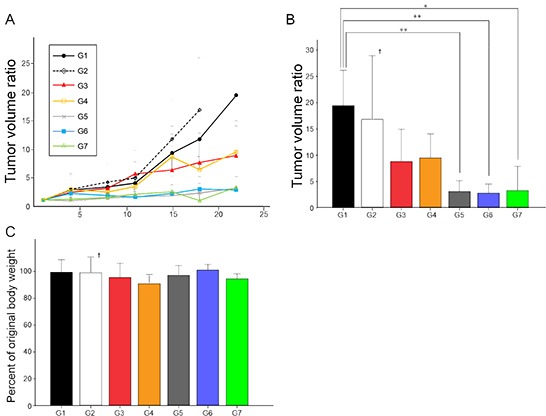
Group 1, control with PBS, i.p.; Group 2, treated with BEZ, 50 mg/kg oral gavage, q5/W for 4 weeks; Group 3, treated with DOX, 2.4 mg/kg, i.p., qW for 4 weeks; Group 4, treated with DOX, 2.4 mg/kg, i.p., qW for 2 weeks; and BEZ, 50 mg/kg, oral gavage, q5/W for 2 weeks; Group 5, treated with S. typhimurium A1-R, 2.5 × 107 CFU, i.p., qW for 4 weeks; Group 6, treated with S. typhimurium A1-R, 2.5 × 107 CFU, i.p., qW for 2 weeks followed by DOX, 2.4 mg/kg, i.p., qW for 2 weeks; and Group 7, treated with S. typhimurium A1-R, 2.5 × 107 CFU, i.p., qW for 2 weeks followed by BEZ, 50 mg/kg, oral gavage, q5/W for 2 weeks. Tumor volume ratio in Group 5 (3.11 ± 2.05, p < 0.01); Group 6 (2.80 ± 1.72, p < 0.01); and Group 7 (3.28 ± 4.62, p < 0.05) were significantly lower than in Group 1 (19.44 ± 6.70). There were not significant differences between any other groups. C. Bar graph shows percentage of original body weight of mice in each group on 22nd day from initial treatment except for Group 2, which was on day-18. Actual weights at these time points were: Group 1: 22.24 ± 1.73; Group 2: 26.13 ± 1.54; Group 3: 22.94 ± 2.01; Group 4: 22.88 ± 1.20; Group 5: 25.32 ± 2.40; Group 6: 25.34 ± 1.52; and Group 7: 23.2 ± 2.21. There were not significant differences between any treated groups and control. *p < 0.05, **p < 0.01. Error bars: ± 1 SD. †The graph of only G2 shows tumor volume ratio on day-18. Error bars: ± 1 SD.
However, in contrast to DOX and BEZ, the FDCS PDOX was sensitive to the tumor-targeting bacterial strain, S. typhimurium A1-R (p < 0.05 at day-22, Group 5) (Figure 3A, 3B). The combination of S. typhimurium A1-R and either DOX (Group 6) or BEZ (Group 7) did not increase the antitumor efficacy of S. typhimurium A1-R (Figure 3), indicating that DOX and BEZ were not active against this tumor. The tumor-volume ratio in Group 5, S. typhimurium A1-R (3.11 ± 2.05, p < 0.01); Group 6, S. typhimurium A1-R and DOX (2.80 ± 1.72, p < 0.01); and Group 7, S. typhimurium A1-R and BEZ (3.28 ± 4.62, p < 0.05) were significantly lower than in Group 1, untreated control (19.44 ± 6.70) (Figure 3B). There were not significant differences between any other groups. Since BEZ alone was inactive, it is not surprising it also had no effect in combination with DOX. Sequential treatment was given with S. typhimurium A1-R followed by either DOX or BEZ. The goal of this experiment was to determine if S. typhimurium A1-R could sensitize the tumor by decoying the quiescent cells in the tumor to begin to cycle and therefore become more responsive to the chemotherapy [20]. However, the tumor was sufficiently sensitive to S. typhimurium A1-R that no further tumor inhibition could be observed in the combination with either an inactive or slightly active drug compared to S. typhimurium A1-R alone.
There were no significant body-weight differences between the groups (Figure 3C). Actual mouse weights at day-22 for all groups, except Group 2 which was determined day-18, were Group 1 (untreated control): 22.24 ± 1.73; Group 2 (BEZ): 26.13 ± 1.54; Group 3 (DOX): 22.94 ± 2.01; Group 4 (BEZ + DOX): 22.88 ± 1.20; Group 5 (S. typhimurium A1-R): 25.32 ± 2.40; Group 6 (S. typhimurium A1-R + DOX): 25.34 ± 1.52; and Group 7 (S. typhimurium A1-R + BEZ): 23.2 ± 2.21.
Histology
The patient's original tumor (Figure 4A) and untreated PDOX (Figure 4B) showed identical histologic features which are characteristic for FDCS, including relatively uniform ovoid-to spindle-shaped cells present in short fascicles and storiform patterns with associated lymphocytes sprinkled throughout the tumor. Similar to the original tumor, the PDOX showed a high rate of mitoses (>20/10 High-power fields [HPFs]) (Figure 4B). The patient's original tumor was positive for CD35 and fascin by immunohistochemistry (data not shown), supporting the diagnosis of FDCS.
Figure 4. Photomicrographs of H&E-stained slides from the original patient tumor and the PDOX-grown tumor.
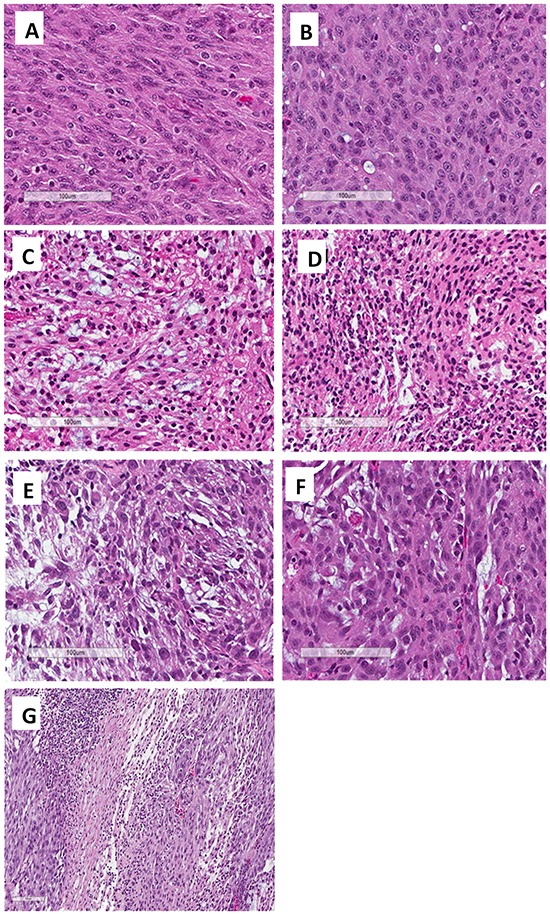
A. The patient's original tumor. B. Untreated PDOX tumor. C. Treated tumor with BEZ (Group 2, 5% necrosis). D. Treated tumor with DOX (Group 3, 20% necrosis). E. Treated tumor with S. typhimurium A1-R (Group 5, 50% necrosis). F. Treated tumor with S. typhimurium A1-R and DOX (Group 6, 20% necrosis). G. Treated tumor with S. typhimurium A1-R and BEZ (Group 7, 60% necrosis). Scale bars: 100 μm.
Review of xenograft sections showed the following approximate amount of necrosis: Group 1: control with PBS (0%, Figure 4B), Group 2: BEZ-235 (5%, Figure 4C), Group 3: DOX (20%, Figure 4D); Group 5: S. typhimurium A1-R (50%, Figure 4E); Group 6: S. typhimurium A1-R and DOX (20%, Figure 4F); Group 7: S. typhimurium A1-R+BEZ (60%, Figure 4G). Group 7 was greatly inhibited by S. typhimurium A1-R. Future studies will examine the extent of necrosis in S. typhimurium A1-R-treated tumors. The BEZ-treated tumors grew extensively and may have outgrown their blood supply and thus became slightly necrotic.
In a previous study, a human patient with advanced leiomyosarcoma was treated with an intra-tumoral injection of Clostridium novyi (C. novyi-NT) spores which reduced the tumor within and surrounding the bone [31], indicating the clinical potential of bacterial therapy of sarcoma. Since S. typhimurium A1-R is a facultative anaerobe, unlike C. novyi-NT which is an obligate anaerobe, it may have more broad application for cancer therapy. S. typhimurium A1-R can greatly potentiate cytotoxic chemotherapy [20]. S. typhimurium A1-R was recently shown to potentiate DOX in a PDOX model of high-grade undifferentiated soft-tissue sarcoma from a striated muscle [25].
In a Phase I clinical trial of patients with metastatic melanoma and renal carcinoma, the S. typhimurium strain tested (VNP20009), attenuated by msbB and purI mutations, was safely administered to patients [32]. The results of the present study suggest S. typhimurium A1-R is a candidate for clinical trial to treat DOX-resistant FDCS.
The findings of the present study are of particular importance since it demonstrates that S. typhimurium A1-R is effective in the PDOX model of FDCS established from a patient who failed DOX therapy and whose PDOX is DOX-resistant.
Previously developed concepts and strategies of highly selective tumor targeting [33–38] can take advantage of bacterial targeting of tumors, including tissue-selective therapy which focuses on unique properties of normal and tumor tissues [33, 38]. S. typhimurium A1-R can possibly overcome de-differentiation of a tumor leading to resistance to targeted chemotherapy, where the targeted protein or pathway may no longer be expressed [38], since S. typhimurium A1-R does not depend on such targets [33, 35]. S. typhimurium A1-R may also be effectively combined with teratogens which could selectively affect cancer cells that are dedifferentiated [34]. Since S. typhimurium A1-R can decoy quiescent cancer cells to begin to cycle, S. typhimurium A1-R could be effectively combined with agents that selectively target proliferating cancer cells [36], where normal cells are protected by agents which induce wild type p53 [37].
MATERIALS AND METHODS
Mice
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4-6 weeks old, were used in this study. All animal studies were conducted with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principals and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. In order to minimize any suffering of the animals, the use of anesthesia and analgesics were used for all surgical experiments. Animals were anesthetized by subcutaneous injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg xylazine, and 0.48 mg/kg acepromazine maleate. The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation when they met the following humane endpoint criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body temperature drop. Animals were housed in a barrier facility on a high efficiency particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet [25].
Patient-derived tumor
A female patient diagnosed with a recurrent extranodal FDCS of the left lower extremity underwent surgical resection. She previously received adjuvant radiotherapy to the left lower extremity following resection of the primary tumor in 2014 and four cycles of chemotherapy with DOX and CTX for her recurrent disease. Chemotherapy was discontinued in April of 2015 due to medical co-morbidities and her inability to tolerate therapy. Surgical resection of the recurrent extra-nodal left lower extremity FDCS was performed by FCE on July 15, 2015. Written informed consent was obtained from the patient as part of a UCLA Institutional Review Board (IRB #10-001857)-approved protocol [25].
Establishment of a PDOX model of FDCS by surgical orthotopic implantation (SOI)
A fresh sample of the FDCS of the patient was obtained and transported immediately to the laboratory at AntiCancer, Inc., on wet ice. The sample was cut into 5-mm fragments and implanted subcutaneously in nude mice. After three weeks, the subcutaneously-implanted tumors grew to more than 10 mm in diameter. The subcutaneously-grown tumors were then harvested and cut into small fragments (3 mm3). After nude mice were anesthetized with the ketamine solution described above, a 5-mm skin incision was made on the right high thigh into the biceps femoris, which was split to make space for the sarcoma tissue fragment. A single tumor fragment was implanted orthotopically into the space to establish the PDOX model. The wound was closed with a 6-0 nylon suture (Ethilon, Ethicon, Inc., NJ, USA) [25].
Preparation and administration of S. typhimurium A1-R
S. typhimurium A1-R expressing GFP (AntiCancer, Inc., San Diego, CA, USA) was grown overnight on LB medium and then diluted 1:10 in LB medium. Bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS. S. typhimuriumA1-R was injected intraperitoneally. A total of 2 × 107 CFU S. typhimurium A1-R in 50 μl PBS was administered intraperitoneally (i.p.) in the follicular dendritic cell sarcoma-bearing mice [25].
Treatment study design in the PDOX model of soft tissue sarcoma
PDOX mouse models were randomized into seven groups of five mice each (Figure 1): Group 1, control with PBS, i.p.; Group 2, treated with BEZ, 50 mg/kg, oral gavage, q5/W for 4 weeks; Group 3, treated with DOX, 2.4 mg/kg, i.p., qW for 4 weeks; Group 4, treated with DOX, 2.4 mg/kg, i.p., qW for 2 weeks; and BEZ, 50 mg/kg, oral gavage, q5/W for 2 weeks; Group 5, treated with S. typhimurium A1-R, 2.5 × 107 CFU, i.p., qW for 4 weeks; Group 6, treated with S. typhimurium A1-R, 2.5 × 107 CFU, i.p., qW for 2 weeks followed by DOX, 2.4 mg/kg, i.p., qW for 2 weeks; and Group 7, treated with S. typhimurium A1-R, 2.5 × 107 CFU, i.p., qW for 2 weeks followed by BEZ, 50 mg/kg, oral gavage, q5/W for 2 weeks. Tumor length, width and mouse body weight were measured twice a week. Tumor volume was calculated with the following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± SD. The tumor valume ratio is defined at the tumor volume at any given time point relative to the initial tumor volume. All treated mice except Group 2 were sacrificed on day-29, and tumors were resected for further histological evaluation. Mice treated with BEZ were sacrificed on day-18 due to outgrowth of the tumors.
Histological examination
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocol. Histological examination was performed with a BHS system microscope. Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada). Grade IV: no viable tumor is detectible [25].
Imaging of tumor-targeted bacteria
The FV1000 confocal microscope (Olympus) [39] was used to image resected tumors for the presence of S. typhimurium A1-R-GFP. The OV100 (Olympus) variable-magnification fluorescence imager [40] was used to image colonies of S. typhimurium A1-R from resected tumors.
Statistical analysis
SPSS statistics version 21.0 was used for all statistical analyses (IBM, New York City, NY, USA). Significant differences for continuous variables were determined using the Mann-Whitney U test. A probability value of P < 0.05 was considered statistically significant [25].
Footnotes
CONFLICTS OF INTEREST
Y.Z. and M.Z. are employees of AntiCancer Inc. T.K., T.M., K.K., K.I. and R.M.H. are unsalaried associates of AntiCancer Inc. R.M.H. is an unsalaried associate of PDOX LLC. There are no other competing financial interests.
Dedication
This paper is dedicated to the memory of A.R. Moossa, M.D. and Sun Lee, M.D.
REFERENCES
- 1.Conry RM. Response of follicular dendritic cell sarcoma to gemcitabine and docetaxel: report of two cases and literature review. Clin Sarcoma Res. 2014;4:6. doi: 10.1186/2045-3329-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalid T, Folman R. Symptoms in cancer patients an unusual tumor: Case 3. Follicular dendritic cell sarcoma. J Clin Oncol. 2005;23:9425–9426. doi: 10.1200/JCO.2004.00.9365. [DOI] [PubMed] [Google Scholar]
- 3.Choi BS, Baek JH, Shin YM, Kim JH, Kim HW, Lee SJ, Cha HJ. Follicular dendritic cell sarcoma: a case report and review of the literature. Cancer Res Treat. 2010;42:121–124. doi: 10.4143/crt.2010.42.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang N, Zhao M, Hoffman RM. Comparison of the selective targeting efficacy of Salmonella typhimurium A1-R and VNP20009 on the Lewis lung carcinoma in nude mice. Oncotarget. 2015;6:14625–14631. doi: 10.18632/oncotarget.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacterial Therapy of Cancer: Methods and Protocols. In: Hoffman RM, editor; Walker John M., editor. Methods in Molecular Biology 1409. Humana Press; (Springer Science+Business Media New York): 2016. [Google Scholar]
- 6.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA. 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Tome Y, Suetsugu A, Zhang L, Zhang N, Hoffman RM, Zhao M. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res. 2012;32:2501–2508. [PubMed] [Google Scholar]
- 10.Zhang Y, Miwa S, Zhang N, Hoffman RM, Zhao M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget. 2015;6:2615–2622. doi: 10.18632/oncotarget.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchugonova A, Zhao M, Zhang Y, Weinigel M, König K, Hoffman RM. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res. 2012;32:4331–4339. [PubMed] [Google Scholar]
- 12.Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle. 2010;9:4518–4524. doi: 10.4161/cc.9.22.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Bouvet M, Hoffman RM. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–1878. [PubMed] [Google Scholar]
- 14.Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, Bouvet M, Hoffman RM. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res. 2010;164:248–255. doi: 10.1016/j.jss.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiroshima Y, Zhao M, Zhang Y, Maawy A, Hassanein MK, Uehara F, Miwa S, Yano S, Momiyama M, Suetsugu A, Chishima T, Tanaka K, Bouvet M, Endo I, Hoffman RM. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle. 2013;12:2774–2780. doi: 10.4161/cc.25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, Uehara F, Miwa S, Yano S, Momiyama M, Suetsugu A, Chishima T, Tanaka K, Bouvet M, Endo I, Hoffman RM. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) J Cell Biochem. 2014;115:1254–1261. doi: 10.1002/jcb.24769. [DOI] [PubMed] [Google Scholar]
- 17.Hiroshima Y, Zhang Y, Murakami T, Maawy AA, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357. doi: 10.18632/oncotarget.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto Y, Miwa S, Zhang Y, Hiroshima Y, Yano S, Uehara F, Yamamoto M, Toneri M, Bouvet M, Matsubara H, Hoffman RM, Zhao M. Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J Cell Biochem. 2014;115:1996–2003. doi: 10.1002/jcb.24871. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto Y, Miwa S, Zhang Y, Zhao M, Yano S, Uehara F, Yamamoto M, Hiroshima Y, Toneri M, Bouvet M, Matsubara H, Tsuchiya H, Hoffman RM. Intraperitoneal administration of tumor-targeting Salmonella typhimurium A1-R inhibits disseminated human ovarian cancer and extends survival in nude mice. Oncotarget. 2015;6:11369–11377. doi: 10.18632/oncotarget.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yano S, Zhang Y, Zhao M, Hiroshima Y, Miwa S, Uehara F, Kishimoto H, Tazawa H, Bouvet M, Fujiwara T, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle. 2014;13:3958–3963. doi: 10.4161/15384101.2014.964115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiroshima Y, Zhang Y, Zhao M, Zhang N, Murakami T, Maawy A, Mii S, Uehara F, Yamamoto M, Miwa S, Yano S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R in combination with Trastuzumab eradicates HER-2-positive cervical cancer cells in patient-derived mouse models. PLoS One. 2015;10:e0120358. doi: 10.1371/journal.pone.0120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Hoffman RM. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106:992–998. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Kishimoto H, Bouvet M, Hoffman RM. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8:870–875. doi: 10.4161/cc.8.6.7891. [DOI] [PubMed] [Google Scholar]
- 24.Miwa S, Zhang Y, Baek K-E, Uehara F, Yano S, Yamamoto M, Hiroshima Y, Matsumoto Y, Kimura H, Hayashi K, Yamamoto N, Bouvet M, Tsuchiya H, Hoffman RM, Zhao M. Inhibition of spontaneous and experimental lung metastasis of soft-tissue sarcoma by tumor-targeting Salmonella typhimurium A1-R. Oncotarget. 2014;5:12849–12861. doi: 10.18632/oncotarget.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, Quan C, Hiroshima Y, Matsuyama R, Chishima T, Tanaka K, Bouvet M, Chawla S, Endo I, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget. 2016;7:12783–12790. doi: 10.18632/oncotarget.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacterial Therapy of Cancer: Methods and Protocols. In: Hoffman RM, editor; Walker John M., editor. Methods in Molecular Biology 1409. Humana Press; (Springer Science+Business Media New York): 2016. [Google Scholar]
- 27.Momiyama M, Zhao M, Kimura H, Tran B, Chishima T, Bouvet M, Endo I, Hoffman RM. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle. 2012;11:628–632. doi: 10.4161/cc.11.3.19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiroshima Y, Zhao M, Zhang Y, Zhang N, Maawy A, Murakami T, Mii S, Uehara F, Yamamoto M, Miwa S, Yano S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS One. 2015;10:e0134324. doi: 10.1371/journal.pone.0134324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manara MC, Nicoletti G, Zambelli D, Ventura S, Guerzoni C, Landuzzi L, Lollini PL, Maira SM, García-Echeverría C, Mercuri M, Picci P, Scotlandi K. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res. 2010;16:530–540. doi: 10.1158/1078-0432.CCR-09-0816. [DOI] [PubMed] [Google Scholar]
- 30.Mita MM, Poplin E, Britten CD, Tap WD, Rubin EH, Scott BB, Berk L, Rivera VM, Loewy JW, Dodion P, Haluska F, Sarantopoulos J, Mita A, Tolcher A. Phase I/IIa trial of the mammalian target of rapamycin inhibitor ridaforolimus (AP23573, MK-8669) administered orally in patients with refractory or advanced malignancies and sarcoma. Ann Oncol. 2013;24:1104–1111. doi: 10.1093/annonc/mds602. [DOI] [PubMed] [Google Scholar]
- 31.Roberts NJ, Zhang L, Janku F, Collins A, Bai RY, Staedtke V, Rusk AW, Tung D, Miller M, Roix J, Khanna KV, Murthy R, Benjamin RS, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014;6:249ra111. doi: 10.1126/scitranslmed.3008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, Freezer LJ, Morton KE, Seipp C, Haworth L, Mavroukakis S, White D, MacDonald S, Mao J, Sznol M, Rosenberg SA. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–52. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today. 2003;8:1104–7. doi: 10.1016/s1359-6446(03)02806-x. [DOI] [PubMed] [Google Scholar]
- 34.Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle. 2005;4:1518–21. doi: 10.4161/cc.4.11.2208. [DOI] [PubMed] [Google Scholar]
- 35.Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia. 2001;15:936–41. doi: 10.1038/sj.leu.2402127. [DOI] [PubMed] [Google Scholar]
- 36.Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20:385–91. doi: 10.1038/sj.leu.2404075. [DOI] [PubMed] [Google Scholar]
- 37.Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget. 2011;2:222–33. doi: 10.18632/oncotarget.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer. 2003;89:1147–51. doi: 10.1038/sj.bjc.6601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchugonova A, Duong J, Zhang N, König K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem. 2011;112:2046–2050. doi: 10.1002/jcb.23122. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66:4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]


