Abstract
Nimotuzumab is a humanized anti-EGFR IgG1 monoclonal antibody and demonstrates a better safety profile than other anti-EGFR antibodies due to its intermediate affinity. Since it was approved in China for the treatment of nasopharyngeal cancer (NPC), it has been widely used in NPC and in many clinical trials for other cancer types. However, the optimal dose and administration frequency of nimotuzumab that should be used and which kind of cancer patients will be more benefited from nimotuzumab is still unknown. In this retrospective study, 205 advanced cancer patients with colorectal cancer, esophageal cancer, head and neck cancer, gastric cancer, non-small cell lung cancer, or other cancers from mainland China, treated with nimotuzumab in combination with chemotherapy, were enrolled. Over 60% of these patients received nimotuzumab > 6 doses and ≥ 400 mg/week as maintenance therapy. It was well tolerated in real-life patients. This report demonstrates that age, sex and previous treatment might be potential predictive factors for survival, and patients received nimotuzumab > 6 doses and > 200 mg/week might benefit more from nimotuzumab therapy. Using these factors for stratification analysis may form a predictive differential clinical strategy for nimotuzumab to maximize the benefit in patients with different epithelial tumors.
Keywords: nimotuzumab, monoclonal antibody, chemotherapy, advanced cancer
INTRODUCTION
Epidermal Growth Factor Receptor (EGFR [HER-1, erbB1]), a transmembrane glycoprotein, is a receptor widely expressed on a variety of tissues such as skin, gastrointestinal tract and has activity in the signaling pathway promoting cell growth, differentiation, proliferation, and inhibition of apoptosis [1, 2]. However, there is well-documented evidence that up-regulation of the EGFR signal transduction pathway is involved in the establishment and spread of tumors of epithelial cell origin [3–5]. EGFR is dysregulated in several malignant tumors located in head and neck, esophageal, gastric, lung, colorectal, and other organs [6], which correlates with increased metastasis, decreased survival, a poor prognosis [7–10] and radiotherapy (RT) and chemotherapy (CT) resistance[11, 12]. Thus, agents that bind to EGFR and inhibit the EGFR pathway would be expected to exert antagonistic biological activity [6, 13]. Currently, EGFR tyrosine kinase inhibitors (gefitinib, erlotinib, lapatinib) and anti-EGFR monoclonal antibodies (cetuximab, nimotuzumab, panitumumab, and matuzumab) have been developed for the treatment of different malignancies.
Nimotuzumab (alternatively referred to as TheraCIM®, Theraloc®, CIMAher®, BIOMAb-EGFR®, Tai Xin Sheng®, OSAG-101 or YMB-1000) is a humanized IgG1 monoclonal antibody targeting the extracellular domain of EGFR. It has demonstrated blocking ability against the binding of EGF and TGF-alpha to EGFR, and has observed inhibitory activity on tumor cell growth, angiogenesis, and apoptosis [14–16]. Further, experimental observations demonstrated that in contrast to other approved anti-EGFR antibodies, the intrinsic properties of nimotuzumab require bivalent binding for stable attachment to the cellular surface, leading nimotuzumab have the maximum clinical benefit and absence of severe dermatological toxicity (high uptake in tumors overexpressing the receptor and low uptake in normal tissues) [17–23]. It has been approved for the treatment of advanced head and neck cancer (H&NC) [24–26], nasopharyngeal cancer (NPC) [27], glioma [28, 29] and esophageal cancer (ESOC) [30] in 30 countries.
Nimotuzumab (trade name in China Tai Xin Sheng®, Registration ID: 2005S02236) was approved in China in 2008 as a drug in combination with RT for a treatment of NPC and was included within Chinese NCCN guideline as a recommended targeted therapy for this indication in 2009.
Post marketing experience in NPC reinforces the safety within Chinese population [31–33]. More than 30,000 patients received this therapy with an excellent safety profile in China [34] and throughout the world [21, 35, 36]. Five phase III clinical trials are ongoing in different tumors from epithelial origin with different schedules of treatment, with the approval of the China Food and Drug Administration (CFDA). For this reason, physicians have used nimotuzumab as an “off-label product” in other cancers of epithelial origin.
After seven years of the first approval in China, the information of several advanced cancer patients who received nimotuzumab in combination with CT in off-label approach has been collected. This retrospective analysis summarizes the safety profile, efficacy and possible predictive factors of this anti-EGFR therapy in Chinese patients with advanced cancers.
RESULTS
Patients' characteristics
Comprising our retrospective study were 205 cancer patients with various diagnoses. Table 1 shows the distribution of patients by tumor-type and schedule treatment. Colorectal cancer (CRC), ESOC, H&NC, gastric cancer (GC), non-small cell lung cancer (NSCLC) and other cancer patients (which consisted of low numbers of breast, pancreatic, bile duct, gallbladder, renal pelvis and ovarian cancer) were included. Patients' characteristics are described in Table 2. In total, 139 patients were male (67.8 %). The majority of patients had stage IV disease (97.6%) when received nimotuzumab and 140 (68.3%) patients were adenocarcinoma (ADC). The 66.8% (137/205) of the patients were younger than 60 years of age. Moreover, 60% of patients underwent surgery, 43.9% (90/205) received RT/chemoradiotherapy (CRT), and 80.5% (165/205) had CT before nimotuzumab.
Table 1. Distribution of patients by tumor localization and schedule treatment.
| Nimotuzumab (mg/week) | 100 | 200 | 250 | 300 | 400 | 500 | 600 | Total (%) |
|---|---|---|---|---|---|---|---|---|
| n (%) | 6 (2.9) | 47 (22.9) | 1 (0.5) | 12 (5.9) | 130 (63.4) | 1 (0.5) | 8 (3.9) | 205 (100) |
| NSCLC | 0 | 5 | 0 | 2 | 16 | 0 | 0 | 23 (11.2) |
| ESOC | 0 | 11 | 0 | 1 | 8 | 0 | 1 | 21 (10.2) |
| CRC | 0 | 12 | 1 | 6 | 48 | 1 | 3 | 71 (34.6) |
| H&NC | 2 | 11 | 0 | 1 | 18 | 0 | 2 | 34 (16.6) |
| GC | 3 | 3 | 0 | 1 | 27 | 0 | 1 | 35 (17.1) |
| Others | 1 | 5 | 0 | 1 | 13 | 0 | 1 | 21 (10.3) |
NSCLC: non small cell lung cancer; ESOC: esophageal cancer; H&NC: head and neck cancer; CRC: colorectal cancer; GC: gastric cancer.
Table 2. Patients demographic and tumor characteristics.
| Characteristic | Total | H&NC | CRC | ESOC | GC | NSCLC | Others | |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Sex | Male | 139(67.8) | 26(76.5) | 48(67.6) | 21(100) | 24(68.6) | 16(69.6) | 4(19) |
| Female | 66(32.2) | 8 (23.5) | 23(32.4) | 0 | 11(31.4) | 7(30.4) | 17(81) | |
| Age (yr) | <60 | 137(66.8) | 23(67.6) | 46(64.8) | 9(42.9) | 23(65.7) | 16(69.6) | 20(95.2) |
| ≥60 | 68(33.2) | 11(32.4) | 25(34.7) | 12(57.1) | 12(34.3) | 7(30.4) | 1(4.8) | |
| Histopathology | ADC | 140(68.3) | 2(5.9) | 71(100) | 0 | 33(94.3) | 14(60.9) | 20(95.2) |
| Non-ADC | 65(31.7) | 32(94.1) | 0 | 21(100) | 2(5.7) | 9(39.1) | 1(4.8) | |
| Clinical stage | III | 5(2.4) | 2(5.9) | 0 | 1(4.8) | 0 | 2(8.7) | 0 |
| IV | 200(97.6) | 32(94.1) | 71(100) | 20(95.2) | 35(100) | 21(91.3) | 21(100) | |
| Previous treatment | ||||||||
| Surgery | NO | 82(40.0) | 21(61.8) | 15(21.1) | 11(52.4) | 20(57.1) | 13(56.5) | 2(9.5) |
| YES | 123(60.0) | 13(38.2) | 56(78.9) | 10(47.6) | 15(42.9) | 10(43.5) | 19(90.5) | |
| RT/CRT | NO | 115(56.1) | 8(23.5) | 47(66.2) | 5(23.8) | 32(91.4) | 13(56.5) | 10(47.6) |
| YES | 90(43.9) | 26(76.5) | 24(33.8) | 16(76.2) | 3(8.6) | 10(43.5) | 11(52.4) | |
| CT | NO | 40(19.5) | 18(52.9) | 9(12.7) | 5(23.8) | 5(14.3) | 1(4.3) | 2(9.5) |
| YES | 165(80.5) | 16(47.1) | 62(87.3) | 16(76.2) | 30(85.7) | 22(95.7) | 19(90.5) | |
H&NC: head and neck cancer; CRC: colorectal cancer; ESOC: esophageal cancer; GC: gastric cancer; NSCLC: non small cell lung cancer; n: number of patients.
ADC: adenocarcinoma; Non-ADC: squamous cell carcinoma or adeno-squamous carcinoma; RT: radiotherapy; CT: chemotherapy; CRT: chemoradiotherapy.
Safety profile
Treatment was well tolerated. The majority of the adverse events (AEs) were classified as mild or moderate (in spite of the causal relationship). No serious AE (SAE) occurred. Although the co-administered CT regimens were different for different indications, the safety profile of nimotuzumab was similar to previously reported studies [37–40]. Table 3 summarizes the System Organ Classification (SOC) of AEs by indication; gastrointestinal disorders (43%) such as nausea, diarrhea, and vomit were the most frequent AEs, followed by some investigations (38.3%) such as white blood cell decreased, neutrophil count decreased and platelet count decreased. Table 4 summarizes the Grade of AEs (381 events) classified by indication. Only 16.8% and 5.2% of patients had AEs in grade 3 and 4 respectively. Supplementary information demonstrates the SOC and Grade of all AEs in different indications. The Grade of all AEs in different nimotuzumab doses (mg/week) are presented in Table 5.
Table 3. System Organ Classification of adverse events.
| System Organ Class | NSCLC | ESOC | CRC | H&NC | GC | Others | Total (%) |
|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Metabolism and nutrition disorders | 1(2.3) | 2(4.7) | 3(2.8) | 3(5.2) | 2(2.3) | 1(2.3) | 12(31.5) |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 0 | 1(1.7) | 1(1.1) | 0 | 2(0.5) |
| Skin and subcutaneous tissue disorders | 3(7.0) | 1(2.3) | 5(4.7) | 2(3.4) | 1(1.1) | 2(4.5) | 14(3.7) |
| General disorders and administration site conditions | 2(4.7) | 2(4.7) | 3(2.8) | 2(3.4) | 6(6.9) | 4(9.1) | 19(5.0) |
| Nervous system disorders | 0 | 1(2.3) | 6(5.7) | 3(5.2) | 2(2.3) | 5(11.4) | 17(4.5) |
| Gastrointestinal disorders | 19(44.2) | 20(46.5) | 53(50.0) | 20(34.5) | 38(43.7) | 14(31.8) | 164(43.0) |
| Vascular disorders | 0 | 0 | 0 | 0 | 1(1.1) | 0 | 1(0.3) |
| Blood and lymphatic system disorders | 0 | 0 | 2(1.9) | 1(1.7) | 3(3.4) | 0 | 6(1.6) |
| Investigations | 18(41.9) | 17(39.5) | 34(32.1) | 26(44.8) | 33(37.9) | 18(40.9) | 146(38.3) |
| Total (%) | 43(11.3) | 43(11.3) | 106(27.8) | 58(15.2) | 87(22.8) | 44(11.5) | 381(100) |
NSCLC: non small cell lung cancer; ESOC: esophageal cancer; H&NC: head and neck cancer; CRC: colorectal cancer; GC: gastric cancer.
No. (%): times and percent of AE occurred in patients of each indication; Total (%): total number of AE times in each indication or each system organ
Table 4. Grade of adverse events classified by indications.
| Indications | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| NSCLC | 13 | 8.2% | 23 | 16.5% | 5 | 7.8% | 2 | 10.0% |
| ESOC | 19 | 12.0% | 11 | 7.9% | 10 | 15.6% | 3 | 15.0% |
| CRC | 41 | 25.9% | 43 | 30.9% | 18 | 28.1% | 4 | 20.0% |
| H&NC | 23 | 14.6% | 19 | 13.7% | 12 | 18.8% | 4 | 20.0% |
| GC | 47 | 29.7% | 28 | 20.1% | 10 | 15.6% | 2 | 10.0% |
| Others | 15 | 9.5% | 15 | 10.8% | 9 | 14.1% | 5 | 25.0% |
| Total | 158 | 100.0% | 139 | 100.0% | 64 | 100.0% | 20 | 100.0% |
| Indication/Total | 158/381 | 41.5 | 139/381 | 36.5 | 64/381 | 16.8 | 20/381 | 5.2 |
NSCLC: non small cell lung cancer; ESOC: esophageal cancer; H&NC: head and neck cancer; CRC: colorectal cancer; GC: gastric cancer
Table 5. Grade of adverse events classified by nimotuzumab doses.
| Doses nimotuzumab (mg/w*) | 100 (n=6) |
200 (n=47) |
250 (n=1) |
300 (n=12) |
400 (n=130) |
500 (n=1) |
600 (n=8) |
Total (n=205) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade of AE | n | Times | n | Times | n | Times | n | Times | n | Times | n | Times | n | Times | n | Times |
| Patients with AE | 6 | 24 | 42 | 94 | 0 | 0 | 9 | 19 | 113 | 219 | 1 | 1 | 8 | 24 | 179 | 381 |
| Patients with No-AE | 0 | 0 | 5 | 0 | 1 | 0 | 3 | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 26 | 0 |
| Grade 1 | 5 | 8 | 23 | 33 | 0 | 0 | 6 | 8 | 59 | 95 | 0 | 0 | 7 | 14 | 100 | 158 |
| Grade 2 | 5 | 10 | 18 | 33 | 0 | 0 | 5 | 5 | 61 | 84 | 1 | 1 | 5 | 6 | 95 | 139 |
| Grade 3 | 1 | 4 | 19 | 26 | 0 | 0 | 4 | 6 | 20 | 26 | 0 | 0 | 2 | 2 | 46 | 64 |
| Grade 4 | 1 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 13 | 14 | 0 | 0 | 1 | 2 | 16 | 20 |
| Grade 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p value (compare with a total) | 0.35 | 0.73 | 0.01 | 0.25 | 0.93 | 0.70 | 0.29 | 1.00 | ||||||||
NSCLC: non small cell lung cancer; ESOC: esophageal cancer; H&NC: head and neck cancer; CRC: colorectal cancer; GC: gastric cancer
n: number of patients in each dosage group; AE: adverse event, if one patient occurred multiple AE in different grade, it was recorded individually. The severity of AE was classified by NCI-CTCAE 4.03.
mg / w: milligrams of antibody (fix doses) administer per week.
Antitumor response
A total of 171 patients (83.4%) were evaluated by RECISIT criteria at least one time after the treatment of nimotuzumab. The antitumor response was reported as objective response rate (ORR, complete response (CR) + partial response (PR)) and disease control rate (DCR, CR+ PR+ stable disease (SD)). The antitumor responses in different indications are shown in Table 6. According to the ORR, H&NC patients were the best responders (55.9%), followed by ESOC patients and those who with CRC (42.9% and 28.2% respectively). In regards to the DCR, all patients reached more than 50%, H&NC was the most efficient (91.2%), followed by NSCLC (78.3%) and CRC (67.6%).
Table 6. Antitumor Response Profile by Pearson Chi-square analysis.
| Factors | n | TOTAL (n=205) | H&NC (n=34) | GC (n=35) | ||||
|---|---|---|---|---|---|---|---|---|
| N(ORR)% | N(DCR)% | N(ORR)% | N(DCR)% | N(ORR)% | N(DCR)% | |||
| Total | 205 | 66(32.2) | 139(67.8) | 19(55.9) | 31(91.2) | 8(22.9) | 19(54.3) | |
| Sex | Male | 139 | 48(34.5) | 102(73.4) | 15(57.7) | 26(100.0) | 6(25.0) | 14(58.3) |
| Female | 66 | 18(27.3) | 37(56.1) | 4(50.0) | 5(62.5) | 2(18.2) | 5(45.5) | |
| p* | 0.445 | 0.002 | 0.187 | 0.005 | 0.193 | 0.028 | ||
| Age | <60y | 137 | 44(32.1) | 92(67.2) | 12(52.2) | 23(100.0) | 7(30.4) | 13(56.5) |
| ≥60y | 68 | 22(32.4) | 47(69.1) | 7(63.6) | 8(72.7) | 1(8.3) | 6(50.0) | |
| p* | 0.515 | 0.240 | 0.220 | 0.032 | 0.285 | 0.863 | ||
| Histology | ADC | 140 | 39(27.9) | 90(64.3) | 2 (100.0) | 2(100.0) | 8 (24.2) | 18(54.6) |
| non-ADC | 65 | 27(41.5) | 49(75.4) | 17 (53.1) | 29(90.6) | 0(0) | 1(50.0) | |
| p* | 0.128 | 0.283 | 0.432 | 0.902 | 0.367 | 0.471 | ||
| Treatment lines | 1st | 40 | 20(50.0) | 30(75.0) | 10(55.6) | 16(88.9) | 3(60.0) | 3(60.0) |
| 2nd | 81 | 26(32.1) | 57(70.4) | 4(44.4) | 8(88.9) | 4(19.0) | 13(61.9) | |
| 3rd | 41 | 13(31.7) | 25(61.0) | 3(100.0) | 3(100.0) | 1(12.5) | 3(37.5) | |
| >3rd | 43 | 7(16.3) | 27(62.8) | 2(50.0) | 4(100.0) | 0(0) | 0(0) | |
| p* | 0.036 | 0.702 | 0.683 | 0.937 | 0.208 | 0.345 | ||
| Nimotuzumab frequency | ≤6 | 73 | 17(23.3) | 39(53.4) | 6 (54.6) | 9(81.8) | 0(0) | 3(23.1) |
| >6 | 132 | 49(37.1) | 100(75.8) | 13 (56.5) | 22(95.7) | 8(36.4) | 16(72.7) | |
| p* | 0.050 | 0.004 | 0.85 | 0.335 | 0.004 | 0.006 | ||
| Nimotuzumab doses (mg/w) | ≤200 | 54 | 20(37.0) | 36(66.7) | 7 (53.9) | 11(84.6) | 1 (16.7) | 4(66.7) |
| >200 | 151 | 46(30.5) | 103(68.2) | 12 (57.1) | 20(95.2) | 7 (24.1) | 15(51.7) | |
| p* | 0.567 | 0.436 | 0.434 | 0.400 | 0.181 | 0.258 | ||
n: number of patients who have received at least one evaluation. p: statistical p value.
Bold text indicates statistically significant P-values (<0.05)
compared between two groups of each factor.
The correlation between different factors (sex, age, histology, previous treatment, treatment lines, doses and frequency of nimotuzumab) and antitumor response were investigated. The difference in DCR between male and female was evident in all patients, with more benefit for men than women had (73.4% vs 56.1%, p=0.002), similar results were found in H&NC and GC (p=0.005 and 0.028, respectively), but no difference was found in NSCLC, ESOC and CRC patients (Data not shown). No obvious difference of ORR was found between men and women in total population or in different tumor types. According to the age, only the DCR of patients with H&NC showed significant difference (p=0.032, 100% vs 72.7% in age <60 years vs ≥ 60 years). Concerning the histology, no significant difference was observed between patients with ADC and non-ADC. The therapeutic schedules (nimotuzumab+ CT) were used as 1st, 2nd, 3rd, and >3rd lines treatment in 40, 81, 41, and 43 patients, and the ORR of the four groups was 50.0%, 32.1%, 31.7%, and 16.3% respectively, with significant difference among groups (P=0.036). However, no difference of treatment lines was found in DCR among groups (p=0.702). The patients received more than six doses have significant improvement on the ORR (23.3% vs. 37.1%, p=0.050) and DCR in total (75.8% vs. 53.4%, p=0.004) and GC (72.7% vs. 23.1%, p=0.006), and ORR in GC (36.4% vs. 0%, p=0.004). In addition, no significant difference was found between previous treatment (surgery, RT/CRT, CT) and antitumor response (data not shown).
Survival
Univariate analysis was performed to determinate the association between factors (sex, age, histology, differentiation, surgery history, RT/CRT history, CT history, treatment lines, nimotuzumab >200mg/week and nimotuzumab >6 doses) and overall survival (OS) or progression free survival (PFS). The analysis for OS showed that the statistically significant variables were age>60 years in H&NC with the hazard ratio (HR) of 4.65(1.29-16.74) (p=0.019), and nimotuzumab > 200mg/week and nimotuzumab >6 doses in GC with the HR of 0.08(0.02-0.39) and 0.21(0.05-0.98) (p=0.002 and 0.047, respectively). Univariate analysis for PFS showed that surgery history and nimotuzumab >6 doses were significantly associated with CRC patients' PFS with the HR of 0.43(0.20-0.93) and 0.39(0.17-0.85) (p=0.037 and 0.020, respectively); male gender was statistically significant related factor for PFS in GC patients with the HR of 3.69(1.08-12.64) (p=0.038). The other clinical and treatment parameters have no correlation with OS or PFS in different tumor types (Data not shown). Further, only those variables found to be significantly associated with outcome in univariate analyses were included in multivariable analyses.
As shown in Table 7A, prior surgery and nimotuzumab >6 doses were identified as independent predictive factors for increased PFS in CRC with an HR of 0.44 (95% CI: 0.20-0.95, p=0.037) and HR of 0.40 (95% CI: 0.18-0.88, p=0.020). A similar pattern can be seen in GC. Male and nimotuzumab > 6 doses were independent predictive factors for increased PFS with HR 0.17 (95% CI: 0.04-0.73, p=0.020) and 0.15 (95% CI: 0.02-0.98, p=0.048). In Table 7B, younger age was a significant independent predictive factor for improved OS of H&NC patients, with HR 0.28 (95% CI: 0.07-1.19, p=0.019). Nimotuzumab >200mg/week was a significant factor related with increased OS in GC, with a HR of 0.11 (95% CI: 0.02-0.59, p=0.01).
Table 7. Factors Related with OS and PFS in Multivariate Cox Regression analysis.
| A: Multivariate analysis for PFS | |||
| Tumor types | Variable | HR (95% CI) | p |
| CRC | Surgery history | 0.44(0.20-0.95) | 0.037 |
| nimo>6 doses | 0.40(0.18-0.88) | 0.020 | |
| GC | Male | 0.17(0.04-0.73) | 0.020 |
| nimo>6 doses | 0.15(0.02-0.98) | 0.048 | |
| B: Multivariate analysis for OS | |||
| Tumor types | Variable | HR (95% CI) | p |
| GC | nimo>200mg/week | 0.11(0.02-0.59) | 0.010 |
| H&NC | Age>60 years | 0.28(0.07-1.19) | 0.019 |
PFS: progression free survival, OS: overall survival
Table 8 and 9 reports the correlation between different factors (sex, age, surgical history, dose and frequency of nimotuzumab) and survival. Age, dose and duration of therapy were also considered to correlate with OS (Table 8). Sex, age, dose and previous treatment (surgery and RT) were related with PFS (Table 9). As shown in Figure 1 and 2, sex, previous treatment, and nimotuzumab > 6 doses and > 200 mg/week might be predictive factors for the likelihood of benefit from nimotuzumab therapy.
Table 8. Overall Survival profile by Log-Rank analysis.
| Factors | OS | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NSCLC | ESOC | CRC | H&NC | GC | |||||||||||||||||
| OS (day) | 95% CI | p | OS (day) | 95% CI | p | OS (day) | 95% CI | p | OS (day) | 95% CI | p | OS (day) | 95% CI | p | |||||||
| General OS | 417 | 209 | 624.7 | 420 | 171 | 669 | 655 | 314 | 996 | 371 | 261 | 481 | 476 | 86 | 866 | ||||||
| Sex | Male | 549 | 85.6 | 1012 | 0.93 | 420 | 171 | 669 | NA | 1393 | 367 | 2419 | 0.38 | 457 | 314 | 600 | 0.07 | 476 | 28.9 | 923 | 0.54 |
| Female | 417 | . | . | NA | NA | NA | 525 | 291 | 759 | 290 | 173 | 407 | 298 | 0 | 703 | ||||||
| Age | <60y | 351 | 201 | 501.4 | 0.58 | . | . | . | 0.48 | 867 | 274 | 1460 | 0.83 | 457 | 354 | 560 | 0.01 | 476 | 0 | 1021 | 0.73 |
| ≥60y | 549 | 4.11 | 1094 | 270 | 18.4 | 522 | 643 | 481 | 805 | 228 | 166 | 290 | 298 | 0 | 612 | ||||||
| Surgery | YES | 417 | 0.197 | 420 | 181.7 | 658.3 | 0.832 | 525 | 293.7 | 756.7 | 0.21 | 388 | 280.9 | 495.1 | 0.42 | 476 | 139.3 | 812.7 | 0.44 | ||
| NO | 278 | 48.4 | 507.6 | . | . | . | 867 | 474.4 | 756.7 | 228 | 20.8 | 435.2 | 166 | . | . | ||||||
| Nimotuzumab frequency | ≤6 | 417 | 104 | 729.9 | 0.22 | 270 | 191 | 349 | 0.12 | 391 | 341 | 441 | 0.32 | 228 | 20.9 | 435 | 0.37 | 77 | . | . | 0.03 |
| >6 | 549 | 168 | 930.3 | . | . | . | 655 | 317 | 993 | 388 | 341 | 435 | 476 | . | . | ||||||
| Nimotuzumab doses (mg/w) | ≤200 | 278 | 9.14 | 546.9 | 0.99 | 270 | . | . | 0.67 | 270 | . | . | 0.94 | 334 | 67.7 | 600 | 0.93 | 77 | 23.1 | 131 | 0.00 |
| >200 | 417 | 254 | 579.7 | 420 | 180 | 660 | 420 | 180 | 660 | 388 | 280 | 496 | 476 | . | . | ||||||
Bold text indicates statistically significant (P-values <0.05)
Table 9. Progression Free Survival profile by Log-Rank analysis.
| Factors | PFS | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NSCLC | ESOC | CRC | H&NC | GC | |||||||||||||||||
| PFS (day) | 95% CI | p | PFS (day) | 95% CI | p | PFS (day) | 95% CI | p | PFS (day) | 95% CI | p | PFS (day) | 95% CI | p | |||||||
| General PFS | 173 | 33.1 | 312.9 | 196 | 0 | 411 | 217 | 181 | 253 | 198 | 102 | 294 | 142 | 18.8 | 265 | ||||||
| Sex | Male | 173 | 112 | 233.9 | 0.23 | 196 | 0 | 411 | NA | 210 | 170 | 250 | 0.76 | 302 | 0 | 635 | 0.04 | . | . | . | 0.03 |
| Female | 89 | 76.2 | 101.8 | NA | NA | NA | 225 | 114 | 336 | 127 | 31.4 | 223 | 127 | 42.4 | 212 | ||||||
| Age | <60y | 104 | 21.9 | 186.1 | 0.04 | 196 | 0 | 408 | 0.56 | 210 | 78.2 | 342 | 0.59 | 198 | 0 | 415 | 0.48 | 253 | 0 | 517 | 0.62 |
| ≥60y | 521 | 225 | 171 | 279 | 151 | 61.6 | 240 | 127 | 12.5 | 242 | |||||||||||
| Surgery | NO | 98 | 0 | 227.6 | 0.34 | 1536 | 0.10 | 171 | 107 | 235 | 0.03 | 169 | 96 | 242 | 0.62 | 142 | 106 | 178 | 0.80 | ||
| YES | 211 | 80.6 | 341.4 | 109 | 0 | 244 | 225 | 188 | 262 | 228 | 0 | 466 | 253 | 30.3 | 476 | ||||||
| Nimotuzumab frequency | ≤6 | 89 | 40.5 | 137.5 | 0.27 | 109 | 28.2 | 190 | 0.07 | 171 | 4.11 | 338 | 0.01 | 228 | 72.7 | 383 | 0.85 | . | . | . | 0.06 |
| >6 | 211 | 146 | 275.8 | 423 | 83.2 | 763 | 225 | 185 | 265 | 198 | 36.8 | 359 | 253 | 103 | 403 | ||||||
| Nimotuzumab doses (mg/w) | ≤200 | 264 | 81.5 | 446.5 | 0.55 | 141 | 0.58 | 163 | 75.5 | 251 | 0.45 | 399 | 85.4 | 713 | 0.30 | . | . | . | 0.06 | ||
| >200 | 173 | 40.1 | 305.9 | 423 | 80.4 | 766 | 217 | 174 | 260 | 169 | 105 | 233 | 253 | 103 | 403 | ||||||
Bold text indicates statistically significant (P-values <0.05)
Figure 1. Potential patient characteristics related with OS and PFS in 5 indications.
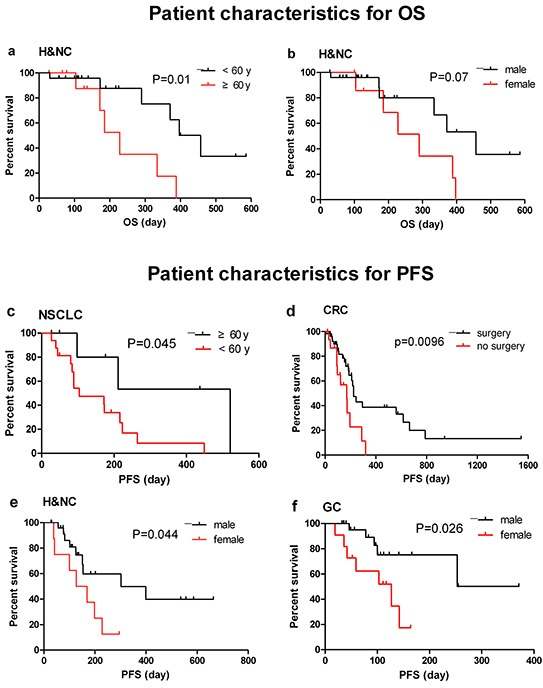
a. OS of H&NC patients by age; b. OS of H&NC patients by sex; c. PFS of NSCLC patients by age; d. PFS of CRC patients by prior surgery history; e. PFS of H&NC patients by sex; f. PFS of GC patients by sex.
Figure 2. Potential treatment parameters related with for OS and PFS in 5 indications.
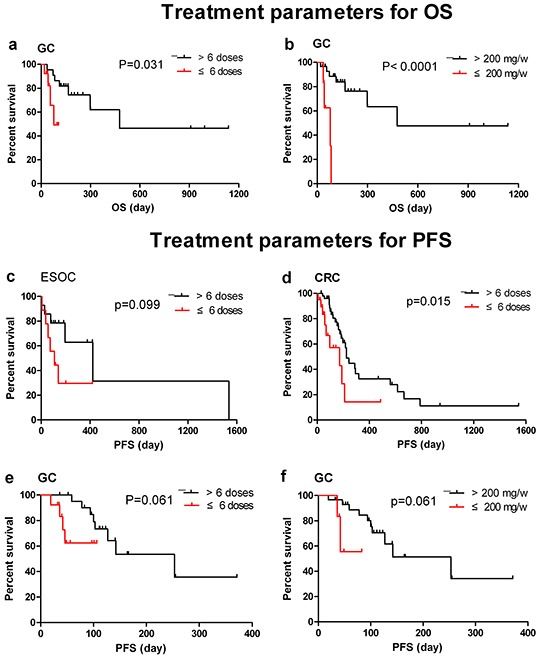
a. OS of GC patients treated by nimotuzumab > or ≤6 doses; b. OS of GC patients treated by nimotuzumab > or ≤ 200mg/week; c. PFS of ESOC patients treated by nimotuzumab > or ≤6 doses; d. PFS of CRC patients treated by nimotuzumab > or ≤ 200mg/week; e. PFS of GC patients treated by nimotuzumab > or ≤6 doses; f. PFS of GC patients treated by nimotuzumab > or ≤ 200mg/week.
The impact of these factors on survival in 5 clinical indications was further illustrated in Figure 3. In the NSCLC arm, there was a trend for longer PFS in patients with age ≥ 60 years and had received more than six doses of nimotuzumab (p=0.071, Figure 3b). In the ESOC arm, the patients without prior surgery had longer PFS when they received more than six doses of nimotuzumab (p=0.046, Figure 3d). In the CRC arm, a significant difference was also observed in PFS when patients had prior surgery and received nimotuzumab over six doses as maintenance treatments (p=0.011, Figure 3f). In the H&NC arm, males with age < 60 years survived longer than others did (p=0.014, Figure 3g). In the GC arm, patients who received more than 200 mg/week and six doses had longer OS than the others (p=0.0005, Figure 3i), and males who received nimotuzumab over six doses had obvious increase in PFS than females who only received nimotuzumab in less than six doses (p<0.0001, Figure 3j).
Figure 3. Factors to predict patient prognosis and response to nimotuzumab therapy in 5 indications.
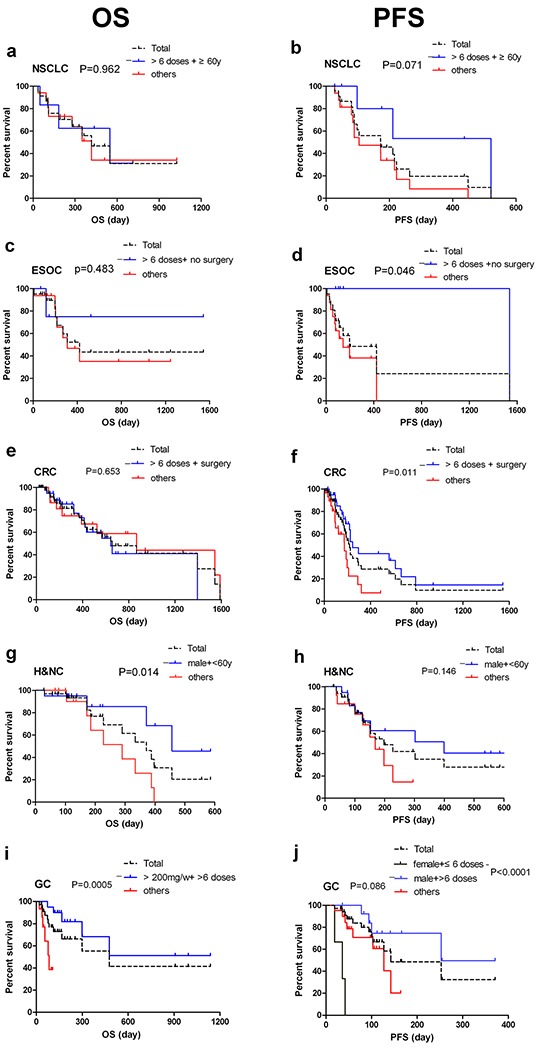
a. OS of NSCLC by nimotuzumab doses and age. Median OS for (>6 doses+ ≥60y) group and others group was 549 days vs 417 days (n=7 vs 16), with HR 0.37 (95% CI 0.10 to 1.16); b. PFS of NSCLC by nimotuzumab doses and age. Median PFS for (>6 doses+ ≥60y) group and others group was 521 days vs 104 days (n=7 vs 16), with HR 0.40 (95% CI 0.15 to 1.10); c. OS of ESOC by frequency of nimotuzumab and prior surgery history. Median OS for (>6 doses+ no surgery) group and others group was undefined vs 308 days (n=5 vs 16), with HR 0.48 (95% CI 0.11 to 2.88); d. PFS of ESOC by frequency of nimotuzumab and prior surgery history. Median PFS for (>6 doses+ no surgery) group and others group was 1536 days vs 141 days (n=5 vs 16), with HR 0.19 (95% CI 0.07 to 0.83); e. OS of CRC by frequency of nimotuzumab and prior surgery history. Median OS for (>6 doses+ surgery) group and others group was 643 vs 867 days (n=42 vs 29), with HR 1.19 (95% CI 0.55 to 2.67); f. PFS of CRC by frequency of nimotuzumab and prior surgery history. Median PFS for (>6 doses+ surgery) group and others group was 244 days vs 173 days (n=42 vs 29), with HR 0.44 (95% CI 0.17 to 0.77). g. OS of H&NC by sex and age. Median OS for (male + <60y) group and others group was 457 vs 290 days (n=20 vs 14), with HR 0.26 (95% CI 0.06 to 0.70); h. PFS of H&NC by sex and age. Median PFS for (male + <60y) group and others group was 399 days vs 169 days (n=20 vs 14), with HR 0.51 (95% CI 0.16 to 1.26); i. OS of GC by doses and frequency of nimotuzumab. Median OS for (>200mg/w+ >6 doses) group and others group was undefined vs 83 days (n=20 vs 15), with HR 0.18 (95% CI 0.01 to 0.23); j. PFS of GC by sex and frequency of nimotuzumab. Median PFS for (male + >6 doses) group and others was 253 days vs 127 days (n=14 vs 21), and median PFS of (female+≤6 doses) group was only 36 days (n=3), with HR 0.08 (95% CI 1.031e-005 to 0.008).
DISCUSSION
Nimotuzumab has shown excellent antitumor activity when combined with RT or CRT in advanced H&NC [21], ESOC [30] and glioma [41] patients. In recent years, some clinical trials in China have been launched to test the clinical benefit of nimotuzumab combined with CT in different types of cancer: ESOC [42–44], NPC [45], GC [46], pancreatic cancer [47], glioma [48] and NSCLC [39, 49, 50]. Ideally, the efficacy of EGFR target agents should be related with some molecular factors. Several biomarkers such as EGFR gene copy number, KRAS mutation, AKT, ERK has been investigated extensively, but results obtained still remain controversial, suggesting potential off-target effects and yet-discovered molecular co-factors [8, 42, 51, 52]. In absence of correlative molecular data, however, oncologists found that some clinical characteristics also could predict the effect of EGFR inhibitors. Examples include patient sex, histology, and smoking history that were found to be associated with the clinical benefit of erlotinib [53]. Our retrospective study was designed to assess the safety and efficacy of nimotuzumab in combination with chemotherapy in advanced tumors from epithelial origin in Chinese patients. The clinical characteristics (age, sex, histology, previous treatment, tumor differentiation, tumor stage) and treatment predictive factors (dose, frequency and treatment lines) were also analyzed.
In this report, treatment with nimotuzumab in combination with CT was well tolerated. No SAE was attributed to nimotuzumab in combination with CT. A total of 381 AEs were collected. In general, the majority of AEs were gastrointestinal disorders (43.0%), laboratory investigations (38.3%), and metabolism and nutrition disorders (31.5%) (Table 3). Skin and subcutaneous tissue disorders only accounted 3.7 % of the AEs. Only 22% AEs were reported as grade 3 or 4 (Table 4).
The addition of nimotuzumab to different chemotherapeutic regimens for different types of tumors did not increase the toxicity of CT. In contrast, SAEs have been reported for other widely used anti-EGFR antibodies already in the market, such as cetuximab and panitumumab. Cetuximab can induce severe acneiform rash in 1-17% patients, severe infusion reactions in approximately 3% of patients as well as cardiopulmonary arrest and sudden death in up to 2% of the patients [54]. Panitumumab engenders dermatologic toxicities in 89% of patients (12% severe) and severe infusion reactions in approximately 1% of patients [55]. In our set of patients, skin and subcutaneous tissue disorders just accounted for 2.6% (10/381) of all the AEs in grade 1 and 2. Studies of nimotuzumab in various tumors also demonstrated remarkable dermatological safety [40, 56]. This favorable toxicity profile can be explained by a kinetic binding model of anti-EGFR antibodies, where intermediate affinity of nimotuzumab (KD = 10−9M) to the receptor, results in a high tumor uptake and low uptake into normal tissues [20, 21].
The excellent safety profile of nimotuzumab allows its long-term use and provides a better quality of life of patients [17]. Over 60% patients received nimotuzuamb over six administrations as maintenance therapy (the maximum number was 60 times) in our study. Patients who received more than 100 mg (200-600 mg) weekly showed a similar safety profile with patients in fewer dosages. No significant difference was found between doses related with the total of AE (Table 5).
Patients with CRC, GC, H&NC, NSCLC and ESOC were selected for efficacy analysis; each indication had more than 20 patients. Most of the patients were in Stage IV (97.6%), 92.2% patients had prior treatment, and 80.5% patients had previously received chemotherapy (>40% patients received at least two regimens before). Surprisingly, it was found that the ORR and DCR arrived 31.7% and 67.3% in total, which makes us believe this could be an alternative therapy even after tumor progression by 1st-3rd lines treatment and possibly beyond.
With regard to the antitumor response, analysis by some risk factors showed that men had better responses than women did (DCR: 73.4% vs 56.1%, p=0.002). Similarly, H&NC patients younger than 60 years had 100% DCR, compared to 72.7% observed in their older counterparts (p=0.032). The therapeutic schedules (nimotuzumab+ CT) that used as 1st line treatment had better response than those treated as 2nd, 3rd and >3rd lines treatment (50.0%, 32.1%, 31.7%, and 16.3% respectively).
Consequently, we performed the univariate and multivariate analysis and Log-Rank test by clinical indications to identify some special sub-populations that may better benefit from the nimotuzumab therapy.
For 34 H&NC patients, 90% in Stage IV and 85% were squamous cell carcinoma. There were 14 cases of NPC, 4 cases of hypopharyngeal cancer, 4 cases of oral cancer, 3 cases tongue cancer, 2 cases of laryngeal cancer, 2 cases of maxillary sinus carcinoma, 1 case parotid gland one, 1 case of lip cancer, and 1 case orbital cancer were included. Sixteen (47.1%) patients received multi-line chemotherapy (including platinum-based regimen, data not shown). Since 2014, the NCCN Guidelines recommended for recurrent, unresectable or metastatic disease in H&NC using different regimens of CT including cisplatin/docetaxel/cetuximab[57], cisplatin/ paclitaxel/cetuximab [58] and cisplatin/gencitabine for non NPC cancer [45]. The ORR of our study was 55.9%, DCR was 91.2%, median PFS was 6.4 months and the median OS 12.4 months, 1-year OS rate was 53.8% (Table 6–8). These results were better than the previous results from CT alone or cetuximab combined with CT regimens in recurrent or metastatic head and neck tumors, with ORR (20% vs 36%, P < 0.001), median PFS (3.3 vs. 5.6 months, P < 0.001) and OS (7.4 vs 10.1 months, P = 0.04 [59]. Multivariate analysis showed that age was a significant predictive factor for the patient's survival (Table 7). Males with age < 60 years survived significant longer than others did (457 days vs 290 days, p=0.014, Figure 3g). Further, patients with NPC in our report showed an ORR in 50% and the 100% of DCR, the median PFS was 6.5 months, 1 year PFS rate was 44.6%, the median OS 12.9 months, 1 year OS at a rate of 66.7% (data not shown). Similar results were previously reported by Chinese oncologists in endemic NPC areas that nimotuzumab in combination with gemcitabine in patients with advanced metastatic NPC obtained an ORR in 61.5% [60] and a combination of nimotuzumab with paclitaxel and cisplatin in the treatment of recurrent NPC had the ORR in 64.28% and DCR in 92.85% [61]. Until this report, no other result was published about the treatment of nimotuzumab combined with CT in advanced H&NC.
Esophageal cancer is one of the most common malignant tumors worldwide and its incidence is increasing. In western countries, esophageal adenocarcinoma in esophagogastric junction cancer has dramatically increased in incidence and now accounts for 60 ~ 70% of esophageal cancer [62], but in China, squamous carcinoma is still the main pathological subtype, accounting for more than 90% [63]. Currently, there is no consensus treatment that is recommended for ESOC. Several phase III trials have not found significant differences in outcome between various CT regimens, such as Irinotecan / 5-FU vs cisplatin / 5FU (PFS 6 vs. 9 months and OS 9 vs. 8.7 months) [64], FOLFIRI vs ECX (Epirubicin/ oxaliplatin/ 5-FU) (OS 9.5 vs. 9.7 months and PFS 5.3 vs. 5.8 months) [65], FLO vs FPL (PFS 5.8 vs. 3.9 months, OS 10.8 vs. 8.8 months) [66]. The median OS remains in 8.7-10.8 months and PFS in 3.9-9 months. All of the 21 ESOC patients analyzed in this retrospective study were male, had squamous cell carcinoma, and were in Stage IV. The median OS was 14 months (420 days), PFS was 6.5 months (196 days), ORR was 42.9%, and DCR was 61.9%, respectively. Our results were similar to a previous study conducted in China, which enrolled 19 patients in advanced stage, treated by nimotuzumab in combination with cisplatin/5FU, the ORR and DCR was 42.1% and 68.4%, respectively [44]. In contrast, cetuximab in combination with cisplatin/5-FU as the first-line therapy for metastatic ESOC had an OS of 9.5 months and DCR in 75% [27]. When Cetuximab combined with irinotecan as the second-line treatment for platinum-resistant gastroesophageal adenocarcinoma, six cases of PR (11%), SD of 37%, median PFS 2.8 months, and OS 6.1 months were obtained [29]. In our study, all the patients had Stage IV disease and 76.2% patients had received prior platinum-based chemotherapy, the efficacy was superior to that of cetuximab. Patients without prior surgery who used more than six doses of nimotuzumab, had a longer PFS than other groups (p=0.046, Figure 3d).
Gastric cancer is the third leading cause of death by cancer in China [67]. Several combination regimens have been used as first-line treatment, including DCF (docetaxel/cisplatin/5-FU) [68], ECF (Epirubicin/cisplatin/5-FU) [69], ECF modification [70], cisplatin/capecitabine [71] and cisplatin/5-FU [66, 72]. However, the median survival has not exceeded 8–13 months [73, 74]. A phase II controlled clinical trial using nimotuzumab in combination with irinotecan as a second-line therapy in advanced gastric cancer patients (83 patients: 40 in nimotuzumab group versus 43 in control group) reported that median PFS was 73.0 versus 85.0 days (P = 0.5668), median OS and RR at 18 months were 250.5 versus 232.0 days (P = 0.9778) and 18.4 versus 10.3%, respectively [37]. With a similar sample size in the present analysis, 35 patients with advanced gastric cancer were treated with nimotuzumab in combination with CT, reached a OS of 15.86 months (476 days) and a PFS of 4.73months (142 days), ORR 22.9% and DCR 54.3%. Furthermore, Patients received more than 200 mg/week in at least six doses had longer OS than the others (p=0.0005), and males who received nimotuzumab >6 doses have significant increase in PFS than females receiving only ≤6 doses of nimotuzumab (p<0.0001). Our results support the possible use of the nimotuzumab in gastric cancer [37].
Twenty-three Patients with advanced NSCLC were analyzed. 91.3% patients were Stage IV and 60.9% were ADC. 22 cases (95.7%) received prior CT and 20 cases (90.9%) received at least 2 CT regimens. In the 22 cases, only 4 patients (18.2%) did not receive targeted therapy. In those cases, the ORR was 13.0%, which was lower than previous reports (25%-35%). However, the DCR was 78.3 %, the OS reached 13.9 months and the PFS was 5.76 months, which were similar to the data has been published [75]. Univariate and multivariate analysis have not found any predictive factors correlated with OS and PFS. The patients older than 60 years of age and treated with nimotuzumab over six doses, had a longer PFS than the others, but this was not a statistically significant difference (521 days vs 104 days, P=0.07). In NSCLC, the relationship between age and survival is inverse with a trend towards statistical significance in univariate analysis. The outcomes of young and old patients with lung cancer have been previously studied, but the results are inconsistent [76, 77]. Most studies compared only the outcomes of younger and older patients, but not the outcomes of different age groups. Our study showed that the median PFS of patients aged ≤ 60 years was significantly shorter than older patients with NSCLC (Table 8 and 9). No significant difference was found in OS. A similar result was obtained in a recent Chinese study[78]. The explanation for the survival difference remains unclear.
Cetuximab has been approval by the Food and Drug Administration (FDA) to treat patients with CRC in combination with FOLFIRI for first-line treatment, in combination with irinotecan in patients who are refractory to irinotecan-based chemotherapy, or as a single agent in patients who have failed oxaliplatin-and irinotecan-based chemotherapy or who are intolerant to irinotecan [54]. Use of cetuximab in combination with FOLFIRI don't reach significant difference concerning with OS but for PFS 8.9 vs. 8.1 months (p=0.036) [54]. In patients with metastatic colorectal cancer treated with CT or best supportive care (BSC), the OS was 6.1 vs. 4.6 months, but when the KARS mutation is taken in account, there is an OS increase for a wide-type KRAS patients, 8.6 vs. 5.0 months. The set of patients analyzed in this report (n=71) were all in Stage IV. 39 patients had a known KRAS gene status and of these, 37 patients (94.9%) were wild-type KRAS. 63 cases (87.5%) had received prior chemotherapy, in which over 50% patients received at least 2 CT regimens. 13 cases received prior immunotherapy with bevacizumab or cetuximab. The general OS for those patients was 21.8 months (655days) and PFS was 7.2 months (217 days), which were significantly longer than previous reports. Multivariate analysis indicated that prior surgery and nimotuzumab> 6 doses were related with PFS significantly. The patients who had prior surgery and received more than six doses of nimotuzumab have better PFS than others (p=0.011). The efficacy of nimotuzumab combined with CT in the treatment of advanced colorectal cancer was slightly better than that of cetuximab, and with mild adverse reactions. This finding warrants further investigation.
It was found that age, patient sex and previous surgery could be factors to consider in antitumor response. Prior surgery in CRC has a positive correlation with PFS (p=0.037) and in ESOC this factor had a negative influence (p=0.012). This factor has no influence on other indications. Patients younger than 60 years appear to have a greater survival benefit to nimotuzumab treatment of H&NC. Nimotuzumab > 6 doses are related with longer PFS in CRC and GC, and Nimotuzumab > 200mg/weekly is related with longer OS in GC. Even in those patients refractory to combination treatment (n=33), the PFS of patients got >6 doses (n=16) was significantly longer than those only received ≤ 6 doses (n=17), with the median PFS of 92±7.9 days vs 41±0.8 days (p=0.002) and the median OS of 655±348.9 days vs 198±67.4 days (p=0.12, data not shown). For H&NC, statistical significance was found to benefit males less than 60 years, while in GC it was found that patients receiving the highest number of doses over 200 mg had a longer survival.
In conclusion, nimotuzumab administered weekly was well tolerated up to 600 mg in Chinese patients. Our results support that >200 mg weekly and more than six in frequency (maintenance therapy) can be the dosing schedule recommended for further clinical studies, especially for the combination of nimotuzumab with chemotherapy. Additionally, it could be possible for combination treatment of nimotuzumab and another targeted therapy or immunomodulatory checkpoint antibody based on its safety profile.
MATERIALS AND METHODS
Patients
Patients with non-resectable, advanced epithelial malignant tumors treated with nimotuzuamb combined with chemotherapy at Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) between May 1, 2010 and August 1, 2015 were indentified. The patients with unknown metastases and poor performance status (ECOG)>2 were not selected. Clinical data of patients was collected, including diagnosis, age, gender, pathological type, tumor stage, tumor grade, pretreatment history (surgery, RT/CRT, CT), recurrence or metastasis time after surgery, metastasis site, history of nimotuzumab (delivery time, dosage, dosing frequency, combined chemotherapy regimens). The retrospective study was conducted in accordance with the Declaration of Helsinki and the International Conference of Harmonization Good Clinical Practices (ICH-GCP).
Treatment
All of the patients received treatment with nimotuzumab in combination with chemotherapy. The antibody was administered by intravenous injection, in 250 mL of saline solution. Doses between 100-600 mg (fixed dose) were administered weekly, in combination with different schedules of chemotherapy, which was depending on the classification of tumors and the corresponding Chinese Guideline recommendations). Due to the safety profile previously reported for this antibody, the clinicians used maintenance therapy (>6 doses with the original dose) in 64.4% (132/205) of patients.
Evaluation
The clinical endpoint of interest was safety, ORR, DCR, PFS and OS in each indication. The information was collected from the clinical historical record of individual patients. All the AEs were collected and graded by National Cancer Institute's Common Toxicity Criteria (NCI CTC) version 4.03. The antitumor response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 and 1.1 guidelines [79, 80]. ORR was calculated as CR+PR, DCR was calculated as CR+PR+SD. OS was defined as the date of the first nimotuzumab/chemotherapy infusion to the date of death or last contact (visit and telephone). PFS was defined as the time from the first injection of nimotuzumab to the date of progression or death.
Statistical analysis
For each indication, the relationship between each variable with ORR or DCR was performed using Pearson Chi-square tests. The log rank test was used to analyze the association between each variable with OS or PFS, with its associated 95% confidence interval (95% CI). Cox's proportional hazard regression models were conducted for multivariate survival analyses. Proportional hazard assumption was evaluated by examining plots of residuals and by including time-dependent covariates in the models. p<0.05 represent significant differences. All the data were analyzed by SPSS software (version 18.0, IBM). GraphPad Prism (version 6.0, GraphPad Software) was used to draft the figure of Kaplan-Meier curve.
SUPPLEMENTARY TABLE
Acknowledgments
We appreciated the support of all participating patients and families. We thank Dr. Chen Shu-lan from the record room of cancer hospital, CAMS for data retrieval. We thank MsC Carmen Elena Viada Gonzalez from Center of Molecular Immunology of Cuba for collaborate with statistical data analysis. In addition, we thank Dr. Jia-de Lu from National University of Singapore for critical review of the manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors have no potential conflicts of interest to disclose.
GRANT SUPPORT
No funding was provided for this work.
REFERENCES
- 1.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–43. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20:1S–13S. [PubMed] [Google Scholar]
- 3.Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31:1203–8. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 4.Johnston JB, Navaratnam S, Pitz MW, Maniate JM, Wiechec E, Baust H, Gingerich J, Skliris GP, Murphy LC, Los M. Targeting the EGFR pathway for cancer therapy. Curr Med Chem. 2006;13:3483–92. doi: 10.2174/092986706779026174. [DOI] [PubMed] [Google Scholar]
- 5.Rocha-Lima CM, Soares HP, Raez LE, Singal R. EGFR targeting of solid tumors. Cancer Control. 2007;14:295–304. doi: 10.1177/107327480701400313. [DOI] [PubMed] [Google Scholar]
- 6.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 7.Itakura Y, Sasano H, Shiga C, Furukawa Y, Shiga K, Mori S, Nagura H. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer. 1994;74:795–804. doi: 10.1002/1097-0142(19940801)74:3<795::aid-cncr2820740303>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Ooft ML, Braunius WW, Heus P, Stegeman I, van Diest PJ, Grolman W, Zuur CI, Willems SM. Prognostic significance of the EGFR pathway in nasopharyngeal carcinoma: a systematic review and meta-analysis. Biomark Med. 2015;9:997–1010. doi: 10.2217/bmm.15.68. [DOI] [PubMed] [Google Scholar]
- 9.Aichler M, Motschmann M, Jutting U, Luber B, Becker K, Ott K, Lordick F, Langer R, Feith M, Siewert JR, Walch A. Epidermal growth factor receptor (EGFR) is an independent adverse prognostic factor in esophageal adenocarcinoma patients treated with cisplatin-based neoadjuvant chemotherapy. Oncotarget. 2014;5:6620–32. doi: 10.18632/oncotarget.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonjic N, Kovac K, Krasevic M, Valkovic T, Ernjak N, Sasso F, Melato M. Epidermal growth factor-receptor expression correlates with tumor cell proliferation and prognosis in gastric cancer. Anticancer Res. 1997;17:3883–8. [PubMed] [Google Scholar]
- 11.Navolanic PM, Steelman LS, McCubrey JA. EGFR family signaling and its association with breast cancer development and resistance to chemotherapy (Review) Int J Oncol. 2003;22:237–52. [PubMed] [Google Scholar]
- 12.Chakravarti A, Chakladar A, Delaney MA, Latham DE, Loeffler JS. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002;62:4307–15. [PubMed] [Google Scholar]
- 13.Cao J, Fang H, Wang B, Ma C, Xu W. Epidermal growth factor receptor as a target for anti-cancer agent design. Anticancer Agents Med Chem. 2010;10:491–503. doi: 10.2174/1871520611009060491. [DOI] [PubMed] [Google Scholar]
- 14.Wang YX, Gao JX, Wang XY, Zhang L, Liu CM. Antiproliferative effects of selective cyclooxygenase-2 inhibitor modulated by nimotuzumab in estrogen-dependent breast cancer cells. Tumour Biol. 2012;33:957–66. doi: 10.1007/s13277-012-0324-4. [DOI] [PubMed] [Google Scholar]
- 15.Akashi Y, Okamoto I, Iwasa T, Yoshida T, Suzuki M, Hatashita E, Yamada Y, Satoh T, Fukuoka M, Ono K, Nakagawa K. Enhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor status. Br J Cancer. 2008;98:749–55. doi: 10.1038/sj.bjc.6604222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crombet-Ramos T, Rak J, Perez R, Viloria-Petit A. Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer. 2002;101:567–75. doi: 10.1002/ijc.10647. [DOI] [PubMed] [Google Scholar]
- 17.Talavera A, Friemann R, Gomez-Puerta S, Martinez-Fleites C, Garrido G, Rabasa A, Lopez-Requena A, Pupo A, Johansen RF, Sanchez O, Krengel U, Moreno E. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res. 2009;69:5851–9. doi: 10.1158/0008-5472.CAN-08-4518. [DOI] [PubMed] [Google Scholar]
- 18.Berger C, Krengel U, Stang E, Moreno E, Madshus IH. Nimotuzumab and cetuximab block ligand-independent EGF receptor signaling efficiently at different concentrations. J Immunother. 2011;34:550–5. doi: 10.1097/CJI.0b013e31822a5ca6. [DOI] [PubMed] [Google Scholar]
- 19.Garrido G, Tikhomirov IA, Rabasa A, Yang E, Gracia E, Iznaga N, Fernandez LE, Crombet T, Kerbel RS, Perez R. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther. 2011;11:373–82. doi: 10.4161/cbt.11.4.14097. [DOI] [PubMed] [Google Scholar]
- 20.Diaz Miqueli A, Blanco R, Garcia B, Badia T, Batista AE, Alonso R, Montero E. Biological activity in vitro of anti-epidermal growth factor receptor monoclonal antibodies with different affinities. Hybridoma (Larchmt) 2007;26:423–31. doi: 10.1089/hyb.2007.0516. [DOI] [PubMed] [Google Scholar]
- 21.Crombet T, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, Iznaga-Escobar N, Figueredo R, Koropatnick J, Renginfo E, Fernández E, Alvárez D, Torres O, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol. 2004;22:1646–54. doi: 10.1200/JCO.2004.03.089. [DOI] [PubMed] [Google Scholar]
- 22.Allan DG. Nimotuzumab: evidence of clinical benefit without rash. Oncologist. 2005;10:760–1. doi: 10.1634/theoncologist.10-9-760. [DOI] [PubMed] [Google Scholar]
- 23.Boland WK, Bebb G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin Biol Ther. 2009;9:1199–206. doi: 10.1517/14712590903110709. [DOI] [PubMed] [Google Scholar]
- 24.Reddy BK, Lokesh V, Vidyasagar MS, Shenoy K, Babu KG, Shenoy A, Naveen T, Joseph B, Bonanthaya R, Nanjundappa Bapsy PP, Loknatha Shetty J, et al. Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol. 2014;50:498–505. doi: 10.1016/j.oraloncology.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez MO, Rivero TC, del Castillo Bahi R, Muchuli CR, Bilbao MA, Vinageras EN, Alert J, Galainena JJ, Rodriguez E, Gracias E, Mulen B, Wilkinson B, de Armas EL, et al. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol Ther. 2010;9:343–9. doi: 10.4161/cbt.9.5.10981. [DOI] [PubMed] [Google Scholar]
- 26.Basavaraj C, Sierra P, Shivu J, Melarkode R, Montero E, Nair P. Nimotuzumab with chemoradiation confers a survival advantage in treatment-naive head and neck tumors over expressing EGFR. Cancer Biol Ther. 2010;10:673–81. doi: 10.4161/cbt.10.7.12793. [DOI] [PubMed] [Google Scholar]
- 27.Huang XD, Yi JL, Gao L, Xu GZ, Jin Jing, Yang WZ, Lu TZ, Wu SZ, Wu RR, Hu WH, Xie WC, Han F, Gao YH, et al. Multi-center phase II clinical trial of humanized anti-epidermal factor receptor monoclonal antibody h-R3 combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma. [Article in Chinese] Zhonghua Zhong Liu Za Zhi. 2007;29:197–201. [PubMed] [Google Scholar]
- 28.Chong DQ, Toh XY, Ho IA, Sia KC, Newman JP, Yulyana Y, Ng WH, Lai SH, Ho MM, Dinesh N, Tham CK, Lam PY. Combined treatment of Nimotuzumab and rapamycin is effective against temozolomide-resistant human gliomas regardless of the EGFR mutation status. BMC Cancer. 2015;15:255. doi: 10.1186/s12885-015-1191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bode U, Massimino M, Bach F, Zimmermann M, Khuhlaeva E, Westphal M, Fleischhack G. Nimotuzumab treatment of malignant gliomas. Expert Opin Biol Ther. 2012:121649–59. doi: 10.1517/14712598.2012.733367. [DOI] [PubMed] [Google Scholar]
- 30.Ramos-Suzarte M, Lorenzo-Luaces P, Lazo NG, Perez ML, Soriano JL, Gonzalez CE, Hernadez IM, Albuerne YA, Moreno BP, Alvarez ES, Callejo IP, Alert J, Martell JA, et al. Treatment of malignant, non-resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapy. Cancer Biol Ther. 2012;13:600–5. doi: 10.4161/cbt.19849. [DOI] [PubMed] [Google Scholar]
- 31.Yan S, Jiang X, Yang J, Yan D, Wang YX. Radiotherapy for nasopharyngeal carcinoma and combined capecitabine and nimotuzumab treatment for lung metastases in a liver transplantation recipient: a case experience of sustained complete response. Cancer Biother Radiopharm. 2012;27:519–23. doi: 10.1089/cbr.2012.1206. [DOI] [PubMed] [Google Scholar]
- 32.Fu ZC, Cheng HH, Li DS, Lin GS, Su Guo, Ying WM, Liu JR. Nimotuzumab combined with IMRT and concurrent cisplatin chemotherapy treatment advanced nasopharyngeal carcinoma. Chin J Cancer Prev Treat. 2011;18:1475–7. [Google Scholar]
- 33.Wu JT, Peng JY, Wu CB, Shen F. Clinical observation of locally advanced nasopharyngeal carcinoma treated by three-dimensional conformal radiation therapy(3DCRT)/intensity-modulated radiation therapy(IMRT) and synchronous cisplatin chemotherapy comined with Nimotuzumab. Chin J of Oncol Prev and Treat. 2014;6:47–51. [Google Scholar]
- 34.Zhao KL, Hu XC, Wu XH, Fu XL, Fan M, Jiang GL. A phase I dose escalation study of Nimotuzumab in combination with concurrent chemoradiation for patients with locally advanced squamous cell carcinoma of esophagus. Invest New Drugs. 2012;30:1585–90. doi: 10.1007/s10637-011-9735-0. [DOI] [PubMed] [Google Scholar]
- 35.Choi HJ, Sohn JH, Lee CG, Shim HS, Lee IJ, Yang WI, Kwon JE, Kim SK, Park MS, Lee JH, Kim JH. A phase I study of nimotuzumab in combination with radiotherapy in stages IIB-IV non-small cell lung cancer unsuitable for radical therapy: Korean results. Lung Cancer. 2011;71:55–9. doi: 10.1016/j.lungcan.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto W, Yoshino T, Takahashi T, Okamoto I, Ueda S, Tsuya A, Boku N, Nishio K, Fukuoka M, Yamamoto N, Nakagawa K. A phase I, pharmacokinetic and pharmacodynamic study of nimotuzumab in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;72:1063–71. doi: 10.1007/s00280-013-2277-8. [DOI] [PubMed] [Google Scholar]
- 37.Satoh T, Lee KH, Rha SY, Sasaki Y, Park SH, Komatsu Y, Yasui H, Kim TY, Yamaguchi K, Fuse N, Yamada Y, Ura T, Kim SY, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer. 2015;18:824–32. doi: 10.1007/s10120-014-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westphal M, Heese O, Steinbach JP, Schnell O, Schackert G, Mehdorn M, Schulz D, Simon M, Schlegel U, Senft C, Geletneky K, Braun C, Hartung JG, et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015;51:522–32. doi: 10.1016/j.ejca.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Qi D, Cui Y, Wang Q, Huang C, Xu J, Yang Y, Xin L, Tian Y, Qi XA. A clinical trial on docetaxel and carboplatin therapy with or without nimotuzumab for the treatment of advanced nonsmall cell lung cancer. J Cancer Res Ther. 2015;11:C32–7. doi: 10.4103/0973-1482.163836. [DOI] [PubMed] [Google Scholar]
- 40.Crombet T, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, Iznaga-Escobar N, Figueredo R, Koropatnick J, Renginfo E, Fernandez E, Alvarez D, Torres O, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol. 2004;22:1646–54. doi: 10.1200/JCO.2004.03.089. [DOI] [PubMed] [Google Scholar]
- 41.Ramos TC, Figueredo J, Catala M, Gonzalez S, Selva JC, Cruz TM, Toledo C, Silva S, Pestano Y, Ramos M, Leonard I, Torres O, Marinello P, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther. 2006;5:375–9. doi: 10.4161/cbt.5.4.2522. [DOI] [PubMed] [Google Scholar]
- 42.Jia J, Cui Y, Lu M, Wang X, Li J, Li J, Li Y, Zhang X, Gao J, Zhou J, Lu Z, Gong J, Yu J, et al. The relation of EGFR expression by immunohistochemical staining and clinical response of combination treatment of nimotuzumab and chemotherapy in esophageal squamous cell carcinoma. Clin Transl Oncol. 2015:1–7. doi: 10.1007/s12094-015-1406-8. [DOI] [PubMed] [Google Scholar]
- 43.Ji YH, Yang XY, Wu JQ, Huo XQ, Li WW, Li GJ, Mu YL, Lu P. Nimotuzumab with cisplatin or fluorouracil on human esophageal squamous cell carcinoma EC1 cells. Eur Rev Med Pharmacol Sci. 2015;19:586–91. [PubMed] [Google Scholar]
- 44.Ling Y, Chen J, Tao M, Chu X, Zhang X. A pilot study of nimotuzumab combined with cisplatin and 5-FU in patients with advanced esophageal squamous cell carcinoma. J Thorac Dis. 2012;4:58–62. doi: 10.3978/j.issn.2072-1439.2011.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin Y, Shi YX, Cai XY, Xia XY, Cai YC, Cao Y, Zhang WD, Hu WH, Jiang WQ. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138:1717–25. doi: 10.1007/s00432-012-1219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu CD. Clinical study of nimotuzumab combined with chemotherapy in the treatment of late stage gastric cancer. Asian Pac J Cancer Prev. 2014;15:10273–6. doi: 10.7314/apjcp.2014.15.23.10273. [DOI] [PubMed] [Google Scholar]
- 47.Su D, Jiao SC, Wang LJ, Shi WW, Long YY, Li J, Bai L. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumour Biol. 2014;35:2313–8. doi: 10.1007/s13277-013-1306-x. [DOI] [PubMed] [Google Scholar]
- 48.Yang QY, Shen D, Sai K, Mu YG, Jiang XB, Zhang XH, Chen ZP. [Nimotuzumab in combination with chemotherapy for patients with malignant gliomas]. [Article in Chinese] Zhonghua Zhong Liu Za Zhi. 2011;33:232–5. [PubMed] [Google Scholar]
- 49.Li LF, Wang HQ, Liu XM, Zhang HL, Qiu LH, Qian ZZ, Li W. [Nimotuzumab in combination with chemotherapy in patients with advanced non-small cell lung cancer]. [Article in Chinese] Zhonghua Zhong Liu Za Zhi. 2011;33:626–8. [PubMed] [Google Scholar]
- 50.Qi DL, Wang HQ, Li Y, Huang CB, Wang QS, Xu L, Yang YZ, Cui Y, Xin L. [Efficacy and adverse effets of nimotuzumab plus palitaxel liposome and carboplatin in the treatment for advanced non-small cell lung cancer]. [Article in Chinese] Zhonghua Zhong Liu Za Zhi. 2012;34:152–5. doi: 10.3760/cma.j.issn.0253-3766.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Sakurada A, Tsao MS. Predictive biomarkers for EGFR therapy. IDrugs. 2009;12:34–8. [PubMed] [Google Scholar]
- 52.Wang CY, Deng JY, Cai XW, Fu XL, Li Y, Zhou XY, Wu XH, Hu XC, Fan M, Xiang JQ, Zhang YW, Chen HQ, Perez R, et al. High EGFR and low p-Akt expression is associated with better outcome after nimotuzumab-containing treatment in esophageal cancer patients: preliminary clinical result and testable hypothesis. Oncotarget. 2015;6:18674–82. doi: 10.18632/oncotarget.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R, Ward C, Mayne K, Trunzer K, Cappuzzo F. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:4113–20. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 54.FDA Label Information of ERBITUX. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125084s262lbl.pdf
- 55.FDA Lable Information of Vectibix (panitumumab) http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125147s080lbl.pdf:FDA
- 56.Crombet T, Torres L, Neninger E, Catala M, Solano ME, Perera A, Torres O, Iznaga N, Torres F, Perez R, Lage A. Pharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer. J Immunother. 2003;26:139–48. doi: 10.1097/00002371-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 57.J. Guigay JF, Dillies A, Sire C, Kerger J. N, Tennevet I, Machiels J. H, Zanetta S, Pointreau Y, Le Moal L. Bozec, Ribere L. Brugel, Henry S. S. Cetuximab, docetaxel, and cisplatin (TPEx) as first-line treatment in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): First results of phase II trial GORTEC 2008-03. J Clin Oncol. 2011;29(suppl) abstr 5567. [Google Scholar]
- 58.Cohen RB. Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC) Cancer Treat Rev. 2014;40:567–77. doi: 10.1016/j.ctrv.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 60.Lu Y, Huang HX, Li GS. Study of nimotuzumab combined with chemotherapy treatment for metastatic nasopharyngeral carcinoma. Zhong Liu Fang Zhi Yan Jiu. 2012;39:449–51. [Google Scholar]
- 61.Jiang Q, Huang JH, Hu B. Clinical observation of nimotuzumab combined with chemotherapy for treatment of nasopharyngeal carcinoma. Chin J Otorhinolaryngology-Skull Base Surgery. 2014;20:372–3. [Google Scholar]
- 62.Brown LM DS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu W, H XS, Jin Y, Li HX, Song LN, Wang SJ, Wang PZ, Chen Y. Analysis of clinicopathologic features of esophageal cancer. Zhong Guo Zhong Liu Lin Chuang. 2008;35:241–4. [Google Scholar]
- 64.Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, Wenczl M, Goker E, Cisar L, Wang K, Bugat R. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–7. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 65.Guimbaud R, Louvet C, Ries P, Ychou M, Maillard E, Andre T, Gornet JM, Aparicio T, Nguyen S, Azzedine A, Etienne PL, Boucher E, Rebischung C, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol. 2014;32:3520–6. doi: 10.1200/JCO.2013.54.1011. [DOI] [PubMed] [Google Scholar]
- 66.Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, Schuch G, Stoehlmacher J, Derigs HG, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–42. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 67.Chen WQ, Zhang SW, Zeng HM, Zheng RS, Zhou XN, Zhao Ping, Wu LY, Li GL, He J. Report of cancer incidence and mortality in China, 2010. Zhong Guo Zhong Liu. 2014;23:1–10. doi: 10.3760/cma.j.issn.0253-3766.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Awad L, Van Cutsem E, Group VS. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3210–6. doi: 10.1200/JCO.2006.08.3956. [DOI] [PubMed] [Google Scholar]
- 69.Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, Price T, Anderson H, Iveson T, Hickish T, Lofts F, Norman A. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20:1996–2004. doi: 10.1200/JCO.2002.08.105. [DOI] [PubMed] [Google Scholar]
- 70.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United K Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 71.Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 72.Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, Thuss-Patience P, Moehler M, Grabowski P, Arnold D, Greten T, Muller L, Rothling N, Peschel C, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2009;20:1667–73. doi: 10.1093/annonc/mdp069. [DOI] [PubMed] [Google Scholar]
- 73.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 74.Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–14. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Ramlau R, Gervais R, Krzakowski M, von Pawel J, Kaukel E, Abratt RP, Dharan B, Grotzinger KM, Ross G, Dane G, Shepherd FA. Phase III study comparing oral topotecan to intravenous docetaxel in patients with pretreated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:2800–7. doi: 10.1200/JCO.2005.03.6491. [DOI] [PubMed] [Google Scholar]
- 76.Chen KY, Chang CH, Yu CJ, Kuo SH, Yang PC. Distribution according to histologic type and outcome by gender and age group in Taiwanese patients with lung carcinoma. Cancer. 2005;103:2566–74. doi: 10.1002/cncr.21087. [DOI] [PubMed] [Google Scholar]
- 77.Hsu CL, Chen KY, Shih JY, Ho CC, Yang CH, Yu CJ, Yang PC. Advanced non-small cell lung cancer in patients aged 45 years or younger: outcomes and prognostic factors. BMC Cancer. 2012;12:241. doi: 10.1186/1471-2407-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Chen SF, Zhen Y, Xiang J, Wu C, Bao P, Luketich J, Hu H, Zhou X, Zhang J, Yao S, Chen HQ. Multicenter analysis of lung cancer patients younger than 45 years in Shanghai. Cancer. 2010;116:3656–62. doi: 10.1002/cncr.25100. [DOI] [PubMed] [Google Scholar]
- 79.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 80.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


