Abstract
Infections remain a significant cause of morbidity and mortality in cancer patients. The differential diagnosis for these patients is often wide, and the timely selection of the right clinical tests can have a significant impact on their survival. However, laboratory findings with current methodologies are often negative, challenging clinicians and laboratorians to continue the search for the responsible pathogen. Novel methodologies are providing increased sensitivity and rapid turnaround time to results but also challenging our interpretation of what is a clinically significant pathogen in cancer patients. This minireview provides an overview of the most common infections in cancer patients and discusses some of the challenges and opportunities for the clinical microbiologist supporting the care of cancer patients.
INTRODUCTION
Cancer is the leading cause of death worldwide, with 8.2 million deaths reported worldwide in 2012 (1). Common treatment options for oncology patients include hematopoietic stem cell transplantation (HSCT), chemotherapeutic drugs, and surgical resection. The first successful HSCT was performed in 1959 by E. Donall Thomas in Cooperstown, NY, with infusion of two acute lymphocytic leukemia (ALL) patients with bone marrow from their disease-free identical twins. Although initially successful, their remission was short-lived, with recurrence of the disease occurring within a few months of the transplant (2). Today, HSCT is a curative therapy for many types of hematologic malignancies and immune deficiency diseases. E. Donall Thomas and Joseph E. Murray received the 1991 Nobel Prize in Physiology or Medicine for their discoveries concerning “organ and cell transplantation in the treatment of human disease.”
Of note for microbiologists, the history of cancer chemotherapy started as a history of antibiotics with Paul Ehrlich's discovery in 1909 of arsphenamine (Salvarsan), the first effective treatment against Treponema pallidum infection. Paul Ehrlich also evaluated early versions of alkylating agents to treat cancer but with little hope that these drugs would be curative. Decades of investigations and trials have resulted in the current modern chemotherapeutic agents that are curative for large groups of either hematologic malignancies or solid tumors when used in conjunction with surgical resection (3).
CANCER AND INFECTIONS
Infections remains a significant cause of death in cancer patients, particularly in HSCT recipients (1). Susceptibility to infections is related to a host's ability to reconstitute their immune system following HSCT and/or chemotherapy treatment. The longer it takes a patient to recover, the higher at risk they are of developing infections. In general, the highest risk of infections occurs in allogeneic HSCT recipients and leukemia patients, and the lowest risk occurs in solid-tumor patients on standard chemotherapy (Table 1) (4). The posttransplant period may be divided into three stages: preengraftment (less than 4 weeks), early postengraftment (3 weeks to 3 months), and late postengraftment (>3 months) stages (Fig. 1). The defect in innate immunity (including breaks in mucosal barriers and decreased granulocyte and monocyte functions) translates into initial susceptibility to bacteria, Candida, and herpes simplex virus (HSV) during the preengraftment or early engraftment period. A delay in the recovery of adaptive immunity in the first 6 months posttransplant predisposes the host to herpesviruses reactivation, fungal infection, and respiratory virus infections (postengraftment period). Approximately 6 months after transplantation, innate immunity is almost completely recovered, while adaptive immunity may take a year or more to reconstitute (Table 1 and Fig. 1) (4, 5). In comparison to HSCT recipients and patients with hematologic malignancies, the risk of infections in solid-tumor patients is lower and primarily related to neutropenia, which often lasts for only a few days (6). This review discusses the laboratory diagnosis of infections in oncology patients, including patients with hematologic malignancies, regardless of their transplant status (e.g., patients with cancer-related immunosuppression, such as acute leukemia patients with leukopenia), solid-tumor patients, and recipients of HSCT.
TABLE 1.
Common risk factors for infections in cancer patients
| Infection | Underlying diseasea | Common risk factorsb | Immune dysfunction | Common pathogen(s) | Comments | Reference(s) |
|---|---|---|---|---|---|---|
| Bacterial | Allo-HSCT, HM, auto-HSCT, ST | Neutropenia, broad-spectrum antibiotics, central venous catheter | Mucosal barrier, cellular immunity, humoral immunity | CNS, Enterococcus spp., viridans streptococci, Gram-negative bacteria, Legionella species, C. difficile | Duration and severity of neutropenia is longer for HM/HSCT than ST | 6, 9 |
| Viral | Allo-HSCT, HM, auto-HSCT, ST | Lymphopenia, GVHD, high corticosteroids, immunosuppressants, D+/R− serostatus, monoclonal antibodies, HLA mismatch donor, cord blood, T-cell depletion, myeloablative conditioning | Cellular immunity | CMV, HSV, EBV, HHV-6, adenovirus, norovirus | Highest risk of CMV or HSV disease in D+/R− seropositive | 6, 29 |
| Fungal | Allo-HSCT, HM, auto-HSCT, ST | Neutropenia, GI mucositis, GVHD, broad-spectrum antibiotics, CMV disease, central venous catheter and port-A catheter, severe neutropenia, corticosteroid, iron overload, T-cell depletion | Mucosal barrier, cellular immunity | Candida, Aspergillus | Non-Aspergillus and PCP are less common but significant causes of high mortality | 6, 58, 59, 65 |
| Parasitic | Allo-HSCT, HM | Corticosteroid, HTLV-1 | Cellular immunity, eosinopenia | Toxoplasma, Strongyloides | Rare, travel history important | 78, 80 |
Allo, allogeneic; HSCT, hematopoietic stem cell transplantation; HM, hematologic malignancies; auto, autologous; ST, solid tumor.
Neutropenia defined as absolute neutrophil count (ANC) of ≤500 cells/μl; profound neutropenia defined as ANC of ≤100 cells/μl; D+, donor serostatus positive; HLA, human leukocyte antigen; HTLV-1, human T-cell lymphotropic virus-1; ST has overall low risk.
FIG 1.
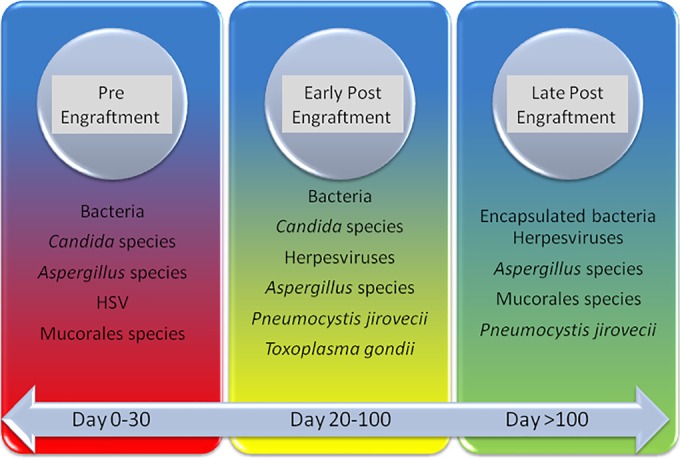
Timing of the most common infections in HSCT recipients and cancer patients.
BACTERIAL INFECTIONS
Gram-positive bacteria.
In a recent multicenter study, bacterial infections accounted for 30% of all infections detected in two cohorts of allogeneic HSCT recipients, bone marrow and peripheral blood stem cell recipients, who were followed over a 2-year period (7). The cumulative bacterial bloodstream infection incidence rates were 52% and 48%, respectively, within the range (12 to 60%) of incidence rates reported in other studies of allogeneic-HSCT (allo-HSCT) recipients (7, 8). The most frequently recovered bacteria were coagulase-negative staphylococci (CNS) and Enterococcus species. This finding is in line with the contemporary epidemiology of increased Gram-positive bacterial infections, which has been attributed in part to the increased use of indwelling devices (7, 9). Although CNS are the most common organisms detected, vancomycin-resistant Enterococcus species (VRE) are more significant pathogens and a frequent cause of bacteremia in HSCT recipients, especially in allo-HSCT recipients (10, 11). Prevalence rates of VRE range between 16 and 27%, with mortality rates of 9% and 20% occurring early in the preengraftment period. Risk factors for developing VRE bacteremia include pretransplant VRE colonization, delayed engraftment, T-cell depletion, or receipt of mismatched peripheral blood stem cells (10, 11).
Gram-negative bacteria.
Worldwide, the incidence of infections caused by Gram-negative bacteria (GNB) in neutropenic cancer patients has increased in recent years, a consequence of injury to the mucosal surface of the gastrointestinal tract from cancer treatment (12, 13). The most commonly reported GNB include Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter species, with rates ranging from 40 to 60% (12, 13). Particularly worrisome is the emergence of carbapenem-resistant Enterobacteriaceae (CRE), extended-spectrum beta-lactamase Enterobacteriaceae (ESBL-E), multidrug-resistant Pseudomonas species, and Acinetobacter species. HSCT patients are at increased risks for GNB infections because of their extended hospitalization, the frequent use of indwelling devices (central venous catheter or urinary catheter), and routine exposure to broad-spectrum antibiotics. The reported mortality rates of CRE and ESBL-E bacteremia in patients with hematologic malignancies can be as high as 56% and 40%, respectively, especially during periods of extended neutropenia (14, 15).
Laboratory diagnosis.
Bacteremia is the most common bacterial infection in cancer patients, and bacterial blood culture remains the primary method of diagnosis. However, low recovery rates for blood cultures, with one review citing recovery rates of 20 to 30% during febrile episodes, present a challenge for establishing a rapid and definitive diagnosis (9). This is particularly important in cancer patients in whom common signs of infections may be missing or indistinguishable from noninfectious syndromes. First, approximately 10 to 50% of solid-tumor patients and >80% of HSCT recipients and patients with hematologic malignancies develop neutropenic fever posttransplant or posttreatment (9). This common syndrome is characterized by the absence of typical signs of infection (e.g., elevated neutrophils), and fever may be the only marker of bacteremia. Therefore, antibacterial prophylaxis (e.g., fluoroquinolone prophylaxis) is routinely used, especially for high-risk patients (6). Second, cancer patients on corticosteroid therapy, which suppresses the inflammatory response, may present with afebrile bacteremia. To circumvent this problem, some institutions caring for these patients utilize surveillance blood cultures, which are collected at predetermined intervals posttransplantation (8). The combination of routine prophylaxis, surveillance cultures, and other host variables translates into even lower sensitivity for blood cultures. New commercially available methodologies, including molecular blood culture panels, peptide nucleic acid fluorescent probes, and mass spectrometry, are additional tools that provide rapid preliminary data from positive blood cultures. This in turn can facilitate the implementation of targeted therapy based on organism identification, institutional antibiogram profile, or identification of a specific resistance marker (e.g., K. pneumoniae carbapenemase [KPC] or VRE). Recent technologies, including amplification methods (e.g., T2 magnetic resonance energy and broad-range PCR/electrospray ionization-mass spectrometry [ESI-MS]) that aim to detect organisms directly in blood samples provide an opportunity to improve on the diagnosis of bacteremia in oncology patients. However, the utility of these new methods still remains to be established.
Legionella species.
Although mostly associated with water outbreaks in hospital or community settings, infections with Legionella species in patients with compromised cellular immunity results in high mortality (16). In one cohort of HSCT patients, neutropenia was identified as the only risk factor, and mortality rates of up to 31%, caused primarily by Legionella pneumophila serotype 1 infections, were reported (17). In cancer patients, non-pneumophila Legionella species are recovered more frequently (50 to 70%), with clinical presentations that may be atypical for Legionella species, including radiological findings suggestive of fungal infections and extrapulmonary diseases (16, 18).
Laboratory diagnosis.
Routine Legionella culture for respiratory specimens may be warranted in cancer patients. However, these cultures require special media and an extended incubation period, which may result in a significant delay in diagnosis. The use of urinary antigen tests provides an additional diagnostic option, but the sensitivities of these tests vary, and because they detect only Legionella pneumophila serotype 1 species, their utility in cancer patients may be limited.
Clostridium difficile.
Due in part to their prolonged immunosuppression, HSCT recipients have an increased risk for Clostridium difficile infections (CDI), especially in the early preengraftment period (Table 1). In a recent multicenter study conducted by members of the Comprehensive Cancer Centers Infection Control Group (C3IC), rates of health care-associated CDI in solid tumors and hematologic malignancy patients were twice the rates reported by the National Healthcare Safety Network for other hospitalized patients (15.8 versus 7.4 per 10,000 patient days, respectively), independent of the method used to detect C. difficile (19). However, CDI incidence rates vary among transplant centers and with the type of HSCT procedure, with higher rates reported in allogeneic HSCT recipients (12.5 to 27.0%) than in autologous HSCT recipients (6.5 to 15%) (20).
Laboratory diagnosis.
Laboratory diagnosis of CDI in cancer patients is particularly challenging. The 2010 Infectious Diseases Society of America (IDSA) guidelines define CDI as the presence of diarrhea (i.e., 3 or more loose stools in ≤24-h period), a positive laboratory stool test for C. difficile toxin, and/or the presence of pseudomembranous colitis, as determined by colonoscopy or histopathology. However, diarrhea is a frequent symptom in many HSCT patients, and with asymptomatic carriage of C. difficile ranging from 7 to 18%, the detection of C. difficile, especially using highly sensitive molecular assays, is problematic in differentiating CDI from other causes of diarrhea, both infectious and noninfectious (21).
Nontuberculous mycobacteria.
The incidence of infections caused by nontuberculous mycobacteria (NTM) in oncology patients is low (0.4 to 5%) but has increased over the last few decades (22, 23). The incidence and epidemiology of NTM species vary across centers, due in part to differences in the frequency of testing, transplant procedures, conditioning regimens, and environmental factors. Although Mycobacterium avium complex is the most commonly recovered NTM group in oncology patients, rapidly growing mycobacteria (RGM) are being recovered with increased frequency. One study reviewed the clinical features of 341 cancer patients positive for RGM (24). Disseminated infection, frequently caused by Mycobacterium abscessus, was the least common presentation and occurred primarily in patients with hematologic malignancies soon after the receipt of chemotherapy treatment. Older patients with solid tumors presented more frequently with pulmonary infections caused by Mycobacterium fortuitum and M. abscessus/M. chelonae. In a large study in pediatric oncology patients, 50% of cases due to RGM occurred in patients with hematologic malignancies and presented as localized catheter-associated infection. Most cases were caused similarly by M. fortuitum and M. abscessus/M. chelonae, with an overall incidence of RGM infections of 2.9 cases per 100,000 patient days (25).
Laboratory diagnosis.
Mycobacterial culture remains the gold standard for diagnosing mycobacterial infection but is limited by its turnaround time of several days to several weeks. A particular challenge in cancer patients is the diagnosis of mycobacterial infections following an incidental finding of acid-fast bacilli (AFB), detected during staining of biopsy samples submitted to rule out malignancies. In many of these cases, insufficient or no tissue samples are submitted for AF culture. Broad-range bacterial sequencing of nucleic acid extracted from formalin-fixed paraffin-embedded (FFPE) tissues has become a necessary tool, not only to rule out primarily M. tuberculosis infections, but also to provide rapid culture-independent identification and identification to the species level of AFB identified in FFPE samples.
VIRAL INFECTIONS
Cytomegalovirus.
Reactivation of latent cytomegalovirus (CMV) in seropositive patients or primary infection in a seronegative HSCT recipient can occur following immunosuppressive therapy resulting in CMV infection or CMV disease. CMV-seropositive HSCT recipients, including those receiving T-cell-depleted grafts or unrelated donor, are particularly at high risk for developing CMV disease until the T-cell response is restored (Table 1). Current management strategies of transplant patients, including antiviral prophylaxis and preemptive therapy, most commonly with ganciclovir or foscarnet, have resulted in a decreased incidence of early (i.e., first 4 months posttransplant) CMV disease, especially CMV pneumonia. In a recent large single-center retrospective analysis, the all-cause mortality of HSCT patients following the onset of CMV pneumonia was still high, with only 30% survival at 6 months (26). When outcomes were compared before and after the use of preemptive therapy, only a modest decrease was observed. This study illustrates the continued challenge of accurately diagnosing CMV disease.
Laboratory diagnosis.
Detection of CMV is accomplished using either antigen tests or molecular assays, with molecular assays providing rapid turnaround time, increased sensitivity, and objective interpretation of results. A challenge of using molecular assays in HSCT patients comes with the interpretation of the significance of a positive test result in certain specimen types. For example, while a qualitative CMV PCR with high sensitivity may be required for the diagnosis of central nervous system infections, a positive result generated using the same assay on a respiratory sample (e.g., bronchoalveolar lavage fluid) may only represent asymptomatic shedding and not be clinically significant. However, since CMV pneumonia remains associated with severe outcomes, results need to be interpreted in the appropriate clinical context.
In general, molecular quantitative CMV tests provide earlier detection of CMV viremia than pp65 antigenemia tests, although the increased sensitivity may result in unnecessary prolonged therapy and increased toxicity. Quantitative assays used in the laboratory for measuring CMV viremia have a significant impact on the clinical management of transplant patients. Since institutional guidelines for initiating therapy rely on specific numerical values generated by the laboratory, a clear understanding of the test performance characteristics, including the specimen type used, the limit of detection, the limit of quantification, the linear range, and the reproducibility of the assay, is necessary for an accurate interpretation of the CMV viral loads. Studies have shown that CMV viral loads measured in whole blood are generally higher than those measured in plasma, as whole blood may contain both free and cell-associated CMV virions. Viral loads in whole blood may therefore overestimate the level of actively replicating viruses (27). Until recently, standardization of the many laboratory-developed quantitative CMV tests was not feasible. The recent availability of the World Health Organization (WHO) international CMV standards and the introduction of commercial in vitro diagnostic (IVD)-cleared assays should result in improved standardization of CMV viral loads obtained from different institutions.
Epstein-Barr virus.
Posttransplant lymphoproliferative disorder (PTLD) is the most common Epstein-Barr virus (EBV)-associated syndrome in HSCT recipients. PTLD represents a group of histopathologically heterogenous disorders, including early lesions (e.g., mononucleosis-like lesions), polymorphic PTLD (polyclonal or monoclonal), and monomorphic PTLD (e.g., lymphoma). PTLD results from the reactivation of EBV in infected B-cell lymphocytes following the loss of EBV-specific T-cell functions due to immunosuppression (Table 1). Incidence rates of PTLD in allogeneic HSCT recipients range from 0.45 to 29%, depending on several factors, e.g., the type of transplant procedure and the conditioning regimens, and generally occur in the postengraftment and late phases following transplantation (Fig. 1). Other clinical manifestations of EBV infections in HSCT include enteritis, hepatitis, encephalitis, and pneumonia (28, 29).
Laboratory diagnosis.
Qualitative EBV molecular tests are sufficient for the diagnosis of many syndromes, such as encephalitis. Quantitative molecular tests in blood samples are the most useful assays for the detection of EBV reactivation and for monitoring of viral load in blood in PTLD. These assays may also be used to distinguish between asymptomatic and low-level transient reactivation, which does not warrant treatment. To date, no standard threshold for implementing EBV treatment exists. Each transplant center sets up an actionable threshold based on institutional experience and taking into consideration the performance characteristics (e.g., limit of quantitation and reportable range) of the quantitative EBV assay in use and the specific patient population. In general, high viral loads are often detected in patients with PTLD, and changes in viral loads of greater than 0.5 log copies/ml between consecutive samples are considered significant. Unlike CMV, there are still no FDA-cleared commercial quantitative molecular tests for measuring EBV viral loads. Studies have shown significant variability in EBV viral loads generated with laboratory-developed tests across centers, which presents a challenge for patients, laboratories, and clinicians alike, as data generated across centers are not easily compared. In 2011, the 1st WHO international standards for EBV became available, providing an opportunity for standardization of laboratory-developed assays.
Herpes simplex viruses.
The risk of HSV infections in cancer patients varies with the underlying disease or chemotherapy treatment. Disseminated HSV infection is now uncommon due to prophylaxis of high-risk patients, and most infections result from reactivation of latent HSV (6). HSV reactivation manifests either as mucocutaneous lesions of the oropharynx, esophagus, or genitals, with rare cases of HSV encephalitis and pneumonia, occurring in less than 1% of HSCT patients (29). Reactivation of HSV generally occurs during period of neutropenia early in the preengraftment period, but it can develop at any time if immune suppression occurs. Less data are available from patients with solid tumors due to their low risk for reactivation. In a recent study that included 45 cancer patients with confirmed, probable, or possible HSV pneumonia, infection resulted in mortality rates of 22%, with identified risk factors including lymphopenia and prior use of corticosteroids (30).
Laboratory diagnosis.
Although direct and indirect fluorescent-antibody assays are commercially available and frequently used for the detection of HSV antigens in clinical specimens, their sensitivity may be too low for diagnosis in transplant patients. Several molecular commercial assays are FDA cleared for the qualitative detection and differentiation of HSV-1 and HSV-2 in cutaneous and mucocutaneous lesions, with sensitivity and specificity ranging between 95 and 100% compared to viral culture and many have a turnaround time of less than 4 h compared to days for culture (Table 2) (31). Currently, one commercial test, the Simplexa HSV-1&2 (Focus Diagnostics), is FDA cleared for the detection and differentiation of HSV-1/2 from cerebrospinal fluid (CSF). The overall sensitivity and specificity of this assay compared to a laboratory-developed test (LDT) using the Roche HSV 1/2 analyte-specific reagent (ASR) were 96.2% and 97.9%, respectively (32). While the gold standard for the diagnosis of HSV central nervous system infection is molecular testing, diagnosis of other HSV syndromes, aside from mucocutaneous lesions, may be challenging, as shedding of HSV occurs frequently in immunocompromised patients, and the detection of viral nucleic acids need to be interpreted in the right clinical context.
TABLE 2.
Laboratory testing and challenges for infection in HSCT and cancer patients
| Infection | Diagnostic methodsa | Commercial molecular methods | Challenges | Opportunitiesb |
|---|---|---|---|---|
| Bacterial | Bacterial culture, urinary antigens, molecular methods | FilmArray blood culture, PNA-FISH (Gram positive, Gram negative), Verigene Gram positive, Verigene Gram negative, MALDI-TOF MS, C. difficilec | Time to bacterial growth, no standard tests for direct from specimen detection, differentiation between infection and disease | Sepsis biomarkers, direct from sample tests, direct from sample susceptibility testing |
| Viral | Viral culture, DFA/IFA, molecular methods | FilmArray respiratory panel, FilmArray gastrointestinal panel, Luminex gastrointestinal panel, Xpert Norovirus, HSV 1/2 (lesions),c Simplexa HSV 1&2 (CSF) | Variability in quantitative PCR assays for herpesviruses, differentiation between infection and disease | WHO IS (EBV and CMV), more WHO IS for standardization of other tests, IVD quantitative assays for herpesviruses, quantitative respiratory virus PCR, mRNA or sensitive and rapid antigen tests |
| Fungal | Fungal culture, β-d-glucans, Aspergillus galactomannan, molecular methods | PNA-FISH (yeast), T2 Biosystems, MALDI-TOF MS | No standardized molecular assays, specificity of fungal antigens | Standardization of PCR, T2 Biosystems, panfungal IVD PCR/sequencing assays |
| Parasitic | Ova and parasites exam, serology, molecular methods | None | Need for multiple stools, depressed antibody response, no standardized molecular assays | Standardization of PCR, IVD Toxoplasma PCR |
DFA, direct fluorescent-antibody assay.
IS, international standards.
Includes several IVD-cleared commercial assays. MALDI-TOF MS, matrix-assisted laser desorption ionization–time of flight mass spectrometry; PNA-FISH, peptide nucleic acid fluorescence in situ hybridization.
Human herpesvirus 6.
In HSCT recipients and hematologic malignancy patients, human herpesvirus 6 (HHV-6) reactivates following immunosuppression or chemotherapy in 50 to 90% of HSCT patients, depending on the source of stem cells, with occurrence anytime posttransplantation (Fig. 1). One of the most commonly reported complications of HHV-6 is encephalitis, which generally occurs in patients with high viral loads, although reports vary depending on the type of transplant (33, 34). In some studies, early reactivation of HHV-6 posttransplantation was associated with delayed neutrophil and platelet engraftment, increased mortality, and the development of acute graft-versus-host disease (GVHD) (35, 36). However, other studies showed no impact from HHV-6 reactivation, and these differences in outcomes may be due to differences in the type of transplant (e.g., cord blood versus HSCT) (37).
Laboratory diagnosis.
Quantitative molecular diagnostics assays are the primary methods for the detection and monitoring of HHV-6 infections. All currently used methods are laboratory-developed assays (Table 2). A diagnostic challenge that is specific to HHV-6 is the differentiation of chromosomally integrated HHV-6 (ciHHV-6) from actively replicating HHV-6. In approximately less than 1% of the population, HHV-6 can integrate into the host telomeres and be transmitted through germ cells, resulting in high viral loads. Detection of HHV-6 in cell-free samples, such as plasma or CSF, may better reflect true infections. Several approaches are used to differentiate between active and latent infections. Whole-blood or plasma PCR with viral loads greater than 106 or 104 copies/ml, respectively, can be indicative of ciHHV-6. Other methods include reverse transcription-PCR (RT-PCR) to detect HHV-6 mRNA, qualitative PCR, or fluorescent in situ hybridization (FISH) on hair follicles, cytogenetics, and immunohistochemistry tests. More recently, ciHHV-6 was detected by digital PCR, in which the ratio of HHV-6 to total cell counts was measured, with a ratio close to 1 indicative of ciHHV-6 (38). A second challenge, which lines up with other quantitative LDT, is the lack of standardization across tests, making it difficult to compare viral loads obtained across centers. To date, WHO international standards for HHV-6 are not yet available.
Adenovirus.
In HSCT recipients, adenovirus infection rates range from 5 to 15%, with clinical disease varying from self-limited infections to severe fatal disseminated disease (39). Mortality rates vary significantly depending on risk factors, such as underlying diseases and the type of transplants (Table 1). In a recent cohort of HSCT patients transplanted with CD34+ selected stem cells, attributable adenovirus mortality rate was 22%, and in patients with adenovirus viremia, the mortality rate was higher, at 44% (40). However, the mortality rate was significantly lower in a cohort of patients with an alemtuzumab-based allogeneic stem cell transplant, where only 1 patient died from disseminated adenovirus infection (41). Following primary exposure, adenovirus establishes latency in lymphoid cells, and infections in cancer patients occur primarily from reactivation. Adenoviruses are associated with a variety of clinical syndromes, including pneumonia, nephritis, hemorrhagic cystitis, hepatitis, and colitis (39).
Laboratory diagnosis.
Quantitative PCR assays are used to monitor adenovirus viremia. In some centers, serial monitoring of adenovirus in plasma at various intervals posttransplant has been instituted, with the goal of earlier detection of adenovirus infection. Other specimen types used to predict adenovirus disease include stool samples, which are primarily used to diagnose adenovirus gastroenteritis. In general, adenovirus enteritis is caused by serotypes 40/41, but in cancer patients, adenovirus enteritis can be caused by any subtype, and in our most recent evaluation, types 40/41 were the least common serotypes recovered in stool samples (our unpublished data). This presents a challenge for the laboratory, as these two subtypes are the only two included in most commercially available gastrointestinal (GI) panels, requiring the laboratory to use either viral culture or adenovirus-specific LDT in addition to GI panels. Furthermore, due to the heterogeneity of adenovirus subtypes, the inclusivity of commercial ASR or LDT may be hard to establish conclusively. Similar to other viral infections, quantitative PCR tests are not standardized, making the establishment of valid actionable threshold challenging.
GASTROINTESTINAL VIRUSES
The most common gastrointestinal (GI) virus evaluated in oncology patients is norovirus. In a retrospective review of allogeneic HSCT adults, norovirus nucleic acid was detected in 90% of symptomatic patients, revealing that in many cases, the infection was underdiagnosed, since presentation could not be distinguished from other causes of diarrhea, including gastrointestinal GVHD (42). Complications of norovirus infections in HSCT recipients can occur and include bowel perforation, aspiration secondary to severe retching, severe weight loss, malnutrition, and sepsis (42, 43). While symptoms resolve within 24- to 48 h in healthy patients, in HSCT recipients and other neutropenic cancer patients, symptoms may last between 7 days and 90 days (43).
The importance of other GI viruses, including astrovirus and sapovirus, is less well established, as methods for routine recovery of these viruses were not available until recently. In a few reports from pediatric oncology patients, sapovirus was recovered infrequently, in <5% of the cases, with diarrhea resolving between 3 and 21 days (44, 45). An astrovirus outbreak in a pediatric hematology and HSCT patients unit revealed that symptoms of astrovirus infections in oncology patients lasted longer and caused more severe dehydration than with sapovirus, and similar to norovirus, shedding could last for several weeks (46). Of note, astrovirus is increasingly being recognized as a neurotropic virus in immunocompromised patients (47).
Laboratory diagnosis.
Molecular methods are the most sensitive laboratory method for the diagnosis of norovirus infections in HSCT recipients and patients with hematologic malignancies. Until recently, a high level of suspicion was required on the part of the clinician to order a specific molecular test for norovirus, as symptoms caused by norovirus cannot be differentiated on clinical presentation alone from other causes of diarrhea, including gut GVHD. This is particularly important, as the management of these two conditions is significantly different, with norovirus infection requiring the attenuation of immunosuppression, while GVHD necessitates increased immunosuppression. The availability of commercially available gastrointestinal syndromic panels that include norovirus has allowed rapid and increased detection of enteritis caused by norovirus. The use of these assays has also provided a useful tool for early recognition and control of outbreaks, which if allowed to go unrecognized can result in significant morbidity and mortality in a high-risk patient (48). A limitation of RT-PCR for noroviruses is the recognition that oncology patients are often chronic shedders, with reports of asymptomatic shedding detected for several months after the resolution of symptoms. Furthermore, unless molecular typing is performed on all positive norovirus samples, distinguishing established and new infections is not easily accomplished.
Similarly, the inclusion of astrovirus and sapovirus on molecular GI panels will allow for routine testing of symptomatic patients and thus provide data on the epidemiology and clinical significance of these viruses in oncology patients that is currently limited.
RESPIRATORY VIRUSES
Although respiratory viral infections in HSCT recipients and oncology patients often remain limited to the upper respiratory tract, these patients are at an increased risk for developing severe complications, including lower respiratory tract infections, airflow obstruction, and bronchiolitis obliterans (49). A recent study highlighted the impact of influenza virus infections in an immunosuppressed patient population that included HSCT recipients and other oncology patients. In this study, all immunosuppressed patients required hospitalization for management of influenza, with 18% requiring admission to the intensive care unit for mechanical ventilation (50).
The incidence of respiratory syncytial virus (RSV) in HSCT recipients is generally higher than that of influenza viruses (2 to 17% versus 1.3 to 2.6%, respectively) (51). Infection with RSV can result in serious complications, similar to those described for influenza viruses. Detection of RSV in the upper respiratory tract is cause for delaying transplant procedures, as 80 to 90% of HCT recipients with upper respiratory RSV infection developed a lower respiratory tract infection (LRTI) within 7 days following the presence of upper respiratory infection (URI) symptoms (51, 52).
Parainfluenza viruses (PIV) are a common cause of respiratory illness in HSCT recipients. The majority of infections are caused by PIV-3. In a recent meta-analysis study, overall rates were 4% (range, 0.2 to 30%), 37% (range, 0 to 74%), and 10% (range, 0 to 31%) for PIV infections, LRTI, and mortality, respectively, in HSCT recipients and patients with hematologic malignancies (53). Rates were higher in allogeneic HSCT recipients than in autologous HSCT recipients. Fewer reports, mostly in small cohorts, have been published on the incidence of human metapneumovirus (hMPV) in cancer patients, with reported incidence rates ranging from 2 to 7% and complications and fatality rates similar to those of RSV, with an approximately 39% mortality rate in a recent study (54).
Laboratory diagnosis.
Timely and accurate detection of respiratory viruses is particularly important in cancer patients and HSCT recipients. In general, diagnosis of respiratory viral infections in these patients is done using molecular methods, owing to their increased sensitivity and rapid turnaround time. Several commercial multiplex assays for the detection of respiratory viruses are now available for testing, primarily using nasopharyngeal swab samples. Many laboratories have validated additional specimen types, especially lower respiratory tract samples, for off-label use with these assays. In addition to well-established pathogenic viruses (influenza viruses and RSV), many panels include viruses, such as picornaviruses (rhinoviruses and enteroviruses) and coronaviruses. These two groups of viruses are frequently recovered among allogeneic HSCT recipients in the first 100 days after transplant and pediatric patients with leukemia, but their impact on morbidity and mortality is not well understood yet (55). An additional challenge with the routine diagnosis of respiratory viruses in transplant patients is their potential for extended shedding. A recent study from our center compared shedding in the culture era to that in the molecular era in both adult and pediatric high-risk oncology patients. Shedding was detected longer for all viruses by PCR, but statistically significant differences were observed in the median duration of shedding for RSV and PIV, at 11 days (range, 5 to 35 days) versus 16 days (range, 5 to 50 days) and 9 days (range, 5 to 41 days) versus 17 days (range, 5 to 45 days), respectively (56). The significance of extended shedding as detected by PCR is unclear, but its impact on patient care is significant, and additional studies are necessary to clearly understand the kinetics of respiratory viruses in oncology patients.
FUNGAL INFECTIONS
Aspergillus species.
Invasive aspergillosis (IA) most commonly presents as a pulmonary infection and remains associated with significant mortality in cancer patients, especially in patients with hematologic malignancies and in allo-HSCT recipients. IA develops during the pre- and early postengraftment periods when neutropenia-increased immunosuppressant therapy and broad-spectrum antibiotics are present (Table 1 and Fig. 1). Aspergillus fumigatus is the most frequently recovered species, but other species often recovered include A. niger, A. flavus, and A. terreus. A. terreus is of special importance due to its increased resistance to amphotericin B and, although rare, its potential to cause true fungemia in patients with hematologic malignancies (57, 58).
Yeast species.
Clinical manifestations of Candida species infection in cancer patients include candidemia, chronic disseminated candidiasis, and mucosal candidiasis. Candidemia may develop early, in the preengraftment or early postengraftment period, and in persistently neutropenic cancer patients remains associated with poor outcomes (59). The increased use of fluconazole prophylaxis has resulted in a significant decrease in invasive candidal disease (from 18% to 7% in one study), especially that caused by Candida albicans (60). Concomitantly, an increase in fluconazole-resistant non-albicans Candida species has been observed in geographically diverse centers (61). C. glabrata is the most common non-albicans Candida species recovered in patients with hematologic malignancies, but other species, including C. krusei and C. parapsilosis, are also observed (60, 62).
Trichosporon species, Geotrichum species, and Rhodotorula species are a few of the rare but emerging opportunistic yeasts causing significant morbidity and mortality in oncology patients. Patients with hematologic malignancies, particularly those with acute leukemia, account for 50 to 90% of reported cases, with a mortality rate ranging from 35 to 77% (63, 64). Identified risk factors for increased mortality included active neutropenia Trichosporon species fungemia and admission to the intensive care unit (ICU) (64).
Non-Aspergillus molds.
Infections caused by Rhizopus species and Mucor species are still uncommon in cancer patients, especially in patients with solid tumors (58). However, the mortality rates associated with these infections remains significantly high, and with the clinical presentation mimicking that of Aspergillus pneumonia, distinction of the two syndromes without laboratory cultures may be challenging. In a recent large study reviewing cases of non-Aspergillus invasive mold infections in allogeneic HSCT recipients, the overall incidence rate was only 1% (124 cases/11,980 patients) for development of infection within 1 year of the transplant. The overall mortality within 1 year of transplant was 78%, which has not changed significantly from rates of 80% reported in the 1990s (65, 66). Mucormycosis infections occur more commonly in the preengraftment period, although higher mortality is associated with infections occurring in the late postengraftment period (Fig. 1). Other significant mold pathogens in transplant patients include Fusarium species and Scedosporium species. Risk factors for these infections include umbilical cord blood transplants and prior CMV infection for Fusarium (Table 1) (65, 67).
Laboratory diagnosis.
Methods used to detect fungi include culture, fungal stains, and fungal antigen assays, such as the Aspergillus galactomannan and the β-d-glucans tests. Each of these assays has advantages and limitations, including low recovery rates and long turnaround time (e.g., culture) or limited specificity (e.g., β-d-glucans). The utility of antigen tests is varied and highly dependent on the patient population, exposure to antifungal prophylaxis, and the responsible mold (68, 69). For example, in patients with hematologic malignancies, the sensitivity of the galactomannan for invasive fungal infection (IFI) caused by A. fumigatus was lower (13%) than that caused by other Aspergillus species (49%) (70). This is challenging, since A. fumigatus is the most common Aspergillus species causing IFI, and many oncology patients are on antifungal prophylaxis. Serial monitoring of serum galactomannan or β-d-glucans can increase sensitivity, although the exact frequency of testing is unknown. A different approach combining serial monitoring of both galactomannan and β-d-glucans shows potential to increase the diagnostic and prognostic value of these tests in monitoring of the treatment response (71). The application of these tests to other specimen types (e.g., bronchoalveolar lavage [BAL] fluid and CSF) is increasingly performed, although the interpretation of the results is not always straightforward. The multitude of available tests underscores the continued challenge of diagnosing IFI. Furthermore, special processing of tissue samples (e.g., mincing instead of grinding) is necessary to increase the recovery of Mucorales, and a high level of suspicion is necessary on the part of clinicians to alert the laboratory. Laboratory-developed molecular assays generally have higher sensitivity than culture but limited clinical specificity and limited range, only targeting a few organisms. Panfungal molecular assays, which target a larger number of clinically relevant fungal pathogens, have also been reported, with sensitivity and specificity ranging from 75 to 100% and 75 to 95% depending on the target and specimen type (72, 73). Of note, the current definition of IFI (proven, probable, and possible) as stated by the Mycoses Study Group and the Cooperative Group of the European Organization for Research and Treatment of Cancer (EORTC) does not yet include the use of molecular tests (74).
Pneumocystis jirovecii.
Implementation of routine prophylaxis with trimethoprim-sulfamethoxazole has significantly reduced the incidence of Pneumocystis jirovecii pneumonia (PCP) from 16% in HSCT recipients to less than 1% (75, 76). Williams and colleagues have recently reviewed PCP data from the Center for International Blood and Marrow Transplant Research (CIBMTR), which includes 66 centers around the world. The overall incidence rate was less than 1%, with rates in allogeneic HSCT recipients double those in autologous HSCT recipients (0.68 versus 0.28%, respectively) (76). Most PCP infections occurred in the postengraftment period, with 50% of cases occurring after 270 days, but up to 25% of cases were observed early, during preengraftment. Although overall incidence was rare, the mortality rate associated with PCP remains high, and as such, PCP continues to be an important fungal infection in cancer patients.
Laboratory diagnosis.
Direct microscopic examination, including calcofluor white stain and direct fluorescent-antibody assay, provides a fairly specific means to identify the organism, but it lacks reliable sensitivity to exclude infection. Molecular methods (e.g., PCR) have demonstrated a significantly higher sensitivity for detecting the presence of P. jirovecii. In HSCT recipients and patients with hematologic malignancies, pulmonary symptoms and radiological findings (e.g., ground-glass opacities) may be suggestive of PCP. However, the prevalence of P. jirovecii infection without disease, often labeled airway “colonization,” remains unclear. Algorithms combining high levels of β-d-glucans (e.g., >500 pg/ml) and positive PCR results are options used to increase the specificity of laboratory diagnostics of PCP. Since β-d-glucans is produced by various fungal pathogens, the exact threshold that provides specificity for PCP in oncology patients remains to be determined.
PARASITIC INFECTIONS
Toxoplasma gondii.
Surveillance studies estimate that the overall seroprevalence of T. gondii in allogeneic HSCT recipients is approximately 10% in the United States and 8 to 16% in Europe (77, 78). T. gondii infections occur most commonly from reactivation of a latent infection following transplantation and less frequently from primary infection or donor-related infections. Among HSCT recipients, the risk of reactivation is higher for Toxoplasma-seropositive allogeneic HSCT recipients and lower in seronegative autologous HSCT recipients. In one review of the existing literature, 73% of toxoplasmosis occurred following reactivation in allo-HSCT patients compared to 41% reactivation in autologous HSCT patients. In allogeneic HSCT recipients, the development of toxoplasmosis disease, primarily central nervous system and disseminated disease, results in attributable mortality rates of 62%, with studies reporting mortality rates as high as 100% (78). In order to prevent the reactivation of T. gondii in HSCT recipients, many transplant centers have implemented a prophylaxis protocol (e.g., trimethoprim-sulfamethoxazole) for seropositive HSCT recipients prior to engraftment, as most cases of toxoplasmosis occurred in the first 90 days posttransplantation (79).
Laboratory diagnosis.
Prompt diagnosis of Toxoplasma reactivation is necessary to decrease mortality rates in high-risk patients. The most common method used is molecular surveillance using nucleic acid amplification tests (e.g., PCR) at various intervals posttransplantation for a predetermined period, depending on the centers and the type of transplants (e.g., cord blood HSCT versus autologous HSCT). Both qualitative and quantitative PCRs are used, and all the available methods are nonstandardized LDTs with different but generally similar performance characteristics (77). The yield of PCR surveillance (i.e., number of reactivating patients) varies widely, and as such, the utility and associated cost of this monitoring approach in HSCT recipients with low seropositive prevalence and on adequate prophylaxis are not always evident. In addition to molecular methods for diagnosis, serology assays (both indirect fluorescent-antibody assay [IFA] and enzyme immunoassay [EIA]) may be used once a patient's immune system has recovered. As Toxoplasma IgM is rarely detected, an important test to distinguish between recent and past infection is the IgG avidity assay, which is most commonly performed at reference laboratories.
Strongyloides stercoralis.
HSCT recipients and hematologic malignancy patients are at increased risk of developing Strongyloides stercoralis hyperinfection syndrome, following administration of a high level of corticosteroids and decrease in cell-mediated immunity. Hyperinfection syndrome results from an increase in the concentration of circulating Strongyloides filariform larvae occurring through autoinfection with parasites acquired from previous exposures. Further complications, including bacterial sepsis, with enteric Gram-negative bacilli (K. pneumoniae and P. aeruginosa) may occur as a result of the filariform larvae moving from the intestinal tract into the circulation. Reported mortality rates for HSCT patients are high, approximately 90%, even after administration of ivermectin. This is due in part to challenges in recognizing and diagnosing the infection. Since infections occur primarily in patients with latent infections, serological screening is recommended for patients from regions of endemicity or for those with the appropriate travel history prior to HSCT. Unlike patients with hematologic malignancies, solid-tumor oncology patients generally do not develop disseminated or hyperinfection syndrome with S. stercoralis. In one study, approximately 50% of cancer patients with Strongyloides infection had solid tumors, and none of these patients developed disseminated infections, even following the administration of high-dose corticosteroids (80).
Laboratory diagnosis.
Timely diagnosis of S. stercoralis can be challenging, requiring a high degree of suspicion. Patients may present with transient mild eosinophilia and a range of nonspecific symptoms, including diarrhea, skin rash, dry cough, dyspnea, and wheezing. The primary method for diagnosis remains the ova and parasites (OVP) exam, performed on stool samples, as well as respiratory specimens, including expectorated sputum, bronchoalveolar lavage fluid, and CSF. However, due to intermittent shedding, multiple stool samples (>3 stools) need to be analyzed to increase the sensitivity, which is 30% for one sample and 100% for 7 samples. To complement microscopy, or in case of negative OVP result, the detection of Strongyloides antibodies may provide the only evidence of infection in the presence of eosinophilia, with sensitivity ranging from 88 to 95%. Serological tests, however, have lower sensitivity in immunocompromised patients and are subject to false-positive results from cross-reaction with antigens from other helminths, and a single positive result cannot distinguish between past and present infections (81). The sensitivity of S. stercoralis detection may be enhanced with the use of culture methods (e.g., the Baermann culture, Harada-Mori filter paper test tube culture, and stool agar culture, with stool agar culture being the simplest to perform [82]). In some instances, S. stercoralis may be detected as an incidental finding on bacterial cultures of stool or respiratory specimens by visualizing the migrating larval tracks.
CONCLUSIONS AND FUTURE PERSPECTIVES
Significant improvements in the conditioning regimen, the sources of stem cells, the type of transplant, and the pre- and posttransplant care protocols have resulted in patients surviving for many years following their procedure. However, the immunosuppression associated with both HSCT and chemotherapy treatments increases the susceptibility of oncology patients to a variety of infectious agents at different stages of their treatment, and as such, infectious complications remain a significant cause of morbidity and mortality in these patients. This minireview provided an overview of some of the most common infections in oncology patients and presented current laboratory diagnostic methods used by clinical microbiologists supporting the care of these patients.
What are the challenges and opportunities in the laboratory diagnosis of HSCT recipients and oncology patients? The significant overlap between infectious and noninfectious causes of syndromes experienced by these patients and the decreased inflammatory and immune responses associated with the procedures used present a significant challenge in knowing when to stop the search for a pathogen or when to continue and throw the proverbial “kitchen sink” at the problem. The increased availability of syndromic panels and other molecular diagnostic tests has significantly improved our ability to identify pathogens with increased sensitivity and speed. These panels are helpful in identifying pathogens which would otherwise require a specific order to rule out (e.g., sapovirus). However, opportunity exists to include pathogens that are emerging in cancer patients (e.g., Microsporidia). These sensitive assays are also challenging us to reconsider what is significant and take a closer look at the host to make that determination. As discussed throughout this minireview, the earlier a pathogen can be identified, the better the outcome for the cancer patient, as targeted therapy and care can be rapidly implemented. We do, however, need a better kitchen sink to further reduce the morbidity and mortality associated with infections postcancer therapy. While panfungal or panbacterial sequencing assays have proven useful, the potential of panpathogen approaches, possible with unbiased whole-genome sequencing (WGS), holds promise for further expanding our diagnostic capabilities. Other non-nucleic acid-based methods including pathogens and/or host biomarkers will be needed to assist in the interpretation and determination of the clinical significant of WGS data. This minireview highlighted a few of the challenges and the many opportunities that clinical microbiologists have in supporting the care of cancer patients.
Biography
Esther Babady, Ph.D., is the Director of Clinical Operations for the Microbiology Laboratory Service and an associate attending in the Department of Laboratory Medicine at Memorial Sloan-Kettering Cancer (MSKCC) in New York City, NY. She also serves as the director of the CPEP clinical microbiology fellowship program at MSKCC. She received her Ph.D. in Biochemistry and Molecular Biology and completed a postdoctoral CPEP fellowship in Clinical Microbiology, both at the Mayo Clinic in Rochester, MN, before joining MSKCC. She is board certified by the American Board of Medical Microbiology and serves on the editorial board of the Journal of Clinical Microbiology. Her research interests include rapid diagnosis of infections in immunocompromised hosts, the development and evaluation of the clinical utility of molecular microbiology assays, and the application of molecular methods for the surveillance and prevention of hospital-acquired infections
REFERENCES
- 1.Pasquini MC, Zhu X. 2015. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides, 2015. Center for International Blood & Marrow Transplant Research, Milwaukee, WI: https://www.cibmtr.org/referencecenter/slidesreports/summaryslides/Pages/index.aspx. [Google Scholar]
- 2.Thomas ED. 1994. The Nobel lectures in immunology. The Nobel Prize for Physiology or Medicine, 1990. Bone marrow transplantation–past, present and future. Scand J Immunol 39:339–345. [DOI] [PubMed] [Google Scholar]
- 3.DeVita VT Jr, Chu E. 2008. A history of cancer chemotherapy. Cancer Res 68:8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 4.Mackall C, Fry T, Gress R, Peggs K, Storek J, Toubert A, Center for International Blood and Marrow Transplant Research (CIBMTR), National Marrow Donor Program (NMDP), European Blood and Marrow Transplant Group (EBMT), American Society of Blood and Marrow Transplantation (ASBMIT), Canadian Blood and Marrow Transplant G (CBMTG), Infectious Disease Society of America (IDSA) Society for Healthcare Epidemiology of America (SHEA), Association of Medical Microbiology and Infectious Diseases Canada (AMMI), Centers for Disease Control and Prevention (CDC). 2009. Background to hematopoietic cell transplantation, including post transplant immune recovery. Bone Marrow Transplant 44:457–462. doi: 10.1038/bmt.2009.255. [DOI] [PubMed] [Google Scholar]
- 5.Antin JH. 2005. Immune reconstitution: the major barrier to successful stem cell transplantation. Biol Blood Marrow Transplant 11:43–45. doi: 10.1016/j.bbmt.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Baden LR, Bensinger W, Angarone M, Casper C, Dubberke ER, Freifeld AG, Garzon R, Greene JN, Greer JP, Ito JI, Karp JE, Kaul DR, King E, Mackler E, Marr KA, Montoya JG, Morris-Engemann A, Pappas PG, Rolston K, Segal B, Seo SK, Swaminathan S, Naganuma M, Shead DA, National Comprehensive Cancer Network. 2012. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 10:1412–1445. [DOI] [PubMed] [Google Scholar]
- 7.Young JA, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C, Knust K, Horowitz MM, Confer DL, Dubberke ER, Pergam SA, Marty FM, Strasfeld LM, Brown JW, Langston AA, Schuster MG, Kaul DR, Martin SI, Anasetti C, Blood Marrow Transplant Clinical Trials Network Trial. 2016. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant 22:359–370. doi: 10.1016/j.bbmt.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kameda K, Kimura S, Akahoshi Y, Nakano H, Harada N, Ugai T, Wada H, Yamasaki R, Ishihara Y, Kawamura K, Sakamoto K, Ashizawa M, Sato M, Terasako-Saito K, Nakasone H, Kikuchi M, Yamazaki R, Kanda J, Kako S, Tanihara A, Nishida J, Kanda Y. 2016. High incidence of afebrile bloodstream infection detected by surveillance blood culture in patients on corticosteroid therapy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 22:371–377. doi: 10.1016/j.bbmt.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 10.Satlin MJ, Soave R, Racanelli AC, Shore TB, van Besien K, Jenkins SG, Walsh TJ. 2014. The emergence of vancomycin-resistant enterococcal bacteremia in hematopoietic stem cell transplant recipients. Leuk Lymphoma 55:2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamboj M, Chung D, Seo SK, Pamer EG, Sepkowitz KA, Jakubowski AA, Papanicolaou G. 2010. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol Blood Marrow Transplant 16:1576–1581. doi: 10.1016/j.bbmt.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montassier E, Batard E, Gastinne T, Potel G, de La Cochetiere MF. 2013. Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. Eur J Clin Microbiol Infect Dis 32:841–850. doi: 10.1007/s10096-013-1819-7. [DOI] [PubMed] [Google Scholar]
- 13.Nesher L, Rolston KV. 2014. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 42:5–13. doi: 10.1007/s15010-013-0525-9. [DOI] [PubMed] [Google Scholar]
- 14.Satlin MJ, Calfee DP, Chen L, Fauntleroy KA, Wilson SJ, Jenkins SG, Feldman EJ, Roboz GJ, Shore TB, Helfgott DC, Soave R, Kreiswirth BN, Walsh TJ. 2013. Emergence of carbapenem-resistant Enterobacteriaceae as causes of bloodstream infections in patients with hematologic malignancies. Leuk Lymphoma 54:799–806. [DOI] [PubMed] [Google Scholar]
- 15.Trecarichi EM, Tumbarello M, Spanu T, Caira M, Fianchi L, Chiusolo P, Fadda G, Leone G, Cauda R, Pagano L. 2009. Incidence and clinical impact of extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect 58:299–307. doi: 10.1016/j.jinf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Sivagnanam S, Pergam SA. 2016. Legionellosis in transplantation. Curr Infect Dis Rep 18:9. doi: 10.1007/s11908-016-0517-x. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson KL, Miceli MH, Tarrand JJ, Kontoyiannis DP. 2008. Legionella pneumonia in cancer patients. Medicine (Baltimore) 87:152–159. doi: 10.1097/MD.0b013e3181779b53. [DOI] [PubMed] [Google Scholar]
- 18.del Castillo M, Lucca A, Plodkowski A, Huang YT, Kaplan J, Gilhuley K, Babady NE, Seo SK, Kamboj M. 2016. Atypical presentation of Legionella pneumonia among patients with underlying cancer: a fifteen-year review. J Infect 72:45–51. doi: 10.1016/j.jinf.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamboj M, Son C, Cantu S, Chemaly RF, Dickman J, Dubberke E, Engles L, Lafferty T, Liddell G, Lesperance ME, Mangino JE, Martin S, Mayfield J, Mehta SA, O'Rourke S, Perego CS, Taplitz R, Eagan J, Sepkowitz KA. 2012. Hospital-onset Clostridium difficile infection rates in persons with cancer or hematopoietic stem cell transplant: a C3IC network report. Infect Control Hosp Epidemiol 33:1162–1165. doi: 10.1086/668023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso CD, Kamboj M. 2014. Clostridium difficile infection (CDI) in solid organ and hematopoietic stem cell transplant recipients. Curr Infect Dis Rep 16:414. doi: 10.1007/s11908-014-0414-0. [DOI] [PubMed] [Google Scholar]
- 21.Donskey CJ, Kundrapu S, Deshpande A. 2015. Colonization versus carriage of Clostridium difficile. Infect Dis Clin North Am 29:13–28. doi: 10.1016/j.idc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Weinstock DM, Feinstein MB, Sepkowitz KA, Jakubowski A. 2003. High rates of infection and colonization by nontuberculous mycobacteria after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 31:1015–1021. doi: 10.1038/sj.bmt.1704043. [DOI] [PubMed] [Google Scholar]
- 23.Gaviria JM, Garcia PJ, Garrido SM, Corey L, Boeckh M. 2000. Nontuberculous mycobacterial infections in hematopoietic stem cell transplant recipients: characteristics of respiratory and catheter-related infections. Biol Blood Marrow Transplant 6:361–369. doi: 10.1016/S1083-8791(00)70012-7. [DOI] [PubMed] [Google Scholar]
- 24.Redelman-Sidi G, Sepkowitz KA. 2010. Rapidly growing mycobacteria infection in patients with cancer. Clin Infect Dis 51:422–434. doi: 10.1086/655140. [DOI] [PubMed] [Google Scholar]
- 25.Apiwattankul N, Flynn PM, Hayden RT, Adderson EE. 2015. Infections caused by rapidly growing mycobacteria spp. in children and adolescents with cancer. J Pediatr Infect Dis 4:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erard V, Guthrie KA, Seo S, Smith J, Huang M, Chien J, Flowers ME, Corey L, Boeckh M. 2015. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation. Clin Infect Dis 61:31–39. doi: 10.1093/cid/civ215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babady NE, Cheng C, Cumberbatch E, Stiles J, Papanicolaou G, Tang YW. 2015. Monitoring of cytomegalovirus viral loads by two molecular assays in whole-blood and plasma samples from hematopoietic stem cell transplant recipients. J Clin Microbiol 53:1252–1257. doi: 10.1128/JCM.03435-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loren AW, Porter DL, Stadtmauer EA, Tsai DE. 2003. Post-transplant lymphoproliferative disorder: a review. Bone Marrow Transplant 31:145–155. doi: 10.1038/sj.bmt.1703806. [DOI] [PubMed] [Google Scholar]
- 29.Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, Ljungman P, Engelhard D, Second European Conference on Infections in Leukemia. 2009. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 43:757–770. doi: 10.1038/bmt.2008.386. [DOI] [PubMed] [Google Scholar]
- 30.Aisenberg GM, Torres HA, Tarrand J, Safdar A, Bodey G, Chemaly RF. 2009. Herpes simplex virus lower respiratory tract infection in patients with solid tumors. Cancer 115:199–206. doi: 10.1002/cncr.24011. [DOI] [PubMed] [Google Scholar]
- 31.Fan F, Stiles J, Mikhlina A, Lu X, Babady NE, Tang YW. 2014. Clinical validation of the Lyra direct HSV 1+2/VZV assay for simultaneous detection and differentiation of three herpesviruses in cutaneous and mucocutaneous lesions. J Clin Microbiol 52:3799–3801. doi: 10.1128/JCM.02098-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binnicker MJ, Espy MJ, Irish CL. 2014. Rapid and direct detection of herpes simplex virus in cerebrospinal fluid by use of a commercial real-time PCR assay. J Clin Microbiol 52:4361–4362. doi: 10.1128/JCM.02623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki J, Numata A, Yamamoto E, Fujii E, Tanaka M, Kanamori H. 2015. Impact of human herpesvirus 6 reactivation on outcomes of allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 21:2017–2022. doi: 10.1016/j.bbmt.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, Kohno K, Kashima K, Ohtsuka E, Kadota J. 2006. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis 193:68–79. doi: 10.1086/498531. [DOI] [PubMed] [Google Scholar]
- 35.Dulery R, Salleron J, Dewilde A, Rossignol J, Boyle EM, Gay J, de Berranger E, Coiteux V, Jouet JP, Duhamel A, Yakoub-Agha I. 2012. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biol Blood Marrow Transplant 18:1080–1089. doi: 10.1016/j.bbmt.2011.12.579. [DOI] [PubMed] [Google Scholar]
- 36.Zerr DM, Boeckh M, Delaney C, Martin PJ, Xie H, Adler AL, Huang ML, Corey L, Leisenring WM. 2012. HHV-6 reactivation and associated sequelae after hematopoietic cell transplantation. Biol Blood Marrow Transplant 18:1700–1708. doi: 10.1016/j.bbmt.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson AL, Dahi PB, Zheng J, Devlin SM, Lubin M, Gonzales AM, Giralt SA, Perales MA, Papadopoulos EB, Ponce DM, Young JW, Kernan NA, Scaradavou A, O'Reilly RJ, Small TN, Papanicolaou G, Barker JN. 2014. Frequent human herpesvirus-6 viremia but low incidence of encephalitis in double-unit cord blood recipients transplanted without antithymocyte globulin. Biol Blood Marrow Transplant 20:787–793. doi: 10.1016/j.bbmt.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedlak RH, Cook L, Huang ML, Magaret A, Zerr DM, Boeckh M, Jerome KR. 2014. Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR. Clin Chem 60:765–772. doi: 10.1373/clinchem.2013.217240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lion T. 2014. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 27:441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YJ, Huang YT, Kim SJ, Maloy M, Tamari R, Giralt SA, Papadopoulos EB, Jakubowski AA, Papanicolaou GA. 2016. Adenovirus viremia in adult CD34+ selected hematopoietic cell transplant recipients: low incidence and high clinical impact. Biol Blood Marrow Transplant 22:174–178. doi: 10.1016/j.bbmt.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sive JI, Thomson KJ, Morris EC, Ward KN, Peggs KS. 2012. Adenoviremia has limited clinical impact in the majority of patients following alemtuzumab-based allogeneic stem cell transplantation in adults. Clin Infect Dis 55:1362–1370. doi: 10.1093/cid/cis689. [DOI] [PubMed] [Google Scholar]
- 42.Roddie C, Paul JP, Benjamin R, Gallimore CI, Xerry J, Gray JJ, Peggs KS, Morris EC, Thomson KJ, Ward KN. 2009. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis 49:1061–1068. doi: 10.1086/605557. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz S, Vergoulidou M, Schreier E, Loddenkemper C, Reinwald M, Schmidt-Hieber M, Flegel WA, Thiel E, Schneider T. 2011. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood 117:5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moser O, Luck S, Dilloo D, Eis-Hubinger AM, Simon A. 2011. Sapovirus as a gastrointestinal pathogen in febrile pediatric patients with cancer. J Med Virol 83:2233–2236. doi: 10.1002/jmv.22219. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan A, Klepper C, Sunkara A, Kang G, Carr J, Gu Z, Leung W, Hayden RT. 2015. Impact of adenoviral stool load on adenoviremia in pediatric hematopoietic stem cell transplant recipients. Pediatr Infect Dis J 34:562–565. doi: 10.1097/INF.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Doef HP, Bathoorn E, van der Linden MP, Wolfs TF, Minderhoud AL, Bierings MB, Wensing AM, Lindemans CA. 2016. Astrovirus outbreak at a pediatric hematology and hematopoietic stem cell transplant unit despite strict hygiene rules. Bone Marrow Transplant 51:747–750. doi: 10.1038/bmt.2015.337. [DOI] [PubMed] [Google Scholar]
- 47.Brown JR, Morfopoulou S, Hubb J, Emmett WA, Ip W, Shah D, Brooks T, Paine SM, Anderson G, Virasami A, Tong CY, Clark DA, Plagnol V, Jacques TS, Qasim W, Hubank M, Breuer J. 2015. Astrovirus VA1/HMO-C: an increasingly recognized neurotropic pathogen in immunocompromised patients. Clin Infect Dis 60:881–888. doi: 10.1093/cid/ciu940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheahan A, Copeland G, Richardson L, McKay S, Chou A, Babady NE, Tang YW, Boulad F, Eagan J, Sepkowitz K, Kamboj M. 2015. Control of norovirus outbreak on a pediatric oncology unit. Am J Infect Control 43:1066–1069. doi: 10.1016/j.ajic.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chemaly RF, Shah DP, Boeckh MJ. 2014. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 59(Suppl 5):S344–S351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Memoli MJ, Athota R, Reed S, Czajkowski L, Bristol T, Proudfoot K, Hagey R, Voell J, Fiorentino C, Ademposi A, Shoham S, Taubenberger JK. 2014. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis 58:214–224. doi: 10.1093/cid/cit725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah DP, Ghantoji SS, Mulanovich VE, Ariza-Heredia EJ, Chemaly RF. 2012. Management of respiratory viral infections in hematopoietic cell transplant recipients. Am J Blood Res 2:203–218. [PMC free article] [PubMed] [Google Scholar]
- 52.Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. 2013. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah DP, Shah PK, Azzi JM, Chemaly RF. 2016. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett 370:358–364. doi: 10.1016/j.canlet.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renaud C, Xie H, Seo S, Kuypers J, Cent A, Corey L, Leisenring W, Boeckh M, Englund JA. 2013. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant 19:1220–1226. doi: 10.1016/j.bbmt.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milano F, Campbell AP, Guthrie KA, Kuypers J, Englund JA, Corey L, Boeckh M. 2010. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 115:2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson L, Brite J, Del Castillo M, Childers T, Sheahan A, Huang YT, Dougherty E, Babady NE, Sepkowitz K, Kamboj M. 2015. Comparison of respiratory virus shedding by conventional and molecular testing methods in patients with haematological malignancy. Clin Microbiol Infect 22:380.e1–380.e7. doi: 10.1016/j.cmi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kontoyiannis DP, Sumoza D, Tarrand J, Bodey GP, Storey R, Raad II. 2000. Significance of aspergillemia in patients with cancer: a 10-year study. Clin Infect Dis 31:188–189. doi: 10.1086/313918. [DOI] [PubMed] [Google Scholar]
- 58.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 59.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marr KA, Seidel K, Slavin MA, Bowden RA, Schoch HG, Flowers ME, Corey L, Boeckh M. 2000. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood 96:2055–2061. [PubMed] [Google Scholar]
- 61.Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. 2012. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 73:45–48. doi: 10.1016/j.diagmicrobio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I. 2008. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112:2493–2499. doi: 10.1002/cncr.23466. [DOI] [PubMed] [Google Scholar]
- 63.Girmenia C, Pagano L, Martino B, D'Antonio D, Fanci R, Specchia G, Melillo L, Buelli M, Pizzarelli G, Venditti M, Martino P, GIMENA Program . 2005. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J Clin Microbiol 43:1818–1828. doi: 10.1128/JCM.43.4.1818-1828.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chitasombat MN, Kofteridis DP, Jiang Y, Tarrand J, Lewis RE, Kontoyiannis DP. 2012. Rare opportunistic (non-Candida, non-Cryptococcus) yeast bloodstream infections in patients with cancer. J Infect 64:68–75. doi: 10.1016/j.jinf.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riches ML, Trifilio S, Chen M, Ahn KW, Langston A, Lazarus HM, Marks DI, Martino R, Maziarz RT, Papanicolou GA, Wingard JR, Young JA, Bennett CL. 2016. Risk factors and impact of non-Aspergillus mold infections following allogeneic HCT: a CIBMTR infection and immune reconstitution analysis. Bone Marrow Transplant 51:277–282. doi: 10.1038/bmt.2015.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. 2000. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis 30:851–856. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 67.Park BJ, Pappas PG, Wannemuehler KA, Alexander BD, Anaissie EJ, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt L, Ito JI, Kauffman CA, Lyon GM, Marr KA, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wingard JR, Walsh TJ, Kontoyiannis DP. 2011. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis 17:1855–1864. doi: 10.3201/eid1710.110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfeiffer CD, Fine JP, Safdar N. 2006. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 42:1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 69.Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, Marchetti O, Third European Conference on Infections in Leukemia (ECIL-3). 2012. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis 54:633–643. doi: 10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]
- 70.Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I. 2009. Utility of galactomannan enzyme immunoassay and (1,3) beta-d-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol 47:129–133. doi: 10.1128/JCM.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neofytos D, Railkar R, Mullane KM, Fredricks DN, Granwehr B, Marr KA, Almyroudis NG, Kontoyiannis DP, Maertens J, Fox R, Douglas C, Iannone R, Kauh E, Shire N. 2015. Correlation between circulating fungal biomarkers and clinical outcome in invasive aspergillosis. PLoS One 10:e0129022. doi: 10.1371/journal.pone.0129022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babady NE, Miranda E, Gilhuley KA. 2011. Evaluation of Luminex xTAG fungal analyte-specific reagents for rapid identification of clinically relevant fungi. J Clin Microbiol 49:3777–3782. doi: 10.1128/JCM.01135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White PL, Wingard JR, Bretagne S, Loffler J, Patterson TF, Slavin MA, Barnes RA, Pappas PG, Donnelly JP. 2015. Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin Infect Dis 61:1293–1303. doi: 10.1093/cid/civ507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ, Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer, Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez M, Fishman JA. 2004. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev 17:770–782, table of contents. doi: 10.1128/CMR.17.4.770-782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams KM, Ahn KW, Chen M, Aljurf MD, Agwu AL, Chen AR, Walsh TJ, Szabolcs P, Boeckh MJ, Auletta JJ, Lindemans CA, Zanis-Neto J, Malvezzi M, Lister J, de Toledo Codina JS, Sackey K, Chakrabarty JL, Ljungman P, Wingard JR, Seftel MD, Seo S, Hale GA, Wirk B, Smith MS, Savani BN, Lazarus HM, Marks DI, Ustun C, Abdel-Azim H, Dvorak CC, Szer J, Storek J, Yong A, Riches MR. 2016. The incidence, mortality and timing of Pneumocystis jiroveci [sic] pneumonia after hematopoietic cell transplantation: a CIBMTR analysis. Bone Marrow Transplant 51:573–580. doi: 10.1038/bmt.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martino R, Bretagne S, Einsele H, Maertens J, Ullmann AJ, Parody R, Schumacher U, Pautas C, Theunissen K, Schindel C, Munoz C, Margall N, Cordonnier C, Infectious Disease Working Party of the European Group for Blood and Marrow Transplantation. 2005. Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin Infect Dis 40:67–78. doi: 10.1086/426447. [DOI] [PubMed] [Google Scholar]
- 78.Gajurel K, Dhakal R, Montoya JG. 2015. Toxoplasma prophylaxis in haematopoietic cell transplant recipients: a review of the literature and recommendations. Curr Opin Infect Dis 28:283–292. doi: 10.1097/QCO.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 79.Isa F, Saito K, Huang YT, Schuetz A, Babady NE, Salvatore S, Pessin M, van Besien K, Perales MA, Giralt S, Sepkowitz K, Papanicolaou GA, Soave R, Kamboj M. 2016. Implementation of molecular surveillance after a cluster of fatal toxoplasmosis at two neighboring transplant centers. Clin Infect Dis 63:565–568. doi: 10.1093/cid/ciw330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Safdar A, Malathum K, Rodriguez SJ, Husni R, Rolston KV. 2004. Strongyloidiasis in patients at a comprehensive cancer center in the United States. Cancer 100:1531–1536. doi: 10.1002/cncr.20120. [DOI] [PubMed] [Google Scholar]
- 81.Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Munoz J. 2013. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7:e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clinical and Laboratory Standards Institute (CLSI). 2005. Procedures for the recovery and identification of parasites from the intestinal tract; approved guideline—2nd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]



