Abstract
Marine biodiversity is recognized by a wide and unique array of fascinating structures. The complex associations of marine microorganisms, especially with sponges, bryozoans, and tunicates, make it extremely difficult to define the biosynthetic source of marine natural products or to deduce their ecological significance. Marine sponges and tunicates are important source of novel compounds for drug discovery and development. Majority of these compounds are nitrogen containing and belong to non-ribosomal peptide (NRPs) or mixed polyketide–NRP natural products. Several of these peptides are currently under trial for developing new drugs against various disease areas, including inflammatory, cancer, neurodegenerative disorders, and infectious disease. This review features pharmacologically active NRPs from marine sponge and tunicates based on their biological activities.
Keywords: marine ecosystem, sponge, tunicates, marine natural products, non-ribosomal peptides, pharmacology
Introduction
Nature provides a wide and structurally diverse array of active biomolecules that have proved vital for the development of novel pharmaceuticals. The marine world, covering more than 70% of the Earth's surface, is the home of tremendous biodiversity. Due to very diverse oceanic environments, marine organisms have developed the capacity to produce unique compounds (Steele, 1985; Mehbub et al., 2014). This rich and unprecedented chemo diversity of marine natural products provides an unlimited resource of novel biomolecules in the field of drug development. The importance of marine metabolites in current drug research is driven by the fact, that during 1981–2002, around half of US FDA-approved drugs consisted of either marine metabolites or their synthetic analogs (Vinothkumar and Parameswaran, 2013). Interestingly, the majority of these natural products involved in clinical or preclinical trials are produced by invertebrates, that is, sponges, tunicates, bryozoans, or molluscs. Sixty per cent of these natural products belong to non-ribosomal peptide (NRP) families, which are biosynthesized by poly-functional mega-synthases called NRP synthetases (NRPSs) (Finking and Marahiel, 2004; Mehbub et al., 2014). The excellent binding properties, low off-target toxicity, and high stability of NRPs make them a promising molecule for development of new therapeutics. Currently, only a handful of NRPs are used as drug (Table 1).
Table 1.
NRPs-based drugs in market.
| Compound | Biosynthetic class of agent | Source | Disease/molecular target | Reference |
|---|---|---|---|---|
| Polymyxin B | Polypeptides | Bacillus polymyxa | Antibiotic/Alters bacterial outer membrane | Paulus and Gray, 1964 |
| Pristinamycin | Depsipeptide | Streptomyces. pristinaespiralis | Antibiotic/protein synthesis inhibitor | de Crécy-Lagard et al., 1997 |
| Gramicidin | Linear pentadecapeptide | Bacillus bovis | Antibiotic/Alters bacterial outer membrane | Kleinkauf and von Döhren, 1995 |
| Bacitracin | Cyclic peptide | Bacillus subtilis | Antibiotic/dephosphorylation of C55-isoprenyl pyrophosphate | Johnson et al., 1945 |
| Capreomycin | Cyclic peptide | Streptomyces capreolus | Antibiotic/protein synthesis inhibitor | Stark et al., 1962 |
| Teicoplanin | Glycopeptide | Actinoplanes teichomyceticus | Antibiotic/inhibit cell wall synthesis | Somma et al., 1984 |
| Vancomycin | Glycopeptide | Amycolatopsis orientalis | Antibiotic/inhibit cell wall synthesis | Van Wageningen et al., 1998 |
| Cephalosporin C | β-lactam | Acremonium sp. | Antibiotic/Alters bacterial outer membrane | Abraham and Newton, 1961 |
| Oritavancin | – | Semi synthetic | Antibiotic/disrupts the cell membrane | Domenech et al., 2009 |
| Bleomycin | Hybrid peptide | Streptomyces verticillus | Antibiotic/inhibition of DNA synthesis | Umezawa et al., 1966 |
| Daptomycin | Lipopeptide | Streptomyces roseosporus | Antibiotic/disrupts the cell membrane | Miao et al., 2005 |
| Cyclosporine A | Cyclic peptide | Tolypocladium inflatum | Immunosuppressant /lower the activity of T cells | Murthy et al., 1999 |
| Actinomycin D | Polypeptide | Streptomyces sp. | Antitumor/inhibit transcription | Waksman and Woodruff, 1940 |
| Romidepsin | Depsipeptide | Chromobacterium violaceum | Antitumor/Histone deacetylase inhibitor | Ueda et al., 1994 |
Marine sponges (Phylum porifera) represent the most primitive multicellular animals, with origins dating back to the Precambrian era (Hentschel et al., 2002). There are about 9000 reported species of sponges and (perhaps twice as many unreported species) available in the ocean (Brusca et al., 1990; Wörheide et al., 2005). These have been broadly categorized in 3 classes : Calcarea (5 orders and 24 families), Demospongiae (15 orders and 92 families), and Hexactinellida (6 orders and 20 families). Till date, more than 5300 different natural products have been isolated from marine sponges, and each year more than 200 additional new metabolites are being discovered (Laport et al., 2009; Mehbub et al., 2014). There are several sponge derived metabolites currently available in market and many in clinical studies (Table 2).
Table 2.
Sponge secondary metabolites that are FDA-approved agents in clinical trial (Mayer et al., 2010; Newman and Cragg, 2016).
| Compound | Biosynthetic class of agent | Source | Disease/molecular target | Clinical status |
|---|---|---|---|---|
| Cytarabine (Ara-C) | Nucleoside | Cryptotethya crypta | Cancer/DNA polymerase | FDA approved |
| Vidarabine (Ara-A) | Nucleoside | C. crypta | Antiviral viral/DNA polymerase I | FDA approved |
| Eribulin mesylate (E7389) | Complex polyketide | Lissodendoryx sp. | Cancer/microtubules | FDA approved |
| Hemiasterlin derivative (E7974) | Modified linear tripeptide (NRPS-PKS) | Cymbastella sp. | Cancer/microtubules | Phase I |
| Discodermolide | Polyketide | Discodermia dissolute | Cancer/microtubules | Phase I |
| Bengamide derivative (LAF389) | Mixed PKS/NRP | Jaspis sp. | Cancer/methionine aminopeptidases | Phase I |
| Spongistatin 1 | Macrocyclic lactone polyether | Hyrtios erecta | Cancer/microtubules | Preclinical |
| Manoalide | Sesterterpene | Luffariella variabilis | Inflammation/inhibition of Phospholipase A2 | Preclinical |
| Salicylhalimides A | Polyketide | Haliclona sp. | Cancer/microtubules | Preclinical |
| Laulimalide | Polyketide | Cacospongia mycofijiensis | Cancer/microtubules | Preclinical |
| Peloruside A | Polyketide | Mycale hentscheli | Cancer/microtubules | Preclinical |
It is proposed that some of the bioactive compounds isolated from sponges are produced by functional enzyme clusters originated from the sponges and their associated microorganisms (Laport et al., 2009; Thomas et al., 2010). It has been observed that bacterial phyla such as Proteobacteria, Nitrospira, Cyanobacteria, Bacteriodetes, Actinobacteria, Chloroflexi, Planctomycetes, Acidobacteria, Poribacteria, and Verrucomicrobia besides members of the domain Archaea are most sponge-associated bacterial community (Hentschel et al., 2002; Olson and McCarthy, 2005). However, fungi and microalgae also symbiotically inhabit sponges. It has been recognized that one host sponge can possess diverse symbionts. For example, unicellular heterotrophic bacteria, unicellular cyanobacteria, and filamentous heterotrophic bacteria all grow together in sponge Theonella swinhoei (Bewley et al., 1996). Likewise, a sponge belonging to Aplysina includes heterogeneous bacteria Bacillus sp., Micrococcus sp., Arthrobacter sp., Vibrio sp., Pseudoalteromonas sp., and so on (Hentschel et al., 2001). Sponge Rhopaloeides odorabile has β-Proteobacteria, γ-Proteobacteria, Cytophaga, Actinobacteria, and green sulfur bacteria (Webster et al., 2001). Besides this, species-specific symbiotic relationship has also been observed. For example, sponge T. swinhoei and δ-proteobacteria have shown a specific association with each other (Schmidt et al., 2000). A species of α-proteobacteria dominates in sponge R. odorabile over various habitats but is not detected from seawater, which strongly suggests that the symbiont is species specific (Lee Y. K. et al., 2001). On the other hand, one symbiont occurs commonly in various sponges from different regions indicating its wide host range (Wilkinson et al., 1981). For example, cyanobacteria Aphanocapsa sp., Phormidium sp., or Oscillatoria spongeliae are found in numerous sponges (Wilkinson, 1978). Symbiotic associations between sponges and marine microorganisms might be involved in nutrient acquisition, stabilization of sponge skeleton, processing of metabolic waste, and secondary metabolite synthesis. It is assumed that symbiotic marine microorganisms harbored by sponges are the original producers of some of these bioactive compounds (Newman and Hill, 2006). For example, antibiotic polybrominated biphenyl ether isolated from the sponge Dysidea herbacea (Demospongiae) are actually produced by endosymbiotic cyanobacterium O. spongeliae (Unson et al., 1994). A symbiotic bacterium Micrococcus sp. produces diketopiperazines previously ascribed to the host sponge Tedania ignis (Stierle et al., 1988). Another symbiotic bacterium Vibrio sp. produces brominated biphenyl ethers formerly attributed to the host sponge Dysidea sp. (Elyakov et al., 1991). Symbiotic bacterium Vibrio sp. produces an anti-Bacillus peptide andrimid that was found in the sponge Hyatella sp. extract (Oclarit et al., 1994). Antimicrobial activity is detected in Micrococcus luteus isolated from the sponge Xestospongia sp. (Bultel-Poncé et al., 1998). Antimicrobial compounds such as quinolones and phosphatidyl glyceride are isolated from a Pseudomonas sp. collected at the surface of the sponge Homophymia sp. (Bultel-Poncé et al., 1999). However, the mutual mechanism between sponge and its microbial associate, in metabolite production, is not well-understood. Thus, it is extremely relevant to highlight the therapeutic potential of various secondary metabolites synthesized by the microbial flora inhabiting sponges. This is because they open up the possibility of providing a continuous supply of the biologically active compounds by laboratory cultivation of the producer (Thomas et al., 2010).
Tunicates include a wide variety of invertebrates that are classified within the Phylum chordata based on presence of a larval notochord during early development. Tunicates contains about 2150 described species that are divided into 4 classes: Ascidiacea (Aplousoobranchia, Phlebobranchia, Stolidobranchia) Thaliacea (Pyrosomida, Doliolida, Salpida), Appendicularia (Larvacea), and Sorberacea (Ruppert and Fox, 2004). Amongst these, Ascidacea (commonly known as the ascidians) are highly studied due to their biologically active metabolites that serve as antineoplastic agents. Geranyl hydroquinone was first ascidian metabolite isolated from Aplidium sp. which displayed chemo protective activity against some forms of leukemia, rous sarcoma, and mammary carcinoma in test animals (Fenical, 1976) (Menna, 2009). Since then, ascidians are known as the source of numerous marine natural products. The biologically active metabolites originated from tunicates which are approved by FDA or in clinical trials along with their biological properties are given in Table 3.
Table 3.
Tunicate secondary metabolites that are FDA-approved agents or in clinical trial (Mayer et al., 2010; Newman and Cragg, 2016).
| Compound | Biosynthetic class of agent | Source | Disease/molecular target area | Clinical status |
|---|---|---|---|---|
| Trabectedin (ET-743) (EU registered only) | NRPS-derived alkaloid | Ecteinascidia turbinata | Cancer/minor groove of DNA | FDA approved |
| Plitidepsin (Aplidine) | Cyclic depsipeptide | Aplidium albicans | Cancer/Rac1 and JNK activation | Phase III |
| Trabectedin analog (PM01183) | NRPS alkaloid | E. turbinata | Cancer/minor groove of DNA, nucleotide excision repair | Phase I |
| Vitilevuamide | NRPS | Didemnum cuculiferum and Polysyncranton lithostrotum | Cancer/microtubules | Preclinical |
To date, significant biological activities, such as antimicrobial, anticancer, neurotoxic, antiprotozoal and their associated cellular targets have been reported for several NRPs from the marine sponges and tunicates. These NRPs have unique structures as compared with those from other sources. It is this attribute that makes marine sponge- and tunicate-derived NRPs highly attractive as potential drug and molecular probes. In this review, we survey the discoveries of NRPs derived from marine sponges and tunicates, which have shown in vivo efficacy or potent in vitro activity against various human diseases. Our objective is to highlight NRPs that have the greatest potential to be clinically useful. The details of sponge- and tunicate-derived NRPs along with biological properties is given Table 4.
Table 4.
Biological activities of NRPs isolated from marine sponges and tunicates.
| NRPs | Chemical class | Origin | Disease/target | Biological active value (IC50/GI50/ID50/ED50) | Reference(s) |
|---|---|---|---|---|---|
| Miraziridine A (1) | Linear penta peptide | Theonella aff. mirabilis | Cancer/inhibit protease cathepsin B | 1.4 μg/mL | Nakao et al., 2000 |
| Haligramides A-B (2–3) | Cyclic hexapeptides | Haliclona nigra | Cancer/A-549 (lung) HCT-15(colon) SF-539 (CNS) SNB-19 (CNS) |
5.17–15.6 μg/mL 3.89–8.82 μg/mL | Rashid et al., 2000 |
| Prepatellamide A (4) | Cyclic peptide | Lissoclinum patella | Cancer/P388 murine leukemia cell lines | 5 μg/mL | Fu et al., 2000 |
| Tamandarins A-B (5–6) | Depsipeptides | Didemnid ascidian | Cancer/pancreatic carcinoma BX- PC3, prostatic cancer DU-145, head and neck carcinoma UMSCC10b |
1.79, 2.00 μg/mL 1.36, 1.53 μg/mL 0.99, 1.76 μg/mL |
Vervoort et al., 2000 |
| Microsclerodermins F–I (7–10) | Cyclic peptides | Microscleroderma sp. | Cancer/HCT-116 cell line | 1.8, 2.4, 1.0, and 1.1 μg/mL | Qureshi et al., 2000 |
| Wainunuamide (11) | Cyclic hexapeptide | Stylotella aurantium | Cancer/A2780 ovarian, K562 leukemia cancer cells | 19.15 and 18.36 μg/mL | Tabudravu et al., 2001 |
| Leucamide A (12) | Cyclic hexapeptide | Leucetta microraphis | Cancer/Tumor cell lines HM02, HepG2, Huh7 |
5.2 μg/mL 5.9 μg/mL 5.1 μg/mL |
Kehraus et al., 2002 |
| Axinellin C (13) | Cyclic octapeptide | S. aurantium | Cancer/A2780 ovarian, K562 leukemia cancer cells | 13.17 and 4.46 mg/mL | Tabudravu et al., 2002 |
| Milnamide D (14) | Linear peptide | Cymbastela sp. | Cancer/HCT-116 cells | 66 nM | Chevallier et al., 2003 |
| Kapakahines E–G (15–17) | – | Cribrochalina olemda | Cancer/P388 murine leukemia cells | 5.0 μg/mL | Nakao et al., 2003 |
| Didmolamides A- B (18–19) | Cyclic hexapeptides | Didemnum molle | Cancer Tumor cell lines (A549, HT29, and MEL28) | 10–20 μg/mL | Rudi et al., 2003 |
| Bistratamides E–J (20- 25) | Cyclic hexapeptides | Lissoclinum bistratum | Cancer/Human colon tumor (HCT-116) cell line | 3, 7.9; 4, 28; 5, 5; 6, 1.7; 7, 9; 8, 1 μg/mL | Perez and Faulkner, 2003 |
| Milnamide C (26) | – | Auletta sp. | Cancer/MDA-MB-435 cancer cells | 3.2 × 10−1 μg/mL | Sonnenschein et al., 2004 |
| Scleritodermin A (27) | Cyclic peptide | Scleritoderma nodosum | Cancer | <2 μM | Schmidt et al., 2004 |
| Microcionamides A-B (28–29) | – | Clathria abietina | Cancer/Human breast tumor cell lines MCF-7 and SKBR-3 |
125 and 98 nM 177 and 172 nM |
Davis et al., 2004 |
| Kendarimide A (30) | Linear peptide | Haliclona sp. | Cancer/KB-C2 cells | – | Aoki et al., 2004 |
| Phakellistatin 14 (31) | Cycloheptapeptide | Phakellia sp. | Cancer/Murine lymphocytic leukemia P388 cell line | 5 μg/mL | Pettit and Tan, 2005 |
| Polytheonamides A-B (32–33) | Polypeptides | T. swinhoei | Cancer/P388 murine leukemia cells | 78, 68 pg/mL | Hamada et al., 2005 |
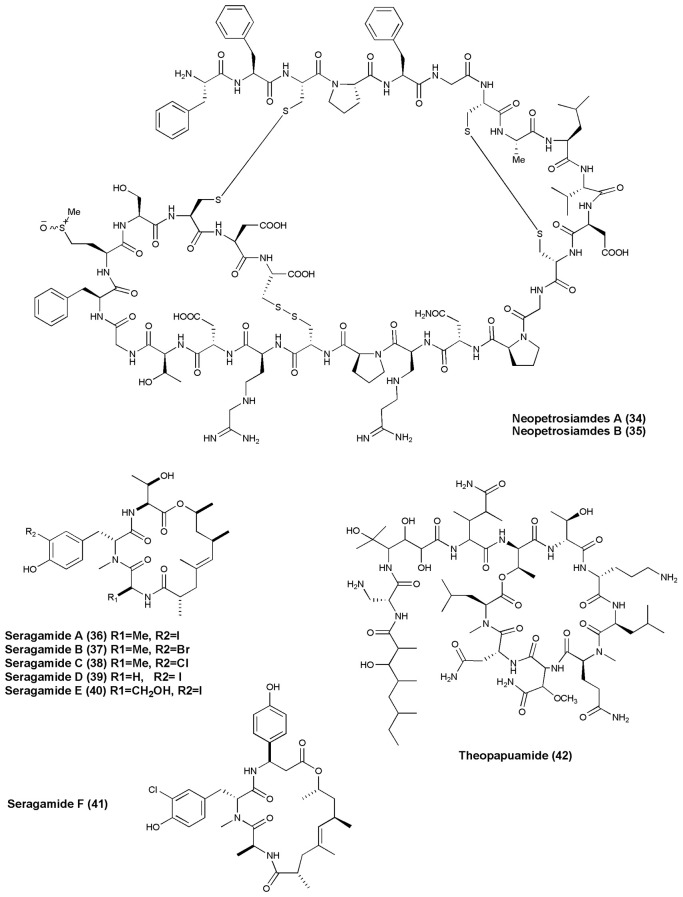
| Neopetrosiamides A- B (34–35) | Tricyclic peptides | Neopetrosia sp. | Cancer | 6 μg/mL | Williams et al., 2005 |
| Seragamides A–F (36–37) | Depsipeptides | Suberites japonicus | Cancer | 0.01, 0.02, 0.01, 0.01, and 0.04 mg/mL | Tanaka et al., 2006 |
| Theopapuamide (38) | Cyclic depsipeptide | T. swinhoei | Cancer/CEM-TART HCT-116 cell lines |
0.5 μM 0.9 μM |
Ratnayake et al., 2006 |
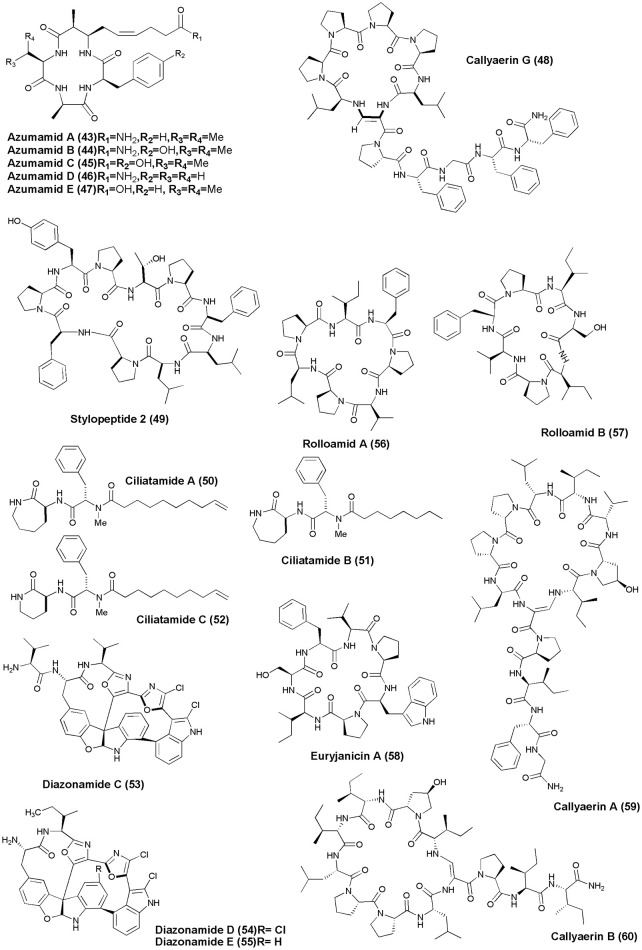
| Azumamide A- E (39–47) | Cyclotetrapeptides | Mycale izuensis | Cancer | – | Maulucci et al., 2007 |
| Callyaerin G (48) | Cyclic peptide | Callyspongia aerizusa | Cancer/Mouse lymphoma cell line (L5178Y) and HeLa cells | 0.53 and 5.4 ug/mL | Ibrahim et al., 2008 |
| Stylopeptide 2 (49) | Cyclodecapeptide | Stylotella sp. | Cancer/BT-549 and HS 578T breast cancer cell lines | – | Brennan et al., 2008 |
| Ciliatamides A-C (50–52) | Lipopeptides | Aaptos ciliata | Cancer/HeLa cells | 50, 4.5, and 50 μg/mL | Nakao et al., 2008 |
| Diazonamides C–E (53–55) | Macrocyclic peptides | Diazona sp. | Cancer/Human tumor cell lines (A549, HT29, MDA-MB 231) |
2.2, 2.9, 8.0 μg/mL 1.8, 2.9, 5.2 μg/mL 2.2, 3.1, 9.0 μg/mL |
Fernández et al., 2008 |
| Rolloamide A- B (56–57) | Cyclic heptapeptides | Eurypon laughlini | Cancer | 0.4−5.8 μM | Williams et al., 2009 |
| Euryjanicin A (58) | Cycloheptapeptide | Prosuberites laughlini | Cancer | – | Vicente et al., 2009 |
| Callyaerin A–F (59–64) and H (65) | Cyclic peptides | C. aerizusa | Cancer/L5178Y cell line | 0.39 and 0.48 μM | Ibrahim et al., 2010 |
| Papuamides E-F (66–67) | Depsipeptides | Melophlus sp. | Cancer/Brine shrimp | 92 and 106 μg/mL | Prasad et al., 2011 |
| Stylissamide X (68) | Octapeptide | Stylissa sp. | Cancer/HeLa cells | 0.1 μM to 10 μM | Arai et al., 2012 |
| Gombamide A (69) | Hexapeptide | Clathria gombawuiensis | Cancer/K562 and A549 cell lines | 6.9 and 7.1 μM | Woo et al., 2013 |
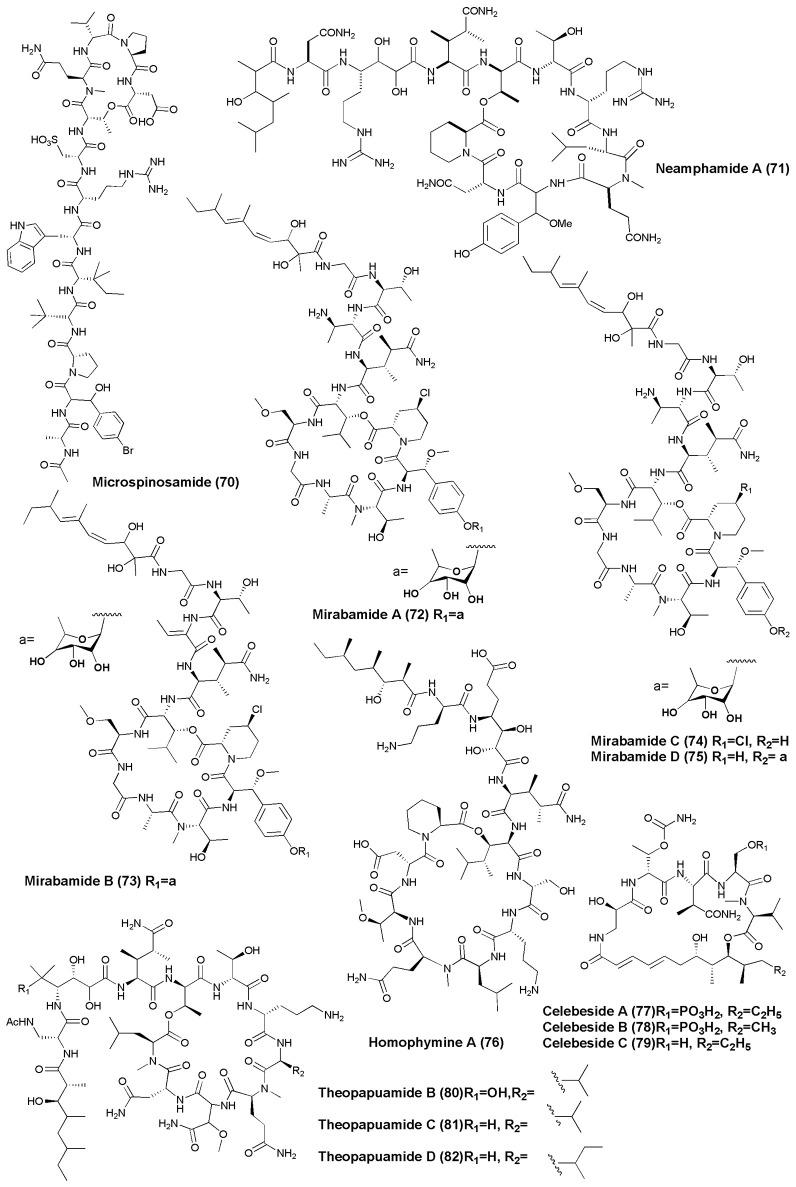
| Microspinosamide (70) | Cyclic depsipeptide | Sidonops microspinosa | HIV | 0.2 μg/mL | Rashid et al., 2001 |
| Neamphamide A (71) | Cyclic depsipeptide | Neamphius huxleyi | HIV | 8 nM | Oku et al., 2004 |
| Mirabamides A-D (72–75) | Cyclic depsipeptide | Siliquariaspongia mirabilis | HIV | 40 and 140 nM, 140 nM and 1.3 μM 190 nM and 3.9 μM |
Plaza et al., 2007 |
| Homophymine A (76) | Cyclodepsipeptide | Homophymia sp. | HIV/PBMC cell line | 75 nM | Zampella et al., 2008 |
| Celebeside A-C (77–79) | Depsipeptides | S. mirabilis | HIV/Colon carcinoma (HCT-116) cells |
2.1 and 4.0 μg/mL 1.9 ± 0.4 μg/mL |
Plaza et al., 2008 |
| Theopapuamides B–D (80–82) | |||||
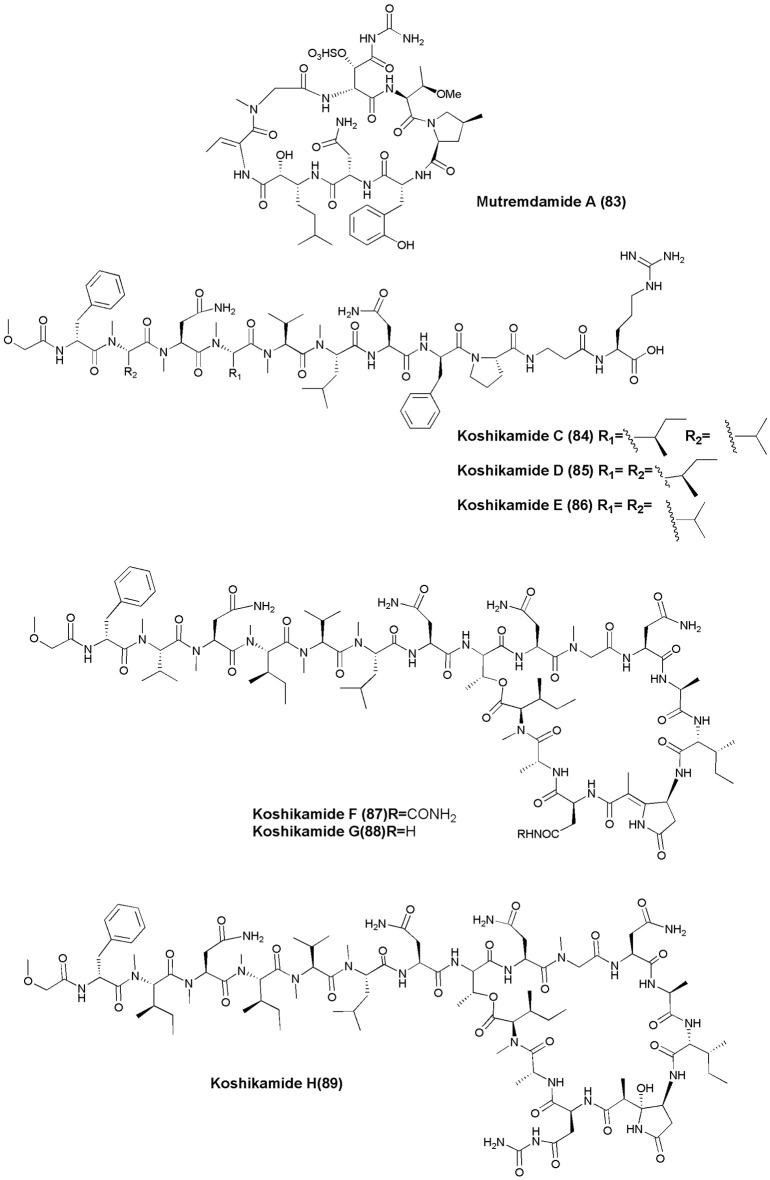
| Mutremdamide A (83) Koshikamides C-H (84–89) | Cyclic depsipeptide | Theonella sp. | HIV | 2.3 and 5.5 μM | Plaza et al., 2010 |
| Ceratospongamide (90) | Cyclic heptapeptide | Sigmadocia symbiotica | Inflammation | 32 nM | Tan et al., 2000 |
| Halipeptin A-B (91–92) | Cyclic depsipeptide | Haliclona sp. | Inflammation | 300 μg/kg (i.p.) | Randazzo et al., 2001 |
| Perthamide C-D (93–94) | Cyclopeptide | T. swinhoei | Inflammation | – | Festa et al., 2009 |
| Solomonamide A- B (95–96) | Cyclic peptide | T. swinhoei | Inflammation | – | Festa et al., 2011 |
| Stylissatin A (97) | Cyclic peptide | Stylissa massa | Murine macrophage RAW264.7 | 87 μM | Kita et al., 2013 |
| Dicynthaurin (98) | – | Halocynthia aurantium | Antimicrobial | – | Lee I. H. et al., 2001 |
| Nagahamide A (99) | Depsipeptide | T. swinhoei | Antibacterial | – | Okada et al., 2002 |
| Plicatamide (100) | Octapeptide | Styela plicata | Antimicrobial | – | Tincu et al., 2003 |
| Callipeltins F-I (101–104) | – | Latrunculia sp. | Antifungal/Candida albicans | 10−4 M | Sepe et al., 2006 |
| Callipeltins J-M (105–108) | – | Latrunculia sp. | Antifungal/C. albicans | 4−10 M | D'Auria et al., 2007 |
| Citronamides A- B (109–110) | – | Citronia astra | Antifungal/Saccharomyces cerevisiae | 8 μg/mL | Carroll et al., 2009 |
| Renieramide (111) | Cyclic tripeptide | Reniera sp. | – | – | Ciasullo et al., 2002 |
| Phoriospongins A- B (112–113) | Depsipeptide | Phoriospongia sp. and Callyspongia bilamellata | Nematocidal/Haemonchus contortus | 100, 194 μg/mL | Capon et al., 2002 |
Anticancer NRPs from marine sponges and tunicates (figures 1–6)
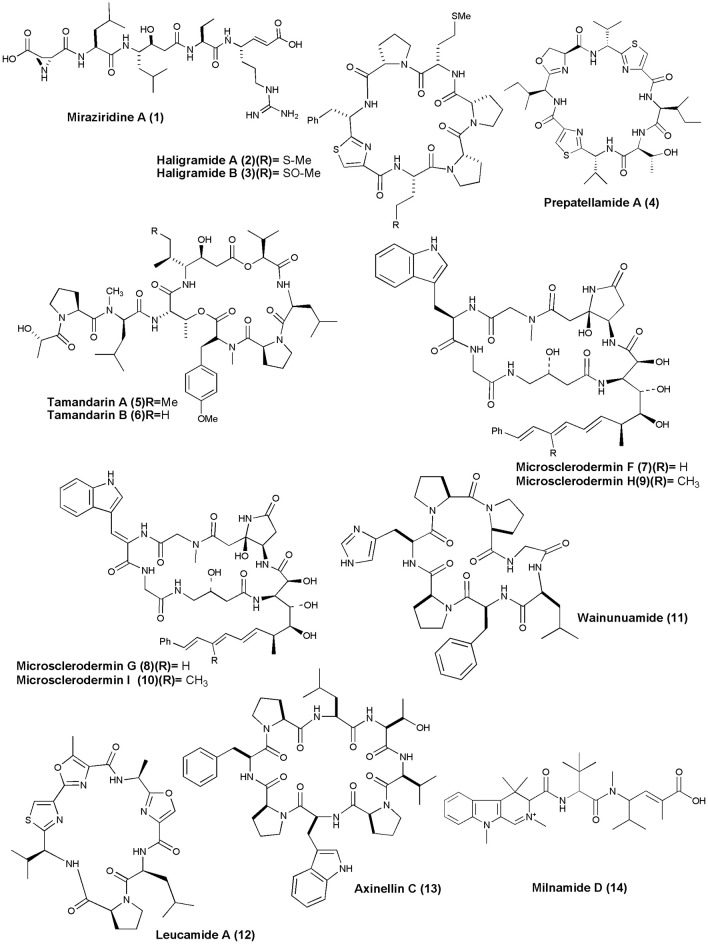
Figure 1.
Structures of anticancer non-ribosomal peptides (1–14).
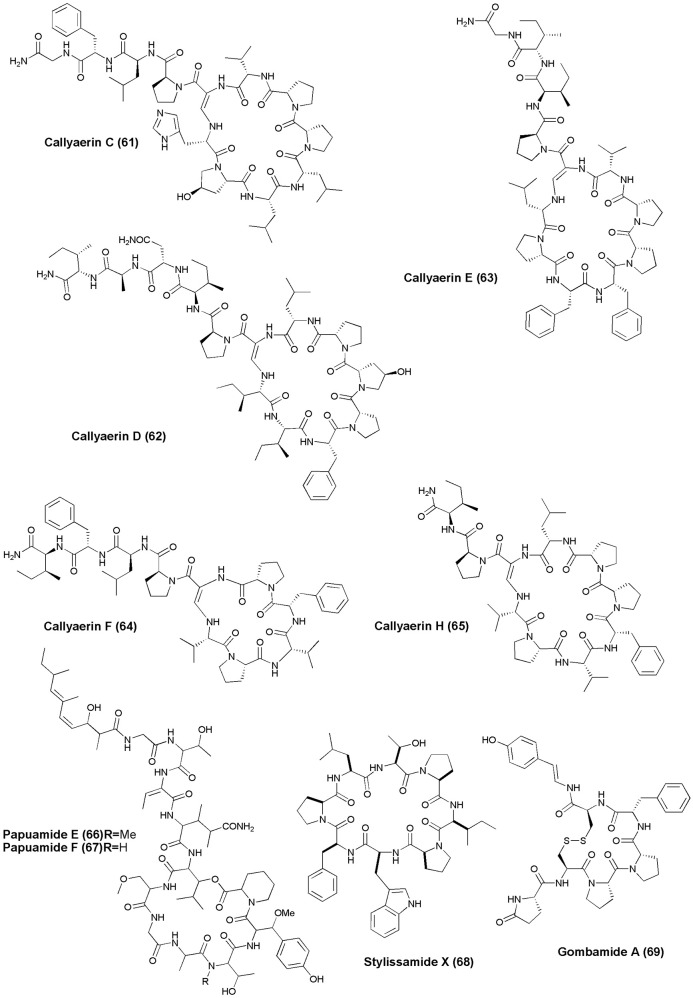
Figure 6.
Structures of anticancer non-ribosomal peptides (61–69).
Sponge Theonella aff. mirabilis was the source of linear penta peptide Miraziridine A (1), which showed inhibitory activity on cathepsin B with an IC50 value of 1.4 μg/mL (Nakao et al., 2000). Two cyclic hexapeptides, Haligramides A (2) and B (3), were isolated from an aqueous extract of the sponge H. nigra. Both compounds exhibited cytotoxicity against various cell lines. Haligramide A exhibited cytotoxicity against A-549, HCT-15, SF-539, SNB-19 cell line with IC50 values of 5.17, 15.62, 9.00, and 9.08 μg/mL, respectively. Haligramide B, was found to be more active than Haligramide A against A-549, HCT-15, SF-539, SNB-19 cell line with IC50 values of 3.89, 8.82, 5.01, and 6.56 μg/mL, respectively (Rashid et al., 2000).
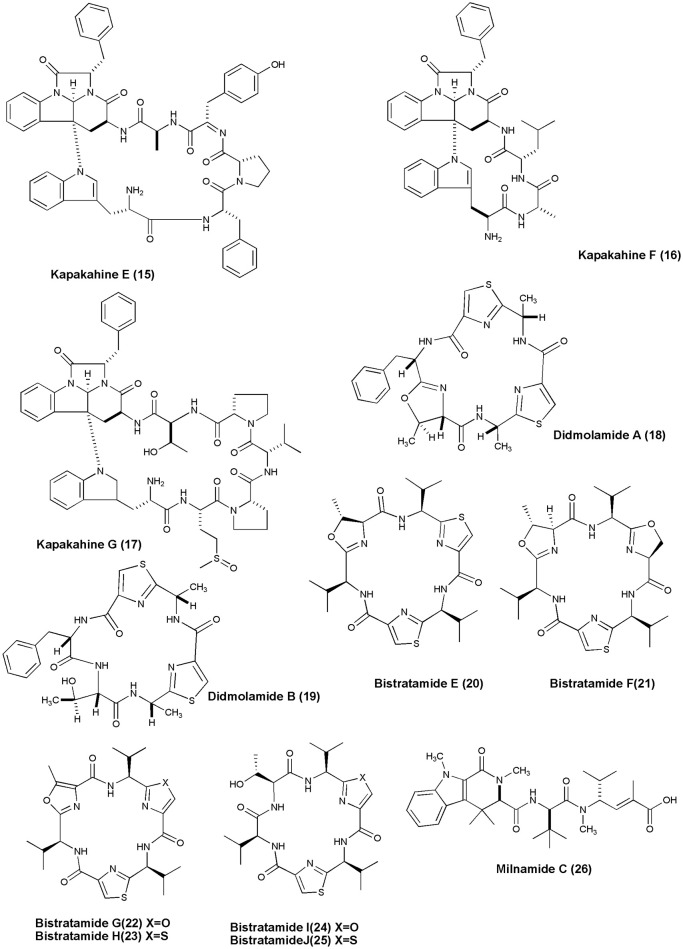
Figure 2.
Structures of anticancer non-ribosomal peptides (15–26).
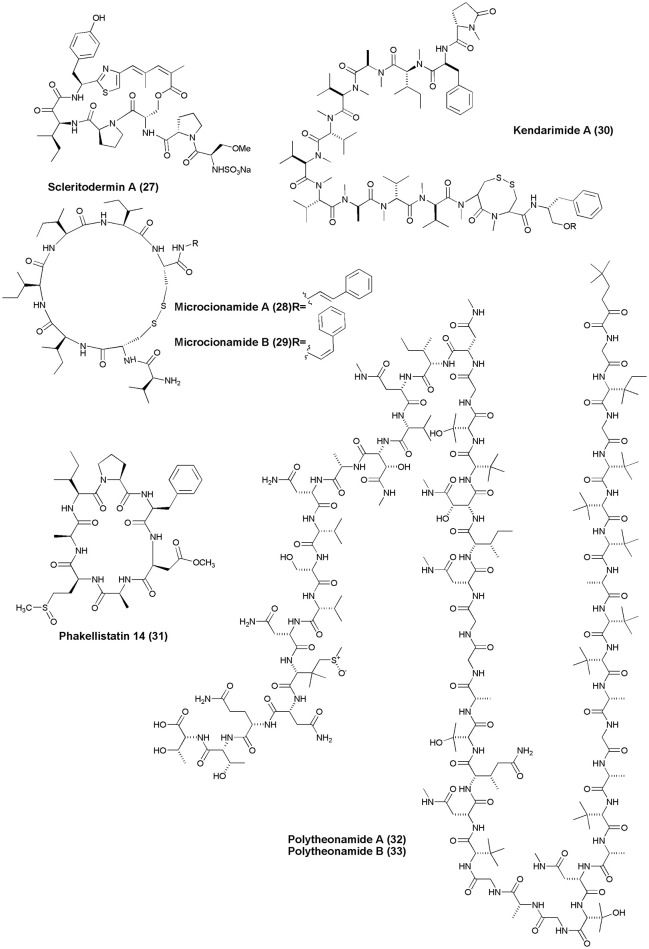
Figure 3.
Structures of anticancer non-ribosomal peptides (27–33).
Figure 4.
Structures of anticancer non-ribosomal peptides (34–42).
Figure 5.
Structures of anticancer non-ribosomal peptides (43–60).
A cyclic peptide, Prepatellamide A (4) was isolated from the cytotoxic extracts of L. patella. The crude extract of this ascidian showed cytotoxicity against P388 murine leukemia cell lines with IC50 = ~5 μg/mL (Fu et al., 2000). Naturally occurring depsipeptides, Tamandarins A and B (5 and 6) were discovered from a Brazilian ascidian of the family Didemnidae and were cytotoxic against various human cancer cell lines including pancreatic carcinoma BX-PC3, IC50 = 1.79, 2.00 μg/mL, prostatic cancer DU-145, IC50 = 1.36, 1.53 μg /mL, head and neck carcinoma UMSCC10b, IC50 = 0.99, 1.7 6 μg/mL, respectively (Vervoort et al., 2000). Microscleroderma sp. from Palau gave 4 new cyclic peptides, Microsclerodermins F–I (7–10), all of which inhibited the growth of C. albicans with MIC value of 1.5, 3, 12, and 25 μg per disk, respectively, and also showed cytotoxicity against the HCT-116 cell line with IC50 value of 1.8, 2.4, 1.0, and 1.1 μg/mL, respectively (Qureshi et al., 2000).
A histidine-containing proline-rich cyclic heptapeptide, Wainunuamide (11), was isolated from the Fijian marine sponge S. aurantium. Compound (11) exhibited weak cytotoxic activity against A2780 ovarian tumor and K562 leukemia cancer cells with ID50 of 19.15 and 18.36 μg/mL, respectively (Tabudravu et al., 2001). The Australian marine sponge L. microraphis was the source of a bioactive cyclic heptapeptide, Leucamide A (12). Compound (12) inhibited the growth of the 3 tumor cell lines HM02 (gastric, GI50 = 5.2 μg/mL), HepG2 (liver, GI50 = 5.9 μg/mL), and Huh7 (liver, GI50 = 5.1 μg/mL) (Kehraus et al., 2002). The Fijian collection of marine sponge S. aurantium gave a proline-rich cyclic octapeptide, Axinellin C (13) (cyclo [Thr1-Val2-Pro3-Trp4-Pro5-Phe6-Pro7-Leu8]). Axinellin C displayed weak cytotoxicity against A2780 ovarian tumor and K562 leukemia cancer cells with ID50s of 13.17 and 4.46 mg/mL, respectively (Tabudravu et al., 2002). The crude extract of a marine sponge Cymbastela sp. (Papua New Guinea) gave a cytotoxic peptide Milnamide D (14). Milnamide D was found to exhibit cytotoxicity against HCT-116 at IC50 value of 66 nM and inhibition of tubulin polymerization at IC50 of 16 μM (Chevallier et al., 2003).
Investigation of marine sponge C. olemda yielded 3 new Kapakahines E−G (15–17). Only kapakahines E was found to display moderate cytotoxicity against P388 murine leukemia cells at IC50 of 5.0 μg/mL (Nakao et al., 2003). Two novel cyclic hexapeptides, Didmolamides A-B (18 and 19) were isolated from ascidian D. molle (Madagascar). Both peptides showed mild cytotoxicity against tumor cell lines (Lung—A549, Colon—HT29, and Skin—MEL28) with IC50 values of 10–20 μg/mL (Rudi et al., 2003). The Philippines ascidian L. bistratum was the source of 6 cyclic hexapeptides, Bistratamides E-J (20–25) which showed weak to moderate activity against the human colon tumor (HCT-116) cell line (IC50's: 3, 7.9; 4, 28; 5, 5; 6, 1.7; 7, 9; 8, 1 μg/mL, respectively) (Perez and Faulkner, 2003). Milnamide C (26) was isolated from Auletta sp., which showed significant activity against MDA-MB-435 breast cancer cells with IC50 values of 3.2 × 10−1 μg/mL (Sonnenschein et al., 2004).
Scleritodermin A (27), a new cyclic peptide, was isolated from the Lithistid Sponge S. nodosum. Scleritodermin A, inhibited tubulin polymerization and demonstrated significant in vitro cytotoxicity against a panel of human tumor cell lines (IC50 < 2 μM), including colon (HCT116), ovarian (A2780), and breast carcinoma cell lines SKBR3 (Schmidt et al., 2004). Microcionamides A (28) and B (29) were isolated from the Philippine marine sponge Clathria (Thalysias) abietina. Both compounds displayed significant cytotoxicity toward human breast tumor cell lines MCF-7 and SKBR-3 with IC50 of 125 and 98 nM for compound (28) and 177 and 172 nM for compound (29), respectively (Davis et al., 2004). Methanol extract of an Indonesian marine sponge Haliclona sp. gave a linear peptide Kendarimide A (30) which reversed P-glycoprotein-mediated multi-drug resistance in mammalian cells (Aoki et al., 2004).
Cycloheptapeptide, Phakellistatin 14 (31), was isolated from Phakellia sp., (Chuuk, Federated States of Micronesia). Compound (31) exhibited cytotoxicity against the murine lymphocytic leukemia P388 cell line at ED50 of 5 μg/mL (Pettit and Tan, 2005). The marine sponge T. swinhoei was found to produce highly cytotoxic polypeptides Polytheonamides A and B (32–33) with 48 amino acid residues. Both compounds were found to be cytotoxic against P388 murine leukemia cells with IC50 values of 78, 68 pg/mL, respectively (Hamada et al., 2005). Two diastereomeric tricyclic peptides Neopetrosiamdes A (34) and B (35) have been isolated from the marine sponge Neopetrosia sp. collected in Papua New Guinea. These peptides inhibited amoeboid invasion of human tumor cells at 6 μg/mL (Williams et al., 2005). Six new depsipeptides, Seragamides A–F (36–41) were isolated from sponge S. japonicas (Okinawan). Except seragamide F, all seragamides have showed multinuclei formation in NBT-T2 cells at 0.01, 0.02, 0.01, 0.01, and 0.04 mg/mL, respectively. Compound (36) also promotes the polymerization of G-actin and stabilizes F-actin filaments (Tanaka et al., 2006). Theonella swinhoei from Papua New Guinea gave a cyclic depsipeptide, Theopapuamide (42). This peptide contains several unusual amino acid residues such as β-methoxyasparagine, 4-amino-5-methyl-2, 3, 5-trihydroxy-hexanoic acid, and also contains an amide linked fatty acid moiety, 3-hydroxy-2, 4, 6-trimethyl-octanoic acid (Htoa) with cytotoxicity against CEM-TART (EC50 = 0.5 μM) and HCT-116 (EC50 = 0.9 μM) cell lines (Ratnayake et al., 2006).
Azumamide A-E (43–47) carboxylic acid containing histone deacetylase (HDAC) inhibitor cyclotetrapeptides were recovered from the sponge M. izuensis. Only compound (47) displayed human histone deacetylase inhibitory activity (Maulucci et al., 2007). An Indonesian sponge C. aerizusa gave a new cyclic peptide named Callyaerin G (48) with cytotoxicity against mouse lymphoma cell line (L5178Y) and HeLa cells with ED50(s) of 0.53 and 5.4 ug/mL, respectively (Ibrahim et al., 2008). The Papua New Guinea marine sponge Stylotella sp. was found to produce a new proline-rich cyclodecapeptide, Stylopeptide 2 (49) which inhibited the growth of BT-549 and HS 578T 2 breast cancer cell lines by 77 and 56%, respectively (Brennan et al., 2008). Bioactive lipopeptides Ciliatamides A-C (50–52) were isolated from the deep-sea sponge A. ciliate. Ciliatamides A-B have showed anti-leishmanial activity at 10 μg/mL with 50 and 45.5% growth inhibition, respectively. Ciliatamides A-C also inhibited growth of HeLa cells with IC50 values of 50, 4.5, and 50 μg/mL, respectively (Nakao et al., 2008).
The marine ascidian Diazona sp. (Indonesia) gave 3 new macrocyclic peptides, Diazonamides C–E (53–55). All the isolated peptides displayed moderate cytotoxicity against a panel of 3 human tumor cell lines (IC50's: A549 = 2.2, 2.9, 8.0 μg/mL; HT29 = 1.8, 2.9, 5.2 μg/mL; MDA-MB-231 = 2.2, 3.1, 9.0 μg/mL) (Fernández et al., 2008). Dominican marine sponge E. laughlini gave 2 cyclic heptapeptides, Rolloamides A (56) and B (57). Rolloamide A displayed significant growth suppression against several cancer cells (prostate, breast, ovarian, glioma, and renal) with IC50's of 0.4–5.8 μM (Williams et al., 2009). Proline-containing cycloheptapeptide, Euryjanicin A (58) was extracted from the marine sponge P. laughlini (Vicente et al., 2009). Bioassay guided extraction of the sponge C. aerizusa (Ambon, Indonesia) revealed 7 new cytotoxic cyclic peptides Callyaerins A–F (59–64) and H (65). All peptides have showed cytotoxicity, however, callyaerins E and H exhibited strong activity against the L5178Y lymphoma cell line with ED50 values of 0.39 and 0.48 μM, respectively (Ibrahim et al., 2010).
An undescribed sponge of the genus Melophlus sp. (Karumolum, Russell Is., Solomon Is.) yielded 2 depsipeptides, Papuamides E (66) and F (67), which were cytotoxic against brine shrimp with LD50 values between 92 and 106 μg/mL (Prasad et al., 2011). A proline-rich octapeptide Stylissamide X (68) isolated from an Indonesian marine sponge of Stylissa sp. inhibited HeLa cells in the concentration range 0.1–10 μM through wound-healing assay (Arai et al., 2012). The marine sponge C. gombawuiensis collected from Korean waters gave a disulphide-linked hexapeptide, Gombamide A (69). Gombamide A showed weak cytotoxic activity against the K562 and A549 cell lines with LC50 values of 6.9 and 7.1 μM, respectively, as well as moderate inhibitory activity against Na+/K+-ATPase with an LC50 value of 17.8 μM (Woo et al., 2013).
Anti-HIV agents (figures 7, 8)
Figure 7.
Structures of non-ribosomal peptides with anti-HIV activity (70–82).
Figure 8.
Structures of non-ribosomal peptides with anti-HIV activity (83–89).
The marine sponge S. microspinosa was the source of a cyclic depsipeptide Microspinosamide (70), inhibited HIV-1 infection with an EC50 value of approximately 0.2 μg/mL (Rashid et al., 2001). A Papua New Guinea collection of the marine sponge N. huxleyi has been shown to produce a new HIV-inhibitory cyclic depsipeptide, Neamphamide A (71). Neamphamide A displayed potent cytoprotective activity against HIV-1 infection with EC50 value ~28 nM (Oku et al., 2004). Four cyclic depsipeptides, Mirabamides A–D (72–75), were isolated from the marine sponge S. mirabilis. Mirabamides A, C and D inhibited HIV-1 fusion (Mirabamides A IC50 values between 40 and 140 nM: Mirabamides C IC50 values between 140 nM and 1.3 μM: and Mirabamides D IC50 values between 190 nM and 3.9 μM). Mirabamides A–C also inhibited the growth of B. subtilis and C. albicans at 1–5 μg/disk (Plaza et al., 2007).
The marine sponge Homophymia sp. was the source of an anti-HIV cyclodepsipeptide, Homophymine A (76). This peptide inhibited the infection of HIV-1 in PBMC cell line with an IC50 of 75 nM (Zampella et al., 2008). Six depsipeptides Celebesides A-C (77–79) and Theopapuamides B-D (80–82) were isolated from an Indonesian sponge S. mirabilis. Compound (77) neutralized HIV-1 with an IC50 value of 1.9 ± 0.4 μg/mL, while the non-phosphorylated analog Celebeside C was inactive at concentrations as high as 50 μg/mL. Theopapuamides A-C displayed cytotoxicity against human colon carcinoma (HCT-116) cells with IC50 values between 2.1 and 4.0 μg/mL, and antifungal activity against wild type and amphotericin B-resistant strains of C. albicans at 1–5 μg/disk (Plaza et al., 2008). The deep-water specimens of T. swinhoei and Theonella cupola (Mutremdiu Reef, Palau) gave sulphated cyclic depsipeptide, Mutremdamide A (83) and 6 N-methylated peptides Koshikamides C–H (84–89). Cyclic koshikamides F and H inhibited HIV-1 entry at IC50 values of 2.3 and 5.5 μM, respectively, while their linear counterparts were inactive (Plaza et al., 2010).
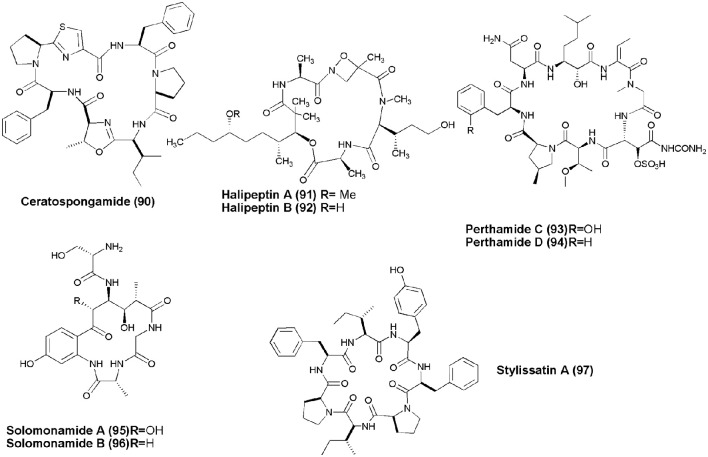
Anti-inflammatory NRPs (figure 9)
Figure 9.
Structures of non-ribosomal peptides with anti-inflammatory activity (90–97).
Marine sponge S. symbiotica collected from Biaro Island, Indonesia, alongwith its symbiont marine red alga (Rhodophyta) Ceratodictyon spongiosum gave thiazole-containing cyclic heptapeptide, Ceratospongamide (90). Compound (90) consists of two l-phenylalanine residues, one (l-isoleucine)-l-methyloxazoline residue, one l-proline residue, and one (l-proline) thiazole residue. The trans-isomer of ceratospongamide exhibits potent inhibition of sPLA2 expression in a cell-based model for anti-inflammation at ED50 32 nM (Tan et al., 2000). Two cyclic depsipeptides, Halipeptins A and B (91,92) were obtained from marine sponge Haliclona sp. Only halipeptins A displayed in vivo potent anti-inflammatory activity (mice at the dose of 300 μg/kg [i.p.]) (Randazzo et al., 2001). A Solomon Lithistid sponge T. swinhoei was the source of 2 new cyclopeptides Perthamides C and D with potent anti-inflammatory (93,94) (Festa et al., 2009). Cyclic peptides, Solomonamides A and B (95,96), were separated out from the marine sponge T. swinhoei; however, only compound (86) showed anti-inflammatory activity (Festa et al., 2011). The marine sponge S. massa produced a cyclic peptide Stylissatin A (97) that inhibited nitric oxide production in LPS-stimulated murine macrophage RAW264.7 cells with an IC50 value of 87 μM (Kita et al., 2013).
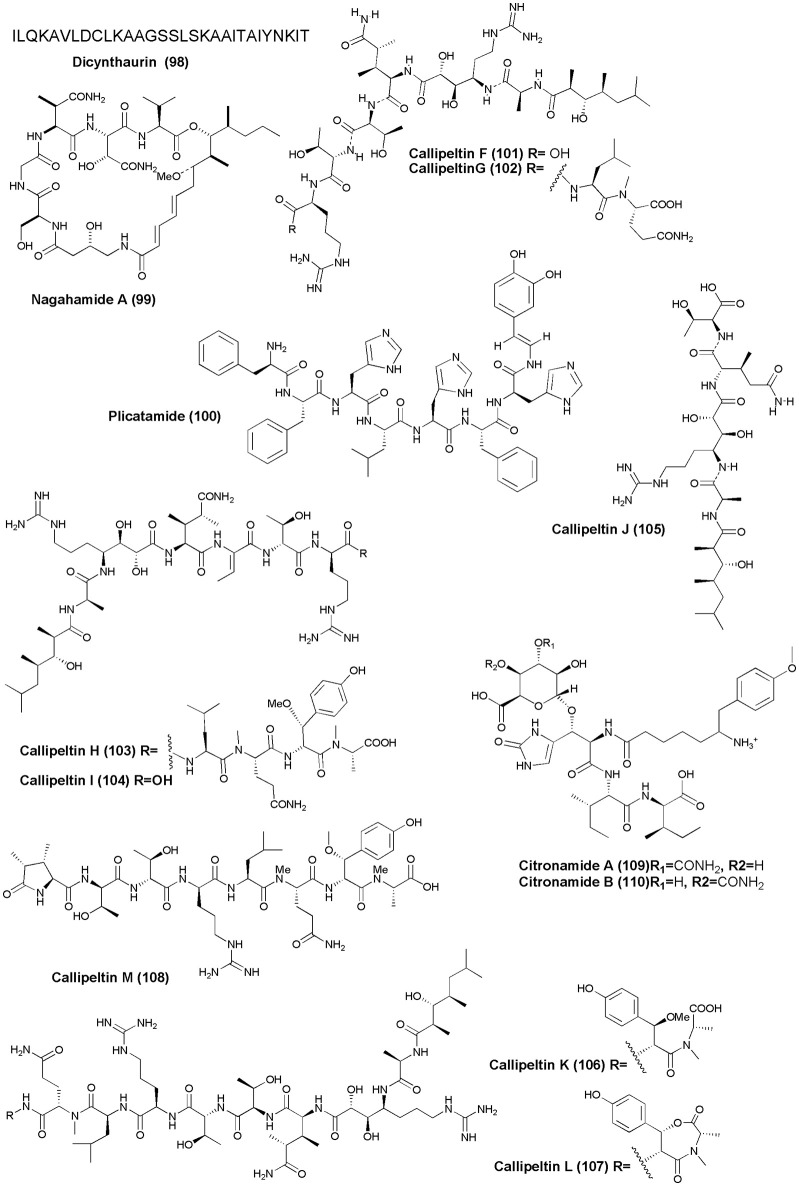
Antimicrobial agents (figure 10)
Figure 10.
Structures of antimicrobial non-ribosomal peptides (98–110).
The solitary tunicate, H. aurantium, was the source of a novel antimicrobial peptide Dicynthaurin (98) (Lee I. H. et al., 2001). An antibacterial depsipeptide, Nagahamide A (99), was discovered from the marine sponge T. swinhoei (Okada et al., 2002). An antimicrobial octapeptide Plicatamide (100) was isolated from S. plicata (Tincu et al., 2003). The marine sponge Latrunculia sp., (Vanuatu Islands) was the source of four new antifungal peptides, Callipeltins F–I (101–104). Callipeltins F–I inhibit the growth of C. albicans (ATCC24433) with a MIC value of 10−4 M (Sepe et al., 2006). Four new peptides, Callipeltins J–M (105–108), were isolated from the marine sponge Latrunculia sp. However, only Callipeltins J and K inhibited the growth of C. albicans with MIC values of ca. 4−10 M (D'Auria et al., 2007). Two new linear tetrapeptides, Citronamides A (109) and B (110), were isolated from the Australian sponge C. astra. Only citronamides A showed moderate antifungal activity against Saccharomyces cerevisiae at MIC value of 8 μg/mL (Carroll et al., 2009).
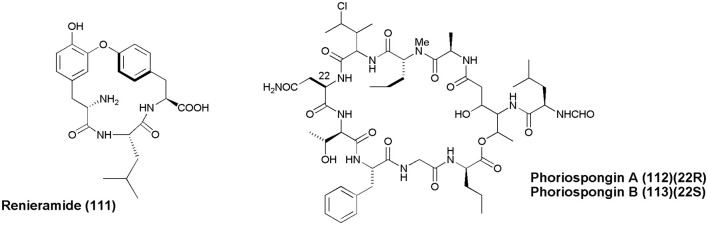
Miscellaneous (figure 11)
Figure 11.
Structures of non-ribosomal peptides with (111–113).
A cyclic tripeptide Renieramide (111) was isolated from Vanuatu collection of sponge Reniera sp. that showed immunomodulating activity in preliminary tests (Ciasullo et al., 2002). Two nematocidal depsipeptides, Phoriospongin A and B (112 and 113), were isolated from Australian marine sponges Phoriospongia sp. and C. bilamellata. Both compounds have displayed significant nematocidal activity against H. contortus (LD99 = 100, 194 μg/mL, respectively) (Capon et al., 2002).
Biological aspects, challenges, and future perspectives
Like their structural diversity, metabolites produced from marine sponges and tunicates bind to a variety of cellular targets to elicit their effects. Numerous articles published in recent years highlighting the significance of these metabolites in disease control, the details of their biological significance from molecular recognition perspective have been rather scarce. Although some promising leads have been obtained, the discovery of their cellular targets, molecular interactions, and adverse effects are lacking. In cases where the therapeutic potential has been reported, details of a proper screening approach to identify nucleic acid or protein targets are missing. However, some established metabolites from these sources (see Tables 1, 2) and their derivatives have been examined extensively and their molecular targets are varied. One of the earliest examples in this class is FDA-approved drug Ara-C (cytarabine), which is known to elicit anticancer properties by inhibiting the functions of DNA polymerase (Furth and Cohen, 1968), which ultimately results in stalling DNA synthesis. Another FDA approved related compound Ara-A(vidarabine), which is known to have antiviral properties (active against herpes simplex and varicella zoster viruses), targets viral DNA polymerase (Chadwick et al., 1978) by functioning as mimic of natural nucleotides. Both Ara-C and Ara-A resemble natural cytidine and adenine nucleosides where the structural differences are in the sugar components of the two (arabinose vs. deoxyribose). The natural nucleoside mimics Ara-A and Ara-C are easily phosphorylated as their triphosphate derivatives by kinases and act as terminators of DNA synthesis. Ara-A is also known to impede 3′-end processing of pre-mRNAs by inhibiting cleavage and polyadenylation (Ghoshal and Jacob, 1991; Rose and Jacob, 1978).
Several other molecules that are either FDA approved or in early stages of clinical trials have been identified as anticancer agents with microtubules as their primary molecular targets. The predominance of natural metabolites being microtubule binding agents has been hypothesized as evolutionary response to predation by plants and animals (Dumontet and Jordan, 2010). Some of these molecules, such as discodermolide, are among the first non-taxane stabilizers of microtubules (Mooberry et al., 2004). The microtubule stabilizers act by enhancing microtubule polymerization at high concentrations. Discodermolide has been known to bind to tubulin dimers in a stoichiometric ratio. Competitive binding experiments have shown that it blocks taxol binding and is a much stronger binder of microtubules than taxol (Kowalski et al., 1997). The microtubule binding of Tau proteins is interfered by discodermolide (Kar et al., 2003). Similarly, laulimalide showed properties very similar to paclitaxel where it helped in enhancing tubulin assembly (Gapud et al., 2004). However, laulimalide modulation of microtubule assembly in C. elegans is dose dependent where it stabilization effects were observed only at concentrations higher than 100 nM (Bajaj and Srayko, 2013).
The antiviral effect of homophymine A has been established by measuring the reverse transcriptase activity in HIV-infected primary peripheral blood mononuclear cells (Zampella et al., 2008). The reverse transcriptase activity is exhibited by 2 classes of molecules: one that directly competes with natural nucleotide triphosphates and the other that either directly blocks the catalytic reactions or by allosteric binding that leads to structural changes in the viral enzyme. Since homophymine A lacks structural features to act as mimics of natural nucleotide triphosphates, it is likely to impede the catalytic activity of the enzyme by direct binding.
A tunicate-derived metabolite trabectedin (ET-743) uses DNA binding to exert its anticancer properties. Trabectin binds to the GC rich regions in the B-DNA where it uses its carbolinamine moiety to form adduct with the exocylic amine (N-2) of guanine (Pommier et al., 1996) and covers 3 base pairs during this process (Marco et al., 2006). Unlike B-DNA minor groove binders, such as Hoechst 33258, which binds snugly along the minor groove curvature with high-affinity (Haq et al., 1997), trabectedin only uses part of its structure to make necessary contacts for the antitumor action (D'Incalci and Galmarini, 2010).
Despite these advances in determining the mode of their binding, a large number of recently discovered metabolites are still not explored to assess it functional capabilities. In the past, well-known anti-retroviral drug zidovudine, which was initially thought to be functionally inert, turned out as excellent therapeutic agent. Such discoveries are possible only when a rational screening design is aimed to asses it full potential as a drug. For example, compounds that have structural regions favorable for protein binding should be screened against all potential protein targets. Similarly, compounds that show preference toward nucleic acid binding should be screened using assays such as competition dialysis that establish a preferential nucleic acid target. Such approaches not only determine the best target for a particular compound but also shed light to its secondary targets, which may be helpful in dealing with toxicity issues. Current target design of marine and tunicate metabolites clearly need to take these approaches.
Some of the metabolites that have weaker binding to a target or have poor bioavailability can be improved by nano-encapsulation techniques. Additionally, DNA binding metabolites can be chemically modified to enhance their affinity using multi-recognition of the target (Willis and Arya, 2010), which has led to remarkable enhancement in the affinity of double,(Arya et al., 2003), triple (Arya and Willis, 2003), and four-stranded DNA helical structures (Ranjan et al., 2013).
Conclusion
Extreme environment of the ocean plays a vital role in exploring and studying marine bio-resources and their bio-actives. The large biodiversity of the sea serves as a huge resource for developing potential drugs with promising pharmacological activities. The significance of marine-derived secondary metabolites has recently been highlighted by introduction of Prialt and Yondelis to the market. In the past three decades, numerous NRPs with unique chemical structures and varied biological activities have been discovered from marine sponges and tunicates as described in this. Some of these exhibit strong potential to be developed as a new drug. However, none of the NRPs highlighted in this review have been successfully marketed as therapeutics. To translate bioactivity of these important metabolites into therapeutically significant outcomes, it is crucial to further unravel their modes of action and measure their toxicity. Since the majority of these studies have been focused on in vitro bioassays and elucidation of the chemical structures only, a complete examination of their biological target selectivity is required. Nevertheless, large-scale production of these NRPs for clinical use is a real challenge. Therefore, environmentally sound and economically feasible alternatives are required. To counter these challenges, many strategies have been established.
Chemical synthesis of NRPs is among the first strategies to be used. However, the structural complexity limits its chemical synthesis and has resulted in only a few successful achievements (e.g., analgesic drug ziconotide; Olivera, 2000). A second strategy uses screening the pharmacological significance of NRPs and subsequently attempting to define the critical pharmacophore that can result in practical drugs based on a marine prototype via chemical synthesis, degradation, modification, or a combination of these. Aquaculture of the source organisms has also been used to secure a sustainable supply of active compounds. However, in most cases, the biomass currently generated is still far from the requirement from an industrial perspective (Mendola, 2000). Identification and large-scale culturing of true producers that are known to thrive within the tissues of marine invertebrates (sponge or tunicate) is an intriguing strategy. However, to date only 5% or less of the symbiotic microbes present in marine specimens can be cultivated under standard conditions. Consequently, molecular approaches such as transfer of biosynthetic gene clusters to a vector suitable for large-scale fermentation could be used to avoid obstacles in culturing symbiotic bacteria. Enzyme technology and solid-phase peptide synthesis offer particularly promising alternatives to generate variety of unique peptides using native peptide as a template. Besides, combinations of chemical synthesis and biosynthetic technologies have potential to accelerate the discovery of novel drugs derived from sponge and their microbial association in future.
Author contributions
SD reviewed the collected information critically. SA collected the relevant information from various sources including databases like Scifider. AA gave the concept of the work.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abraham E., Newton G. (1961). The structure of cephalosporin C. Biochem. J. 79:377. 10.1042/bj0790377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S., Cao L., Matsui K., Rachmat R., Akiyama S.-I., Kobayashi M. (2004). Kendarimide A, a novel peptide reversing P-glycoprotein-mediated multidrug resistance in tumor cells, from a marine sponge of Haliclona sp. Tetrahedron 60, 7053–7059. 10.1016/j.tet.2003.07.020 [DOI] [Google Scholar]
- Arai M., Yamano Y., Fujita M., Setiawan A., Kobayashi M. (2012). Stylissamide X, a new proline-rich cyclic octapeptide as an inhibitor of cell migration, from an Indonesian marine sponge of Stylissa sp. Bioorg. Med. Chem. Lett. 22, 1818–1821. 10.1016/j.bmcl.2011.10.023 [DOI] [PubMed] [Google Scholar]
- Arya D. P., Willis B. (2003). Reaching into the major groove of B-DNA: synthesis and nucleic acid binding of a Neomycin-Hoechst 33258 conjugate. J. Am. Chem. Soc. 125, 12398–12399. 10.1021/ja036742k [DOI] [PubMed] [Google Scholar]
- Arya D. P., Xue L., Tennant P. (2003). Combining the best in triplex recognition: synthesis and nucleic acid binding of a BQQ-Neomycin conjugate. J. Am. Chem. Soc. 125, 8070–8071. 10.1021/ja034241t [DOI] [PubMed] [Google Scholar]
- Bajaj M., Srayko M. (2013). Laulimalide induces dose-dependent modulation of microtubule behaviour in the C. elegans embryo. PLoS ONE 8:e71889. 10.1371/journal.pone.0071889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley C., Holland N., Faulkner D. (1996). Two classes of metabolites from Theonella swinhoei are localized in distinct populations of bacterial symbionts. Experientia 52, 716–722. 10.1007/BF01925581 [DOI] [PubMed] [Google Scholar]
- Brennan M. R., Costello C. E., Maleknia S. D., Pettit G. R., Erickson K. L. (2008). Stylopeptide 2, a proline-rich cyclodecapeptide from the sponge Stylotella sp. J. Nat. Prod. 71, 453–456. 10.1021/np0704856 [DOI] [PubMed] [Google Scholar]
- Brusca R. C., Brusca G. J., Haver N. J. (1990). Invertebrates. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Bultel-Poncé V., Berge J.-P., Debitus C., Nicolas J.-L., Guyot M. (1999). Metabolites from the sponge-associated bacterium Pseudomonas species. Mar. Biotechnol. 1, 384–390. 10.1007/PL00011792 [DOI] [PubMed] [Google Scholar]
- Bultel-Poncé V., Debitus C., Berge J.-P., Cerceau C., Guyot M. (1998). Metabolites from the sponge-associated bacterium Micrococcus luteus. J. Mar. Biotechnol. 6, 233–236. [PubMed] [Google Scholar]
- Capon R. J., Ford J., Lacey E., Gill J. H., Heiland K., Friedel T. (2002). Phoriospongin A and B: two new nematocidal depsipeptides from the Australian marine sponges Phoriospongia sp. and Callyspongia bilamellata. J. Nat. Prod. 65, 358–363. 10.1021/np010329d [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Duffy S., Avery V. M. (2009). Citronamides A and B, tetrapeptides from the australian sponge Citronia astra. J. Nat. Prod. 72, 764–768. 10.1021/np800832w [DOI] [PubMed] [Google Scholar]
- Chadwick R. G., Bassendine M. F., Crawford E. M., Thomas H. C., Sherlock S. (1978). Hbsag-positive chronic liver disease: inhibition of DNA polymerase activity by vidarabine. Br. Med. J. 2, 531–533. 10.1136/bmj.2.6136.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C., Richardson A. D., Edler M. C., Hamel E., Harper M. K., Ireland C. M. (2003). A new cytotoxic and tubulin-interactive milnamide derivative from a marine sponge Cymbastela sp. Org. Lett. 5, 3737–3739. 10.1021/ol035476c [DOI] [PubMed] [Google Scholar]
- Ciasullo L., Casapullo A., Cutignano A., Bifulco G., Debitus C., Hooper J., et al. (2002). Renieramide, a cyclic tripeptide from the Vanuatu sponge Reniera n. sp. J. Nat. Prod. 65, 407–410. 10.1021/np010383u [DOI] [PubMed] [Google Scholar]
- D'Auria M. V., Sepe V., D'Orsi R., Bellotta F., Debitus C., Zampella A. (2007). Isolation and structural elucidation of callipeltins J–M: antifungal peptides from the marine sponge Latrunculia sp. Tetrahedron 63, 131–140. 10.1016/j.tet.2006.10.032 [DOI] [Google Scholar]
- Davis R. A., Mangalindan G. C., Bojo Z. P., Antemano R. R., Rodriguez N. O., Concepcion G. P., et al. (2004). Microcionamides A and B, bioactive peptides from the Philippine sponge Clathria (Thalysias) abietina. J. Org. Chem. 69, 4170–4176. 10.1021/jo040129h [DOI] [PubMed] [Google Scholar]
- de Crécy-Lagard V., Blanc V., Gil P., Naudin L., Lorenzon S., Famechon A., et al. (1997). Pristinamycin I biosynthesis in Streptomyces pristinaespiralis: molecular characterization of the first two structural peptide synthetase genes. J. Bacteriol. 179, 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incalci M., Galmarini C. M. (2010). A review of trabectedin (ET-743): a unique mechanism of action. Mol. Cancer Therap. 9, 2157–2163. 10.1158/1535-7163.MCT-10-0263 [DOI] [PubMed] [Google Scholar]
- Domenech O., Francius G., Tulkens P. M., Van Bambeke F., Dufrêne Y., Mingeot-Leclercq M.-P. (2009). Interactions of oritavancin, a new lipoglycopeptide derived from vancomycin, with phospholipid bilayers: effect on membrane permeability and nanoscale lipid membrane organization. Biochim. Biophys. Acta 1788, 1832–1840. 10.1016/j.bbamem.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Dumontet C., Jordan M. A. (2010). Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 9, 790–803. 10.1038/nrd3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyakov G., Kuznetsova T., Mikhailov V., Maltsev I., Voinov V., Fedoreyev S. (1991). Brominated diphenyl ethers from a marine bacterium associated with the sponge Dysidea sp. Experientia 47, 632–633. 10.1007/BF01949894 [DOI] [Google Scholar]
- Fenical W. (1976). Geranyl hydroquinone, a cancer-protective agent from the tunicate Aplidium species. Food Drugs Sea Proc. 4, 388–394. [Google Scholar]
- Fernández R., Martín M. J., Rodríguez-Acebes R., Reyes F., Francesch A., Cuevas C. (2008). Diazonamides C–E, new cytotoxic metabolites from the ascidian Diazona sp. Tetrahedron Lett. 49, 2283–2285. 10.1016/j.tetlet.2008.02.012 [DOI] [Google Scholar]
- Festa C., De Marino S., Sepe V., D'Auria M. V., Bifulco G., Débitus C., et al. (2011). Solomonamides A and B, new anti-inflammatory peptides from Theonella swinhoei. Org. Lett. 13, 1532–1535. 10.1021/ol200221n [DOI] [PubMed] [Google Scholar]
- Festa C., De Marino S., Sepe V., Monti M. C., Luciano P., D'auria M. V., et al. (2009). Perthamides C and D, two new potent anti-inflammatory cyclopeptides from a Solomon Lithistid sponge Theonella swinhoei. Tetrahedron 65, 10424–10429. 10.1016/j.tet.2009.10.026 [DOI] [Google Scholar]
- Finking R., Marahiel M. A. (2004). Biosynthesis of nonribosomal peptides1. Annu. Rev. Microbiol. 58, 453–488. 10.1146/annurev.micro.58.030603.123615 [DOI] [PubMed] [Google Scholar]
- Fu X., Su J., Zeng L. (2000). Prepatellamide A, a new cyclic peptide from the ascidian Lissoclinum patella. Sci. China Ser. B Chem. 43, 643–648. 10.1007/BF02969512 [DOI] [Google Scholar]
- Furth J. J., Cohen S. S. (1968). Inhibition of mammalian DNA polymerase by the 5′-triphosphate of 1-β-d-arabinofuranosylcytosine and the 5′-triphosphate of 9-β-d-arabinofuranosyladenine. Cancer Res. 28, 2061–2067. [PubMed] [Google Scholar]
- Gapud E. J., Bai R., Ghosh A. K., Hamel E. (2004). Laulimalide and paclitaxel: a comparison of their effects on tubulin assembly and their synergistic action when present simultaneously. Mol. Pharmacol. 66, 113–121. 10.1124/mol.66.1.113 [DOI] [PubMed] [Google Scholar]
- Ghoshal K., Jacob S. T. (1991). Ara-ATP impairs 3′-end processing of pre-mRNAs by inhibiting both cleavage and polyadenylation. Nucleic Acids Res. 19, 5871–5875. 10.1093/nar/19.21.5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T., Matsunaga S., Yano G., Fusetani N. (2005). Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J. Am. Chem. Soc. 127, 110–118. 10.1021/ja045749e [DOI] [PubMed] [Google Scholar]
- Haq I., Ladbury J. E., Chowdhry B. Z., Jenkins T. C., Chaires J. B. (1997). Specific binding of Hoechst 33258 to the d(CGCAAATTTGCG)2 duplex: calorimetric and spectroscopic studies1. J. Mol. Biol. 271, 244–257. [DOI] [PubMed] [Google Scholar]
- Hentschel U., Hopke J., Horn M., Friedrich A. B., Wagner M., Hacker J., et al. (2002). Molecular evidence for a uniform microbial community in sponges from different oceans. Appl. Environ. Microbiol. 68, 4431–4440. 10.1128/AEM.68.9.4431-4440.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel U., Schmid M., Wagner M., Fieseler L., Gernert C., Hacker J. (2001). Isolation and phylogenetic analysis of bacteria with antimicrobial activities from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. FEMS Microbiol. Ecol. 35, 305–312. 10.1111/j.1574-6941.2001.tb00816.x [DOI] [PubMed] [Google Scholar]
- Ibrahim S. R., Edrada-Ebel R., Mohamed G. A., Youssef D. T., Wray V., Proksch P. (2008). Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. ARKIVOC 12, 164–171. 10.3998/ark.5550190.0009.c18 [DOI] [Google Scholar]
- Ibrahim S. R., Min C. C., Teuscher F., Ebel R., Kakoschke C., Lin W., et al. (2010). Callyaerins A–F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg. Med. Chem. 18, 4947–4956. 10.1016/j.bmc.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Johnson B. A., Anker H., and Meleney, F. L. (1945). Bacitracin: a new antibiotic produced by a member of the B. subtilis group. Science 102, 376–377. 10.1126/science.102.2650.376 [DOI] [PubMed] [Google Scholar]
- Kar S., Florence G. J., Paterson I., Amos L. A. (2003). Discodermolide interferes with the binding of tau protein to microtubules. FEBS Lett. 539, 34–36. 10.1016/S0014-5793(03)00181-9 [DOI] [PubMed] [Google Scholar]
- Kehraus S., König G. M., Wright A. D., Woerheide G. (2002). Leucamide A: a new cytotoxic heptapeptide from the Australian sponge Leucetta microraphis. J. Org. Chem. 67, 4989–4992. 10.1021/jo020058r [DOI] [PubMed] [Google Scholar]
- Kita M., Gise B., Kawamura A., Kigoshi H. (2013). Stylissatin A, a cyclic peptide that inhibits nitric oxide production from the marine sponge Stylissa massa. Tetrahedron Lett. 54, 6826–6828. 10.1016/j.tetlet.2013.10.003 [DOI] [Google Scholar]
- Kleinkauf H., von Döhren H. (1995). The nonribosomal peptide biosynthetic system—on the origins of structural diversity of peptides, cyclopeptides and related compounds. Antonie Van Leeuwenhoek 67, 229–242. 10.1007/BF00873687 [DOI] [PubMed] [Google Scholar]
- Kowalski R. J., Giannakakou P., Gunasekera S. P., Longley R. E., Day B. W., Hamel E. (1997). The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol. Pharmacol. 52, 613–622. [PubMed] [Google Scholar]
- Laport M., Santos O., Muricy G. (2009). Marine sponges: potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 10, 86–105. 10.2174/138920109787048625 [DOI] [PubMed] [Google Scholar]
- Lee I. H., Lee Y. S., Kim C. H., Kim C. R., Hong T., Menzel L., et al. (2001). Dicynthaurin: an antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. Biochim. Biophys. Acta 1527, 141–148. 10.1016/S0304-4165(01)00156-8 [DOI] [PubMed] [Google Scholar]
- Lee Y. K., Lee J.-H., Lee H. K. (2001). Microbial symbiosis in marine sponges. J. Microbiol. 39, 254–264. [Google Scholar]
- Marco E., David-Cordonnier M.-H., Bailly C., Cuevas C., Gago F. (2006). Further insight into the DNA recognition mechanism of trabectedin from the differential affinity of its demethylated analogue ecteinascidin ET729 for the triplet DNA binding site CGA. J. Med. Chem. 49, 6925–6929. 10.1021/jm060640y [DOI] [PubMed] [Google Scholar]
- Maulucci N., Chini M. G., Di Micco S., Izzo I., Cafaro E., Russo A., et al. (2007). Molecular insights into azumamide E histone deacetylases inhibitory activity. J. Am. Chem. Soc. 129, 3007–3012. 10.1021/ja0686256 [DOI] [PubMed] [Google Scholar]
- Mayer A. M., Glaser K. B., Cuevas C., Jacobs R. S., Kem W., Little R. D., et al. (2010). The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol. Sci. 31, 255–265. 10.1016/j.tips.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Mehbub M. F., Lei J., Franco C., Zhang W. (2014). Marine sponge derived natural products between 2001 and 2010: trends and opportunities for discovery of bioactives. Mar. Drugs 12, 4539–4577. 10.3390/md12084539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendola D. (2000). Aquacultural production of bryostatin 1 and ecteinascidin 743, in Drugs from the Sea, ed Fusetani N.(Basel: Karger; ), 120–133. 10.1159/000062482 [DOI] [Google Scholar]
- Menna M. (2009). Antitumor potential of natural products from Mediterranean ascidians. Phytochem. Rev. 8, 461–472. 10.1007/s11101-009-9131-y [DOI] [Google Scholar]
- Miao V., Coeffet-Legal M.-F., Brian P., Brost R., Penn J., Whiting A., et al. (2005). Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology 151, 1507–1523. 10.1099/mic.0.27757-0 [DOI] [PubMed] [Google Scholar]
- Mooberry S. L., Randall-Hlubek D. A., Leal R. M., Hegde S. G., Hubbard R. D., Zhang L., et al. (2004). Microtubule-stabilizing agents based on designed laulimalide analogues. Proc. Natl. Acad. Sci. U.S.A. 101, 8803–8808. 10.1073/pnas.0402759101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M. R., Mohan E., Sadhukhan A. (1999). Cyclosporin-A production by Tolypocladium inflatum using solid state fermentation. Process Biochem. 34, 269–280. 10.1016/S0032-9592(98)00095-8 [DOI] [Google Scholar]
- Nakao Y., Fujita M., Warabi K., Matsunaga S., Fusetani N. (2000). Miraziridine A, a novel cysteine protease inhibitor from the marine sponge Theonella aff. mirabilis 1. J. Am. Chem. Soc. 122, 10462–10463. 10.1021/ja001859j [DOI] [Google Scholar]
- Nakao Y., Kawatsu S., Okamoto C., Okamoto M., Matsumoto Y., Matsunaga S., et al. (2008). Ciliatamides A−C, bioactive lipopeptides from the deep-sea sponge Aaptos ciliata. J. Nat. Prod. 71, 469–472. 10.1021/np8000317 [DOI] [PubMed] [Google Scholar]
- Nakao Y., Kuo J., Yoshida W. Y., Kelly M., Scheuer P. J. (2003). More kapakahines from the marine sponge Cribrochalina olemda. Org. Lett. 5, 1387–1390. 10.1021/ol026830u [DOI] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Newman D. J., Hill R. T. (2006). New drugs from marine microbes: the tide is turning. J. Ind. Microbiol. Biotechnol. 33, 539–544. 10.1007/s10295-006-0115-2 [DOI] [PubMed] [Google Scholar]
- Oclarit J., Okada H., Ohta S., Kaminura K., Yamaoka Y., Iizuka T., et al. (1994). Anti-bacillus substance in the marine sponge, Hyatella species, produced by an associated Vibrio species bacterium. Microbios 78, 7. [PubMed] [Google Scholar]
- Okada Y., Matsunaga S., van Soest R. W., Fusetani N. (2002). Nagahamide A, an antibacterial depsipeptide from the marine sponge Theonella swinhoei 1. Org. Lett. 4, 3039–3042. 10.1021/ol0262791 [DOI] [PubMed] [Google Scholar]
- Oku N., Gustafson K. R., Cartner L. K., Wilson J. A., Shigematsu N., Hess S., et al. (2004). Neamphamide A, a new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi. J. Nat. Prod. 67, 1407–1411. 10.1021/np040003f [DOI] [PubMed] [Google Scholar]
- Olivera B. M. (2000). ω- Conotoxin MVIIA: from marine snail venom to analgesic drug, in Drugs from the Sea, ed Fusetani N.(Basel: Karger; ), 74–85. 10.1159/000062483 [DOI] [Google Scholar]
- Olson J. B., McCarthy P. J. (2005). Associated bacterial communities of two deep-water sponges. Aquat. Microb. Ecol. 39, 47–55. 10.3354/ame039047 [DOI] [Google Scholar]
- Paulus H., Gray E. (1964). The biosynthesis of polymyxin B by growing cultures of Bacillus polymyxa. J. Biol. Chem. 239, 865–871. [PubMed] [Google Scholar]
- Perez L. J., Faulkner D. J. (2003). Bistratamides EJ, modified cyclic hexapeptides from the Philippines ascidian Lissoclinum bistratum. J. Nat. Prod. 66, 247–250. 10.1021/np0204601 [DOI] [PubMed] [Google Scholar]
- Pettit G. R., Tan R. (2005). Isolation and structure of Phakellistatin 14 from the Western Pacific marine sponge Phakellia sp., 1. J. Nat. Prod. 68, 60–63. 10.1021/np040092w [DOI] [PubMed] [Google Scholar]
- Plaza A., Bifulco G., Keffer J. L., Lloyd J. R., Baker H. L., Bewley C. A. (2008). Celebesides A−C and theopapuamides B−D, depsipeptides from an Indonesian sponge that inhibit HIV-1 entry. J. Org. Chem. 74, 504–512. 10.1021/jo802232u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza A., Bifulco G., Masullo M., Lloyd J. R., Keffer J. L., Colin P. L., et al. (2010). Mutremdamide A and koshikamides C−H, peptide inhibitors of HIV-1 entry from different Theonella species. J. Org. Chem. 75, 4344–4355. 10.1021/jo100076g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza A., Gustchina E., Baker H. L., Kelly M., Bewley C. A. (2007). Mirabamides A–D, depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV-1 fusion. J. Nat. Prod. 70, 1753–1760. 10.1021/np070306k [DOI] [PubMed] [Google Scholar]
- Pommier Y., Kohlhagen G., Bailly C., Waring M., Mazumder A., Kohn K. W. (1996). DNA Sequence- and structure-selective alkylation of guanine N2 in the DNA minor groove by ecteinascidin 743, a potent antitumor compound from the caribbean tunicate Ecteinascidia turbinata. Biochemistry 35, 13303–13309. 10.1021/bi960306b [DOI] [PubMed] [Google Scholar]
- Prasad P., Aalbersberg W., Feussner K.-D., Van Wagoner R. M. (2011). Papuamides E and F, cytotoxic depsipeptides from the marine sponge Melophlus sp. Tetrahedron 67, 8529–8531. 10.1016/j.tet.2011.08.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A., Colin P. L., Faulkner D. J. (2000). Microsclerodermins F–I, antitumor and antifungal cyclic peptides from the lithistid sponge Microscleroderma sp. Tetrahedron 56, 3679–3685. 10.1016/S0040-4020(00)00286-6 [DOI] [Google Scholar]
- Randazzo A., Bifulco G., Giannini C., Bucci M., Debitus C., Cirino G., et al. (2001). Halipeptins A and B: two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J. Am. Chem. Soc. 123, 10870–10876. 10.1021/ja010015c [DOI] [PubMed] [Google Scholar]
- Ranjan N., Davis E., Xue L., Arya D. P. (2013). Dual recognition of the human telomeric G-quadruplex by a Neomycin-anthraquinone conjugate. Chem. Commun. 49, 5796–5798. 10.1039/c3cc42721h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid M. A., Gustafson K. R., Boswell J. L., Boyd M. R. (2000). Haligramides A and B, two new cytotoxic hexapeptides from the marine sponge Haliclona nigra. J. Nat. Prod. 63, 956–959. 10.1021/np000051+ [DOI] [PubMed] [Google Scholar]
- Rashid M. A., Gustafson K. R., Cartner L. K., Shigematsu N., Pannell L. K., Boyd M. R. (2001). Microspinosamide, a new HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa 1. J. Nat. Prod. 64, 117–121. 10.1021/np0002379 [DOI] [PubMed] [Google Scholar]
- Ratnayake A. S., Bugni T. S., Feng X., Harper M. K., Skalicky J. J., Mohammed K. A., et al. (2006). Theopapuamide, a cyclic depsipeptide from a Papua New Guinea lithistid sponge Theonella swinhoei. J. Nat. Prod. 69, 1582–1586. 10.1021/np060229d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. M., Jacob S. T. (1978). Selective inhibition of RNA polyadenylation by Ara-ATP in vitro: a possible mechanism for antiviral action of Ara-A. Biochem. Biophys. Res. Commun. 81, 1418–1424. 10.1016/0006-291X(78)91294-9 [DOI] [PubMed] [Google Scholar]
- Rudi A., Chill L., Aknin M., Kashman Y. (2003). Didmolamide A and B, two new cyclic hexapeptides from the marine ascidian Didemnum molle. J. Nat. Prod. 66, 575–577. 10.1021/np020531w [DOI] [PubMed] [Google Scholar]
- Ruppert E., Fox R. (2004). Invertebrate Zoology: A Functional Evolutionary Approach (of RD Barnes' Invertebrate Zoology). Belmont, CA: Brooks/Cole. [Google Scholar]
- Schmidt E. W., Obraztsova A., Davidson S., Faulkner D., Haygood M. (2000). Identification of the antifungal peptide-containing symbiont of the marine sponge Theonella swinhoei as a novel δ-proteobacterium,“Candidatus Entotheonella palauensis”. Mar. Biol. 136, 969–977. 10.1007/s002270000273 [DOI] [Google Scholar]
- Schmidt E. W., Raventos-Suarez C., Bifano M., Menendez A. T., Fairchild C. R., Faulkner D. J. (2004). Scleritodermin A, a cytotoxic cyclic peptide from the lithistid sponge Scleritoderma nodosum. J. Nat. Prod. 67, 475–478. 10.1021/np034035z [DOI] [PubMed] [Google Scholar]
- Sepe V., D'Orsi R., Borbone N., D'auria M. V., Bifulco G., Monti M. C., et al. (2006). Callipeltins F–I: new antifungal peptides from the marine sponge Latrunculia sp. Tetrahedron 62, 833–840. 10.1016/j.tet.2005.10.058 [DOI] [Google Scholar]
- Somma S., Gastaldo L., Corti A. (1984). Teicoplanin, a new antibiotic from Actinoplanes teichomyceticus nov. sp. Antimicrob. Agents Chemother. 26, 917–923. 10.1128/AAC.26.6.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenschein R. N., Farias J. J., Tenney K., Mooberry S. L., Lobkovsky E., Clardy J., et al. (2004). A further study of the cytotoxic constituents of a milnamide-producing sponge. Org. Lett. 6, 779–782. 10.1021/ol036446c [DOI] [PubMed] [Google Scholar]
- Stark W., Higgens C., Wolfe R., Hoehn M., McGuire J. (1962). Capreomycin, a new antimycobacterial agent produced by Streptomyces capreolus sp. n. Antimicrob. Agents Chemother. 1962, 596–606. [Google Scholar]
- Steele J. H. (1985). A comparison of terrestrial and marine ecological systems. Nature 313, 355–358. 10.1038/313355a0 [DOI] [Google Scholar]
- Stierle A., Cardellina I. I. J., Singleton F. (1988). A marine Micrococcus produces metabolites ascribed to the sponge Tedania ignis. Experientia 44, 1021–1021. 10.1007/BF01939910 [DOI] [PubMed] [Google Scholar]
- Tabudravu J., Morris L. A., Kettenes-Van Den Bosch J. J., Jaspars M. (2001). Wainunuamide, a histidine-containing proline-rich cyclic heptapeptide isolated from the Fijian marine sponge Stylotella aurantium. Tetrahedron Lett. 42, 9273–9276. 10.1016/S0040-4039(01)01993-1 [DOI] [Google Scholar]
- Tabudravu J. N., Morris L. A., Kettenes-van den Bosch J. J., Jaspars M. (2002). Axinellin C, a proline-rich cyclic octapeptide isolated from the Fijian marine sponge Stylotella aurantium. Tetrahedron 58, 7863–7868. 10.1016/S0040-4020(02)00898-0 [DOI] [Google Scholar]
- Tan L. T., Williamson R. T., Gerwick W. H., Watts K. S., McGough K., Jacobs R. (2000). cis, cis-and trans, trans-Ceratospongamide, new bioactive cyclic heptapeptides from the Indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica. J. Org. Chem. 65, 419–425. 10.1021/jo991165x [DOI] [PubMed] [Google Scholar]
- Tanaka C., Tanaka J., Bolland R. F., Marriott G., Higa T. (2006). Seragamides A–F, new actin-targeting depsipeptides from the sponge Suberites japonicus Thiele. Tetrahedron 62, 3536–3542. 10.1016/j.tet.2006.01.099 [DOI] [Google Scholar]
- Thomas T. R. A., Kavlekar D. P., Lokabharathi P. A. (2010). Marine drugs from sponge-microbe association—a review. Mar. Drugs 8, 1417–1468. 10.3390/md8041417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tincu J. A., Menzel L. P., Azimov R., Sands J., Hong T., Waring A. J., et al. (2003). Plicatamide, an antimicrobial octapeptide from Styela plicata hemocytes. J. Biol. Chem. 278, 13546–13553. 10.1074/jbc.M211332200 [DOI] [PubMed] [Google Scholar]
- Ueda H., Nakajima H., Hori Y., Fujita T., Nishimura M., Goto T., et al. (1994). FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J. Antibiot. 47, 301–310. 10.7164/antibiotics.47.301 [DOI] [PubMed] [Google Scholar]
- Umezawa H., Maeda K., Takeuchi T., Okami Y. (1966). New antibiotics, bleomycin A and B. J. Antibiot. 19, 200. [PubMed] [Google Scholar]
- Unson M., Holland N., Faulkner D. (1994). A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue. Mar. Biol. 119, 1–11. 10.1007/BF00350100 [DOI] [Google Scholar]
- Van Wageningen A. A., Kirkpatrick P. N., Williams D. H., Harris B. R., Kershaw J. K., Lennard N. J., et al. (1998). Sequencing and analysis of genes involved in the biosynthesis of a vancomycin group antibiotic. Chem. Biol. 5, 155–162. 10.1016/S1074-5521(98)90060-6 [DOI] [PubMed] [Google Scholar]
- Vervoort H., Fenical W., Epifanio R. D. A. (2000). Tamandarins A and B: new cytotoxic depsipeptides from a Brazilian ascidian of the family Didemnidae. J. Org. Chem. 65, 782–792. 10.1021/jo991425a [DOI] [PubMed] [Google Scholar]
- Vicente J., Vera B., Rodríguez A. D., Rodríguez-Escudero I., Raptis R. G. (2009). Euryjanicin A: a new cycloheptapeptide from the Caribbean marine sponge Prosuberites laughlini. Tetrahedron Lett. 50, 4571–4574. 10.1016/j.tetlet.2009.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinothkumar S., Parameswaran P. (2013). Recent advances in marine drug research. Biotechnol. Adv. 31, 1826–1845. 10.1016/j.biotechadv.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Waksman S. A., Woodruff H. B. (1940). Bacteriostatic and bactericidal substances produced by a soil actinomyces. Exp. Biol. Med. 45, 609–614. 10.3181/00379727-45-11768 [DOI] [Google Scholar]
- Webster N. S., Wilson K. J., Blackall L. L., Hill R. T. (2001). Phylogenetic diversity of bacteria associated with the marine sponge Rhopaloeides odorabile. Appl. Environ. Microbiol. 67, 434–444. 10.1128/AEM.67.1.434-444.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C. (1978). Microbial associations in sponges. III. Ultrastructure of the in situ associations in coral reef sponges. Mar. Biol. 49, 177–185. 10.1007/BF00387117 [DOI] [Google Scholar]
- Wilkinson C. R., Nowak M., Austin B., Colwell R. R. (1981). Specificity of bacterial symbionts in Mediterranean and Great Barrier Reef sponges. Microb. Ecol. 7, 13–21. 10.1007/BF02010474 [DOI] [PubMed] [Google Scholar]
- Williams D. E., Austin P., Diaz-Marrero A. R., Soest R. V., Matainaho T., Roskelley C. D., et al. (2005). Neopetrosiamides, peptides from the marine sponge Neopetrosia sp. that inhibit amoeboid invasion by human tumor cells. Org. Lett. 7, 4173–4176. 10.1021/ol051524c [DOI] [PubMed] [Google Scholar]
- Williams D. E., Yu K., Behrisch H. W., Van Soest R., Andersen R. J. (2009). Rolloamides A and B, cytotoxic cyclic heptapeptides isolated from the Caribbean marine sponge Eurypon laughlini. J. Nat. Prod. 72, 1253–1257. 10.1021/np900121m [DOI] [PubMed] [Google Scholar]
- Willis B., Arya D. P. (2010). Triple recognition of B-DNA by a Neomycin−Hoechst 33258−Pyrene conjugate. Biochemistry 49, 452–469. 10.1021/bi9016796 [DOI] [PubMed] [Google Scholar]
- Woo J.-K., Jeon J.-E., Kim C.-K., Sim C. J., Oh D.-C., Oh K.-B., et al. (2013). Gombamide A, a cyclic thiopeptide from the sponge Clathria gombawuiensis. J. Nat. Prod. 76, 1380–1383. 10.1021/np4003367 [DOI] [PubMed] [Google Scholar]
- Wörheide G., Solé-Cava A. M., Hooper J. N. (2005). Biodiversity, molecular ecology and phylogeography of marine sponges: patterns, implications and outlooks. Integr. Comp. Biol. 45, 377–385. 10.1093/icb/45.2.377 [DOI] [PubMed] [Google Scholar]
- Zampella A., Sepe V., Luciano P., Bellotta F., Monti M. C., D'Auria M. V., et al. (2008). Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J. Org. Chem. 73, 5319–5327. 10.1021/jo800583b [DOI] [PubMed] [Google Scholar]