Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) infection is a global health care problem. Large studies (e.g., >25,000 patients) show that active surveillance testing (AST) followed by contact precautions for positive patients is an effective approach for MRSA disease control. With this approach, the clinical laboratory will be asked to select what AST method(s) to use and to provide data monitoring outcomes of the infection prevention interventions. This minireview summarizes evidence for MRSA disease control, reviews the involvement of the laboratory, and provides examples of how to undertake a program cost analysis. Health care organizations with total MRSA clinical infections of >0.3/1,000 patient days or bloodstream infections of >0.03/1,000 patient days should implement a MRSA control plan.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) has been a major health care problem for more than 50 years (1). The latest U.S. national data indicate that rates of infection are slowly decreasing but that the disease risk remains substantial (2), including an estimated 59,000 MRSA cases resulting in 9,670 deaths during 2012 (3). With the most recent published disease trends being from 2012, the Centers for Disease Control and Prevention (CDC) has concluded that “MRSA remains an important public health problem and more remains to be done to further decrease risks of developing these infections” (2). Given this information, it is very likely that clinical microbiology laboratories will be actively involved with MRSA control programs. The Joint Commission requires the clinical microbiology laboratory to provide needed support for infection prevention and control (4). Furthermore, the CDC has published data suggesting that an immediate, coordinated national infection control and antibiotic stewardship effort to reduce health care-associated infections (HAIs) from carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Pseudomonas aeruginosa, invasive MRSA, and Clostridium difficile would result in 619,000 fewer HAIs over 5 years (5). All these indicate that clinical microbiology laboratories will be actively involved in infection control for many years to come.
Laboratory support of infection prevention and control can have many roles, including pathogen detection, surveillance, data analysis, support of outbreak investigation, and monitoring the outcome of intervention(s) designed to lower health care-associated infections (6). The purpose of this minireview is to use very large published studies on MRSA control reported after 2005 in order to assist the laboratory in implementing surveillance programs relating to MRSA disease prevention and control when the decision to do this has been made at its health care organization. We will review the clinical impact of major approaches to MRSA disease control in order to predict the likelihood of future intervention strategies, highlight the laboratory role for each of these, suggest an approach to decision making for active surveillance testing (AST) through assessment of economic benefit, and describe a general approach for monitoring disease burden (including the outcome of any intervention). Based on the large published studies, we will present reasonable disease reduction target(s) that can be expected from an effective MRSA control program and demonstrate how the clinical microbiology laboratory can help prepare a MRSA disease risk assessment for presentation to the administrators of the infection prevention and control program that is useful in determining if MRSA control efforts are adequate. We have recently published a comprehensive review of the molecular diagnostic tests available for detection of MRSA (7), so this description will not be part of the current review. However, with these two sources, our goal is that the clinical laboratory can actively and expertly support MRSA infection prevention efforts when asked to do so.
METHODS
For our literature review, we used PubMed.gov (MEDLINE) with the terms “MRSA,” “Transmission,” and “Prevention” to search the medical literature from 2005 through March 2016. Articles selected for review were those clinical studies or models incorporating at least 25,000 patients or model simulations. Only models using original data were included. Individual hospital reports that were also part of large health care organization studies were not included so as not to report the same data twice. The reason for selecting a high threshold for patient encounters assessed was to evaluate those reports with sufficiently robust data sets so that the information reported was highly likely to be reproducible and generalizable.
As part of this review we have also updated our own data to encompass 10 full years of MRSA surveillance (D. Schora, B. Smith, A. Robicsek, R. Thomson, and L.R. Peterson. The impact of MRSA admission nasal surveillance on nosocomial infection: ten years of testing, presented at Microbe, Boston, MA, 16–20 June 2016). Data were reviewed from 1 August /2003 to 31 July 2015. Yearly MRSA nosocomial infection rates were determined with the Carefusion MedMined Nosocomial Infection Marker for blood, respiratory tract, urinary tract, and wound infections, which we have validated as a reliable method to determine MRSA clinical infection rates (8). The data for patient days were gathered from finance reports and included observation days. Statistical analysis was done as previously described (9). Briefly, infection rates were compared between the 3 study periods using Poisson models, implemented via SAS PROC GENMOD with Poisson distribution, log link function, and log of patient days as offsets. The clinical MRSA HAI infection count was the dependent variable, and the independent variable was the study period (i.e., 2003 baseline as period 1, 2004 intensive care unit [ICU] surveillance as period 2, or 2005 to 2015 hospital-wide surveillance as period 3). Aggregate HAI MRSA rates were further analyzed with a segmented Poisson regression model (8).
RESULTS
In addition to core infection control practices at health care organizations, such as monitoring hand hygiene performance, there are three major approaches to MRSA infection prevention that have been systematically studied. These are (i) universal isolation (using contact precautions), (ii) universal decolonization (source control), and (iii) AST (universal or targeted) with either isolation of MRSA carriers, decolonization of these patients, or both. Following is a discussion of large data sets studying each approach.
Clinical impact of MRSA control practices. (i) Universal isolation.
During 2012, Harris and colleagues performed a 9-month prospective, cluster-randomized study of patients in the intensive care units (ICUs) at 20 hospitals across the United States where they compared use of contact precautions (gown and glove isolation) for everyone versus standard practice (10). They measured the changes in acquisition of MRSA and in infection from this pathogen. The primary laboratory involvement was culture of admission and discharge surveillance swab samples for MRSA and vancomycin-resistant enterococci (VRE), in addition to the usual role of performing cultures to detect infection when clinically needed (10). A total of 26,180 patients were included in the study. There was no change in VRE acquisition but a statistically significant lowering of MRSA acquisition was found (from 10.03 to 6.0 acquisitions per 1,000 days at risk for the intervention units compared to a change from 6.98 to 5.94 acquisitions per 1,000 days at risk in control units; P = 0.046). However, there was no difference in the rate of ICU infections between the intervention and control groups (10), and in fact the HAI rate rose during the intervention period for all measured infections (central line-associated blood stream infections [CLABSI], ventilator-associated pneumonia [VAP], and catheter-associated urinary tract infection [CAUTI]). These results are summarized in Table 1.
TABLE 1.
Comparison of outcome measures with various approaches to MRSA control
| Intervention studied | Population (no. of patients) | Outcome measureda | Results | Significance | Reference or report |
|---|---|---|---|---|---|
| Universal glove and gown isolation | Cluster-randomized trial in 20 ICUs (26,180) | Acquisition of MRSA (secondary); HAI (secondary) | Reduction from 10.03 to 6 MRSA acquisitions/1,000 patient days for intervention; 6.98 to 5.94 for control; no change in HAI | P = 0.046 for reduced acquisition of MRSA colonization; all HAI rates increased for intervention (P = 0.16 to 0.68) | 10 |
| Universal decolonization with mupirocin and chlorhexidine | Cluster-randomized trial in 74 ICUs (122,464) | Rate of MRSA BSI (primary); rate of MRSA clinical culture (secondary) | Change from 0.46 to 0.49 MRSA BSI/1,000 patient days for group 1, from 0.47 to 0.56 for group 2, and from 0.58 to 0.38 for group 3; reduction from 3.4 to 3.1 MRSA clinical cultures/1,000 patient days for group 1, 4.3 to 3.2 for group 2, and 3.4 to 2.1 for group 3 | P = 0.11 for change in MRSA BSI; for comparison of intervention on MRSA clinical cultures, P = 0.09 for group 2 vs 1, P = 0.003 for group 3 vs 1, and P = 0.16 for group 2 vs 3 | 11 |
| Universal AST (PCR) with isolation and decolonization of MRSA patients | Before-after design in 3 affiliated hospitals (65,369) | Rate of all MRSA clinical infections (primary) | Rate of MRSA clinical infection was 0.89 cases/1,000 patient days during baseline period, 0.74/1,000 during ICU testing period, and 0.39 during universal admission AST period | No significant difference between baseline and ICU periods (P = 0.15); difference was significant between baseline and universal AST period (P < 0.001) | 9 |
| Universal AST (PCR) with isolation of MRSA patients | Before-after design in 153 affiliated hospitals (1,934,598) | Rate of all MRSA clinical infections (primary) and rate of MRSA transmission (primary) | Rate of MRSA disease in the ICU changed from 1.64 cases/1,000 patient days at beginning to 0.62 at the end, and the non-ICU rate went from 0.47 to 0.26 cases/1,000 patient days; rate of transmission in the ICU changed from 3.02 cases/1,000 patient days at beginning to 2.5 at the end with the non-ICU rate going from 2.54 to 2 cases/1,000 patient days | Change in both clinical infection and transmission was significant in the ICU and non-ICU populations (P < 0.001 for all comparisons) | 13 |
| Universal AST (PCR) with isolation of MRSA patients | Before-after design in 153 affiliated hospitals (2,382,952) | Rate of all MRSA clinical infections (primary) and rate of MRSA transmission (primary) | Rate of MRSA disease in the ICU changed from 0.54 cases/1,000 patient days at beginning to 0.46, and the non-ICU rate went from 0.29 to 0.16 cases/1,000 patient days, with the combined rate changing from 0.33 to 0.21 clinical infections/1,000 patient days; rate of transmission in the ICU changed from 2.54 cases/1,000 patient days at beginning to 2.36 at the end with the non-ICU rate going from 2.27 to 1.96 cases/1,000 patient days | The change in both clinical infection and transmission was not significant in the ICU, but was in the non-ICU and overall populations (P < .001 for these latter comparisons) | 14 |
| Universal (culture) or targeted (PCR) AST with isolation and decolonization vs enhanced hand hygiene | Before-after-before quasi-experimental design in 33 surgical units at 10 hospitals in 9 countries (126,750) | Rate of all MRSA clinical infections (primary) and rate of MRSA surgical site infections (primary) | Only significant reductions in MRSA infections were for clean surgical patients (n = 43,166 procedures); AST and decolonization had 15% reduction in disease per month, with 18% in the combined arm; after multivariable analysis, AST and decolonization provided a 17% reduction per month in total MRSA infection | No impact from enhanced hand hygiene; for clean surgery, significance of AST plus decolonization was P = 0.019, combined with hand hygiene was it P = 0.007, and multivariable MRSA reduction was P = 0.041 | 15 |
| Targeted AST (culture) vs universal AST with contact precautions isolation of positives | Before-after-before quasi-experimental design in 3 hospitals (147,975) | Rate of newly identified nosocomial MRSA patients (clinical infection and AST results combined; primary); MRSA nosocomial infection (secondary) | Rate of nosocomial MRSA was 0.42 cases/1,000 patient days with targeted AST and 0.48 during universal AST; MRSA BSI was 1.8 and 2.1 cases/100,000 patient days, respectively; MRSA detection rate prevalence was 0.98% during targeted and 2.6% during universal AST periods | No significant differences found for MRSA transmission; nonsignificant reduction of MRSA nosocomial infection of 0.011 cases/1,000 patient days during universal AST | 17 |
| Universal and targeted AST (PCR) with isolation with or without decolonization | Before-after design in 4 affiliated hospitals (501,129) | Rate of all MRSA clinical infections (primary) | Final rate of MRSA clinical infection was 0.23 cases/1,000 patient days during AST period; the fourth hospital was added during the 10-yr period and achieved the same level of MRSA HAI as the original three hospitals after 3 yr (Fig. 1) | Difference was significant between aggregate baseline and AST periods at P < 0.001; targeted, risk-based screening began in January 2012 | New data in current report |
HAI, hospital-acquired infection; BSI, bloodstream infection.
(ii) Universal decolonization.
Huang and colleagues conducted a multicenter, cluster-randomized trial in 43 hospitals (mainly community hospitals) with 74 ICUs over 18 months. Including baseline and intervention periods, a total of 122,464 patients were randomly assigned to 1 of 3 infection control strategies. These were MRSA AST (culture-based) followed by isolation of positive patients in group 1, surveillance followed by isolation and decolonization of positive patients in group 2, and decolonization (universal) of all patients in group 3 (11). The primary laboratory involvement was culture of AST swab samples for MRSA in groups 1 and 2, in addition to the usual role of performing cultures to detect infection when clinically needed (11). Rates of bloodstream infection (BSI) caused by any pathogen were significantly lower in both groups 2 and 3 than in group 1, with group 3 significantly lower than group 2. However, there were no significant differences in the rates of BSI caused by MRSA among any of the 3 groups. A comparison of the groups for the rate of any clinical MRSA infection found only group 3 to be superior to group 1; there was no significant difference in the rates of MRSA clinical infections between groups 2 and 3. Examination of the supplementary data showed that preventing BSI from coagulase-negative staphylococci (CNS) was the main driver of the reduction in BSI in the ICU (12). For a pairwise group comparison of results after removal of BSI caused by CNS, the rate ratio between group 3 versus group 2 was 0.9234 (95% confidence interval, 0.73 to 1.17; P = 0.50). A summary of the results is found in Table 1.
(iii) Active surveillance testing.
Robicsek and colleagues published the first large study of this intervention using a before-after quasi-experimental approach (9). This was a three-hospital study over 45 months evaluating all-admission (universal) AST with isolation and decolonization of nasal MRSA-positive patients. Real-time PCR testing was used for nasal MRSA surveillance on 65,369 patients during three study periods (12-month baseline, 12-month ICU-only testing, and 18-month universal admission testing). The laboratory involvement was real-time PCR of AST swab samples for MRSA and performance of clinical cultures to detect infection (9). The primary outcome was a change in clinical MRSA disease. They found no significant reduction in MRSA clinical disease during the ICU testing period and a 69.6% disease reduction with universal admission testing (Table 1). While there appears to be a reduction in disease during 2004 (the ICU testing period) in Fig. 1 that includes these early data, it is important to examine the original published report where this apparent reduction was not significant and recognize the fact that during the ICU testing year, the rate of MRSA clinical disease was actually increasing at the end of this focused surveillance trial (9). A statistically significant and sustained reduction in MRSA disease did not occur until all admission testing (e.g., universal admission testing) began in 2005 (9). When adding our additional years of this practice, we included 501,129 more patients; in the final 2 years (calendar years 2014 to 2015); this approach realized a rate of MRSA HAI at 0.23 infections per 1,000 patient days (Fig. 1; Table 1).
FIG 1.
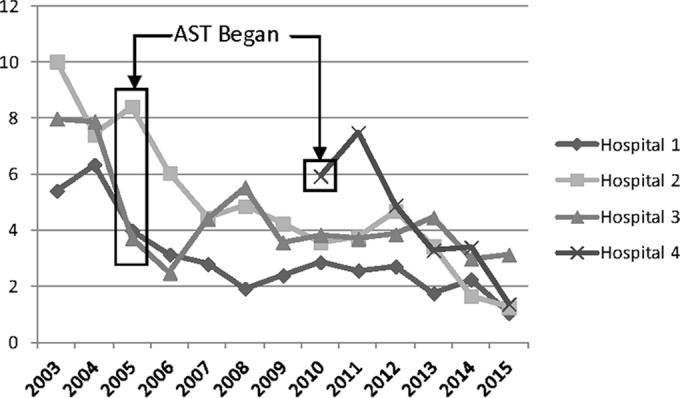
Rate of MRSA nosocomial infection (per 10,000 patient days). When calculated from baseline for each hospital, the aggregate P value was <0.001.
Jain and colleagues reported the next large study, which was done in the U.S. Veterans Affairs (VA) health care system (13) and also used a before-after quasi-experimental design. This investigation included 153 hospitals nationwide and took place from October 2007 through June 2010; it included 1,934,598 admissions, transfers, and discharges (13). Patients underwent universal AST (primarily real-time PCR) with isolation (no decolonization) of those who were MRSA positive. The laboratory involvement was real-time PCR (admission) or culture (discharge) of AST swab samples for MRSA and culture testing for infection (13). The primary measured outcome was a change in clinical infection and in the rate of MRSA transmission. There was a 62% reduction in MRSA disease within the ICU setting and 45% fewer MRSA infections hospital-wide (P < 0.001 for both). The results are summarized in Table 1. The VA has also published an update on their results through June 2012 that includes another 2,382,952 admissions, transfers, and discharges (14). They have seen continued improvement relating to MRSA transmission and disease (Table 1).
A large multicenter, multinational trial that studied approaches to reduce MRSA in surgical patients was reported by Lee and colleagues (15). The study included 10 hospitals in 9 countries using 33 surgical wards with a total of 126,750 patients and was designed as a prospective trial with a 6-month baseline, a 12-month intervention, and 6-month washout study periods. The interventions were enhanced hand hygiene training and MRSA AST with contact precautions and decolonization of positive carriers (15). AST was conducted with chromogenic agar culture for universal surveillance and PCR for targeted (risk-based) surveillance. For patients undergoing clean surgery, there was a significant reduction in MRSA clinical infection for the AST and decolonization (15% per month) arm and for the combined AST and decolonization plus enhanced hand hygiene arm (18% per month). After multivariable analysis, the only significant reduction in clinical MRSA nosocomial infection was for patients undergoing clean surgery in the AST and decolonization (17% per month) arm (15). The results are summarized in Table 1. Noteworthy is the fact that the lead investigators in this report earlier published a widely recognized article indicating that AST was not useful in preventing MRSA surgical infections (16), but the prior population studied was from a single site with a somewhat smaller population sample (n = 21,754), indicating the importance of focusing on results from larger (and preferably multisite) studies in order to extrapolate the results to a broader patient population.
The most recent large report is from Canada where Roth and colleagues compared the impact of risk-based AST (e.g., targeted) with that of universal testing (17). This study included three hospitals in the Ottawa hospital system and was designed as a before-after quasi-experimental investigation. The interventions were a baseline of 24 months using targeted AST followed by 20 months of universal AST. Testing was done by pooling swab samples from various body sites in a broth culture with PCR performed on the broth after incubation (17). AST was done within 48 h of admission, and the median length of hospital stay was 3 days. Other than a nearly 3-fold increased detection of MRSA-colonized patients at the time of admission, there were no significant differences in MRSA nosocomial infection or bacteremia comparing targeted or universal AST (17). The results are summarized in Table 1. One reason that no change in MRSA disease was seen is that the rate of clinical infection (<0.5 total infection and <0.03 MRSA BSI per 1,000 patient days) was very low throughout the study so that no intervention was needed.
(iv) Key implications of MRSA control practices for laboratories.
Based on the data discussed, there are two practices with the potential to lower MRSA clinical infection rates: universal decolonization (11) and active surveillance testing (9, 13–15). AST, combined with contact precaution isolation or with isolation plus decolonization, was reported to achieve the lowest endpoint rates of clinical MRSA disease: between 0.21 and 0.48 clinical infections per 1,000 patient days for 5,032,023 enrollees (9, 13–14, 17), compared to 2.1 cases per 1,000 patient days for universal decolonization in a 122,464-patient study (11). The low AST disease rate findings are consistent with a recent report from The Netherlands, a country using “search and destroy” since 1988, where 0.11 cases of clinical infection per 1,000 patient days (57 infections in 527,267 patient days) were found during a study from 2008 to 2013 (18). Thus, it would be expected that many health care organizations will use some form of MRSA prevention and control approach that will heavily involve the laboratory.
Financial impact of MRSA control practices.
The optimal way to approach assessment of the financial impact of infection prevention and control interventions has been controversial, but much has been learned during the considerable focus on MRSA disease. Potential costs to consider are those that occur during the primary hospitalization plus those related to MRSA infection following hospital discharge. Nelson and colleagues recently reported on the impact of a MRSA nosocomial infection (HAI) following discharge in the VA health care system (19). Over 3 years, they studied 369,743 patients from 123 hospitals, of whom 3,599 (1%) had a positive culture for MRSA >48 h after admission. They determined the associated health care cost within 1 year of discharge (19), using a comparison to matched patients. Those with a positive MRSA culture had $776 in additional outpatient cost and $12,167 in additional inpatient expense (19). Extrapolating this additional $12,943 cost of care after discharge from the 1% of patients with a MRSA HAI to all admissions suggests that $129.43 could be spent on each patient admitted for preventing a MRSA clinical infection to still have a cost-effective prevention initiative.
A novel approach for determining the financial impact of a MRSA HAI during hospital admission has also been reported by the VA health care system. In this evaluation, Nelson and colleagues determined the cost only after the MRSA HAI occurred and compared that result to more typical methods for this type of analysis such as when the entire hospitalization is included (against matched patients) and the cost of MRSA infection when matched to the cost for other patients based on the time to onset of infection (20). The data set was 114 VA hospitals from 1 October 2007 to 30 September 2010 and included 121,520 patients in the post-HAI analysis. The calculation showed that the total excess costs for the MRSA HAI were 32% and 12% higher for the two traditional methods and gave a figure of $24,015 when only costs after infection were counted (20). Interestingly, when we assessed the cost of a MRSA infection using a model that eliminated all matched controls with an inpatient stay of less than 8 days (the median time to onset of a MRSA HAI in our facilities), our total cost assessment was nearly the same at $23,783 (21). Therefore, the excess cost of a MRSA HAI can reasonably be approximated at $24,000 when one is contemplating the benefit of a successful MRSA prevention and control program in the United States.
Huang and colleagues reported a very in-depth cost analysis on the multicenter ICU trial (REDUCE MRSA) discussed earlier where they developed a table for specifically calculating the cost of any particular MRSA prevention and control intervention (22), based on the data collected in their earlier report (11). Since the table created contained virtually all costs associated with an inpatient control program, it is straightforward for the microbiology laboratory and the infection control and prevention department at any hospital to develop a Microsoft Excel spreadsheet to determine both the cost and likely benefit of a MRSA control program. Examples of such spreadsheets are shown in Fig. 2 and 3. Figure 2 can be used to determine the cost of a potential AST program. It is important to understand that if universal surveillance is the initial AST intervention, one will quickly learn the risk factors for colonization in one's own patient population, and then a prediction rule can be developed so that even though all patients are “screened (by a computer algorithm)” at admission, only a portion who are at higher risk actually need to be tested for MRSA colonization (23). This is noted in line B of Fig. 2 where even though the table is constructed for 1,000 patients, only 500 are actually tested using a developed prediction rule. We implemented a prediction rule that was developed from our own universal surveillance program in January 2012 and now only test 50% of admissions, even though all are screened for MRSA risk using the prediction model (23). As can be seen from Fig. 1, the downward trend of MRSA clinical disease continues after this practice change. In the legend to Fig. 2 is a note on the cost of a universal surveillance program if there is no decolonization. This is included because the VA health care system does not decolonize patients (13, 14), and we recently completed a trial in one of our hospitals indicating that decolonization was not needed when contact precautions were in practice (27). Figure 3 depicts a spreadsheet that can be used to calculate the cost benefit of reducing MRSA clinical disease. Again there is a comparison of the results from universal decolonization and universal surveillance with a third column for entering local data. Use of Fig. 2 and 3 to estimate the benefit of a MRSA prevention and control program can provide a reliable estimate of costs and benefits before a decision is made on which intervention(s) to pursue.
FIG 2.
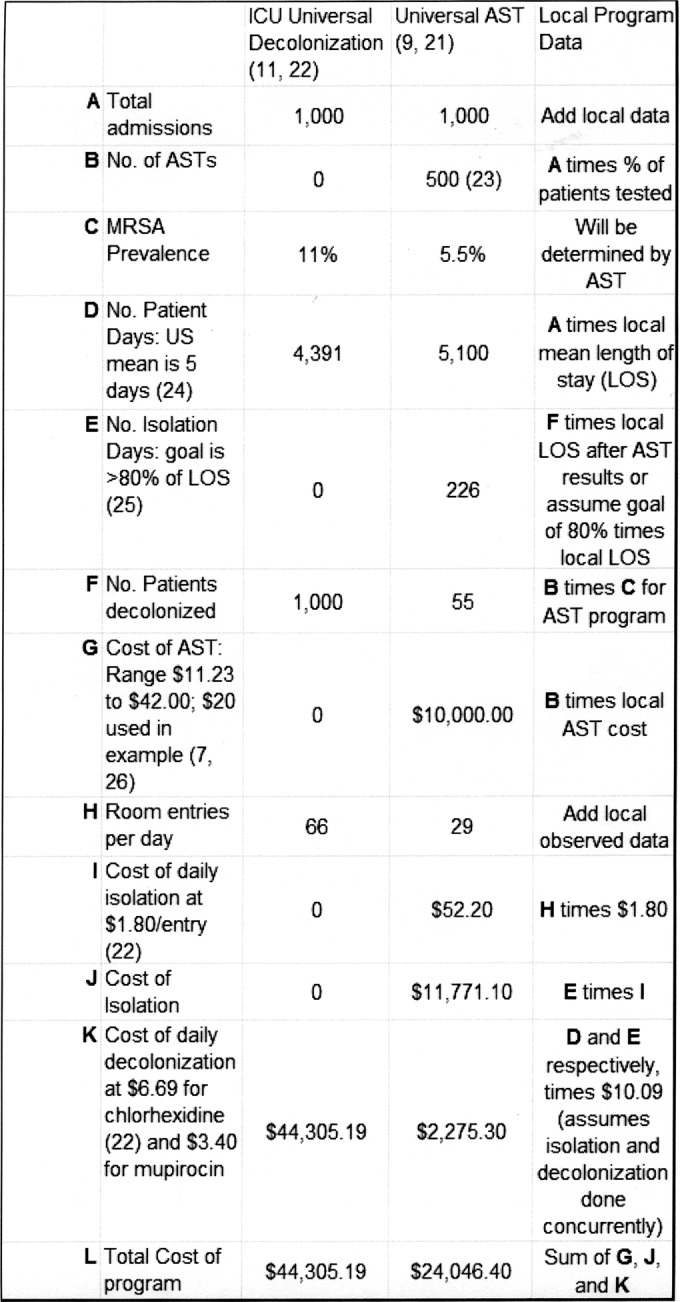
Sample table for calculating cost of a MRSA control program. The mean price for mupirocin ($16.99 per 22 g, 2% tube) was obtained from http://www.goodrx.com/mupirocin. Note that the total cost of the AST program with no colonization is $21,771.10. See cited references 7, 9, 11, 21, 22, 23, 24, 25, and 26.
FIG 3.
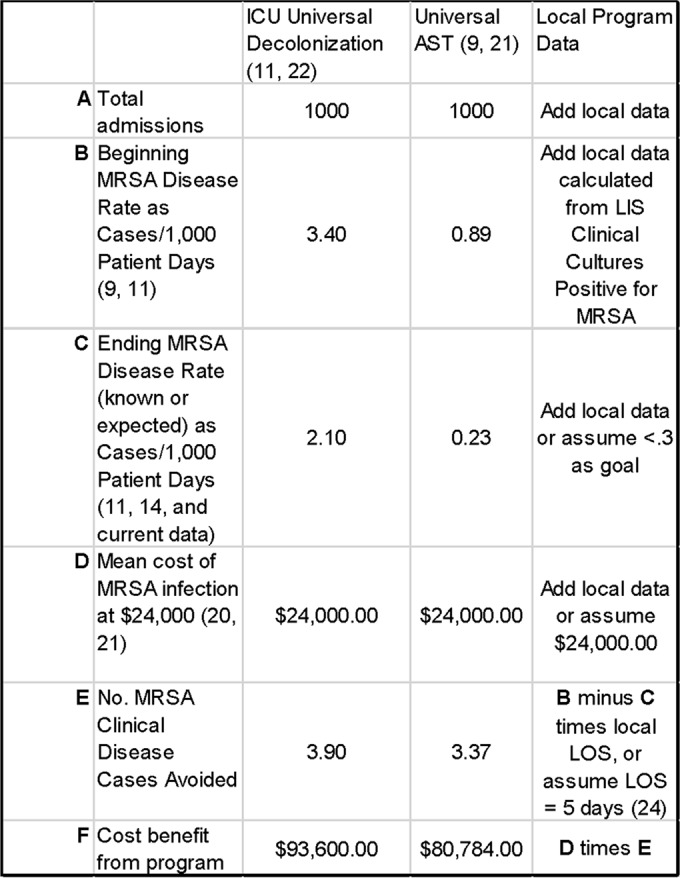
Sample table for calculating cost benefit of a MRSA control program.
MRSA control modeling, federal mandates, and the likely future.
A very interesting model for MRSA control was reported by Bootsma and colleagues, who investigated a one-hospital model and a three-hospital model that evaluated the impact of rapid diagnostic testing (results within a few hours) on MRSA control, based mainly on their own data (28). In a setting with no MRSA control, the prevalence of MRSA-colonized patients within the hospital would saturate at 15%. Most interestingly, they found that isolation of MRSA carriers identified by clinical cultures was insufficient to adequately interrupt MRSA transmission (and control clinical disease) and that the prevalence would plateau at 5%. Our own experience confirms their modeling; isolation of patients with clinical MRSA infection has been the practice for decades, and our hospital prevalence when tested in 2004 was 8.5% (21), even exceeding the model estimate and implying that our isolation efficacy was insufficient (28). In the model, application of contact precautions to those clinically infected combined with AST of patients at risk for MRSA had the impact of lowering MRSA prevalence. Concerted application of AST in a setting of high nosocomial endemicity reduced the nosocomial prevalence to <1% within 6 years (28). This model suggests a two-phase process where a rapid reduction in MRSA is seen initially, followed by a continued decline to a very low level of MRSA prevalence, as seen from our NorthShore data in Fig. 1. Rapid diagnostic testing increased the program feasibility and decreased the prevalence faster than did culture (28).
Another impetus to reduce MRSA disease is the recent action of the Center for Medicare and Medicare Services (CMS). On 1 October 2016 (the beginning of U.S. fiscal year [FY] 2017) CMS adopted the assessment of hospital-onset MRSA bacteremia as a measure in the safety domain and increased domain measure weight to 20%, with the fining of hospitals having too high a disease rate (29). These fines are substantial and will place considerable pressure on U.S. hospitals to reduce or eliminate MRSA nosocomial bacteremia (and eventually all MRSA HAIs). Since AST is one of the core measures for prevention and control in the Society for Health Epidemiologists of America (SHEA) guide for reducing MRSA clinical disease when routine practices are not sufficient (30), this will place increasing pressure on health care organizations to act, and substantial involvement by the clinical microbiology laboratory is very likely.
Laboratory monitoring of MRSA clinical disease rates and suggested disease targets.
The microbiology laboratory has unique access to data for its health care organization that enables the rapid calculation of MRSA disease rates. Virtually all laboratory information systems (LIS) used in U.S. hospitals have the capability of determining the annual number of MRSA-positive clinical cultures from specimens submitted more than 2 days after admission to the hospital. If one organizes these data so that each positive patient is only included once per month, then the laboratory has the data necessary for an accurate estimate of the clinical disease rate (8, 11). The same can be done for estimating MRSA BSI by simply collecting only blood culture data, if that is the desired metric. One caution for use of BSI data is that application of a care bundle for prevention of CLABSI can markedly reduce BSI from central venous catheters (31, 32), so that the desired level of MRSA BSI should be very low with good catheter care and a MRSA control program (our organization's rate has remained under the proposed 0.03/1,000 patient days target for 9 years). Once the numerator is determined, only the number of inpatient days is needed, typically available from the finance department and often published on the hospital website in its annual report. Then, the calculation of the MRSA clinical disease rate estimate (disease per 1,000 patient days) is straightforward using the formula: total positive clinical cultures (or MRSA BSI) divided by total inpatient days times 1,000 days. A target threshold for separating adequate versus inadequate MRSA prevention and control in U.S. hospitals (based on articles discussed in this review) is shown in Table 2.
TABLE 2.
Proposed target thresholds for MRSA clinical culture rate (e.g., clinical disease rate) for U.S. hospitals
Once the decision has been made to introduce an AST program, the laboratory will be tasked with choosing the format of MRSA surveillance testing to adopt. Key to the decision process is selection of a testing system with sufficient sensitivity and result reporting time so that a combination of these factors will enable placing patients in contact precautions for 80% of the time they are in the hospital (9, 25, 26). This is critical since the most expensive AST program is one where testing is done, but MRSA clinical disease is not reduced, an outcome to be avoided. The other factors important for the decision are test cost and performance specificity. The test cost directly impacts the expense of the assay to the laboratory and the specificity of the assay impacts the cost of the program to the health care organization (7, 33). For example, a test with high cost and high specificity may actually be less expensive overall than one with low cost and low specificity since low specificity leads to unnecessary patient isolation, which can add as much as $30,000 of unnecessary isolation cost for every 10,000 tests done for each 1% loss of test specificity (33). More discussion of the potential economic and patient safety impacts of test specificity is provided by Brukner and colleagues, who point out that some commercial assays have unacceptably low specificity (which can be improved) that can lead to adverse outcomes, especially when colonization prevalence is low (34).
SUMMARY
Over the past decade several large studies from the United States, Europe, and Canada have addressed key approaches for improving patient safety by reducing the risk of health care-associated MRSA clinical disease. With more than 5 million patients studied in these investigations, the data indicate that AST with application of contact precautions (with or without decolonization of MRSA-colonized persons) and universal decolonization of all patients are the two likely strategies for achieving clinical disease reduction, with AST providing the lowest rate of MRSA infection. Very low rates of clinical disease can be achieved with a successful program. Health care organizations should assess their rate of MRSA clinical infection and if all potential body infection sites have a rate of >0.3/1,000 patient days or bloodstream infection a rate of >0.03/1,000 patient days, then implementation of a MRSA prevention and control strategy should be undertaken.
ACKNOWLEDGMENTS
L. R. Peterson had full access to all of the data in the report and takes responsibility for the integrity of the data and the accuracy of the data analysis. Manuscript concept and design: L. R. Peterson, D. M. Schora. Acquisition, analysis, or interpretation of data: L. R. Peterson, D. M. Schora. Drafting of the manuscript: L. R. Peterson, D. M. Schora. Critical revision of the manuscript for important intellectual content: L. R. Peterson, D. M. Schora, Statistical analysis: L. R. Peterson.
We declare no conflict of interest that pertains to this report. L.R.P. has received speaking honoraria from Becton Dickinson, Cepheid, Roche, and CareFusion and research funding from Becton Dickinson, Cepheid, Nanosphere, 3M, GeneWEAVE and Roche.
Biographies

Lance R. Peterson, M.D., F.A.S.C.P., F.I.D.S.A., F.A.A.M., F.S.H.E.A., is the Director of Microbiology and Infectious Diseases Research, Vice-Chair of Pathology for Research, and Epidemiologist at NorthShore University HealthSystem in Evanston, Illinois. He is also a Clinical Professor at the Pritzker School of Medicine of the University of Chicago, in Chicago, Illinois, and a staff member in the Clinical Microbiology and Infectious Disease Divisions at Evanston Hospital. After receiving a B.S. degree from the University of Minnesota in Minneapolis in 1971, Dr. Peterson was awarded an M.D. from the University of Minnesota in 1972. He then completed an internship at St. Paul-Ramsey Medical Center, a residency in Internal Medicine at the University of Minnesota Hospitals, and a fellowship in Infectious Diseases at the VA Medical Center, all in the Twin Cities of Minnesota. He has directed the clinical microbiology laboratories at the Minneapolis VA Medical Center and Northwestern Memorial Hospital in Chicago. At both medical centers, he was also a consultant in infectious diseases. He has worked on projects involving Staphylococcus aureus, infection control, and antibiotic resistance since the beginning of his career.

Donna M. Schora, M.T.(A.S.C.P.), is a Clinical Microbiologist in the Department of Microbiology and Infectious Diseases Research at NorthShore University HealthSystem in Evanston, Illinois. Donna received her bachelor of science degree in Clinical Laboratory Sciences at Marquette University, Milwaukee, Wisconsin. She began her clinical career in the Microbiology Laboratory at Northwestern Memorial Hospital in Chicago, Illinois. She held several supervisory positions there, including Technical Supervisor as well as the oversight of the mycology, mycobacteriology, and antimicrobial susceptibility testing laboratories. She left the clinical laboratory in 1999 to join the CDC-sponsored Prevention Epicenter at Northwestern Memorial Hospital as the Clinical Microbiologist. In 2002, she joined the team at Evanston Northwestern Healthcare (now NorthShore University HealthSystem), where her current focus is epidemiologic research, performing validation and outcome management for new, innovative tests and methodologies designed for the rapid, accurate detection of nosocomial pathogens.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. NorthShore University HealthSystem funded this work by paying the salaries of the authors.
REFERENCES
- 1.Wise RI, Ossman EA, Littlefield DR. 1989. Personal reflections on nosocomial staphylococcal infections and the development of hospital surveillance. Rev Infect Dis 11:1005–1019. doi: 10.1093/clinids/11.6.1005. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2016. Methicillin-resistant Staphylococcus aureus (MRSA) infections. http://www.cdc.gov/mrsa/tracking/ Accessed 10 March 2016.
- 3.Centers for Disease Control and Prevention. 2012. Active bacterial core surveillance (ABCs) report, emerging infections program network, methicillin-resistant Staphylococcus aureus, 2012. http://www.cdc.gov/abcs/reports-findings/survreports/mrsa12.pdf.
- 4.Joint Commission Resources. 2016. Standard IC.01.02.01. In 2016 laboratory accreditation standards. Joint Commission Resources, Oakbrook Terrace, IL. [Google Scholar]
- 5.Slayton RB, Toth D, Lee BY, Tanner W, Bartsch SM, Khader K, Wong K, Brown K, McKinnell JA, Ray W, Miller LG, Rubin M, Kim DS, Adler F, Cao C, Avery L, Stone NT, Kallen A, Samore M, Huang SS, Fridkin S, Jernigan JA. 2015. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities–United States. MMWR Morb Mortal Wkly Rep 64:826–831. doi: 10.15585/mmwr.mm6430a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson LR, Wright MO. 2010. Chapter 8, 21st century clinical microbiology laboratory support for healthcare-associated infection control and prevention. In SHEA practical handbook for healthcare epidemiologists, 3rd ed Slack, Inc., Thorofare, NJ. [Google Scholar]
- 7.Mangold KA, Peterson LR. 2016. Chapter 15, Molecular detection of Staphylococcus aureus colonization and infection, p 169–184. In Persing DH, Tenover FA, Hayden RT, Ieven M, Miller MB, Nolte FS, Tang Y-W, van Belkum A (ed), Molecular microbiology: diagnostic principles and practice, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 8.Peterson KE, Hacek DM, Robicsek A, Thomson RB Jr, Peterson LR. 2012. Electronic surveillance for infectious disease trend analysis following a quality improvement intervention. Infect Control Hosp Epidemiol 33:790–795. 22759546. doi: 10.1086/666625. [DOI] [PubMed] [Google Scholar]
- 9.Robicsek A, Beaumont JL, Paule SM, Hacek DM, Thomson RB Jr, Kaul KL, King P, Peterson LR. 2008. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 148:409–418. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]
- 10.Harris AD, Pineles L, Belton B, Johnson JK, Shardell M, Loeb M, Newhouse R, Dembry L, Braun B, Perencevich EN, Hall KK, Morgan DJ, Benefits of Universal Glove and Gown (BUGG) Investigators, Shahryar SK, Price CS, Gadbaw JJ, Drees M, Kett DH, Muñoz-Price LS, Jacob JT, Herwaldt LA, Sulis CA, Yokoe DS, Maragakis L, Lissauer ME, Zervos MJ, Warren DK, Carver RL, Anderson DJ, Calfee DP, Bowling JE, Safdar N. 2013. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 310:1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, Lankiewicz J, Gombosev A, Terpstra L, Hartford F, Hayden MK, Jernigan JA, Weinstein RA, Fraser VJ, Haffenreffer K, Cui E, Kaganov RE, Lolans K, Perlin JB, Platt R, CDC Prevention Epicenters Program, AHRQ DECIDE Network and Healthcare-Associated Infections Program. 2013. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BA, Peterson LR. 2013. Universal decolonization in the ICU: the new panacea? Medscape, 20 September 2013. http://www.medscape.com/viewarticle/811217.
- 13.Jain R, Kralovic SM, Evans ME, Ambrose M, Simbartl LA, Obrosky DS, Render ML, Freyberg RW, Jernigan JA, Muder RR, Miller LJ, Roselle GA. 2011. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 14.Evans ME, Kralovic SM, Simbartl LA, Freyberg RW, Obrosky DS, Roselle GA, Jain R. 2013. Veterans Affairs methicillin-resistant Staphylococcus aureus prevention initiative associated with a sustained reduction in transmissions and health care-associated infections. Am J Infect Control 41:1093–1095. doi: 10.1016/j.ajic.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Lee AS, Cooper BS, Malhotra-Kumar S, Chalfine A, Daikos GL, Fankhauser C, Carevic B, Lemmen S, Martínez JA, Masuet-Aumatell C, Pan A, Phillips G, Rubinovitch B, Goossens H, Brun-Buisson C, Harbarth S, MOSAR WP4 Study Group. 2013. Comparison of strategies to reduce meticillin-resistant Staphylococcus aureus rates in surgical patients: a controlled multicentre intervention trial. BMJ Open 3:e003126. doi: 10.1136/bmjopen-2013-003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harbarth S, Fankhauser C, Schrenzel J, Christenson J, Gervaz P, Bandiera-Clerc C, Renzi G, Vernaz N, Sax H, Pittet D. 2008. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA 299:1149–1157. doi: 10.1001/jama.299.10.1149. [DOI] [PubMed] [Google Scholar]
- 17.Roth VR, Longpre T, Taljaard M, Coyle D, Suh KN, Muldoon KA, Ramotar K, Forster A. 2016. Universal vs risk factor screening for methicillin-resistant Staphylococcus aureus in a large multicenter tertiary care facility in Canada. Infect Control Hosp Epidemiol 37:41–48. doi: 10.1017/ice.2015.230. [DOI] [PubMed] [Google Scholar]
- 18.Souverein D, Houtman P, Euser SM, Herpers BL, Kluytmans J, Den Boer JW. 2016. Costs and benefits associated with the MRSA search and destroy policy in a hospital in the region Kennemerland, The Netherlands. PLoS One 11:e0148175. doi: 10.1371/journal.pone.0148175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson RE, Jones M, Liu CF, Samore MH, Evans ME, Graves N, Lee B, Rubin MA. 2015. The impact of healthcare-associated methicillin-resistant Staphylococcus aureus infections on post-discharge healthcare costs and utilization. Infect Control Hosp Epidemiol 36:534–542. doi: 10.1017/ice.2015.22. [DOI] [PubMed] [Google Scholar]
- 20.Nelson RE, Samore MH, Jones M, Greene T, Stevens VW, Liu CF, Graves N, Evans MF, Rubin MA. 2015. Reducing time-dependent bias in estimates of the attributable cost of health care-associated methicillin-resistant Staphylococcus aureus infections: a comparison of three estimation strategies. Med Care 53:827–534. doi: 10.1097/MLR.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 21.Peterson LR, Hacek DM, Robicsek A. 2007. 5 million lives campaign. Case study: an MRSA intervention at Evanston Northwestern Healthcare. Jt Comm J Qual Patient Saf 33:732–738. [DOI] [PubMed] [Google Scholar]
- 22.Huang SS, Septimus E, Avery TR, Lee GM, Hickok J, Weinstein RA, Moody J, Hayden MK, Perlin JB, Platt R, Ray GT. 2014. Cost savings of universal decolonization to prevent intensive care unit infection: implications of the REDUCE MRSA trial. Infect Control Hosp Epidemiol 35(Suppl 3):S23–S31. doi: 10.1086/677819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robicsek A, Beaumont JL, Wright MO, Thomson RB Jr, Kaul KL, Peterson LR. 2011. Electronic prediction rules for methicillin-resistant Staphylococcus aureus colonization. Infect Control Hosp Epidemiol 32:9–19. doi: 10.1086/657631. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. Hospital utilization (in non-Federal short stay hospitals). http://www.cdc.gov/nchs/fastats/hospital.htm Accessed 20 March 2016.
- 25.Peterson LR, Diekema DJ. 2010. To screen or not to screen for methicillin-resistant Staphylococcus aureus. J Clin Microbiol 48:683–689. doi: 10.1128/JCM.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paule SM, Mehta M, Hacek DM, Gonzalzles TM, Robicsek A, Peterson LR. 2009. Chromogenic media vs real-time PCR for nasal surveillance of methicillin-resistant Staphylococcus aureus: impact on detection of MRSA-positive persons. Am J Clin Pathol 131:532–539. doi: 10.1309/AJCP18ONZUTDUGAQ. [DOI] [PubMed] [Google Scholar]
- 27.Peterson LR, Wright MO, Beaumont JL, Komutanon V, Patel PA, Schora DM, Schmitt BH, Robicsek A. 2016. Nonimpact of decolonization as an adjunctive measure to contact precautions for the control of methicillin-resistant Staphylococcus aureus transmission in acute care. Antimicrob Agents Chemother 60:99–104. doi: 10.1128/AAC.02046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bootsma MCJ, Diekmann O, Bonten MJM. 2006. Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc Natl Acad Sci U S A 103:5620–5625. doi: 10.1073/pnas.0510077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hospital-Acquired Condition Reduction Program. 2015. CMS to improve quality of care during hospital inpatient stays. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2014-Fact-sheets-items/2014-08-04-2.html.
- 30.Calfee DP, Salgado CD, Milstone AM, Harris AD, Kuhar DT, Moody J, Aureden K, Huang SS, Maragakis LL, Yokoe DS. 2014. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 35:772–796. doi: 10.1086/676534. [DOI] [PubMed] [Google Scholar]
- 31.Wright MO, Tropp J, Schora DM, Dillon-Grant M, Peterson K, Boehm S, Robicsek A, Peterson LR. 2013. Continuous passive disinfection of catheter hubs prevents contamination and bloodstream infection. Am J Infect Control 41:33–38. doi: 10.1016/j.ajic.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. 2011. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One 6:e15452. doi: 10.1371/journal.pone.0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel PA, Robicsek A, Grayes A, Schora DM, Peterson KE, Wright MO, Peterson LR. 2015. Evaluation of multiple real-time PCR tests on nasal samples in a large MRSA surveillance program. Am J Clin Pathol 143:652–658. doi: 10.1309/AJCPMDY32ZTDXPFC. [DOI] [PubMed] [Google Scholar]
- 34.Brukner I, Oughton M, Giannakakis A, Kerzner R, Dascal A. 2013. Significantly improved performance of a multitarget assay over a commercial SCCmec-based assay for methicillin-resistant Staphylococcus aureus screening: applicability for clinical laboratories. J Mol Diagn 15:577–580. doi: 10.1016/j.jmoldx.2013.04.009. [DOI] [PubMed] [Google Scholar]


