Abstract
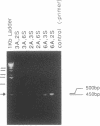
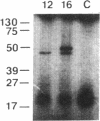
UDP-GlcNAc:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I (GnT I; EC 2.4.1.101) catalyzes an essential first step in the conversion of high-mannose N-glycans to hybrid and complex N-glycans. Cloning of the gene encoding this enzyme was carried out by mixed oligonucleotide-primed polymerase chain reaction amplification of rabbit liver single-stranded cDNA using sense and antisense 20- to 24-base-pair (bp) primers. A rabbit liver library in phage lambda gt10 yielded a 2.5-kilobase (kb) cDNA with a 447-amino acid coding sequence. None of the nine asparagine residues were in an Asn-Xaa-(Ser or Thr) sequence, indicating that the protein is not N-glycosylated. There is no sequence homology to other previously cloned glycosyltransferases, but GnT I appears to have a domain structure typical of these enzymes--i.e., a short amino-terminal domain, a transmembrane domain, a "neck" region, and a large carboxyl-terminal catalytic domain. RNA was transcribed off the 2.5-kb cDNA, and in vitro translation with rabbit reticulocyte lysate yielded a 52-kDa protein with GnT I activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appert H. E., Rutherford T. J., Tarr G. E., Wiest J. S., Thomford N. R., McCorquodale D. J. Isolation of a cDNA coding for human galactosyltransferase. Biochem Biophys Res Commun. 1986 Aug 29;139(1):163–168. doi: 10.1016/s0006-291x(86)80094-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendiak B., Schachter H. Control of glycoprotein synthesis. Kinetic mechanism, substrate specificity, and inhibition characteristics of UDP-N-acetylglucosamine:alpha-D-mannoside beta 1-2 N-acetylglucosaminyltransferase II from rat liver. J Biol Chem. 1987 Apr 25;262(12):5784–5790. [PubMed] [Google Scholar]
- Bendiak B., Schachter H. Control of glycoprotein synthesis. Purification of UDP-N-acetylglucosamine:alpha-D-mannoside beta 1-2 N-acetylglucosaminyltransferase II from rat liver. J Biol Chem. 1987 Apr 25;262(12):5775–5783. [PubMed] [Google Scholar]
- Brockhausen I., Carver J. P., Schachter H. Control of glycoprotein synthesis. The use of oligosaccharide substrates and HPLC to study the sequential pathway for N-acetylglucosaminyltransferases I, II, III, IV, V, and VI in the biosynthesis of highly branched N-glycans by hen oviduct membranes. Biochem Cell Biol. 1988 Oct;66(10):1134–1151. doi: 10.1139/o88-131. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- D'Agostaro G., Bendiak B., Tropak M. Cloning of cDNA encoding the membrane-bound form of bovine beta 1,4-galactosyltransferase. Eur J Biochem. 1989 Jul 15;183(1):211–217. doi: 10.1111/j.1432-1033.1989.tb14915.x. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brands R., Rothman J. E. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985 Feb;40(2):463–472. doi: 10.1016/0092-8674(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Bovine colostrum UDP-N-acetylglucosamine:alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. Separation from UDP-N-acetylglucosamine:alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase II, partial purification, and substrate specificity. J Biol Chem. 1980 May 25;255(10):4885–4893. [PubMed] [Google Scholar]
- Hollis G. F., Douglas J. G., Shaper N. L., Shaper J. H., Stafford-Hollis J. M., Evans R. J., Kirsch I. R. Genomic structure of murine beta-1,4-galactosyltransferase. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1069–1075. doi: 10.1016/0006-291x(89)90782-1. [DOI] [PubMed] [Google Scholar]
- Joziasse D. H., Shaper J. H., Van den Eijnden D. H., Van Tunen A. J., Shaper N. L. Bovine alpha 1----3-galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J Biol Chem. 1989 Aug 25;264(24):14290–14297. [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lammers G., Jamieson J. C. The role of a cathepsin D-like activity in the release of Gal beta 1-4GlcNAc alpha 2-6-sialyltransferase from rat liver Golgi membranes during the acute-phase response. Biochem J. 1988 Dec 1;256(2):623–631. doi: 10.1042/bj2560623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R. D., Rajan V. P., Ruff M. M., Kukowska-Latallo J., Cummings R. D., Lowe J. B. Isolation of a cDNA encoding a murine UDPgalactose:beta-D-galactosyl- 1,4-N-acetyl-D-glucosaminide alpha-1,3-galactosyltransferase: expression cloning by gene transfer. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8227–8231. doi: 10.1073/pnas.86.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R. D., Rivera-Marrero C. A., Ernst L. K., Cummings R. D., Lowe J. B. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:beta-D-Gal(1,4)-D-GlcNAc alpha(1,3)-galactosyltransferase cDNA. J Biol Chem. 1990 Apr 25;265(12):7055–7061. [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Masibay A. S., Qasba P. K. Expression of bovine beta-1,4-galactosyltransferase cDNA in COS-7 cells. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5733–5737. doi: 10.1073/pnas.86.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri K. A., Appert H. E., Fukuda M. N. Identification of the full-length coding sequence for human galactosyltransferase (beta-N-acetylglucosaminide: beta 1,4-galactosyltransferase). Biochem Biophys Res Commun. 1988 Dec 15;157(2):657–663. doi: 10.1016/s0006-291x(88)80300-0. [DOI] [PubMed] [Google Scholar]
- Moremen K. W. Isolation of a rat liver Golgi mannosidase II clone by mixed oligonucleotide-primed amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5276–5280. doi: 10.1073/pnas.86.14.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu H., Sinha S., Brew K., Okayama H., Qasba P. K. Cloning and sequencing of cDNA of bovine N-acetylglucosamine (beta 1-4)galactosyltransferase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4720–4724. doi: 10.1073/pnas.83.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y., Pegg W., Paulsen H., Schachter H. Control of glycoprotein synthesis. Purification and characterization of rabbit liver UDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I. J Biol Chem. 1988 Jun 15;263(17):8270–8281. [PubMed] [Google Scholar]
- Oppenheimer C. L., Eckhardt A. E., Hill R. L. The nonidentity of porcine N-acetylglucosaminyltransferases I and II. J Biol Chem. 1981 Nov 25;256(22):11477–11482. [PubMed] [Google Scholar]
- Oppenheimer C. L., Hill R. L. Purification and characterization of a rabbit liver alpha 1 goes to 3 mannoside beta 1 goes to 2 N-acetylglucosaminyltransferase. J Biol Chem. 1981 Jan 25;256(2):799–804. [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Pierce M., Arango J. Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-beta (1,6)Man-alpha (1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem. 1986 Aug 15;261(23):10772–10777. [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Russo R. N., Shaper N. L., Shaper J. H. Bovine beta 1----4-galactosyltransferase: two sets of mRNA transcripts encode two forms of the protein with different amino-terminal domains. In vitro translation experiments demonstrate that both the short and the long forms of the enzyme are type II membrane-bound glycoproteins. J Biol Chem. 1990 Feb 25;265(6):3324–3331. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986 Mar;64(3):163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Schachter H., Brockhausen I., Hull E. High-performance liquid chromatography assays for N-acetylglucosaminyltransferases involved in N- and O-glycan synthesis. Methods Enzymol. 1989;179:351–397. doi: 10.1016/0076-6879(89)79138-2. [DOI] [PubMed] [Google Scholar]
- Schachter H., Narasimhan S., Gleeson P., Vella G. Control of branching during the biosynthesis of asparagine-linked oligosaccharides. Can J Biochem Cell Biol. 1983 Sep;61(9):1049–1066. doi: 10.1139/o83-134. [DOI] [PubMed] [Google Scholar]
- Shaper J. H., Hollis G. F., Shaper N. L. Evidence for two forms of murine beta-1,4-galactosyltransferase based on cloning studies. Biochimie. 1988 Nov;70(11):1683–1688. doi: 10.1016/0300-9084(88)90303-3. [DOI] [PubMed] [Google Scholar]
- Shaper N. L., Hollis G. F., Douglas J. G., Kirsch I. R., Shaper J. H. Characterization of the full length cDNA for murine beta-1,4-galactosyltransferase. Novel features at the 5'-end predict two translational start sites at two in-frame AUGs. J Biol Chem. 1988 Jul 25;263(21):10420–10428. [PubMed] [Google Scholar]
- Shaper N. L., Shaper J. H., Meuth J. L., Fox J. L., Chang H., Kirsch I. R., Hollis G. F. Bovine galactosyltransferase: identification of a clone by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1573–1577. doi: 10.1073/pnas.83.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper N. L., Wright W. W., Shaper J. H. Murine beta 1,4-galactosyltransferase: both the amounts and structure of the mRNA are regulated during spermatogenesis. Proc Natl Acad Sci U S A. 1990 Jan;87(2):791–795. doi: 10.1073/pnas.87.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Moritz R. L., Begg G. S., Rubira M. R., Nice E. C. Micropreparative procedures for high sensitivity sequencing of peptides and proteins. Anal Biochem. 1989 Mar;177(2):221–236. doi: 10.1016/0003-2697(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Moritz R. L., Nice E. E., Grego B. A high-performance liquid chromatography procedure for recovering subnanomole amounts of protein from SDS-gel electroeluates for gas-phase sequence analysis. Eur J Biochem. 1987 May 15;165(1):21–29. doi: 10.1111/j.1432-1033.1987.tb11189.x. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Moritz R. L., Rubira M. R., Van Snick J. Murine hybridoma/plasmacytoma growth factor. Complete amino-acid sequence and relation to human interleukin-6. Eur J Biochem. 1988 Sep 1;176(1):187–197. doi: 10.1111/j.1432-1033.1988.tb14267.x. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Larsen R. D., Mattox S., Lowe J. B., Cummings R. D. Transfer and expression of a murine UDP-Gal:beta-D-Gal-alpha 1,3-galactosyltransferase gene in transfected Chinese hamster ovary cells. Competition reactions between the alpha 1,3-galactosyltransferase and the endogenous alpha 2,3-sialyltransferase. J Biol Chem. 1990 Apr 15;265(11):6225–6234. [PubMed] [Google Scholar]
- Tosi M., Young R. A., Hagenbüchle O., Schibler U. Multiple polyadenylation sites in a mouse alpha-amylase gene. Nucleic Acids Res. 1981 May 25;9(10):2313–2323. doi: 10.1093/nar/9.10.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]
- Yamamoto F., Marken J., Tsuji T., White T., Clausen H., Hakomori S. Cloning and characterization of DNA complementary to human UDP-GalNAc: Fuc alpha 1----2Gal alpha 1----3GalNAc transferase (histo-blood group A transferase) mRNA. J Biol Chem. 1990 Jan 15;265(2):1146–1151. [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Ohkura T., Kobata A. Enzymatic basis for the structural changes of asparagine-linked sugar chains of membrane glycoproteins of baby hamster kidney cells induced by polyoma transformation. J Biol Chem. 1985 Apr 10;260(7):3963–3969. [PubMed] [Google Scholar]