Abstract
Introduction
Sensitive and specific assessment of the hepatic graft metabolism after liver transplantation (LTX) is essential for early detection of postoperative dysfunction implying the need for consecutive therapeutic interventions.
Objectives
Here, we assessed circulating liver metabolites of the cholesterol pathway, amino acids and acylcarnitines and evaluated their predictive value on early allograft dysfunction (EAD) and clinical outcome in the context of LTX.
Methods
The metabolites were quantified in the plasma of 40 liver graft recipients one day pre- and 10 days post-LTX by liquid chromatography/tandem mass spectrometry (LC–MS/MS). Plant sterols as well as cholesterol and its precursors were determined in the free and esterified form; lanosterol in the free form only. Metabolites and esterification ratios were compared to the model for early allograft function scoring (MEAF) which is calculated at day 3 post-LTX from routine parameters defining EAD.
Results
The hepatic esterification ratio of all sterols, but not amino acids and acylcarnitine concentrations, showed substantial metabolic disturbances post-LTX and correlated to the MEAF. In ROC analysis, the low esterification ratio of β-sitosterol and stigmasterol from day 1 and of the other sterols from day 3 were predictive for a high MEAF, i.e. EAD. Additionally, the ratio of esterified β-sitosterol and free lanosterol were predictive for all days and the esterification ratio of the other sterols at day 3 or 4 post-LTX for 3-month mortality.
Conclusion
Low ratios of circulating esterified sterols are associated with a high risk of EAD and impaired clinical outcome in the early postoperative phase following LTX.
Electronic supplementary material
The online version of this article (doi:10.1007/s11306-016-1129-z) contains supplementary material, which is available to authorized users.
Keywords: Liver transplantation, Sterol metabolism, MEAF
Introduction
Liver transplantation (LTX) is a life-saving treatment option for patients suffering from a variety of liver diseases including acute and chronic liver failure, hepatocellular carcinoma and fulminant hepatitis. Over the past 50 years, significant improvements in immunosuppression, surgical technique and postoperative management of liver allograft recipients have paved the way for the clinical success of LTX which has finally become a standard therapy and clinical routine.
Primary graft dysfunction after LTX is a critical event and may display a broad clinical range from reversible graft dysfunction, known as early allograft dysfunction (EAD), up to an irreversible dysfunctional state called primary non function which represents an indication for urgent re-transplantation (Uemura et al. 2007). EAD has been shown to have negative impact on both, patient and graft outcome. Early and accurate assessment of graft function following LTX is critical for timely therapeutic intervention resulting in reduced mortality and reduced numbers of re-transplantation. Moreover, sensitive and specific assessment of the hepatic metabolism after LTX remains essential for early detection of postoperative dysfunction and consecutive adjustment of the therapeutic regime. In clinical routine, liver cell function is assessed by the activity of in part specific enzymes such as alanine and aspartate aminotransferase (ALT, AST), bilirubin and parameters of the coagulation system such as the international normalized ratio of prothrombin (INR) to define EAD (Olthoff et al. 2010; Lee et al. 2014). Recently, a model for the quantitative assessment of EAD after LTX has been validated (model for early allograft function scoring, MEAF) (Pareja et al. 2015). This score, based on the parameters ALT, bilirubin and INR calculated at day 3 post-LTX, provides improved graft function assessment than the single parameters (Pareja et al. 2015) while at the same time being a predictor of both recipient and graft survival. However, all single parameters used to calculate the MEAF might be influenced by the ischemic period during organ preservation, liver graft quality, coagulation factor substitutions and blood or plasma transfusions, as well as nutrition. Thus, evaluation of the actual parenchymal function and precise distinction between a toxic and hypoxic liver injury can be challenging (Hickman et al. 1997; Shaked et al. 1997; Tanaka et al. 2000; Sear 2002) since routine parameters may rather reflect pre- and perioperative liver cell injury due to ischemia and organ preservation than the residual metabolic capacity of the transplanted liver.
To characterize new postoperative parameters that might assess more adequately or even earlier EAD compared to the MEAF, we here examined various liver specific metabolic pathways including cholesterol synthesis and secretion, sterol esterification, branched-chain and aromatic amino acids and fatty acid beta-oxidation by liquid chromatography/tandem mass spectrometry (LC–MS/MS). Free and esterified sterols, amino acids and acylcarnitines were determined together with the routine parameters by high throughput methods 1 day pre- and 10 days post-LTX and related to clinical outcome and the MEAF.
The change of sterol concentrations before and after LTX was studied in patients with end-stage primary biliary cirrhosis and acute liver failure (Nikkilä et al. 1992, 2005, 2008). Here, we focused on sterol esterification, which depends strongly on liver function and was already discussed as a marker for it many years ago (Jones et al. 1971; Simon et al. 1974). However, sterol esterification has not been studied in liver function after transplantation. Conversion of free sterols into sterol esters is catalyzed by the lecithin cholesterol acyltransferase (LCAT). This enzyme is synthesized in the liver and its activity is a sensitive parameter of liver disease (Borowsky et al. 1980; Breier et al. 1983). Using our LC–MS/MS approach, we are able to determine sterol esterification by simultaneous quantification of the free and esterified sterol including cholesterol, endogenous and non-endogenous sterols.
Materials and methods
Subjects
A total of 40 Caucasian men (n = 29) and women (n = 11) undergoing LTX were consecutively recruited from July 2008 to July 2009. Table 1 summarizes baseline and hospitalization characteristics of patients pre-LTX. 27 out of the 40 patients (27/40) had alcoholic liver disease (ALD) as baseline liver disease. 20/27 of these patients were men. Alcohol abuse is a mostly problem of men: two-thirds of people with alcohol use disorders are men (Deleuran et al. 2015). We did not record the personalized diet of each patient. The patients were fed according to the following suggestions: (i) patients without any complication: post-LTX oral light diet, after 2–3 days stepwise increasing caloric food; (ii) patients which could not be fed orally within the first 1–4 days post-LTX but afterwards: Ringer‘s solution i.v., after 4 days oral light diet and increasing caloric food; patients with complications and probably no oral nutrition after 4–5 days will be possible: enteral nutrition according to the patients demand.
Table 1.
Baseline and hospitalization characteristics of the patients pre-LTX
| Parameter (unit) | Number (%) | Mean ± SD or median (interquartile range) |
|---|---|---|
| Recipient information | ||
| Number of patients | 40 | |
| Age (years) | 53.68 ± 8.35 | |
| Gender (male) | 29 (72.5) | |
| Baseline liver disease | ||
| Alcoholic liver disease (ALD) | 27 (67.5) | |
| Hepatitis B/C | 4 (10.0) | |
| Hemochromatosis | 2 (5.0) | |
| Acute liver failure (ALF) | 3 (7.5) | |
| Posttransplant-liver complication | 2 (5.0) | |
| Biliar disease | 2 (5.0) | |
| Tumor (HCC) | 13 (32.5) | |
| MELD | 19.50 (12.75–35.25) | |
| MEAF | 4.72 (3.25–6.11) | |
| Transplantation before study transplantation | 3 (7.3) | |
| Mortality after 10 days | 0 (0) | |
| Mortality after 30 days | 1 (2.5) | |
| Mortality after 3 months | 3 (7.5) | |
| Organ failure after 30 days | 2 (5.0) | |
| Organ failure after 3 months | 5 (12.5) | |
| Organ failure after 18 months | 8 (20.0) | |
| Length of hospital stay (days) | 30.50 (21–39.25) | |
| Length of ICU stay (days) | 13.00 (8.00–20.00) | |
| Donor information | ||
| Age (years) donor | 54.58 ± 17.59 | |
| Gender (male) donor | 22 (55.0) | |
| Gender mismatch | ||
| No | 25 (62.5) | |
| Male (D) → female (R) | 4 (10.0) | |
| Female(D) → male (R) | 11 (27.5) | |
| BMI (kg/m2) | 26.18 (23.38–29.39) | |
| Surgical information | ||
| CIT (min) | 597 ± 146 | |
| Arterial anastomosis delay (min) | 60.000 (50.00–60.00) | |
Mean and standard deviation (SD), for non-normally distributed parameters median and interquartile ranges are given
BMI body mass index, CIT cold ischemic time, D donor, ICU intermediate care unit, HCC hepatocellular carcinoma, MEAF model for early allograft function scoring, MELD model for end-stage liver disease, R recipient
Three patients had to undergo re-transplantation after their first LTX. Here, only the data after re-transplantation were included. Two out of these three patients underwent a LTX 2 and 6 months before the second LTX because of thrombosis of the liver artery. In both cases blood was not collected after the first LTX. After the second LTX the determined parameters of both patients were between the 25th and 75th percentile of all patients. The third patient received the second LTX 4 days after the first LTX because of acute liver failure. This is one of the patients who died within 3 months after LTX.
Blood samples
1–24 h pre-LTX (day 0) and each day post-LTX until day 10 (days 1–10) peripheral venous blood was collected. 80.0 % of all blood samples were collected between 4 and 7 am. and 9.1 % between 7 and 10 am. Because all patients were in the intensive care unit (ICU) post-LTX, taking blood samples depended on the treatment needs. Thus, the remaining 10.9 % of blood samples could not have been taken within the time frame 4–10 am. The blood was centrifuged immediately at 2500×g for 20 min to obtain EDTA-plasma. Samples were stored at −80 °C and thawed only once. To generate dried blood specimen, EDTA whole blood was dropped on filter paper (grade 903; GE Healthcare, Munich, Germany) dried at room temperature for 3 h and afterward stored in foil-barrier ziploc bags and desiccant packets (Whatman GmbH, Dassel, Germany) at –80 °C till analysis.
Scores
The model used for end-stage liver disease (MELD) score was the crude MELD score. It was calculated with the following formula after recruitment of the patients (Kamath and Kim 2007): MELD = [0.957 ln(creatinine) + 0.378 ln(bilirubin) + 1.120 ln(INR) + 0.643] × 10 (United Network for Organ Sharing, UNOS; MELD Calculator; accessed March 30, 2015). MEAF was calculated with the following formula (Pareja et al. 2015) with max3POD = maximum variable values during the first 3 postoperative days: MEAF = score ALTmax3POD + score INRmax3POD + score bilirubin3POD. The MEAF could not be determined for two patients. Clinical outcome as 3-month mortality (n = 3 patients), 3-month mortality/organ failure (n = 5 patients), 12- and 18-month mortality (n = 5 patients) were collected retrospectively.
Chemical and reagents
Methanol and isopropanol were purchased from Merck (Darmstadt, Germany) and acetyl chloride (p.a.) from Sigma-Aldrich (Steinheim, Germany). Water (HPLC grade) was obtained from J. T. Baker (Deventer, Netherlands). AA and AC reference isotope labeled standard kits (NSK A, NSK B; Cambridge Isotope Laboratories, Andover, USA) were used as internal standard. 3N butanolic HCl was made in-house using butanol (spectroscopy grade, Merck Darmstadt, Germany). Dried blood controls for amino acids and acylcarnitines (Level 1 and Level 2) were obtained from Chromsystems (Munich, Germany).
Clinical laboratory characteristics
All analyzed parameters are summarized in Supplemental Table 1. ALT and AST, gamma glutamyl transferase (GGT), glutamate dehydrogenase (GLDH), creatinine and bilirubin serum concentrations were analyzed using Cobas 6000 and 8000 analyzers (Roche, Mannheim, Germany). The prothrombin assay was performed in citrate plasma to determine the INR using an ACL TOP 700 System (Instrumentation Laboratory, Lexington, USA).
LC–MS/MS analysis
Sterol analysis included the free and esterified plant sterols brassicasterol (BR), campesterol (CA), β-sitosterol (SI) and stigmasterol (ST), the cholesterol biosynthesis precursors lanosterol (LA) as well as the sum parameter including desmosterol, zymosterol, 7-dehydrocholesterol (DEZY7DHC), and cholesterol itself (CH). They were analyzed by LC–MS/MS (Lembcke et al. 2005; Becker et al. 2015). 25 µl of the supernatants were injected onto the analytical column (Chromolith SpeedROD RP-18e, 50 × 4.6 mm, monolithic column, Merck KGaA, Darmstadt, Germany). An API 4000 triple quadrupole mass spectrometer with an atmospheric pressure photoionization source (AB Sciex, Darmstadt, Germany) was used. The quantification of free and esterified sterols was performed according to ISO DIN 17025 and ISO DIN 15189 including an external 4-point calibration with three quality controls at different concentrations. Data were acquired using Analyst software (version 1.5.1). The sterol esterification ratio was calculated with the following formula: esterified sterol/(free sterol + esterified sterol) × 100.
Analysis of amino acids and acylcarnitines was performed according to published protocols (Ceglarek et al. 2002; Brauer et al. 2011). Briefly, 3 mm dried blood spots were extracted with 100 µl of the internal standard solution and centrifugated at 3000×g for 10 min at room temperature. After butanolic esterification, samples were analyzed by liquid chromatography tandem mass spectrometry (API 2000, Sciex, Darmstadt, Germany).
Statistical analyzes
Before statistical analysis, non-normally distributed parameters were logarithmically transformed to approximate normal distribution. Mean and standard deviation (SD), for non-normally distributed parameters median and the interquartile range (25th–75th percentile) were used. All statistical computations were performed using SPSS version 20.0 (IBM, Ehningen, Germany). p values less than 5 % were considered as significant.
Receiver Operating Characteristic (ROC) analyzes were used to assess the diagnostic power of the new parameters for discrimination between patients. The observed area under the ROC curve (AUC) was tested against the null-hypothesis (AUC = 0.5). Optimal cut-offs for the evaluated parameters were derived from the ROC curves by maximizing the sum of sensitivity and specificity (Youden-Index).
Results
Clinical and anthropometric characteristics of patients are summarized in Table 1. The study group included 40 LTX patients with a mean age of 53.7 ± 1.3 (35–69) years. Alcoholic liver disease (ALD) was the predominant underlying liver disease, followed by hepatitis B/C.
The esterification ratio of sterols showed substantial disturbances pre- and post-LTX
Substantial metabolic disturbances occurred immediately after LTX as seen in the time course of the esterification ratio of all sterols (Fig. 1) and of routine parameters, suitable to follow-up LTX, as ALT, AST, bilirubin, GGT, glutamate dehydrogenase (GLDH), and prothrombin time (PT). No or only low disturbances post-LTX were seen in circulating amino acids as well as acylcarnitines (data not shown).
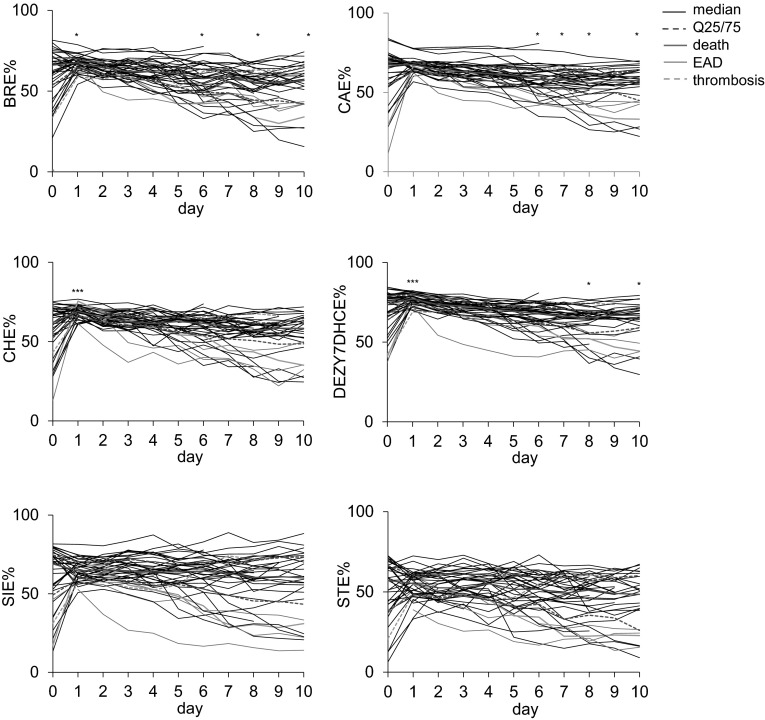
Fig. 1.
Time course of the ratio of esterified sterols The median and the 25 and 75 % interquartile ranges (Q25/75) are shown in red. The patients who died within 3 months (n = 3) and the patients with organ failure (n = 2) are shown in pink and in green, respectively. The continuous green line represents a patient with EAD and high MEAF whereas the green broken line indicates a patient who lost the liver 2 months post-LTX due to thrombosis of the hepatic artery. The differences between day 0 pre-LTX to each day post-LTX are indicated (Wilcoxon signed-rank test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001)
The ratio of esterified sterols prior to LTX varied considerably for each sterol among the patients (Fig. 1); e.g. of CA between 11.8 and 83.9 %. Immediately post-LTX the esterification ratio of all sterols increased especially in patients with a low esterification ratio pre-LTX. The esterification ratio of all sterols post-LTX did not correlate to the cold ischemic time of the donor liver (data not shown). At day 1 the ratio of each esterified sterol, apart from ST, was higher than 50 % in all patients. From day 2 post-LTX the ratio of esterified sterols decreased continuously in at least half of the patients. Thus, we included the difference in the ratio of esterified sterols between day one to each other day after LTX into further analysis. In part, the difference in the esterification ratio between day 1 and 2 post-LTX was even more significant. At day 10, the ratio of esterified sterols varied among the patients partly as pre-LTX. For BR, CA and DEZY7DHC the median esterification ratio was even higher pre-LTX compared to that of day 10 post-LTX.
We found no significant differences between men and women concerning age and all routine and sterol data. Additionally, we found no differences between the 25 % youngest and 25 % oldest women. Thus, it is likely that the hormonal status/menopause had no effect on the results.
The esterification ratio of sterols correlates inversely to the MEAF
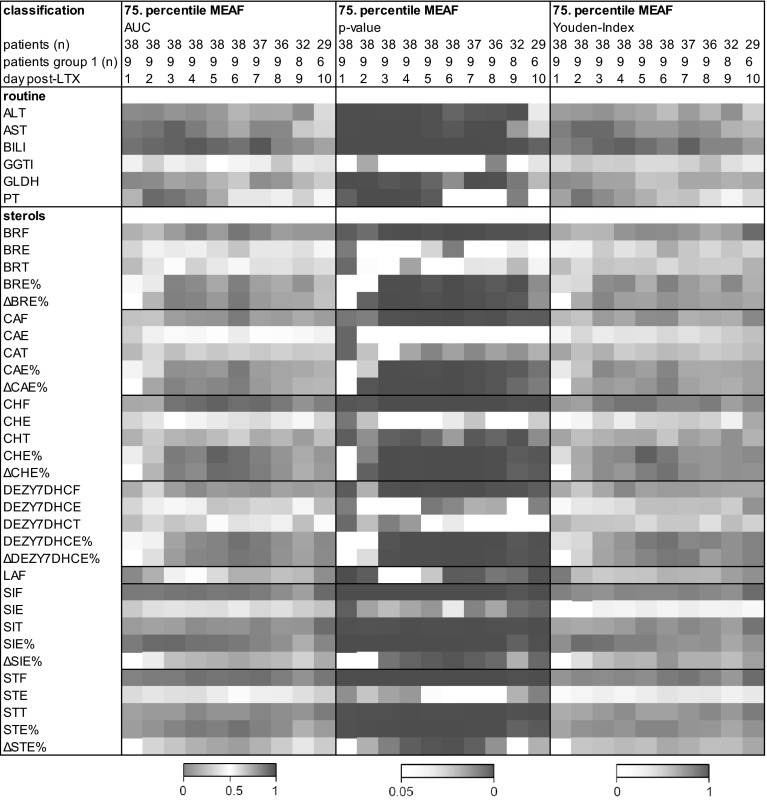
Recently, the MEAF was identified to be most suitable for modeling EAD in patients undergoing LTX (Pareja et al. 2015). The score includes ALT, bilirubin and INR. Consequently, these parameters correlated to the MEAF (data not shown). Interestingly, the esterification ratio of the sterols correlated inversely to the MEAF almost every day except day 1, i.e. the higher the MEAF the lower the esterification ratio. The amount of free sterols and the MEAF correlated slightly. Supplemental Fig. S1a shows exemplarily that the data for free (SIF), esterified (SIE), total (SIT), and the ratio of esterified SI (SIE %) correlated to ALT, bilirubin, INR and MEAF whereas figure S1b illustrates the correlation between the esterification ratio of each sterol to the MEAF at days 3, 6, and 9 post-LTX. Finally receiver operating characteristic (ROC) analysis was performed. As binary classifier the 75 % percentile of the MEAF, e.g. the 25 % of the patients with a MEAF ≥ 6.10 were compared to the others. The low esterification ratio from day 1 of SI and ST and from day 3 of all the other sterols was predictive for a high MEAF (Fig. 2). Thus, high ratios of circulating esterified sterols probably predict a low risk of EAD. The Youden-Index, defining the maximum potential effectiveness and the cut-off of the esterification ratio, was calculated (Fig. 2). The highest Youden-Index was 0.82 for SIE % at day 2 post-LTX (cut-off 59.10 %) and 0.90 for CHE % at day 5 post-LTX (cut-off 57.80 %).
Fig. 2.
Sterols as predictors for a high MEAF receiver operating characteristic (ROC) analysis to verify which parameter predicts a high MEAF. The 75 % percentile of the MEAF was used as binary classifier, i.e. the 25 % of the patients with a MEAF ≥ 6.10 were compared to the others. Values for the area under the curve (AUC; 0–1), p values (<0.05–0) and the Youden-Index (0–1) are shown in heat maps. The Youden-Index, defining the maximum potential effectiveness of the parameter, was calculated from the AUC curves
The other non-routine parameters such as amino acids and acylcarnitines either did not correlate or randomly correlated to the MEAF and are thus not predictive (Fig. S2).
The ratio of esterified sterols predicts clinical outcome
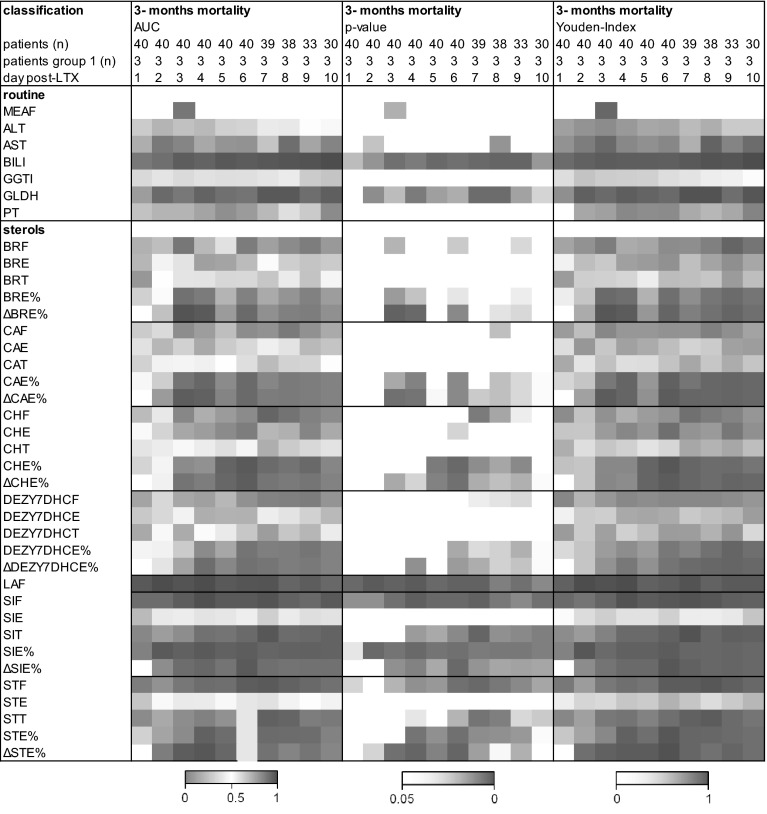
Finally, we evaluated the prognostic value of the assessed metabolic parameters on clinical outcome. Patients who died within the first three months after LTX very often showed esterification ratios of the sterols below the median of all patients or even the lowest values on many days (Fig. 1). The two patients who lost the organ had different profiles: the one with EAD was comparable to patients who died within 3 months whereas the patient who lost the liver 2 months post-LTX due to thrombosis of the hepatic artery with no signs of EAD and a low MEAF always had high esterification ratios of the sterols (Fig. 1). In the ROC analysis 3, 12, and 18 month mortality were used as binary classifiers. In Fig. 3, the AUC and the level of significance for 3-month mortality are given for each day. Among the routine parameters, bilirubin and GLDH were predictive on nearly all days as was the MEAF determined at day 3. Among the newly evaluated parameters, the sterols were especially interesting. Free LA (LAF), free ST (STF) and free and the ratio of esterified SI (SIF, SIE %) were predictive on all days post-LTX. The ratio of esterified as well as difference in the ratio of esterified BR (BRE, ΔBRE %) and CA (CAE, ΔCAE %) were predictive from day 3 post-LTX. For 12, and 18 month mortality the same results tended to be seen as for 3-month mortality, albeit at a lower or no level of significance. Finally, the Youden-Index was calculated (Fig. 3). The highest Youden-Index was 0.95 for SIE % (cut-off 57.52 %) and 1.00 for LAF at day 2 post-LTX (cut-off 0.27 mg/l) which are better than that of the MEAF calculated at day 3 post-LTX.
Fig. 3.
Sterols as predictors for 3 month mortality Receiver operating characteristic (ROC) analyzes to verify which parameter predicts clinical outcome. 3 month mortality was used as binary classifier. Explanations see Fig. 2
Circulating amino acids were not informative (Fig. S3). In tendency, several short- and medium-chain acylcarnitines showed predictive AUC levels, but they never or only on single days reached the level of significance.
Discussion
Here, we identified a significant predictive value for the esterification ratio of circulating sterols on EAD and 3-month mortality in patients undergoing LTX.
Circulating sterols are composed of cholesterol, an essential component of membranes by regulating the fluidity of the bilayer, several precursors of cholesterol as lanosterol which are markers for cholesterol synthesis, and plant sterols which are exclusively derived from dietary sources (Kuksis 2001). Intracellular esterification and the subsequent packing into cytoplasmic lipid droplets detoxifies and sequesters sterols until they are required. The hydrophobic sterols are transported in circulation via lipoproteins to the peripheral cells (Huang et al. 2015).
The esterification ratio of sterols depends on two enzymes. Sterol esterification for synthesis of chylomicrons and very low-density lipoproteins (VLDLs) to transport synthesized sterols from the liver in the circulation is catalyzed by acyl-coenzyme A: cholesterol acyltransferase 2 (ACAT2) (Rogers et al. 2015). The LCAT, secreted by the liver into the blood, influences sterol esterification in circulating lipoproteins (Glomset and Verdery 1977; Dobiasova and Frohlich 1999; Jonas 2000). LCAT activity itself is a sensitive parameter for the severity of the liver disease (Borowsky et al. 1980; Breier et al. 1983). Thus, the esterification ratio of circulating sterols correlates to the number of intact hepatocytes. Overall, the plasma concentration of LCAT in normal liver function showed only minor variation (Kunnen and Van 2012) and varied only slightly in adult humans with age, gender, and smoking (Albers et al. 1982).
In our study, the ratio of each esterified sterol was significantly different between the patients pre-LTX with variances around 50–70 %. Of note was the marked increase in the esterification ratio immediately post-LTX for all sterols in patients that had shown low esterification ratios pre-LTX. From day two post-LTX the esterification ratio of all sterols started decreasing in more than 50 % of the patients. This uniform time course for all sterols suggests either that during LTX highly esterified sterols, typical for healthy individuals (Temel et al. 2003) have been transfused or that the LCAT activity of the donor liver is not disturbed immediately after LTX. Consistently, the ratio of all esterified sterols correlated strongly to routine parameters defining EAD and variables of the MEAF (Olthoff et al. 2010; Lee et al. 2014; Pareja et al. 2015). The low esterification ratio of SI and ST from day 1 and of the other sterols from day 3 was predictive to be among the 25 % of the patients with the highest MEAF. Although based on a small study cohort, these data suggest that the low esterification ratio of sterols is predictive for EAD.
Circulating plant sterols such as BR, CA, SI and ST solely result from dietary intake (Gylling et al. 2014; Ikeda 2015) and cannot be metabolized in the human organism except by being esterified predominantly in the liver (Chang et al. 2006). In our study, the esterification ratio of SI from day 1 and of BR and CA from day 3 as well as the difference in the esterification ratio day 1–2 of ST predicted a 3-month mortality after LTX. Interestingly, the free form of SI and ST was also predictive for a 3-month mortality from day 1 until day 10 post-LTX. The same association could be found for free LA. LA is a non-CH sterols that are active precursors in the cellular CH biosynthesis. Their CH-normalized concentrations are surrogate markers reflecting endogenous de novo synthesis of CH in the liver (Miettinen et al. 1990). The fact that free LA, SI, ST also predict 3-month mortality suggests that disturbances in hepatic cholesterol synthesis and cholesterol efflux by biliary excretion impact cholesterol homeostasis.
In addition, we also evaluated whether systemic levels of circulating amino acids were predictive for EAD and clinical outcome post-LTX. Altered amino acid metabolism is a hallmark of liver disease. Acute liver failure (ALF) is characterized by elevated levels of the circulating aromatic amino acids (AAAs) Phe, Trp and Tyr as well as Met. In contrast, reports on the concentrations of circulating branched-chain amino acids (BCAAs) Leu/Iso and Val have been conflicting (Blonde-Cynober et al. 1999; Honda et al. 2004). In chronic liver failure (CLF), decreased circulating BCAA and slightly elevated AAAs concentration have been reported consistently (Fischer et al. 1976; Watanabe et al. 1982). In the present study, the circulating level of all amino acids and the Fisher ratio, i.e. the ratio of the BCAAs to AAAs, did not correlate to the MEAF. Of note was the prediction of mortality for some amino acids and of organ failure only on day 1 after LTX which might be caused by blood transfusion or nutritional effects linked to caloric substitution. Overall, the patients with an unfavorable time course post-LTX received a different nutritional regimen compared to the patients with an uncomplicated healing. Probably the different scheme in nutrition explains the missing correlation between liver function and plasma amino acids and acylcarnitines. However, sterol esterification is not influenced by nutrition.
Finally, we also examined the carnitine shuttle and beta-oxidation of fatty acids. l-carnitine transports fatty acids into the mitochondria for subsequent β-oxidation, a process which results in its esterification to form acylcarnitine derivatives. As such, the endogenous carnitine pool is comprised of l-carnitine and various short-, medium- and long-chain acylcarnitines (McCoin et al. 2015). Thus, acylcarnitines play a decisive role in free fatty acid (FFA) oxidation, responsible for the transportation of acyl-coenzyme A into mitochondria, especially in liver and muscle cells. Acute liver failure with microvesicular steatosis is the consequence of severe impairment of mitochondrial beta-oxidation (Pessayre et al. 1999; Jaeschke et al. 2002). However, we did not find any correlation between l-carnitine/acyl carnitines and any of the routine parameters defining EAD and the MEAF. Long-chain acyl carnitines are sensitive biomarkers of acetaminophen (APAP)-induced hepatotoxicity in mouse models and human children (Bhattacharyya et al. 2013, 2014). High doses of APAP, the most widely used drug for the treatment of pain and fever, is the major cause of acute liver failure (ALF). In our study, none of the patients with acute liver failure had abused. Long-chain acylcarnitines accumulated, whereas free carnitine, medium and short-chain acylcarnitines decreased with the severity of the non-malignant chronic liver diseases, accompanied with corresponding alterations in enzyme activities of carnitine palmitoyl transferase 2 (CPT2) (Zhou et al. 2012).
In summary, we present promising data showing that sterols and especially their esterification ratios are suitable markers to follow up LTX and to predict EAD and clinical outcome. Our results clearly indicate the diagnostic potential of circulating sterols in the context of liver function assessment and justify further in-depth exploration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations of the determined parameters are given in Table S1. Each sterol was abbreviated with two letters (e. g. β-sitosterol: SI). The free, esterified and total concentration, the esterification ratio and the difference in the esterification ratio are given as SIF, SIE, SIT, SIE and ΔSIE %, respectively. Supplementary material 1 (DOC 142 kb)
Abbreviations
- ALD
Alcoholic liver disease
- AUC
Area under the curve
- EAD
Early allograft dysfunction
- LC–MS/MS
Liquid chromatography–mass tandem spectrometry
- LCAT
Lecithincholesterol acyltransferase
- LTX
Liver transplantation
- MEAF
Model for early allograft function scoring,
- MELD
Model for end-stage liver disease
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Footnotes
Uta Ceglarek and Kathleen Kresse have equally contributed to this work.
References
- Albers JJ, Bergelin RO, Adolphson JL, Wahl PW. Population-based reference values for lecithin-cholesterol acyltransferase (LCAT) Atherosclerosis. 1982;43(2–3):369–379. doi: 10.1016/0021-9150(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Becker S, Rohnike S, Empting S, Haas D, Mohnike K, et al. LC-MS/MS-based quantification of cholesterol and related metabolites in dried blood for the screening of inborn errors of sterol metabolism. Analytical and Bioanalytical Chemistry. 2015;407(17):5227–5233. doi: 10.1007/s00216-015-8731-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Pence L, Beger R, Chaudhuri S, McCullough S, et al. Acylcarnitine profiles in acetaminophen toxicity in the mouse: comparison to toxicity, metabolism and hepatocyte regeneration. Molecular Diversity Preservation International. 2013;3(3):606–622. doi: 10.3390/metabo3030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Yan K, Pence L, Simpson PM, Gill P, et al. Targeted liquid chromatography-mass spectrometry analysis of serum acylcarnitines in acetaminophen toxicity in children. Biomarkers in Medicine. 2014;8(2):147–159. doi: 10.2217/bmm.13.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonde-Cynober F, Aussel C, Cynober L. Abnormalities in branched-chain amino acid metabolism in cirrhosis: Influence of hormonal and nutritional factors and directions for future research. American Journal of Clinical Nutrition. 1999;18(1):5–13. doi: 10.1016/S0261-5614(99)80043-0. [DOI] [PubMed] [Google Scholar]
- Borowsky SA, Perlow W, Baraona E, Lieber CS. Relationship of alcoholic hypertriglyceridemia to stage of liver disease and dietary lipid. Digestive Diseases and Sciences. 1980;25(1):22–27. doi: 10.1007/BF01312728. [DOI] [PubMed] [Google Scholar]
- Brauer R, Leichtle AB, Fiedler GM, Thiery J, Ceglarek U. Preanalytical standardization of amino acid and acylcarnitine metabolite profiling in human blood using tandem mass spectrometry. Journal of Metabolomics. 2011;7:344–352. doi: 10.1007/s11306-010-0256-1. [DOI] [Google Scholar]
- Breier C, Lisch HJ, Braunsteiner H. Lipoproteins, HDL-apolipoproteins, activities of hepatic lipase and lecithin-cholesterol acyltransferase in the plasma of patients with post-alcoholic end-stage liver cirrhosis. Klinische Wochenschrift. 1983;61(18):929–931. doi: 10.1007/BF01537534. [DOI] [PubMed] [Google Scholar]
- Ceglarek U, Muller P, Stach B, Buhrdel P, Thiery J, et al. Validation of the phenylalanine/tyrosine ratio determined by tandem mass spectrometry: Sensitive newborn screening for phenylketonuria. Clinical Chemistry and Laboratory Medicine. 2002;40(7):693–697. doi: 10.1515/CCLM.2002.119. [DOI] [PubMed] [Google Scholar]
- Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annual Review of Cell and Developmental Biology. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- Deleuran T, Vilstrup H, Becker U, Jepsen P. Epidemiology of alcoholic liver disease in Denmark 2006–2011: A population-based study. Alcohol Alcoholism. 2015;50(3):352–357. doi: 10.1093/alcalc/agv003. [DOI] [PubMed] [Google Scholar]
- Dobiasova M, Frohlich JJ. Advances in understanding of the role of lecithin cholesterol acyltransferase (LCAT) in cholesterol transport. Clinica Chimica Acta. 1999;286(1–2):257–271. doi: 10.1016/S0009-8981(99)00106-0. [DOI] [PubMed] [Google Scholar]
- Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, et al. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery. 1976;80(1):77–91. [PubMed] [Google Scholar]
- Glomset JA, Verdery RB. Role of LCAT in cholesterol metabolism. Exposés Annuels de Biochimie Médicale. 1977;33:137–142. [PubMed] [Google Scholar]
- Gylling H, Plat J, Turley S, Ginsberg HN, Ellegard L, et al. Plant sterols and plant stanols in the management of dyslipidemia and prevention of cardiovascular disease. Atherosclerosis. 2014;232(2):346–360. doi: 10.1016/j.atherosclerosis.2013.11.043. [DOI] [PubMed] [Google Scholar]
- Hickman PE, Potter JM, Pesce AJ. Clinical chemistry and post-liver-transplant monitoring. Clinical Chemistry. 1997;43(8 Pt 2):1546–1554. [PubMed] [Google Scholar]
- Honda T, Fukuda Y, Nakano I, Katano Y, Goto H, et al. Effects of liver failure on branched-chain alpha-keto acid dehydrogenase complex in rat liver and muscle: comparison between acute and chronic liver failure. Journal of Hepatology. 2004;40(3):439–445. doi: 10.1016/j.jhep.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Huang LH, Elvington A, Randolph GJ. The role of the lymphatic system in cholesterol transport. Frontiers in Pharmacology. 2015;6:182. doi: 10.3389/fphar.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda I. Factors affecting intestinal absorption of cholesterol and plant sterols and stanols. Journal of Oleo Science. 2015;64(1):9–18. doi: 10.5650/jos.ess14221. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, et al. Mechanisms of hepatotoxicity. Toxicological Sciences. 2002;65(2):166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- Jonas A. Lecithin cholesterol acyltransferase. Biochimica et Biophysica Acta. 2000;1529(1–3):245–256. doi: 10.1016/S1388-1981(00)00153-0. [DOI] [PubMed] [Google Scholar]
- Jones DP, Sosa FR, Shartsis J, Shah PT, Skromak E, et al. Serum cholesterol esterifying and cholesteryl ester hydrolyzing activities in liver diseases: relationships to cholesterol, bilirubin, and bile salt concentrations. Journal of Clinical Investigation. 1971;50(2):259–265. doi: 10.1172/JCI106490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- Kuksis A. Plasma non-cholesterol sterols. Journal of Chromatography A. 2001;935(1–2):203–236. doi: 10.1016/S0021-9673(01)01226-2. [DOI] [PubMed] [Google Scholar]
- Kunnen S, Van EM. Lecithin:cholesterol acyltransferase: Old friend or foe in atherosclerosis? Journal of Lipid Research. 2012;53(9):1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DD, Singh A, Burns JM, Perry DK, Nguyen JH, et al. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver Transplantation. 2014;20(12):1447–1453. doi: 10.1002/lt.23985. [DOI] [PubMed] [Google Scholar]
- Lembcke J, Ceglarek U, Fiedler GM, Baumann S, Leichtle A, et al. Rapid quantification of free and esterified phytosterols in human serum using APPI-LC-MS/MS. Journal of Lipid Research. 2005;46(1):21–26. doi: 10.1194/jlr.C400004-JLR200. [DOI] [PubMed] [Google Scholar]
- McCoin CS, Knotts TA, Adams SH. Acylcarnitines–Old actors auditioning for new roles in metabolic physiology. Nature Reviews Endocrinology. 2015;11(10):617–625. doi: 10.1038/nrendo.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. American Journal of Epidemiology. 1990;131(1):20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- Nikkilä K, Hockerstedt K, Miettinen TA. Liver transplantation modifies serum cholestanol, cholesterol precursor and plant sterol levels. Clinica Chimica Acta. 1992;208(3):205–218. doi: 10.1016/0009-8981(92)90077-4. [DOI] [PubMed] [Google Scholar]
- Nikkilä K, Miettinen TA, Hockerstedt KV, Isoniemi H. Sterol parameters as markers of liver function in primary biliary cirrhosis before and after liver transplantation. Transplant International. 2005;18(2):221–225. doi: 10.1111/j.1432-2277.2004.00002.x. [DOI] [PubMed] [Google Scholar]
- Nikkilä K, Nissinen MJ, Gylling H, Isoniemi H, Miettinen TA. Serum sterols in patients with primary biliary cirrhosis and acute liver failure before and after liver transplantation. Journal of Hepatology. 2008;49(6):936–945. doi: 10.1016/j.jhep.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplantation. 2010;16(8):943–949. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- Pareja E, Cortes M, Hervas D, Mir J, Valdivieso A, et al. A score model for the continuous grading of early allograft dysfunction severity. Liver Transplantation. 2015;21(1):38–46. doi: 10.1002/lt.23990. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Mansouri A, Haouzi D, Fromenty B. Hepatotoxicity due to mitochondrial dysfunction. Cell Biology and Toxicology. 1999;15(6):367–373. doi: 10.1023/A:1007649815992. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Liu J, Song BL, Li BL, Chang CC, et al. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. Journal of Steroid Biochemistry and Molecular Biology. 2015;151:102–107. doi: 10.1016/j.jsbmb.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear JW. Assessment of liver function: its application to outcome from liver transplantation. British Journal of Anaesthesia. 2002;88(6):757–760. doi: 10.1093/bja/88.6.757. [DOI] [PubMed] [Google Scholar]
- Shaked A, Nunes FA, Olthoff KM, Lucey MR. Assessment of liver function: pre- and peritransplant evaluation. Clinical Chemistry. 1997;43(8 Pt 2):1539–1545. [PubMed] [Google Scholar]
- Simon JB, Kepkay DL, Poon R. Serum cholesterol esterification in human liver disease: Role of lecithin-cholesterol acyltransferase and cholesterol ester hydrolase. Gastroenterology. 1974;66(4):539–547. [PubMed] [Google Scholar]
- Tanaka E, Inomata S, Yasuhara H. The clinical importance of conventional and quantitative liver function tests in liver transplantation. Journal of Clinical Pharmacology and Therapeutics. 2000;25(6):411–419. doi: 10.1046/j.1365-2710.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- Temel RE, Gebre AK, Parks JS, Rudel LL. Compared with Acyl-CoA:Cholesterol O-acyltransferase (ACAT) 1 and lecithin:cholesterol acyltransferase, ACAT2 displays the greatest capacity to differentiate cholesterol from sitosterol. The Journal of Biological Chemistry. 2003;278(48):47594–47601. doi: 10.1074/jbc.M308235200. [DOI] [PubMed] [Google Scholar]
- Uemura T, Randall HB, Sanchez EQ, Ikegami T, Narasimhan G, et al. Liver retransplantation for primary nonfunction: Analysis of a 20-year single-center experience. Liver Transplantation. 2007;13(2):227–233. doi: 10.1002/lt.20992. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Hayashi S, Higashi T, Obata T, Sakata T, et al. Characteristics change in serum amino acid levels in different types of hepatic encephalopathy. Gastroenterologia Japonica. 1982;17(3):218–223. doi: 10.1007/BF02775999. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang Q, Yin P, Xing W, Wu Z, et al. Serum metabolomics reveals the deregulation of fatty acids metabolism in hepatocellular carcinoma and chronic liver diseases. Analytical and Bioanalytical Chemistry. 2012;403(1):203–213. doi: 10.1007/s00216-012-5782-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations of the determined parameters are given in Table S1. Each sterol was abbreviated with two letters (e. g. β-sitosterol: SI). The free, esterified and total concentration, the esterification ratio and the difference in the esterification ratio are given as SIF, SIE, SIT, SIE and ΔSIE %, respectively. Supplementary material 1 (DOC 142 kb)