Abstract
In the screening of marine mangrove derived fungi for lovastatin productivity, endophytic Aspergillus luchuensis MERV10 exhibited the highest lovastatin productivity (9.5 mg/gds) in solid state fermentation (SSF) using rice bran. Aspergillus luchuensis MERV10 was used as the parental strain in which to induce genetic variabilities after application of different mixtures as well as doses of mutagens followed by three successive rounds of genome shuffling. Four potent mutants, UN6, UN28, NE11, and NE23, with lovastatin productivity equal to 2.0-, 2.11-, 1.95-, and 2.11-fold higher than the parental strain, respectively, were applied for three rounds of genome shuffling as the initial mutants. Four hereditarily stable recombinants (F3/3, F3/7, F3/9, and F3/13) were obtained with lovastatin productivity equal to 50.8, 57.0, 49.7, and 51.0 mg/gds, respectively. Recombinant strain F3/7 yielded 57.0 mg/gds of lovastatin, which is 6-fold and 2.85-fold higher, respectively, than the initial parental strain and the highest mutants UN28 and NE23. It was therefore selected for the optimization of lovastatin production through improvement of SSF parameters. Lovastatin productivity was increased 32-fold through strain improvement methods, including mutations and three successive rounds of genome shuffling followed by optimizing SSF factors.
Keywords: Aspergillus luchuensis, Genome shuffling, Lovastatin, Mutation, Solid state fermentation
Lovastatin is a member of the statins family, a safe and effective competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, the rate-limiting enzyme of cholesterol biosynthesis. It reduces cholesterol in the management and prevention of cardiovascular disease [1,2]. Over the past decade, statins have been shown to have wide range of activities, such as anti-proliferative, anti-angiogenic, radiosensitizing, pro-apoptotic properties, anti-metastasis properties (in lymphoma, melanoma, and colon tumors), and neuroprotective ability as a probable therapeutic or preventive agent in Alzheimer's disease, as well as antimitotic and antibacterial activities [3,4,5,6,7].
Numerous fungal genera produce lovastatin, including Aspergillus, Penicillium, Monascus, Paecilomyces, Trichoderma, Scopulariopsis, Doratomyces, Phoma, Phythium, Gymnoascus, Hypomyces, and Pleurotus [8]. Marine mangroves are well known to be hosts for a large community of associated endophytic fungi without inducing disease symptoms. Endophyte microbes are becoming targets of the continuing search for an economical, continuous, and manageable supply of the pharmacologically active compounds by laboratory cultivation of the endophytic producer outside the hosts [9].
In recent years, a new breeding technique in industrial microorganisms is induction of genetic variabilities followed by genome shuffling. The advantage of this method is that the genetic reconstruction can be achieved with the tested strains without knowing its genetic basis, making it a highly effective technique. This introduces the advantage of concurrent genetic variations at various locations throughout the whole genetic material without requiring genome sequences data [10]. This technique has also been successfully applied to enhance ayamycin and xylanase productivity from marine derived-microorganisms Nocardia sp. ALAA 2000 and Aspergillus sp. NRCF5, respectively as well as antimycotic and anti-hepatitis C virus (HCV) metabolites produced by mangrove fungi [9,11,12]. Solid state fermentation (SSF) is a method where by various agricultural wastes and polymers can be used for growing fungal species. Previous research has shown that several fungal strains are capable of producing statin pharmaceuticals in SSF using various wastes [8].
To our knowledge, there are no reports on the overproduction of lovastatin in mangrove-derived fungi through strain improvement via genome shuffling followed by optimizing SSF factors using natural solid wastes as substrates, as a cheap and renewable source for statin drugs.
MATERIALS AND METHODS
Lovastatin-producing endophytic mangrove fungi (wild strains)
The marine endophytic fungal strains were obtained previously [9]. Lovastatin-producing endophytic fungi were grown and preserved on potato dextrose agar (PDA) medium for 7 days at 28℃ until conidia formation.
Fermentation procedure
In a 500-mL Erlenmeyer flask, ten grams of solid substrate (wheat straw, wheat bran, rice husk, rice bran, corncob, sugarcane bagasse, potato peel, orange epicarp, lemon epicarp, cauliflower leaf waste, groundnut shell, saw dust, and tea dust) were adjusted to initial moisture content of 60% and pH 6 with particle size of 0.8mm, sterilized, and then inoculated with 106 spore/mL of the fungal strain under study. The fermentation was carried out at 28℃ for 5 days.
Lovastatin extraction and quantitative analysis
The fermented materials were dried at 50℃, powdered, and then extracted with ethyl acetate (pH 3.0) in 250-mL Erlenmeyer flasks for 2 hr in a rotary shaker at 200 rpm at room temperature. Then the combination was centrifuged at 10,000 rpm for 10 min and supernatant was filtered through membrane filter with a pore size of 0.45 µm. This supernatant was kept for further analysis. To 1 mL of the supernatant, 1 mL of trifluroacetic acid (1%) was added and incubated for 10 min (lactonization of the hydroxyl acid form of lovastatin). From the above solution, 0.5 mL was taken and diluted 10 times with methanol. Its absorbance was read at 238 nm by UV-visible spectrophotometer [13]. Standard stock solution of lovastatin, and the calibration curve was created by plotting drug concentration versus the absorbance values measured by UV spectrophotometer [13,14]. Lovastatin production was further established via the laboratory analytical technique thin layer chromatography (TLC). TLC plates were spotted with extracts along with the standard lovastatin. Loaded TLC plates were dipped into a solvent system containing toluene and ethanol in the ratio of 8 : 2. These plates were exposed to a UV lamp in order to visualize the spots [15].
Application of mutagenesis and genome shuffling technique
The hyper-lovastatin producer strain, Aspergillus luchuensis MERV10, was selected for enhancement of lovastatin production by induction of mutations. Conidia from a week-old PDA slants were suspended in sterile normal saline solution (0.85% NaCl) containing 0.1% Tween-80 to disperse spore clumps. A suspension containing 108 conidia/mL was treated with two combination methods of mutations for strain improvement. The first combination consisted of UV (254 nm) for 10min at a distance of 20 cm as a physical mutagen, followed by 150 µg/mL N-methyl-N-nitro-N-nitrosoguanidine (NTG) added into the suspension and left for 60 min as chemical mutagen. The second combination consisted of two chemical mutagens: 150 µg/mL NTG and 250 µg/mL ethidium bromide (EtBr) in 0.85% NaCl for 60 min. The suspension of surviving spores was diluted and spread onto PDA medium supplemented with 0.1% Triton X-100, to limit the growth of the fungal colonies, and 50 µg/mL lovastatin, then incubated at 28℃ for 2~7 days. Colonies that appeared were chosen randomly and transferred to slants with PDA medium containing 50 µg/mL lovastatin to be tested for lovastatin productivity, and hyper-lovastatin productivity mutants were then selected for further study [11,16].
To test the effect of genome shuffling on lovastatin productivity, four mutants induced from A. luchuensis MERV10 by various mutagenic treatments were selected. Protoplasts were prepared by enzymatic hydrolysis of mycelial suspensions using the procedure of El-Bondkly [11], and El-Bondkly and El-Gendy [16]. Equal numbers of protoplasts from these mutants were mixed, centrifuged, and resuspended in 35% (w/v) polyethylene glycol 6000 solution containing 0.05 M glycine-NaOH buffer, pH 7.5, and 0.05M CaCl2. After gently shaking at 30℃ for 10 min to allow the protoplasts to fuse, the fused protoplasts were diluted appropriately in an osmatically balanced phosphate buffer, and plated on the hypertonic regeneration medium. This is the same as the protoplast medium, with 0.7M KCl, 0.1% (v/v) Triton X-100, 20.0 g/L agar, and 100 µg/mL lovastatin [11,16]. The plates were incubated at 28℃ until colonies grew on the surface of plates. The colonies that found under these conditions were measured for lovastatin productivity, and those with the highest productivity were selected for the subsequent round of genome shuffling. Three successive rounds of genome shuffling were carried out using the same method; after each round, the lovastatin concentrations selected for further treatment were increased.
Biomass estimation and optimization of SSF factors for lovastatin production
Biomass estimation in SSF of each fermented substrate was recorded according to Augustine et al. [17]. The lovastatin yield parameters were optimized under SSF and the parameters optimized earlier were used in subsequent trials. These parameters were natural substrate wastes, supplementation of wheat bran with exogenous additives (such as glycerol, fructose, glucose, sucrose, lactose, maltose, peptone, yeast extract, beef extract, malt extract, soybean meal, Na-glutamate, corn steep solid, Tween-80, butyrolactone, dodecane, and sodium acetate at a concentration of 1% as well as ZnSO4 and MgSO4 at a concentration of 0.2%). The initial pH values were 3, 4, 5, 5.5, 6, 6.5, 7, and 8, respectively. Incubation temperatures were 25℃, 28℃, 30℃, 35℃, and 40℃, respectively. Ratio of substrate mass to flask volume was 0.5 : 50, 1.0 : 50, 2.0 : 50, 2.5 : 50, and 3.0 : 50, respectively. Typical time course of lovastatin production was 1~10 days. Inoculum size was 102, 103, 104, 105, 106, 107, 108, and 109 spore/mL, respectively. Initial moisture level was 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, and 90%, respectively. Particle size was 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, and 2.0 mm, respectively.
Determination of minimum inhibition, bactericidal and fungicidal concentration (MIC, MBC, and MFC) of lovastatin against several bacterial and fungal strains
Antimicrobial activities of lovastatin were evaluated against different reference strains, including standard bacteria such as Escherichia coli ATCC-8739, E. coli ATCC-35218, E. coli ATCC-11775, Staphylococcus aureus MRSA ATCC-6538, S. aureus MRSA ATCC-33591, S. aureus MRSA ATCC-33592, S. epidermidis MRS ATCC-700568, S. epidermidis MRS ATCC-700570, S. hominis MRS ATCC-51624, and S. hominis MSS ATCC-700564. The MIC and MFC values of lovastatin against fungi, including Mucor miehei NRRL-3420, Penicillium chrysogenum NRRL-1951, Alternaria alternata NRRL-54028, Candida parapsilosis NRRL-YB-597, C. vaccinii NRRL Y-17684, C. magnoliae NRRL Y-2024, Rhizopus oryzae NRRL-395, R. oligosporus NRRL-2710, Fusarium graminearum NRRL-31084, Botrytis cinerea ATCC-56594, and B. preuss ATCC-18042 were also determined. The MICs were measured for all standard bacterial and fungal strains according to the National Committee for Clinical Laboratory Standards (NCCLS). MIC was defined as the lowest concentration at which no growth occurred. Plates were read in duplicate, and the highest MIC value was recorded. The MBC and MFC of each antifungal agent was determined as described previously [18,19].
RESULTS AND DISCUSSION
Screening of wild type strains for lovastatin productivity
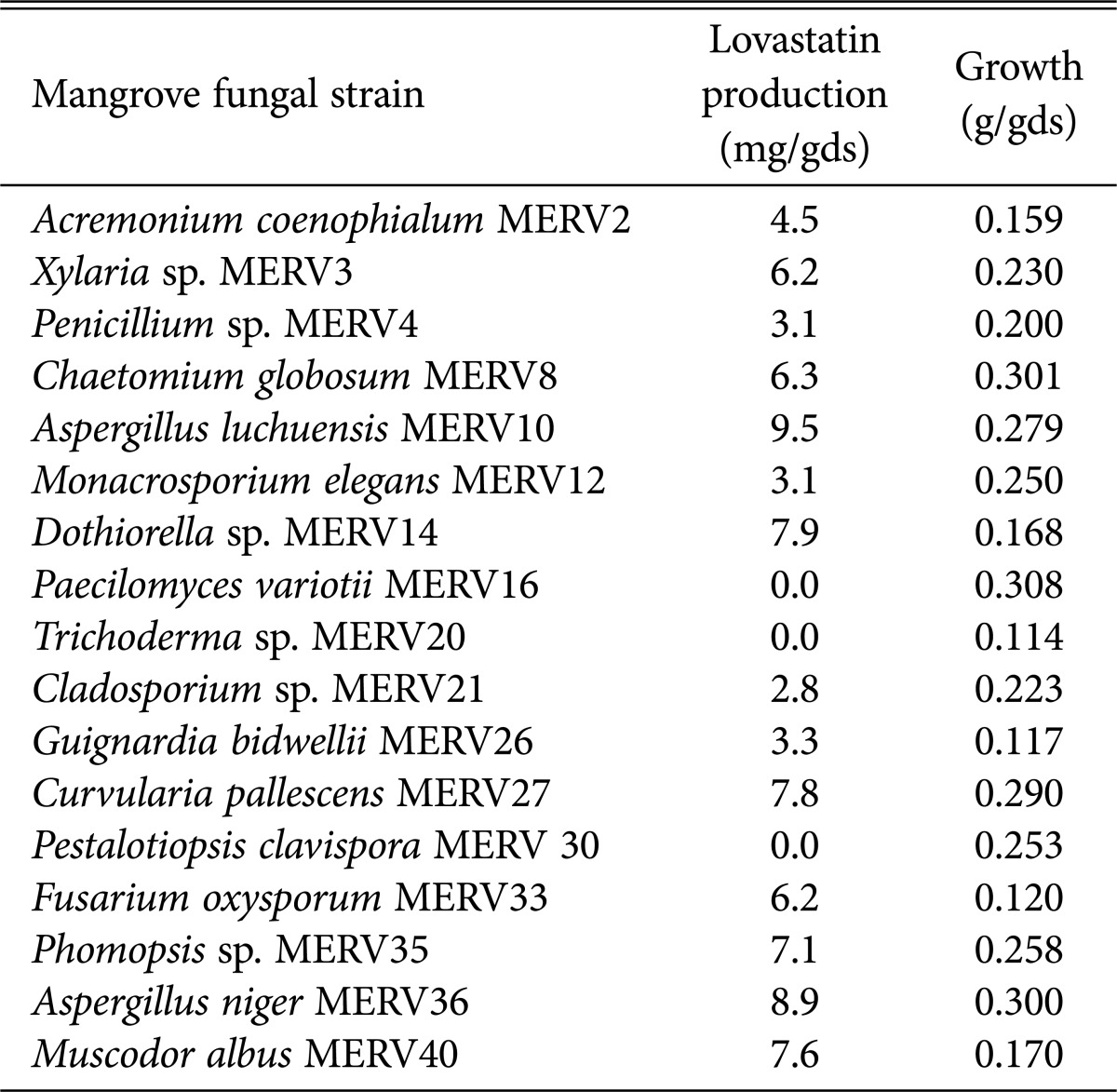
Different mangrove fungal species (17 strains) (Table 1) were obtained from a previous study [9] and evaluated for lovastatin productivity by TLC analysis, followed by further estimation at 238 nm. The results showed that 82.35% (14 isolates) were positive for lovastatin production. Among them, A. luchuensis MERV10 showed the highest lovastatin activity (9.5 mg/gds) using rice bran in SSF; it was then selected for improvement studies (Table 1). Endophytic microorganisms produced varieties of bioactive products with unique structure, including alkaloids, benzopyranones, chinones, flavonoids, phenolic acids, quinones, steroids, terpenoids, tetralones, xanthones, and others [20]. Furthermore, as reported by Osman et al. [14] and Chaynika and Srividya [15], lovastatin production was detected in a wide range of fungi, such as A. oryzae, A. terreus, A. niger, Doratomyces stemonitis, Paecilomyces variotii, P. citrinum, P. chrysogenum, P. janthinellum, P. citrinum, P. spinulosum, Scopulariopsis brevicaulis, Trichoderma viride, and Mycelia sterile; of these, A. terreus was the best lovastatin producer. The results proved that the fungus, A. luchuensis MERV10 is an excellent source of lovastatin productivity, as well as a potential microorganism for gene transfer to improve the lovastatin yield.
Table 1. Screening of different mangrove endophytic fungal strains for lovastatin production using rice bran in solid state fermentation.
Induction of genetic variabilities in A. luchuensis MERV10
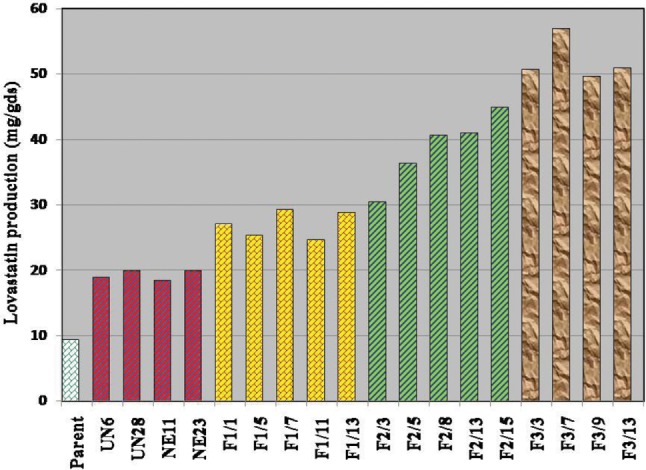
Aspergillus luchuensis MERV10 (9.5 mg/gds of lovastatin under SSF using rice bran) was subjected to a rational mutation-selection program based on resistance to lovastatin (50 µg/mL). Two different combination methods were used to generate populations of mutants. The colonies thus obtained were selected on 50 µg/mL lovastatin and screening for lovastatin productivity. As shown in Fig. 1, the lovastatin productivity of different A. luchuensis mutants varied widely in comparison with the production of the original culture. A total 58 mutants were selected and tested for their ability in lovastatin production; of these, 32 were induced after treatment with the physical-chemical combination. The remaining 26 mutants were induced after the chemical-chemical combination treatment. Lovastatin production was distributed in three classes. Out all the tested mutants, 8 (13.79%) showed lovastatin production located in the original strain production (class A). Forty mutants (68.97%) yielded more lovastatin than the wild strain, ranging from 10.0 to 15.0 mg/gds (class B) (Fig. 1). Ten mutants (17.24%) produced the highest yield of lovastatin, from 15.1 to 20.0 mg/gds (class C) (Fig. 1). The mutants with the highest lovastatin productivity, UN6, UN28, NE11, and NE23 (19.0, 20.0, 18.5, and 20.0 mg/gds, respectively), were selected as the initial mutants for the first round of genome shuffling (Fig. 2). Prabhakar et al. [21] induced mutations in A. terreus KLV28 using ethyl methylsulphonate (EMS) and UV; their isolated mutant mu21 produced the highest yield of lovastatin. Furthermore, A. terreus that was subjected to EMS or UV mutagenesis led to mutants JPM-EMS2 and JPM-UV2, which yielded lovastatin in significant quantity: 948.5 and 1,553.02 mg/L, respectively [22].
Fig. 1. Histogram representing the classes of lovastatin production (mg/gds) and the number of Aspergillus luchuensis MERV10 isolates were obtained after induction of mutations and application of three rounds of genome shuffling.
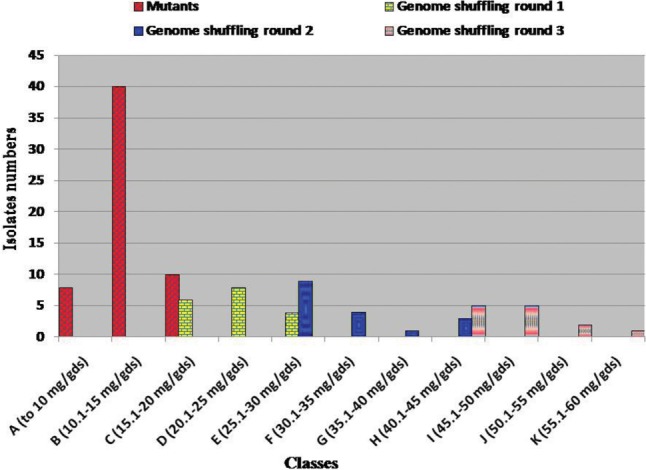
Fig. 2. Improvement of lovastatin production was achieved after induction of mutations and application of three rounds of genome shuffling compared to the original strain Aspergillus luchuensis MERV10.
Application of genome shuffling technique for enhancement of lovastatin production
Application of the genome shuffling technique increased genetic diversity and gene doses by homologous recombination in the chosen mutant populations. The four selected mutants (UN6, UN28, NE11, and NE23) produced at least 94.74% more lovastatin than the original strain (9.5 mg/gds). Protoplast from each of these mutants was prepared, and all were pooled in equal ratio. After protoplast fusion, colonies that appeared on the regeneration medium supplemented with 100 µg/mL lovastatin were selected for fermentation in SSF using rice bran.
After the first round of genome shuffling, 18 isolates were isolated and tested for lovastatin productivity. All produced more lovastatin than the wild strain A. luchuensis MERV10. Their production ranged from 18.9 to 29.4 mg/gds (classes C, D, and E) (Fig. 1). Five fusants, F1/1, F1/5, F1/7, F1/11, and F1/13, showed greater increase in the yield of lovastatin (27.1, 25.4, 29.4, 24.7, and 28.9 mg/gds, respectively) (Fig. 2). These fusants were then applied as the starter isolates for the second round of genome shuffling. This produced 17 colonies that were selected as resistant to 150 µg/mL lovastatin and assayed for lovastatin production. Their lovastatin yield ranged from 25.7 to 45.0 mg/gds (classes E, F, G, and H) (Fig. 1). The fusants with the highest lovastatin productivity, F2/3, F2/5, F2/8, F2/13, and F2/15, exhibited a further enhancement in the production of lovastatin (30.5, 36.4, 40.7, 41.0, and 45.0mg/gds, respectively) (Fig. 2). These recombinants were subjected to a third round of genome shuffling. This produced 13 isolates that were resistant to 200 µg/mL lovastatin. These were tested for lovastatin productivity, which ranged from 45.0 to 57.0 mg/gds (classes H, I, J, and K) (Fig. 1). The results showed that fusants F3/3, F3/7, F3/9, and F3/13 exhibited lovastatin productivity equal to 50.8, 57.0, 49.7, and 51.0 mg/gds, respectively (Fig. 2). Recombinant strain F3/7 yielded 57.0 mg/gds of lovastatin, which is 6-fold and 2.85-fold higher than the original strain A. luchuensis MERV10 (9.5 mg/gds) and the highest mutants UN28 or NE23 (20.0 mg/gds), respectively. It was therefore selected for the optimization of lovastatin production under SSF.
The genome shuffling technique has been applied in many previous studies. After four rounds of genome shuffling, El-Bondkly [11] isolated the highest xylanase producer, recombinant R4/31, which produced 427.5 U/mL (612.5% more than the initial strain Aspergillus sp. NRCF5). Kavitha et al. [23] applied interspecific protoplast fusion between wild strains of Aspergillus terreus and Aspergillus flavus, which maximized lovastatin production under SSF. These studies approved that genome shuffling is an effective tool, building on genetic material recombination without any previous information about genome formation. To our
Lovastatin productivity optimization in SSF by recombinant strain F3/7 of A. luchuensis MERV10
Effect of different natural solid substrates and additives
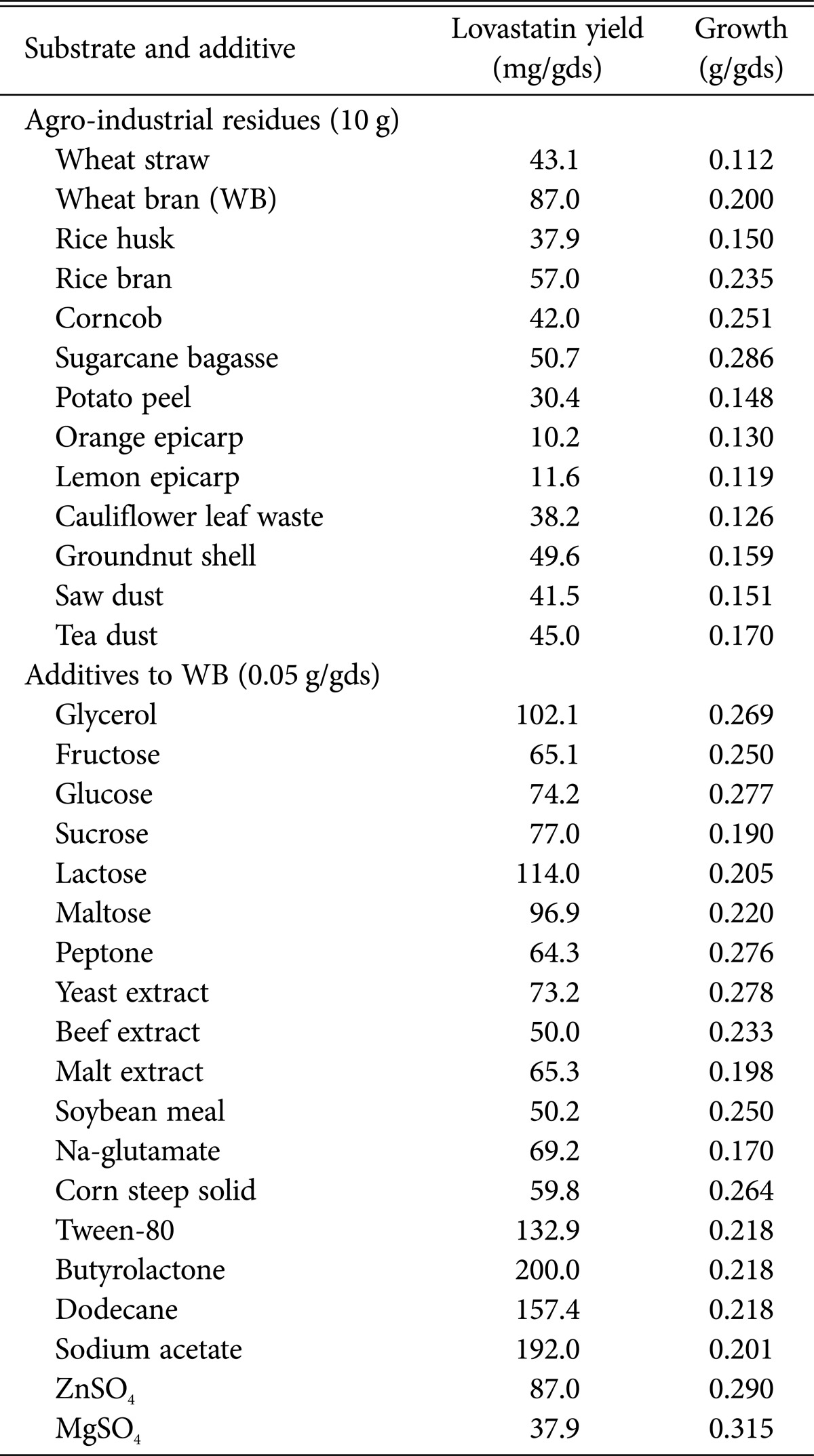
In our search to formulate a low-cost, effective production medium that supports the production of lovastatin from A. luchuensis recombinant strain F3/7, a variety of easily available and inexpensive agro-industrial residues were used and evaluated. The order of substrate suitability (Table 2) was wheat bran (87.0 mg/gds) > rice bran (57.0 mg/gds) > sugarcane bagasse (50.7 mg/gds) > groundnut shell (49.6 mg/gds) > tea dust (45.0 mg/gds) > wheat straw (43.1 mg/gds) > corncob (42.0 mg/gds) > saw dust (41.5 mg/gds) > cauliflower leaf waste (38.2 mg/gds) > rice husk (37.9 mg/gds) > potato peel (30.4mg/gds) > lemon epicarp (11.6mg/gds) > orange epicarp (10.2mg/gds). Based on this screening study, wheat bran was chosen as the most suitable waste for further investigation of lovastatin optimization under SSF by recombinant strain F3/7. In line with our results, various species of Aspergillus such as A. terreus UV1718, and A. terreus 4, and other fungal species like Monascus sp., P. funiculosum, and P. ostreatus proved their ability to produce the maximum yield of lovastatin using wheat bran as the favored substrate [24,25,26,27,28]. On the other hand, Raghunath et al. [29] report that rice bran was the most suitable substrate, inducing the highest lovastatin production by endophytic A. niger. Osman et al. [14] report that among thewaste materials they tested, including molasses, apple waste, strawberry waste, bagasse, wheat bran, and corn meal, bagasse proved to be the most appropriate waste for lovastatin productivity by A. terreus, followed by strawberry waste.
Table 2. Effect of different nutritional factors on lovastatin production by the recombinant strain F3/7 of Aspergillus luchuensis MERV10.
Different carbon sources were used to enhance lovastatin productivity along with the wheat bran. As shown in Table 2, although wheat bran can support lovastatin production to 87.0 mg/gds, wheat bran medium supplemented with lactose, followed by glycerol and maltose, increased lovastatin production by recombinant strain F3/7 by 1.31-fold, 1.17-fold, and 1.11-fold, respectively, but supplementation with readily metabolizable substrates such as glucose and fructose resulted in suppression of lovastatin productivity (74.18 and 65.13mg/gds, respectively). Our results are in disagreement with Jaivel and Marimuthu [30] who reported a lower yield of lovastatin in SSF by A. terreus when lactose was incorporated into solid substrate, while lactose was found to be the best inducer for lovastatin productivity by A. terreus ATCC20542 and A. fischeri [31,32].
Different nitrogen sources were also added to improve lovastatin production, along with the wheat bran supplemented with lactose (Table 2). However, all of these additional nitrogen sources, including peptone, yeast extract, beef extract, malt extract, soybean meal, Na-glutamate, and corn steep solid, showed negative effect on lovastatin production by 26.15%, 15.92%, 42.53%, 24.94%, 42.32%, 20.49%, and 31.23%, respectively. It is essential to strike an appropriate equilibrium between carbon and nitrogen sources for achieving maximum lovastatin yield, as these two elements control the biomass and metabolite productivity [14]. Growth of A. terreus and lovastatin production were greatly affected by the C :N ratio in the medium. The highest C : N ratio supported the best fermentation conditions for lovastatin productivity [28,33].
Conversely, wheat bran medium supplemented with butyrolactone followed by sodium acetate, dodecane, and Tween-80 improved lovastatin production by fusant F3/7 strain by 2.30-fold, 2.21-fold, 1.81-fold, and 1.53-fold, respectively (Table 2). On the other hand, the mineral ZnSO4 had no effect on lovastatin production, whereas MgSO4 reduced it by 56.41%. Our data support the data previously obtained by Xu et al. [34] with Monascus ruber, Bizukojc and Ledakowicz [35] with A. terreus and Osman et al. [14] with various fungi. All of these researchers reported that these supplementations produce better lovastatin yield by performing as a precursor for statin synthesis.
Initial pH and incubation temperature effects
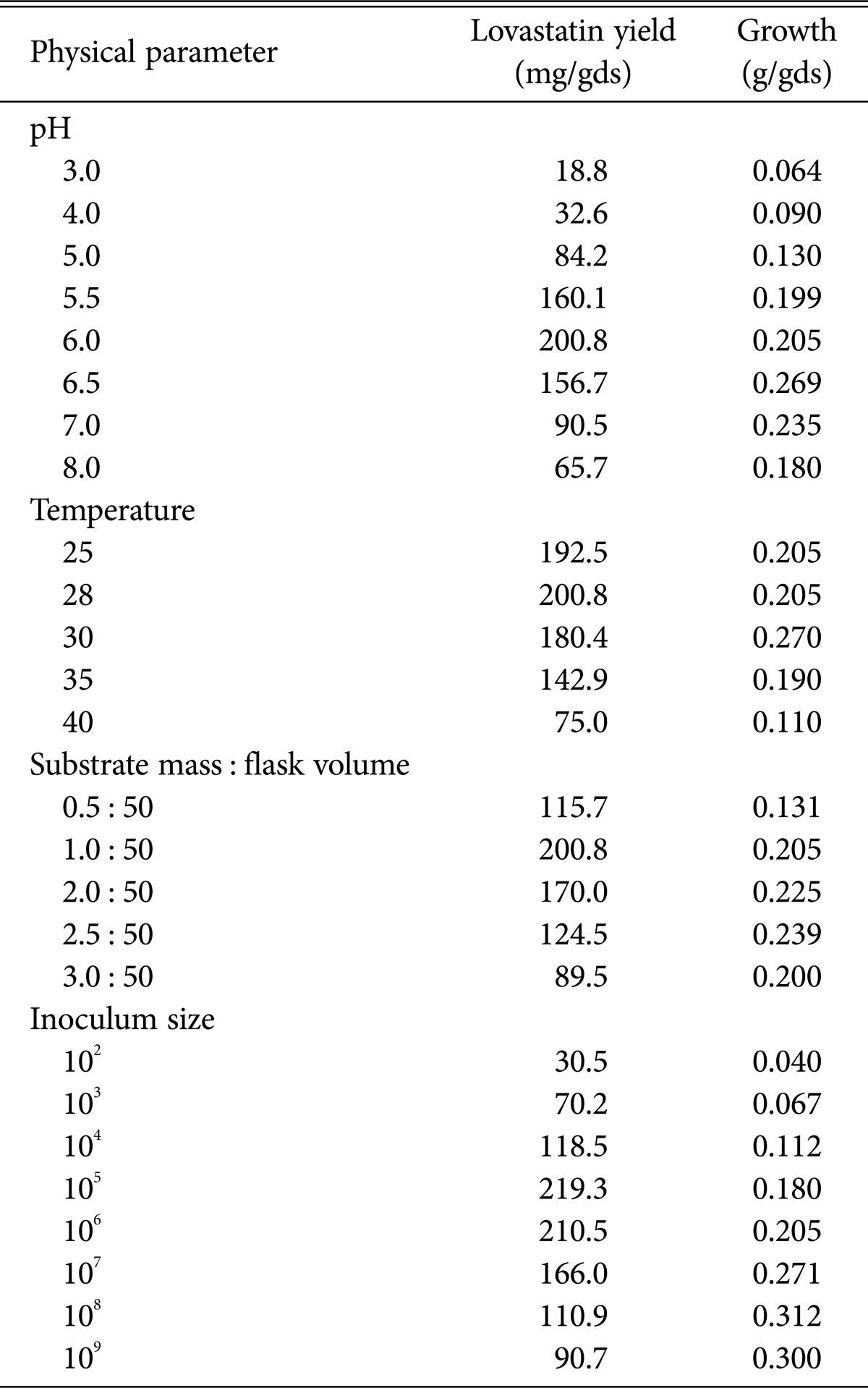
As presented in Table 3, the initial pH of the substrates affected the peak lovastatin productivity. The maximum production of lovastatin was at a pH of 6.0. Lovastatin yield at this optimal pH was about 10.68-fold and 3.06-fold more than at pH values of 3.0 and 8.0, respectively. The pH range from 5 to 6 provided optimum conditions for lovastatin yield by A. terreus, A. fischerii and A. flavus. Similarly, the optimum initial pH for lovastatin yield in SSF is close to neutral pH, with minor differences that could be attributed to the kinds of substrates and microbes used in the fermentation procedure [23,32,36]. Manzoni and Rollini [37] statedthat by adjusting pH and gradually adding the carbon source, lovastatin productivity by filamentous fungi could improve fivefold. On the other hand, Jahromi et al. [38] indicated that the initial pH element has no significant effect on lovastatin yield, because they found no significant alteration in lovastatin yield by A. terreus ATCC 20542 and A. terreus ATCC 74135 at pH of 5 to 8.
Table 3. Effect of different physical factors on lovastatin production by the recombinant strain F3/7 of Aspergillus luchuensis MERV10.
The incubation temperature is one of the significant factor affecting microbial activity and thus the production of bioactive compounds. Recombinant strain F3/7, when incubated at temperatures of 25℃, 28℃, 30℃, 35℃, and 40℃, showed maximum yield of lovastatin (200.8 mg/gds) at 28℃ in wheat bran medium (Table 3). At temperatures lower or higher than the optimum, less lovastatin production was observed. May other sources report 28℃ as the best for lovastatin production by A. terreus, A. fischerii, and endophytic A. niger [32,36,39].
Effect of substrate mass to flask volume ratio
The effect of various ratios of wheat bran mass to flask volume (0.5 : 50, 1 : 50, 2 : 50, 2.5 : 50, and 3 : 50) on lovastatin production was evaluated. Maximum yield of lovastatin (200.8 mg/gds) was detected in the flasks containing 10 g of wheat bran in a 500-mL Erlenmeyer flask (1 : 50) (Table 3). Lower or higher substrate mass per flask gave lower lovastatin yields. The level of substrate per unit area of working volume of the flask influences the porosity and aeration of the substrate [40]. A ratio of 1 : 50 of substrate mass to flask volume ratio has been reported as providing the maximum lovastatin production from the recombinant strain of the wild strains A. terreus and A. flavus [23], but contrary to the present study, a much higher ratio of 1 : 10 was recommended by Gulyamova et al. [28] as the optimum ratio for lovastatin production by A. terreus.
Typical time course of lovastatin production
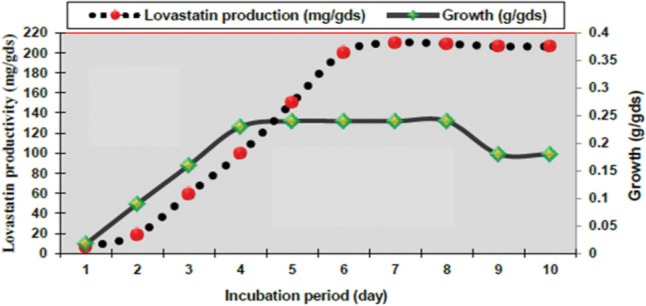
The time course experiment revealed a steady increase in lovastatin production after the first day of fermentation (6.2 mg/gds) up to the seventh day (210.0 mg/gds), with no significant effect on the rate of lovastatin yield, indicating the onset of the stationary phase, which in turn favored the lovastatin production (Fig. 3). Similar results were reported by Samiee et al. [41] and Dewi et al. [42] on lovastatin production by A. terreus which reached a peak after 7 days of fermentation under SSF. Other researchers have found that incubation times of 6~10 days were optimum for lovastatin yield by several fungi [29]. While the biosynthesis of lovastatin reached its maximum on the 11th day of growth in A. terreus 4, A. terreus 20, and Aspergillus sp. no. 76 strains by SSF using oat, wheat bran, and wheat bran, respectively [15,28]. Pansuriya and Singhal [25] found that lovastatin yield by A. terreusin SSF improved until day 3 of fermentation, with no further improvement subsequently.
Fig. 3. Effect of different incubation periods on lovastatin production by recombinant fusant F3/7 of Aspergillus luchuensis MERV10 under solid state fermentation.
Effects of inoculum size, initial moisture content, and particle size
The effect of inoculum size on lovastatin yield was estimated by changing the levels of inoculum from 102 to 109 spore/gds, in 10 g of wheat bran at 60% moisture content. Maximum productivity after 7 days of incubation (219.3 mg/gds) was detected with 105 spore/gds (Table 3). Any additional increase in spore density leads to gradual decrease in lovastatin productivity. Conversely, Jahromi et al. [38] and Pansuriya and Singhal [25] reported that inoculum sizes did not affect lovastatin productivity by the two strains of A. terreus but using inoculum lower than 5 × 107 spore/mL can decrease the productivity of lovastatin.
Moisture level is the most critical factor in SSF; it often determines the success of a process. Solid substrate moistened at 50% initial moisture level afforded high lovastatin productivity (245.9mg/gds) at the seventh day of fermentation. Moisture content greater or less than 50% was not favorable for the highest lovastatin production. This may be because of the adequate amount of oxygen and water present within the substrate. Jahromi et al. [38] suggest that 50% moisture content is optimal for lovastatin yield by two strains of A. terreus. Solid substrate with 60% moisture content resulted in maximum lovastatin production in A. flavipes and A. terreus [23] and mevastatin using P. citrinum NCIM 768 under SSF [43] but moisture content up to 75% enhanced lovastatin production by endophytic A. niger [39].
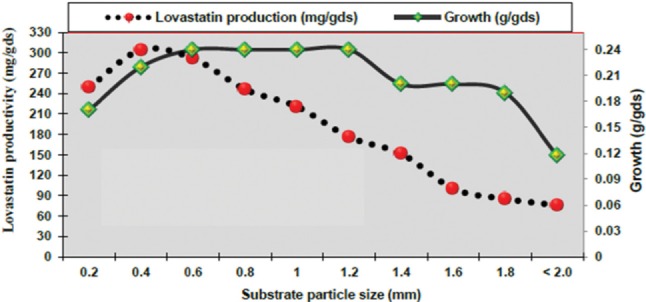
From data in Fig. 4, the particle size is a critical factor in SSF of wheat bran. Lovastatin production was increased from 76 to 304mg/gds when particle size decreased from 2.0 to 0.4 mm. As reported by Jahromi et al. [38], there are two different effects of particle size on SSF process at any given moisture content. The first is that smaller particle size increases the surface region of solid materials for the attachment and growth of the fungi; the second is that smaller particle size decreases inter-space between particles and gas phase oxygen transfer. The results obtained are in accordance with several authors, such as Valera et al. [24] who found that increasing the particle size of wheat bran as substrate from 0.4 to 1.1 mm in SSF led to reduction of lovastatin yield by A. flavipes. On the other hand, Wei et al. [44] found that ground rice at 420 µm reduced lovastatin production by A. terreus and Raghunath et al. [29] suggest an optimum particle size of solid substrate between 1.4 and 2 mm for maximum lovastatin production by A. terreus ATCC 74135.
Fig. 4. Effect of substrate particle size on lovastatin production by recombinant fusant F3/7 of Aspergillus luchuensis MERV10 under solid state fermentation.
Determination of MIC, MBC, and MFC against several bacterial and fungal strains
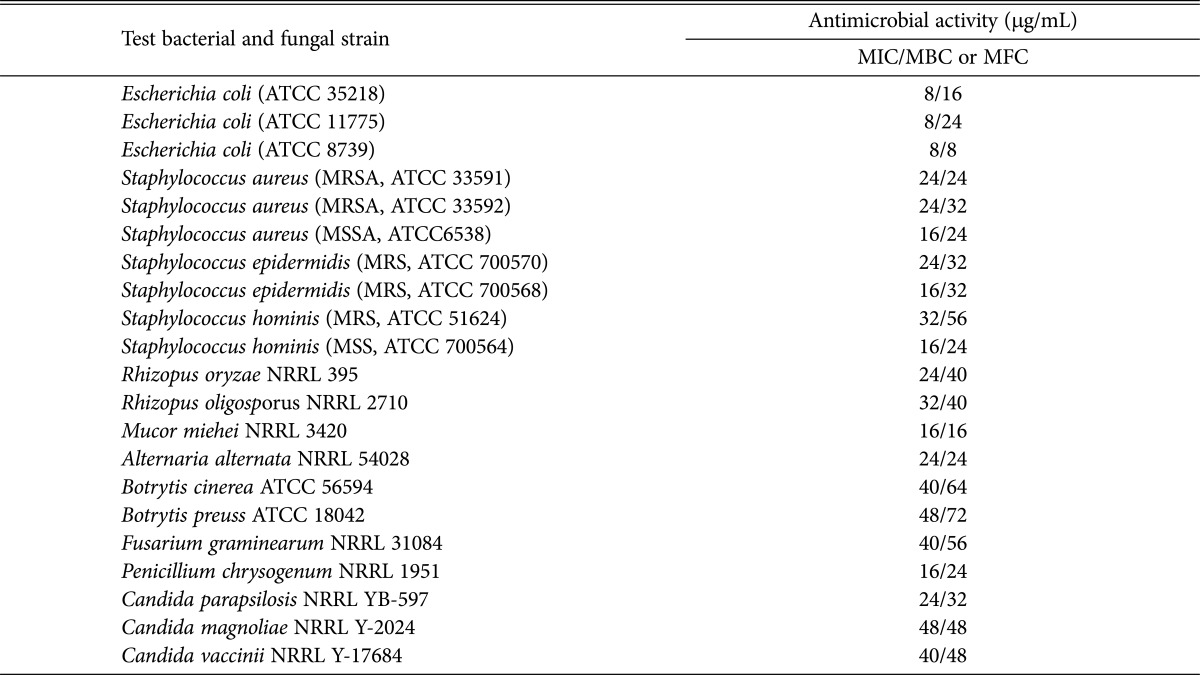
Results presented in Table 4 show that lovastatin is able to induce varying degrees of antimicrobial activities against several standard bacterial and fungal strains. The antibacterial activity was dependent on the standard strains as follow: E. coli ATCC-8739 (8/8 µg/mL), E. coli ATCC-35218 (8/16 µg/mL), E. coli ATCC-11775 (8/24 µg/mL), S. aureus MRSA ATCC-6538 (16/24 µg/mL), S. aureus MRSA ATCC-33591 (24/24 µg/mL), S. aureus MRSA ATCC-33592 (24/32 µg/mL), S. epidermidis MRSA ATCC-700568 (16/32 µg/mL), S. epidermidis MRSA ATCC-700570 (24/32 µg/mL), S. hominis MRS ATCC-51624 (32/56 µg/mL), and S. hominis MSS ATCC-700564 (16/24 µg/mL). The MIC and MFC of lovastatin against fungi, in ascending order (Table 4) were as follows: M. miehei NRRL-3420 (16/16 µg/mL), P. chrysogenum NRRL-1951 (16/24 µg/mL), A. alternata NRRL-54028 (24/24 µg/mL), C. parapsilosis NRRL-YB-597 (24/32 µg/mL), R. oryzae NRRL-395 (24/40 µg/mL), R. oligosporus NRRL-2710 (32/40 µg/mL), C. vaccinii NRRL Y-17684 (40/48 µg/mL), C. magnoliae NRRL Y-2024 (48/48 µg/mL), F. graminearum NRRL 31084 (40/56 µg/mL), B. cinerea ATCC56594 (40/64 µg/mL), and B. preuss ATCC-18042 (48/72 µg/mL). The antimicrobial activities of statins were demonstrated against many standard and clinical strains such as methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) strains [45], different gram-positive and gram-negative bacteria [19,46], M. racemosus; Saccharomyces cerevisiae, pathogenic Candida sp. and Cryptococcus neoformans and opportunistic human pathogenic fungus C. albicans [7]. Chamilos et al. [5] reported that lovastatin had considerable fungicidal activity in vitro against all of the different Zygomycetes isolates tested, and was more active against Mucor sp. than Rhizopus and Cunninghamella sp.
Table 4. Antibacterial and antimycotic activities of lovastatin.
MIC, minimum inhibition concentration; MBC, minimum bactericidal concentration; MFC, minimum fungicidal concentration.
This study recommends a feasible and effective low-cost fermentation strategy that result in a marked enhancement of the production of lovastatin, a cholesterol-lowering drug, from genetically modified endophytic mangrove fungal strain A. luchuensis MERV10. The results of this study also support gene transfer through genome shuffling technique, followed by enhancement and optimization of procedure parameters, as effective methods to enhance production of microbial products of biotechnological importance. Further investigations are necessary to determine the relationship between these bioactive compounds, the producing strain, and the host mangrove plant during this intimate interaction.
References
- 1.Osmak M. Statins and cancer: current and future prospects. Cancer Lett. 2012;324:1–12. doi: 10.1016/j.canlet.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Syed MB, Rajasimman M. Fermentative production and optimization of mevastatin in submerged fermentation using Aspergillus terreus. Biotechnol Rep. 2015;6:124–128. doi: 10.1016/j.btre.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadalizadeh E. Antimicrobial effect and immunomodulation of atorvastatin. Life Sci J. 2012;9:4013–4016. [Google Scholar]
- 4.Brower V. Of cancer and cholesterol: studies elucidate anticancer mechanisms of statins. J Natl Cancer Inst. 2003;95:844–846. doi: 10.1093/jnci/95.12.844. [DOI] [PubMed] [Google Scholar]
- 5.Chamilos G, Lewis RE, Kontoyiannis DP. Lovastatin has significant activity against Zygomycetes and interacts synergistically with voriconazole. Antimicrob Agents Chemother. 2006;50:96–103. doi: 10.1128/AAC.50.1.96-103.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clendening JW, Penn LZ. Targeting tumor cell metabolism with statins. Oncogene. 2012;31:4967–4978. doi: 10.1038/onc.2012.6. [DOI] [PubMed] [Google Scholar]
- 7.Gyetvai A, Emri T, Takács K, Dergez T, Fekete A, Pesti M, Pócsi I, Lenkey B. Lovastatin possesses a fungistatic effect against Candida albicans, but does not trigger apoptosis in this opportunistic human pathogen. FEMS Yeast Res. 2006;6:1140–1148. doi: 10.1111/j.1567-1364.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Radha KV, Lakshmanan D. A review: lovastatin production and applications. Asian J Pharm Clin Res. 2013;6:21–26. [Google Scholar]
- 9.El-Gendy MM, El-Bondkly AM, Yahya SM. Production and evaluation of antimycotic and antihepatitis C virus potential of fusant MERV6270 derived from mangrove endophytic fungi using novel substrates of agroindustrial wastes. Appl Biochem Biotechnol. 2014;174:2674–2701. doi: 10.1007/s12010-014-1218-2. [DOI] [PubMed] [Google Scholar]
- 10.Petri R, Schmidt-Dannert C. Dealing with complexity: evolutionary engineering and genome shuffling. Curr Opin Biotechnol. 2004;15:298–304. doi: 10.1016/j.copbio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 11.El-Bondkly AM. Molecular identification using ITS sequences and genome shuffling to improve 2-deoxyglucose tolerance and xylanase activity of marine-derived fungus, Aspergillus sp. NRCF5. Appl Biochem Biotechnol. 2012;167:2160–2173. doi: 10.1007/s12010-012-9763-z. [DOI] [PubMed] [Google Scholar]
- 12.El-Gendy MM, El-Bondkly AM. Genome shuffling of marine derived bacterium Nocardia sp. ALAA 2000 for improved ayamycin production. Antonie Van Leeuwenhoek. 2011;99:773–780. doi: 10.1007/s10482-011-9551-8. [DOI] [PubMed] [Google Scholar]
- 13.Mielcarek J, Naskrent M, Grobelny P. Photochemical properties of simvastatin and lovastatin induced by radiation. J Therm Anal Calorim. 2009;96:301–305. [Google Scholar]
- 14.Osman ME, Khattab OH, Zaghlol GM, Abd El-Hameed RM. Screening for the production of cholesterol lowering drugs (Lovastatin) by some fungi. Aust J Basic Appl Sci. 2011;5:698–703. [Google Scholar]
- 15.Chaynika P, Srividya S. Bioprospecting of lovastatin producing fungi isolated from soil samples. Int Res J Biol Sci. 2014;3:42–46. [Google Scholar]
- 16.El-Bondkly AM, El-Gendy MM. Cellulase production from agricultural residues by recombinant fusant strain of a fungal endophyte of the marine sponge Latrunculia corticata for production of ethanol. Antonie Van Leeuwenhoek. 2012;101:331–346. doi: 10.1007/s10482-011-9639-1. [DOI] [PubMed] [Google Scholar]
- 17.Augustine A, Joseph I, Raj RP. Biomass estimation of Aspergillus niger S14 a mangrove fungal isolate and A. oryzae NCIM 1212 in solid-state fermentation. J Mar Biol Assoc India. 2006;48:139–146. [Google Scholar]
- 18.Espinel-Ingroff A, Fothergill A, Peter J, Rinaldi MG, Walsh TJ. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J Clin Microbiol. 2002;40:3204–3208. doi: 10.1128/JCM.40.9.3204-3208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masadeh M, Mhaidat N, Alzoubi K, Al-azzam S, Alnasser Z. Antibacterial activity of statins: a comparative study of atorvastatin, simvastatin, and rosuvastatin. Ann Clin Microbiol Antimicrob. 2012;11:13. doi: 10.1186/1476-0711-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molina G, Pimentel MR, Bertucci TC, Pastore GM. Application of fungal endophytes in biotechnological processes. Chem Eng Trans. 2012;27:289–294. [Google Scholar]
- 21.Prabhakar M, Lingappa K, Babu V, Amena S, Vishalakshi N, Mahesh D. Characterization of physical factors for optimum lovastatin production by Aspergillus terreus KLVB28 mu21 under solid state fermentation. J Recent Adv Appl Sci. 2012;27:01–05. [Google Scholar]
- 22.Sreedevi K, Venkateswaa Rao J, Lakshmi N, Fareedullah M. Strain improvement of Aspergillus terreus for the enhanced production of lovastastin, a HMG-CoA reductase inhibitor. J Microbiol Biotechnol Res. 2011;1:96–100. [Google Scholar]
- 23.Kavitha V, Janani B, Angayarkanni J. Optimization of process parameters for lovastatin production from red gram bran by solid state fermentation. Int J Sci Res. 2012;3:1413–1418. [Google Scholar]
- 24.Valera HR, Gomes J, Lakshmi S, Gururaja R, Suryanarayan S, Kumar D. Lovastatin production by solid state fermentation using Aspergillus flavipes. Enzyme Microb Technol. 2005;37:521–526. [Google Scholar]
- 25.Pansuriya RC, Singhal RS. Response surface methodology for optimization of production of lovastatin by solid state fermentation. Braz J Microbiol. 2010;41:164–172. doi: 10.1590/S1517-838220100001000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy DS, Latha DP, Latha KP. Production of lovastatin by solid state fermentation by Penicillium funiculosum NCIM 1174. Drug Invent Today. 2011;3:75–77. [Google Scholar]
- 27.Lakshmanan D, Radha KV. An effective quantitative estimation of lovastatin from Pleurotus ostreatus using UV and HPLC. Int J Pharm Pharm Sci. 2012;4:462–464. [Google Scholar]
- 28.Gulyamova TG, Ruzieva DM, Nasmetova SM, Sattarova RS, Lobanova KV, Abdulmyanova LA, Rasulova GA. Lovastatin production by Aspergillus terreus in solid state and submerged fermentations. Int J Eng Sci Technol. 2013;5:19–24. [Google Scholar]
- 29.Raghunath R, Radhakrishna A, Manikandan N, Nathiya K, Palaniswamy M. Optimized production of lovastatin through solid state fermentation by endophytic fungi. Int J Pharm Bio Sci. 2012;3:562–570. [Google Scholar]
- 30.Jaivel N, Marimuthu P. Optimization of lovastatin production in solid state fermentation by Aspergillus terreus. Int J Eng Sci Technol. 2010;2:2730–2733. [Google Scholar]
- 31.Lai LS, Hung CS, Lo CC. Effects of lactose and glucose on production of itaconic acid and lovastatin by Aspergillus terreus ATCC 20542. J Biosci Bioeng. 2007;104:9–13. doi: 10.1263/jbb.104.9. [DOI] [PubMed] [Google Scholar]
- 32.Chanakya P, Latha PM, Manipati Srikanth M. Solid state fermentation for the production of lovastatin by Aspergillus fischerii. Res J Pharm Sci Biotechnol. 2011;1:9–13. [Google Scholar]
- 33.Bizukojc M, Ledakowicz S. Biosynthesis of lovastatin and (+)-geodin by Aspergillus terreus in batch and fed-batch culture in the stirred tank bioreactor. Biochem Eng J. 2008;42:198–207. [Google Scholar]
- 34.Xu BJ, Wang QJ, Jia XQ, Sung CK. Enhanced lovastatin production by solid state fermentation of Monascus ruber. Biotechnol Bioprocess Eng. 2005;10:78–84. [Google Scholar]
- 35.Bizukojc M, Ledakowicz S. Physiological, morphological and kinetic aspects of lovastatin biosynthesis by Aspergillus terreus. Biotechnol J. 2009;4:647–664. doi: 10.1002/biot.200800289. [DOI] [PubMed] [Google Scholar]
- 36.Kumar MS, Jana SK, Senthil V, Shashanka V, Kumar SV, Sadhukhan AK. Repeated fed-batch process for improving lovastatin production. Process Biochem. 2000;36:363–368. [Google Scholar]
- 37.Manzoni M, Rollini M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biotechnol. 2002;58:555–564. doi: 10.1007/s00253-002-0932-9. [DOI] [PubMed] [Google Scholar]
- 38.Jahromi MF, Liang JB, Ho YW, Mohamad R, Goh YM, Shokryazdan P. Lovastatin production by Aspergillus terreus using agro-biomass as substrate in solid state fermentation. J Biomed Biotechnol. 2012;2012:196264. doi: 10.1155/2012/196264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raghunath R, Radhakrishna A, Angayarkanni J, Palaniswamy M. Production and cytotoxicity studies of lovastatin from Aspergillus niger PN2: an endophytic fungi isolated from Taxus baccata. Int J Appl Biol Pharm Technol. 2012;3:342–351. [Google Scholar]
- 40.El-Gendy MM. Production of glucoamylase by marine endophytic Aspergillus sp. JAN-25 under optimized solidstate fermentation conditions on agro residues. Aust J Basic Appl Sci. 2012;6:41–54. [Google Scholar]
- 41.Samiee SM, Moazami N, Haghighi S, Mohseni FA, Mirdamadi S, Bakhtiari MR. Screening of lovastatin production by filamentous fungi. Iran Biomed J. 2003;7:29–33. [Google Scholar]
- 42.Dewi RT, Artanti N, Mulyani H, Lotulung PD, Minarti Production of lovastatin and sulochrin by Aspergillus terreus using solid state fermentation. Makara J Technol. 2011;15:1–4. [Google Scholar]
- 43.Mahesh N, Balakumar S, Indumathi P, Ayyadurai A, Vivek R. Production and optimization of mevastatin using Penicillium citrinum NCIM 768. J Microb Biochem Technol. 2012;4:001–004. [Google Scholar]
- 44.Wei PL, Xi ZN, Cen PL. Lovastatin production by Aspergillus terreus in solid-state fermentation. J Zhejiang Univ Sci A. 2007;8:1521–1526. [Google Scholar]
- 45.Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. J Antimicrob Chemother. 2008;61:362–364. doi: 10.1093/jac/dkm496. [DOI] [PubMed] [Google Scholar]
- 46.Welsh AM, Kruger P, Faoagali J. Antimicrobial action of atorvastatin and rosuvastatin. Pathology. 2009;41:689–691. doi: 10.3109/00313020903305860. [DOI] [PubMed] [Google Scholar]