Abstract
Trichoderma species are able to persist on living sapwood and leaves of cacao (Theobroma cacao) in an endophytic relationship. In this research, we evaluated the ability of Trichodema asperellum introduced at the incision site in the bark for side grafting with the concentration of 4 g/10 mL, 4 g/100 mL, and 4 g/1,000 mL (suspended in water) in suppressing vascular streak dieback (VSD) incidence and promoting growth of side grafts in the field. The incidence of VSD in two local clones of cacao, MCC1 and M04, without application of T. asperellum was 71.2% and 70.1% at 21 wk after grafting, respectively. However, when the two clones were treated with a concentration of 4 g/10 mL T. asperellum, the incidence was 20.6% and 21.7%, respectively, compared to 29.1% and 20.9% at 4 g/100 mL and 18.2% and 15.6% at 4 g/1,000 mL. By comparing to the control, the treatment with the same concentrations of T. asperellum listed above, the total number of stomata in MCC1 decreased by 41.9%, 30.2%, and 14.0% and in M04 by 30.5%, 21.9%, and -2.5% (exception), respectively. Otherwise, the total area of stomata opening increased by 91.4%, 99.7%, and 28.6% in MCC1 and by 203.8%, 253.5%, and 35.9% in M04, respectively. Furthermore, the number of buds and branches treated with a mixture concentration on the the two clones increased by 90.7% and 21.7%, respectively. These data showed that the application of T. asperellum to cacao scions while grafting can decrease VSD incidence in side grafts and increase growth of grafts in addition to decreasing total number of stomata, increasing total area of opened stomata, and increasing number of buds and branches.
Keywords: Branches, Buds, Endophytic relationship, Stomata opening area, VSD incidence
Vascular streak dieback (VSD) caused by the basidiomycete (Ceratoasidiales) fungus, Ceratobasidium theobromae (Talbot and Keane) [1,2], is an important disease in cacao growing areas extending from New Britain Island, Papua New Guinea in the east to Hainan Island, China in the north and to Kerala State, India in the west [1]. In Indonesia, the disease was reported for the first time in Sabatik Island, East Kalimantan in 1983. Then in 1984, VSD was found in Southeast Sulawesi and Maluku and in 1985 in West Java, Central Java, East Java, North Sumatra, West Kalimantan, and North Sulawesi [3]. In the early 2000s, this disease also caused serious losses in cacao plantations in South Sulawesi, West Sulawesi, Central Sulawesi, Gorontalo, Bali, Papua, and West Sumatra [2,4,5,6]. Therefore, VSD became widely distributed in the country, to a similar extent as Phytophthora pod rot disease. Basidiospores of the fungus released and dispersed by the wind during high humidity periods in rainy season infect young leaves. In the leaf, the fungus colonizes xylem vessels and then moves into the midrib, petiole, and branch. This infection cause the death of shoot apex, branches and in susceptible clone can kill a mature cacao tree [2,7]. Production losses due to VSD was estimated at 30,000 tonnes annually which was 5.3% of the Asian cacao production in 1998 [8]. The use of infected plant material, besides air-borne spores of the fungus are apparently responsible for long distance distribution of the disease.
Pruning, the use of healthy plant material, and the propagation of resistant cacao clones are so far the known methods of controlling VSD infestation. Frequent pruning of infected branches about 30~40 cm below the end visibly streaking symptoms delay the disease build up [9,10], but this method may inhibit cacao growth, and is labor intensive and expensive. In actual conditions where the infestation of VSD is very widespread, it is difficult to find healthy plant material for use in cacao rejuvenation and rehabilitation. Although resistant cacao clones such as S1, S2, Sca 6, KEE 2, DRC 15, and KKM 22 are available in the field [11,12], some of these clones are not preferable for farmers. This can limit the use of resistant clone in the management of VSD. We have investigated using endophytes as a durable, plant-based, biocontrol alternative for management of VSD disease in the short and or long-term period.
Trichoderma species are nonpathogenic free living organisms associated with the roots of plants as opportunistic, avirulent plant symbionts [13]. The critical characteristic of this association is Trichoderma penetration into the root system, where they can persist. Recent studies on cacao show that Trichoderma is also capable of persisting on living sapwood and leaves in an endophytic association [14,15,16]. In the presence of Trichoderma on cacao tissue, the VSD incidence decreased when the pathogen was inoculated by foliar spraying and seedling grafted with scion infected by pathogen. In addition, Trichoderma have added ability of enhancing cacao shoot-grafts on seedlings [16].
The result mentioned above was based on research carried out in green house where the inoculation of pathogen occurred one time, while in the field the inoculation of the pathogen could be multiple due to more sources of inoculum. The use of scions infested by C. theobromae or infection of the fungus on side grafts already grown in field can cause the failure of side graft growth and, therefore, the objective of the present research was to increase the survival and growth of side graft, and also protect them from severe infestation of the disease, by application of a Trichoderma asperellum isolate to the scion prior to attachment to the exposed cambium at the site of side grafting.
MATERIALS AND METHODS
Source and preparation of Trichoderma for treatment
A strain of T. asperellum shown to be the most effective in controlling of VSD in the green house was chosen for this study: T. asperellum strain ART-4/G.J.S. 09-1559 [16]. This strain is held in the collection of the Cocoa Research Group Laboratory, Faculty of Agriculture, Hasanuddin University, Makassar 90245 Indonesia and in the collection of the Systematic Mycology and Microbiology Laboratory, U.S. Department of Agriculture, Agricultural Research Service, Beltsville, MD, USA. To obtain sufficient inocula for field application, the strain was cultured on potato dextrose agar (PDA) medium, incubated at room temperature (25℃ to 27℃) for 5 days and then five agar plugs with spores and mycelia were transferred to 100 g of rice grain medium in a plastic bag. The plastic bag was then sealed and the most of the air removed with a fine sterile needle to reduce the aeration of the T. asperellum culture. After 6 or 7 days of incubation at the same temperature as above, the culture was harvested, dried at a temperature of 35℃ to 40℃ for 2 hr, and then air dried for several hours. The product was ready to apply in the field after grinding to form a smooth powder.
Field assessment
To evaluate the efficacy of T. asperellum against VSD disease, the fungus was introduced through the connection site of side grafting on adult cacao tree in the field area infested severely by the disease. Healthy scions about 1 cm thick, 7 cm long, brown in color, and with three until four buds were selected. Their leaves were removed leaving around half a centimeter of the petioles. On the thick end of this scion, a wedge was formed by making two slanted cuts, one long and one short and placed in a suspension of T. asperellum for 10 min. After air drying, the scion was inserted into cuts in the bark made horizontally and vertically down to the cambium level 1 m from the ground, then attached by a cord wrapped around the trunk. The graft was covered with transparent plastic for 20~25 days. The quantity of T. asperellum isolate used was based on the amount used by Hakkar et al. [17], consisting of 4 g suspended in 10 mL, 100 mL, and 1,000mL of sterile water.
The experiment was established with a randomized block design with two clones MCC1 and M04 located in each plot, treated with varying concentrations of T. asperellum as mentioned above, and a control with just water used. Each treatment for each clone consisted of 18 side grafts, providing a total, including the control of 72 side grafts and for two clones, 144 side grafts. This field trial was located on a farm where infestation of cocoa trees by VSD was very high.
The impact of T. asperellum treatments was evaluated by VSD incidence in grafted shoots and, also by the growth of side grafts, stomata number per square centimeter of leaf, measurement of length, width and area of stomata opening, and amount of chlorophyll. VSD incidence was determined on side grafts by using the formula of I = a/b where I is incidence, a is number of leaves showing VSD symptom and b is the total of leave observed in one side grafts. The youngest fully expanded leaf on cacao branches of 24-week-old was selected at AM 8:00 and used for calculation of stomata density, measurement of stomata pore length, width and area, and chlorophyll content. A drop of nail polish was applied to the surface of the leaf and after drying, this drop was covered with clear packing tape. The packing tape was then pressed firmly for about 30 sec, peeled gently, and the impression stuck to a clean slide and observed under the light microscope. The area of stomata opening in suare micrometer was calculated by using the formula of A = πRW × RL, where A is area, π is constanta, Rw is width radius and RL is length radius. Chlorophyll content was measured with a CCM 200 plus device and calculated by the formula of Silla et al. [18]. The stomata number, stomata size, and chlorophyll content was obtained from 12 side grafts or 48 leaves per treatment as well as the control for the MCC1 and M04 clones.
In addition, side graft growth was also evaluated for the number of shoots on each side graft. This evaluation was done for 30 side grafts treated with Trichoderma and 30 non-treated side grafts.
Trichoderma isolation
Re-isolation of T. asperellum was done after the new scion and leaves developed on side grafts at 16 wk, 20 wk, and 24 wk after grafting. Leaves, petioles and scion were sampled: in each case, the leaves were cut using a surface sterilized knife into 1 cm2 pieces, while the petioles and the branch into 1 cm sections. These were sterilized in 2% sodium hypochlorite, 70% ethanol and vigorously washed several times in sterile water before being placed onto 20 mL PDA medium in 9 cm diameter Petri dishes [19]. All Petri dishes were incubated at room temperature and examined every day for the presence of Trichoderma.
Analysis
VSD incidence, density of stomata per square centimeter, length, width, and area of stomata opening, and chlorophyll content per square centimeter were analyzed without any data transformation. Least significant difference was then used for evaluating significant differences between the treatment means.
RESULTS
VSD incidence
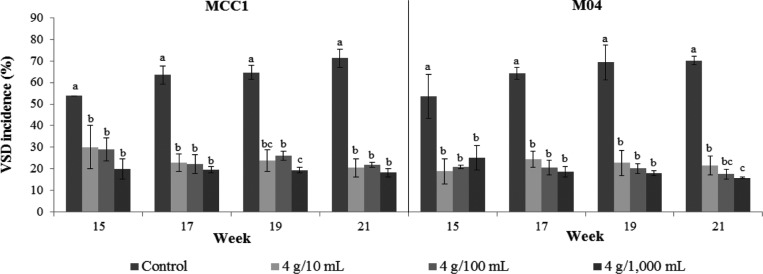
The buds on side graft of MCC1 and M04 clones started to sprout 3~4 wk after inserting the scion to the trunk. The symptoms of VSD on leaves of untreated side grafts appeared after 12 wk, while on leaves of side grafts treated by T. asperellum with either 4 g/10 mL, 4 g/100 mL or 4 g/1,000 mL appeared after 13 wk. The incidence of VSD both on MCC1 and M04 without application of T. asperellum tended to increase with time, reaching 53.9% and 53.5%, respectively, 15 wk after inserting the scion and 71.2% and 70.1% at 21 wk. On the contrary, the incidence of VSD on these two clones treated tended to decrease with time. On MCC1 and M04 treated with a concentration of 4 g/10 mL, VSD incidence was 30.0% and 18.8% at 15 wk and 20.6% and 21.7% at 21 wk, respectively. At a concentration of 4 g/100 mL, the incidence was 29.1% and 20.9% at 15 wk and 22.0% and 17.6% at 21 wk, respectively. At a concentration of 4 g/1,000mL, the incidence was 19.9% and 25.1% at 15 wk and 18.2% and 15.9% at 21 wk, respectively (Fig. 1). In these two clones, the VSD incidence on control was significantly different (p ≤ 0.05) with T. asperellum treatment of 4 g/10 mL, 4 g/100 mL, and 4 g/1,000 mL, while between treatment was not significantly different, except on M04 at 21 wk (Fig. 1).
Fig. 1. The incidence of vascular streak dieback (VSD) on side grafts of MCC1 and M04 clones after treatment by Trichoderma asperellum at concentrations of 4 g/10 mL, 4 g/100 mL, and 4 g/1,000 mL applied to scions prior to side grafting. Means of incidence in the same time followed by same letter are not significantly different according to least significant difference (p ≤ 0.05).
Density, pore length, pore width, and pore area of stomata
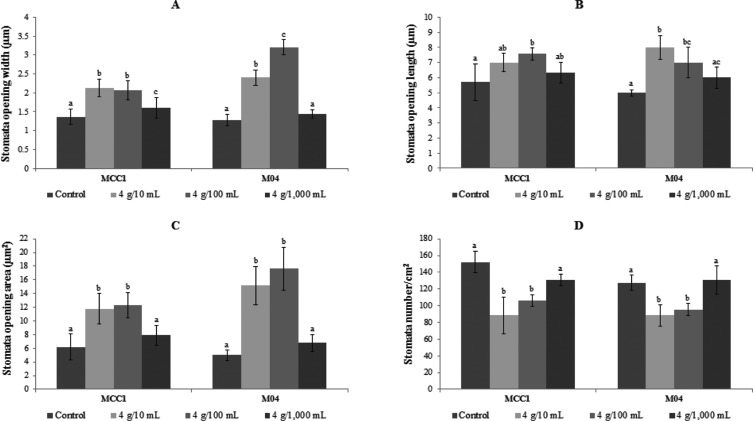
The density of stomata on leaves of MCC1 cacao clone was higher than that on leaves of M04 clone and this density was reduced when the two clones were treated with T. asperellum at grafting with concentrations of 4 g/10mL and 4 g/100 mL, and 4 g/1,000 mL, except in M04 was increased slightly when treated with a concentration of 4 g/1,000 mL. Leaves treated with the three concentrations (4 g/10 mL, 4 g/100 mL, and 4 g/1,000 mL) had a stomata number per square centimeter of 88.5, 106.2, and 130.9 for MCC1 and 88.5, 99.5, and 130.6 for M04, compared to 152.2 and 127.4, for the control leaves of MCC1 and M04, respectively (Fig. 2). In the two clones above, the number of stomata in the control was significantly different (p ≤ 0.05) from those following T. asperellum treatments of 4 g/10 mL and 4 g/100 mL but was not following treatment of 4 g/1,000 mL.
Fig. 2. Stomata opening width (A), stomata opening length (B), stomata opening area (C), and total number of stomata (D) on MCC1 and M04 clones leaf 24 wk after treatment by Trichoderma asperellum through connection site of side grafting with concentration of 4 g/10mL, 4 g/100mL, and 4 g/1,000mL compared with control. Means of each parameter followed by same letter in same clone are not significantly different according to least significant difference (p ≤ 0.05).
The stomata opening length of MCC1 cacao leaves was longer than that of M04 cacao leaves, while the stomata opening width was apparently not difference. When side graft of two clones treated by T. asperellum with concentration of 4 g/10mL, 4 g/100mL, and 4 g/1,000mL, the length, the width, and the area of stomata opening increase compared to control. This length on MCC1 treated was 7.0 µm, 7.6 µm, and 6.3 µm or incresing of 22.8%, 33.3%, and 10.5% and on M04 was 8.0 µm, 7.0 µm, and 6.0 µm or increasing of 60.0%, 40%, and 20%, respectively (Fig. 2). The width on MCC1 was 2.1 µm, 2.1 µm, and 1.6 µm or increasing 40.0%, 40.0%, and 6.7% and on M04 was 2.4 µm, 3.2 µm, and 1.5 µm or increasing of 84.6%, 146.2%, and 15.4%, respectively (Fig. 2). While the area of stomata opening on MCC1 was 11.8 µm2, 12.3 µm2, and 7.9 µm2 or increasing 91.4%, 99.7%, and 28.6% and on M04 was 15.2 µm2, 17.6 µm2, and 6.8 µm2 or increasing 203.8%, 253,5%, and 35.9%, respectively. Both on MCC1 and M01 clones, the average of stomata opening area on control was significantly different (p ≤ 0.05) with Trichoderma treatment of 4 g/10 mL and 4 g/100 mL, but not with 4 g/1,000 mL.
Chlorophyll content
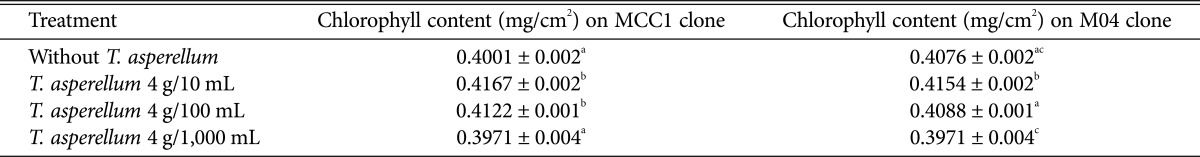
The impact of T. asperellum on chlorophyll content in cacao leaves depand on clone and T. asperellum concentration. On MCC1 clone, the chlorophyll content was increased when this clone was treated by T. asperellum concentaration of 4 g/10 mL and 4 g/100 mL with 0.42 mg/cm2 and 0.41 mg/cm2 while on M04 just by concentration of 4 g/10 mL with 0.42 mg/cm2, respectively. All of these chlorophyll content was significantly different (p ≤ 0.05) with control having 0.40 mg/cm2 and 0.41 mg/cm2 chlorophyll, respectively. With T. asperellum concentration of 4 g/1,000 mL, the cholophyll content was same with control on MCC1 and on M04 clone was decreased to 0.40 mg/cm2 (Table 1).
Table 1. Chlorophyll content (mg/cm2) on MCC1 and M04 clone leaves 24 wk after treatment of scions with three concentrations Trichoderma asperellum in suspension, prior to attachment at the incision site in bark as aside graft.
Means in the same column followed by same letter are not significantly different according to least significant difference (p ≤ 0.05).
Side graft shoots and branches
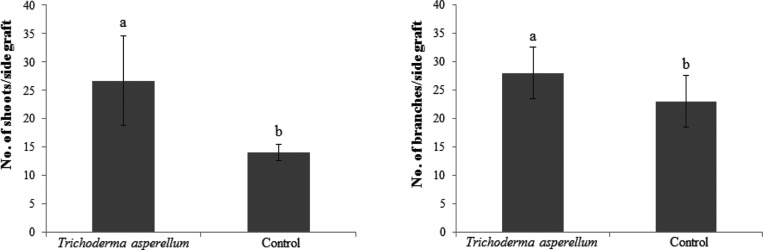
Application of T. asperellum increased the number of shoots and branches formed on side grafts. The average number of shoots on treated side grafts was 26.7 and on untreated side grafts was 14 and this difference was significant (p ≤ 0.05). The average number of branches on treated side graft treated was 28 and untreated side graft nontreated was 23 and the difference was also significant (Fig. 3).
Fig. 3. Average number of shoots and branches formed on cacao side grafts 24 wk after treament by Trichoderma asperellum. Means of shoot and branche number followed by same letter are not significantly different according to least significant difference (p ≤ 0.05).
Re-isolation of T. asperellum
No T. asperellum was found on leaves, petioles and branches after 16, 20, or 24 wk after application of this fungus at the connection site of side grafting.
DISCUSSION
The results reported here showed that VSD incidence on young MCC1 (previously called M01) and M04 reached 71.2% and 70.1% at 21 wk, respectively. These clones are considered to be susceptible to Ceratobasidium theobromae infection. The source of inoculum possibly was the fungus on the infected mother tree and other infected cacao trees in the field and/or the infected scion itself. Since we selected scions for side grafting in field areas infested by VSD, it was possible that these scions harboured the fungus. T. asperellum inoculated through the connection site for side grafting in the field had a positive impact, decreasing VSD disease and also increasing side grafts growth. Symptoms of VSD on leaves were significantly decreased, but we were not able to re-isolate T. asperellum from leaves, petioles and new branches. This suggests that the Trichoderma moved to other parts of the cacao tree, probably to the roots as has been described for other Trichoderma spp. [20]. A study of cacao seedlings treated by T. asperellum showed that this fungus occurred on leaves and stems in the first and the second month after foliar spraying and after this time was recovered dominantly on roots [21]. Beneficial relationships between plants and microorganisms often occur in the rhizosphere [22] and the mode of action here suggest an indirect action where the stimulation of resistance to infection of leaves by C. theobromae, the pathogen of VSD originated from the root, besides direct action as has been described for other strains of Trichoderma [23,24,25]. In case of indirect action, Trichoderma releases damage-associated molecular patterns and expresses a microbial-associated molecular patterns (MAMPs) resulting from the activity of its cell wall degrading enzyme on pathogens or plants, with a further consequence of elicitation of defense responses [26,27]. MAMPs secreted by Trichoderma responsible for defense responses include polygalacturonases, xylanases, cellulases, cerato-platanins, swollenins, avirulence proteins and lysine motif (LysM) domains, peptaibols, 6-pentyl pyrones, and trichothecenes [20].
There was not much variation in response to the three concentration of T. asperellum included in this study. On MCC1 clone, VSD suppression at the three different concentrations reached 69.1~74.4% and on M04 clone reached 69.0~77.3%. Therefore, we consider that the application of Trichoderma through site of side grafting in field was relatively effective and a concentration of 4 g/1,000 mL was more efficient due to ease of preparation of the suspension and the higher number of scions that can be treated fom one suspension.
In addition to plant defense response, the presence of Trichoderma on plants could up-regulate some proteins, while down-regulating other proteins. The largest number of up-regulated proteins is involved in carbohydrate metabolism, with substantial increases in proteins involved in photosynthesis [28,29]. This cross-talk between Trichoderma and plants plays a role in the phytohormonal network leading to an improvement of plant growth and development [30]. The increase of phytohormones such as auxins and the decrease of cytokinins and abscisic acid are probable the responsible for plant growth and development promotion [31,32]. Beside proteins and hormons, certains polyamine increase in Trichoderma-colonized plant. Polyamines are organic polycation such as putrescine (Put), spermidine (Spd), and spermine (Spm) involved in various developmental processes [33].
We suggest in our case, the change of stomata density, opening size and opening area on cacao leaves treated by T. asperellum have an impact on cacao growth and development. Almost at all concentrations of T. asperellum, the stomata density decreased. Lower stomata densities have been reported in resistant cacao clones [12], although balanced with significant increase of length and width of stomata opening. If we see the total size of opening, the application of T. asperellum with three different concentrations increased the area of these opening, this would contribute to increase the photosynthesis by more uptake of CO2 and could be advantageous to promote side graft growth. Polyamines regulate stomata closely correlated with the improvement of photosynthesis, possibly due to the greater amount of CO2 available for its fixation by photosynthetic enzyme [34]. In our work, the number of chlorophyll increased notably on MCC1 clone when T. asperellum was applied in high concentration. Several studies have shown that chloroplasts contain high activities of polyamine biosynthetic enzymes and transglutaminase (Tgase) catalyzing the covalent binding of polyamines to proteins (Table 1) [35,36]. In response to stress, enzymes are also involved in regulation of photosynthesis [37] including disease infection.
We conclude that the increase of stomata opening are both through augmentation of opening length and width and stomata number is correlated with growth promotion of side grafts. This suggestion was also supported by more buds and branches formed when treated with T. asperellum. However, more or larger stomata may also lead to dehydration in long term, therefore stomata must function in a way to optimize dry matter production by balancing photosinthesis and transpiration. For this reason, small concentration of T. asperellum (4 g/1,000 mL) is apparantly sufficient to support the growth of side grafts and can also efficiently inhibit VSD disease.
An Indonesian government program of cacao rehabilitation involving the complete replacement of non productive, dead or diseased cocoa is underway to increase cocoa productivity in the centers of Indonesia cacao plantings and side grafting is an important propagation method. Our research offered new insights to the application of a biological agent using the simple method of application at the site of side grafting in the field. The treated side grafts resist to systemic disease, VSD which is difficult to control. In addition, many scions fail to grow and develop due to several factors including infection by disease.We showed that application of T. asperellum may help growth and survival of side grafts. The simplicity of the application method permits this treatment of T. asperellum through site of side grafting to be used on a big scale, and, therefore, would be suitable in rehabilitation programs. So far, the application of T. asperellum through side graft using only low concentrations have been conducted on more than 15,000 trees and we observe the side graft have grown well with healthier leaves, compared to untreated grafts, with lower infestation rates of VSD.
ACKNOWLEDGEMENTS
The authors are grateful to the Ford Foundation and World Cocoa Foundation who have supported this research throughout.
References
- 1.Guest D, Keane PJ. Vascular-streak dieback: a new encounter disease of cacao in Papua New Guinea and Southeast Asia caused by the obligate basidiomycete Oncobasidium theobromae. Phytopathology. 2007;97:1654–1657. doi: 10.1094/PHYTO-97-12-1654. [DOI] [PubMed] [Google Scholar]
- 2.Samuels GJ, Ismaiel A, Rosmana A, Junaid M, Guest D, McMahon P, Keane P, Purwantara A, Lambert S, Rodriguez-Carres M, et al. Vascular streak dieback of cacao in Southeast Asia and Melanesia: in planta detection of the pathogen and a new taxonomy. Fungal Biol. 2012;116:11–23. doi: 10.1016/j.funbio.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Pawirosoemardjo S, Purwantara A. Symptoms of vascular streak dieback disease on cocoa in Indonesia. Menara Perkebunan. 1989;57:74–78. [Google Scholar]
- 4.Rosmana A. Vascular streak dieback: a new disease on cocoa in Sulawesi; Prosiding Seminar Ilmiah dan Pertemuan Tahunan PEI dan PFI XVI Komda Sul-Sel; 2005 May 27; Makassar, Indonesia. pp. 1–7. [Google Scholar]
- 5.Syahnen MS. Recomendations of VSD disease control on cocoa in Pasaman, West Sumatra [Internet] Medan: BBPPTP Medan; 2013. [Google Scholar]
- 6.BBPPTP Ambon. Distribution map of VSD (Oncobasidium theobromae) infestation Q 1 2014 in working Ambon. Ambon: BBPPTP Ambon; 2014. [Google Scholar]
- 7.Keane PJ. Epidemiology of vascular-streak dieback of cocoa. Ann Appl Biol. 1981;98:227–241. [Google Scholar]
- 8.Taylor M. The World Cocoa Situation; International Forum in Cocoa; 1998 Oct 28-29; Lima, Peru. London: LMC Internationall; 1998. [Google Scholar]
- 9.Febriantomo A. Experience of VSD (vascular-streak dieback) disease control in Kendeng Lembu field; Prosiding Simposium Kakao 2012; 2012 Nov 5-8; Padang, Indonesia. pp. 148–159. [Google Scholar]
- 10.Susilo AW, Anita-Sari I. Relationship between the shoot characteristics and plant resistance to vascular-streak dieback on cocoa. Pelita Perkebunan. 2014;30:181–189. [Google Scholar]
- 11.Susilo AW, Mawardi S, Sudarsianto Yield performance of the cocoa (Theobroma cacao L.) clones of Sca 6 and DRC 15 resistant to vascular-streak dieback. Pelita Perkebunan. 2009;25:76–87. [Google Scholar]
- 12.Prawoto AA, Santoso TI, Marifah, Hartanto L, Sutikno Terpene profile, leaf anatomy, and enzyme activity of resistant and susceptible cocoa clones to vascular streak dieback disease. Pelita Perkebunan. 2013;29:197–209. [Google Scholar]
- 13.Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species: opportunistic avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 14.Samuels GJ, Ismaiel A. Trichoderma evansii and T. lieckfeldtiae: two new T. hamatum-like species. Mycologia. 2009;101:142–156. doi: 10.3852/08-161. [DOI] [PubMed] [Google Scholar]
- 15.Bailey BA, Strem MD, Wood D. Trichoderma species form endophytic associations within Theobroma cacao trichomes. Mycol Res. 2009;113:1365–1376. doi: 10.1016/j.mycres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Rosmana A, Samuels GJ, Ismaiel A, Ibrahim ES, Chaverri P, Herawati Y, Asman A. Trichoderma asperellum: a dominant endophyte species in cacao grown in Sulawesi with potential for controlling vascular streak dieback disease. Trop Plant Pathol. 2015;40:19–25. [Google Scholar]
- 17.Hakkar AA, Rosmana A, Rahim MD. Control of Phytophthora pod rot disease on cacao using endophytic fungi Trichoderma asperellum. J Fitopatol Indonesia. 2014;11:139–144. [Google Scholar]
- 18.Silla F, González-Gil A, González-Molina ME, Mediavilla S, Escudero A. Estimation of chlorophyll in Quercus leaves using a portable chlorophyll meter: effects of species and leaf age. Ann For Sci. 2010;67:108. [Google Scholar]
- 19.Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA. Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci U S A. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermosa R, Rubio MB, Cardoza RE, Nicolás C, Monte E, Gutiérrez S. The contribution of Trichoderma to balancing the costs of plant growth and defense. Int Microbiol. 2010;16:69–80. doi: 10.2436/20.1501.01.181. [DOI] [PubMed] [Google Scholar]
- 21.Nasaruddin Endophytism of Trichoderma and its impact on growth of cocoa shoot graft; Sulawesi Interternational Seminar on Cocoa; 2013 Jun 26-28; Makassar, Indonesia. [Google Scholar]
- 22.Zamioudis C, Pieterse CM. Modulation of host immunity by beneficial microbes. Mol Plant Microbe Interact. 2012;25:139–150. doi: 10.1094/MPMI-06-11-0179. [DOI] [PubMed] [Google Scholar]
- 23.Bailey BA, Bae H, Strem MD, Crozier J, Thomas SE, Samuels GJ, Vinyard BT, Holmes KA. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biol Control. 2008;46:24–35. [Google Scholar]
- 24.Bae H, Roberts DP, Lim HS, Strem MD, Park SC, Ryu CM, Melnick RL, Bailey BA. Endophytic Trichoderma isolates from tropical environments delay disease and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms. Mol Plant Microbe Interact. 2011;24:336–351. doi: 10.1094/MPMI-09-10-0221. [DOI] [PubMed] [Google Scholar]
- 25.Atanasova L, Druzhinina IS, Jaklitsch WM. Two hundred Trichoderma species recognized on the basis of molecular phylogeny. In: Mukherjee PK, Horwitz BA, Singh US, Mukherjee M, Schmoll M, editors. Trichoderma: biology and applications. Boston (MA): CABI; 2013. pp. 10–42. [Google Scholar]
- 26.Marra R, Ambrosino P, Carbone V, Vinale F, Woo SL, Ruocco M, Ciliento R, Lanzuise S, Ferraioli S, Soriente I, et al. Study of the three-way interaction between Trichoderma atroviride, plant and fungal pathogens by using a proteomic approach. Curr Genet. 2006;50:307–321. doi: 10.1007/s00294-006-0091-0. [DOI] [PubMed] [Google Scholar]
- 27.Shoresh M, Harman GE. The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: a proteomic approach. Plant Physiol. 2008;147:2147–2163. doi: 10.1104/pp.108.123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfano G, Ivey ML, Cakir C, Bos JI, Miller SA, Madden LV, Kamoun S, Hoitink HA. Systemic modulation of gene expression in tomato by Trichoderma hamatum 382. Phytopathology. 2007;97:429–437. doi: 10.1094/PHYTO-97-4-0429. [DOI] [PubMed] [Google Scholar]
- 29.Harman GE, Björkman T, Ondik K, Shoresh M. Changing paradigms on the mode of action and uses of Trichoderma spp. for biocontrol. Outlooks Pest Manag. 2008;19:24–29. [Google Scholar]
- 30.Hermosa R, Viterbo A, Chet I, Monte E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology. 2012;158:17–25. doi: 10.1099/mic.0.052274-0. [DOI] [PubMed] [Google Scholar]
- 31.Chowdappa P, Mohan Kumar SP, Jyothi Lakshmi M, Upreti KK. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol Control. 2013;65:109–117. [Google Scholar]
- 32.Martínez-Medina A, Del Mar Alguacil M, Pascual JA, Van Wees CM. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J Chem Ecol. 2014;40:804–815. doi: 10.1007/s10886-014-0478-1. [DOI] [PubMed] [Google Scholar]
- 33.Brotman Y, Lisec J, Méret M, Chet I, Willmitzer L, Viterbo A. Transcript and metabolite analysis of the Trichoderma-induced systemic resistance response to Pseudomonas syringae in Arabidopsis thaliana. Microbiology. 2012;158:139–146. doi: 10.1099/mic.0.052621-0. [DOI] [PubMed] [Google Scholar]
- 34.Shu S, Guo SR, Yuan LY. A review: polyamines and photosynthesis. In: Najafpour MM, editor. Advances in photosynthesis: fundamental aspects. Rijeka: InTech; 2013. pp. 439–464. [Google Scholar]
- 35.Andreadakis A, Kotzabasis K. Changes in the biosynthesis and catabolism of polyamines in isolated plastids during chloroplast photodevelopment. J Photochem Photobiol B. 1996;33:163–170. [Google Scholar]
- 36.Della Mea M, Di Sandro A, Dondini L, Del Duca S, Vantini F, Bergamini C, Bassi R, Serafini-Fracassini D. A Zea mays 39-kDa thylakoid transglutaminase catalyses the modification by polyamines of light-harvesting complex II in a light-dependent way. Planta. 2004;219:754–764. doi: 10.1007/s00425-004-1278-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang LJ, Fan L, Loescher W, Duan W, Liu GJ, Cheng JS, Luo HB, Li SH. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010;10:34. doi: 10.1186/1471-2229-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]