Abstract
Human prion diseases are neurodegenerative disorders caused by abnormally folded prion proteins in the central nervous system. These proteins can be detected using the quaking-induced conversion assay. Compared with other bioassays, this assay is extremely sensitive and was used in the present study to determine prion distribution in sporadic Creutzfeldt-Jakob disease patients at autopsy. Although infectivity of the sporadic form is thought to be restricted within the central nervous system, results showed that prion-seeding activities reach 106/g from a 50% seeding dose in non-neuronal tissues, suggesting that prion-seeding activity exists in non-neural organs, and we suggested that non-neural tissues of 106/g SD50 did not exist the infectivity.
Keywords: Prion, Prion-seeding activity, SD50, Non-neural tissue, Creutzfeldt-Jakob disease
Highlights
-
•
Prion-seeding activities reach 106/g from a 50% seeding dose in non-neuronal tissues.
-
•
Results suggest that prion-seeding activity exists in neural and non-neural organs.
A major problem for the diagnosis and management of human prion diseases is the lack of rapid and high-sensitive assays to measure low prion levels. Recent studies have tried to measure prion concentrations in non-neuronal tissues, but prion levels were not sufficient. Therefore, we developed the RT-QuIC method to measure prion-seeding activity in the non-neuronal, human tissues. The SD50 levels in the spleen, kidney, lung, and liver were 5.0–6.5, with different SD50 levels in the individual cases.
1. Introduction
Transmissible spongiform encephalopathies, also called ‘prion diseases,’ are caused by abnormally accumulated prion protein (PrP-res) in the central nervous system (CNS). The causative agent is thought to be solely composed of amyloid prion proteins and is not inactivated by standard and popular procedures. Iatrogenic Creutzfeldt-Jakob disease (CJD) can be caused by the reuse of neurosurgical instruments or contamination of biomaterials, such as dura mater graft material (Thadani et al., 1988, Bernoulli et al., 1977, Will and Matthews, 1982). However, extensive investigations have concluded that accidental prion transmission, as well as sporadic CJD (sCJD), which is the idiopathic form of CJD, is not likely the consequence of spontaneous somatic mutations. The proteinase K (PK)-resistant (PK-res) PrP in sCJD has been shown to be limited to cases with biological materials from the CNS or cornea. Additionally, in sCJD, prion infectivity has not been detected in extracerebral organs in studies using animal models, suggesting that infectious prions are restricted to the CNS. However, recent studies used Western blotting analysis to detect PrP-res in the spleen of a sCJD patient, although the PrPSc levels were lower by a factor of approximately 10− 4 than in brain tissue (Glatzel et al., 2003). These studies highlight the need to elucidate prion distribution in humans to reduce the risk of accidental prion infection. Various studies have already detected PK-resistant PrP in peripheral tissues in natural and experimental sCJD cases. For instance, Glatzel M, et al. (Glatzel et al., 2003) used Western blotting to show the presence of PrPSc in the spleen, as well as in the muscle of some sCJD patients. Additionally, Rubenstains et al. detected PrPSc in tonsil and lymph node tissues of sCJD patients (Rubenstein & Chang, 2013). Experimentally, Herzog et al. infected nonhuman primates with the sCJD agent (among others) to investigate the involvement of peripheral organs (Herzog et al., 2005). They also confirmed the presence of PrPSc in lymphoreticular organs and muscles.
A technique recently developed for in vitro amplification of prions, the RT-QuIC (quaking-induced conversion) assay, is a highly sensitive and specific technique that can detect small amounts of prion-seeding activity (Takatsuki et al., 2015). In our study, the detection limit of the RT-QuIC assay was approximately 0.12 fg of PrP-res (Herzog et al., 2005). Combined with an endpoint dilution, it was possible to quantify prion-seeding activity and the 50% seeding dose (SD50), which correlated well with PK-res PrP. These results encouraged us to re-evaluate the human prion in various tissues in sCJD patients.
2. Materials & Methods
2.1. Patients
A total of four female patients were diagnosed with classical-type sCJD, which was histopathologically confirmed after autopsy. To avoid contamination of brain tissue, other organs were separately harvested. The tissue specimens were immediately stored at − 80 °C until further use. Western blotting analysis of the PK-res PrP fragment and genotyping at codon 129 of the PRNP gene was conducted as previously described by the reference laboratory of the Japan CJD Surveillance Unit. Non-CJD tissues were purchased from Proteo Genex (Culver City, CA, USA). The protocol was approved by the Ethics Committee of Nagasaki University Hospital (ID: 100428423) and the use of specimens was also granted ethical approval by the Japan CJD Surveillance Unit. The study was registered with the University Hospital Medical Information Network (ID: UMIN000003301). Informed consent was obtained from patient families and/or patients.
2.2. Tissue Homogenate Preparation
Brains, spleens, kidneys, lungs, livers, and adrenal glands were subjected to RT-QuIC for evaluating SD50. To prevent contamination of brain tissues into other samples, we used single-use disposable tubes and beads, and all procedures were performed on different days. Tissue samples of brain, spleen, kidney, lung, liver, and adrenal gland were homogenized in 10% (w/v) ice-cold phosphate-buffered saline supplemented with a protease inhibitor mixture (Roche, Mannheim, Germany) using a multi-bead shocker (Yasui Kikai, Osaka, Japan). The samples were clarified by centrifugation at 6000 rpm for 2 min and stored at − 80 °C.
2.3. Western Blotting of Precipitated PrPSc Collected Using the Ultra-Centrifugal Concentration Method in Tissues of Sporadic CJD Patients and the Heathy Subjects
Brain (1 mg) or tissue (100 mg) homogenates from normal persons or CJD patients were lysed in the same volume of 2 × cell lysis buffer (100 mM Tris-HCl at pH 7.5, 300 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate). Samples were digested with PK concentration (finial concentration) at 50 μg/ml and incubated at 37 °C for 30 min. After that, samples were mixed with 4 mM pefabloc SC (Roche) to inactivate PK. The tissue homogenates (100 mg) were centrifuged at 6000 rpm for 3 min to remove cell debris. The supernatants (1 ml) from tissues were transferred to the bottom of a 5-ml ultra-centrifuge tube and 4 ml of phosphate-buffered saline (PBS) was overlaid. The samples were ultra-centrifuged at 100,000 × g at room temperature for 1 h. After centrifugation, the supernatants were removed and the pellets were re-suspended with sample buffer and boiled at 95 °C for 10 min. The samples were separated to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto a PVDF membrane (Millipore) in transfer buffer containing 20% methanol. The proteins were blocked with 5% skim milk in TBS-T for 60 min. To detect PrP, the membrane was incubated with anti-PrP antibody (3F4, primary antibody) and anti-mouse IgG antibody conjugated with horseradish peroxidase (HRP) as the secondary antibody. The PrP-res were imaged using enhanced chemiluminescence (ECL)-plus reagents (Amersham) and a CCD camera gel imager (ATTO).
2.4. Endpoint RT-QuIC (Takatsuki et al., 2015, Wilham et al., 2010)
Purification of recombinant human PrP (rHuPrP: residues 23–231, codon 129 M) was performed as previously described (Takatsuki et al., 2015). After purification, rHuPrP was stored at − 80 °C. Brain homogenates (BHs) (10% [w/v]) were serially diluted (10-fold) with artificial cerebrospinal fluid (A-CSF) containing 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 0.2 ng/ml BSA, and 0.05% glucose. rHuPrP, suspended in 95 μl of RT-QUIC buffer (500 mM NaCl, 50 mM PIPES pH 7.0, 10 μM Thioflavin T (ThT), and1 mM EDTA), was loaded into each well of a 96-well plate and mixed with 5 μl of brain sample, and then the assay was monitored for 40 h. Four to eight replicates of each diluted sample were measured. The SD50 was calculated using the Spearman–Kärber method. The same organs from two healthy subjects were used as negative controls in all RT-QuIC assay experiments using eight wells.
2.5. Bioassay
To evaluate the 50% lethal dose (LD50) in a brain sample from a sCJD patient (#1), we conducted a bioassay using a knock-in transgenic mice expressing the human-mouse chimeric PrP (Ki-ChM mouse) (Taguchi et al., 2003). The Ki-ChM mouse line was kindly provided by Dr. Kitamoto and his colleagues and was maintained in our institute. At 4 weeks of age, the Ki-ChM mice were intracerebrally inoculated with 20 μl of brain homogenate, following a serial dilution with PBS, into the right parietal lobe. All animal experiments were approved by the committee of Nagasaki University in accordance with the Guidelines for Animal Experimentation of Nagasaki University and conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
2.6. PrP-Immunohistochemistry in Extra-Neural Tissues of sCJD Patients and the Heathy Subjects
Formalin-fixed brain tissues were treated with formic acid (99% for human tissues or 60% for mouse tissues) for 1 h to inactivate the infectivity, and embedded in paraffin. Tissue sections were pretreated by hydrolytic autoclaving before PrP-immunohistochemistry. The anti-PrP monoclonal antibody 3F4 (Signet, Dedham, MA, USA) was used as the primary antibody for human sections, and anti-PrP antiserum PrP-N was used as the primary antibody for mouse sections. Goat-anti-mouse immunoglobulin polyclonal antibody labeled with peroxidase-conjugated dextran polymer, EnVision + (Dako), and anti-rabbit EnVision + were used as secondary antibodies.
2.7. Phosphotungstic Acid Precipitation (PTA)
We performed the concentration of PrPSc according to previous reports (Glatzel et al., 2003).
3. Results
3.1. Analysis of Brain Tissues from Patients with Sporadic CJD the Heathy Subjects by Bioassay and Endpoint RT-QuIC Assay (Fig. 1)
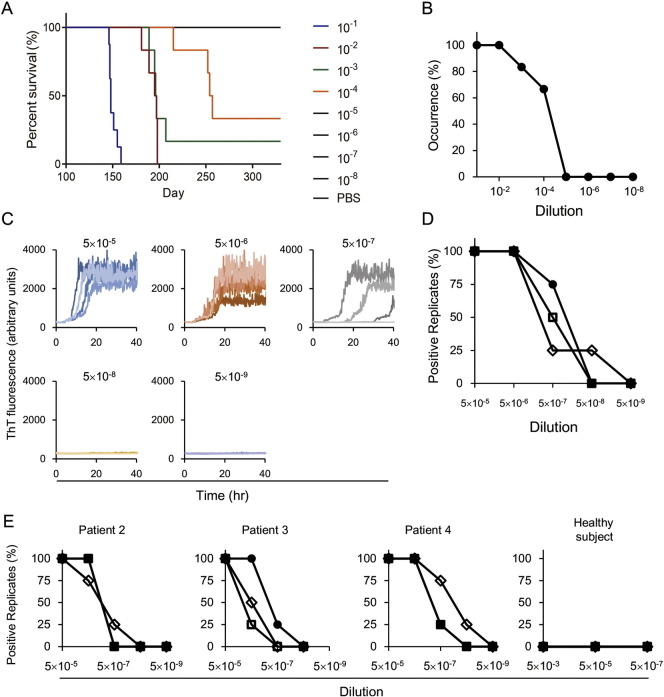
Fig. 1.
Analysis of brain tissues from patients with sporadic CJD using bioassay and endpoint RT-QuIC assay. (A, B) Transmission of human prions to Ki-ChM mice. (A) Survival curve for Ki-ChM mice inoculated with a serially diluted brain homogenate from Patient #1. (B) Endpoint titration of brain tissue from a sCJD patient (Patient #1) using the bioassay. Each value represents the percentage of occurrence of symptoms caused by prion disease. (C, D) Endpoint RT-QuIC assay of brain tissue from Patient #1. (C) Brain tissue was diluted and the endpoint RT-QuIC assay was used to evaluate prion-seeding activity. (D) Each value represents the percentage of positive reactions for each dilution rate. The experiments were performed in triplicate for each sample. (E) Endpoint RT-QuIC assay of brain tissues from patients with sCJD (Patients #2–4). The experiments were performed in triplicate for each sample.
Ki-ChM mice inoculated with 10− 1 to 10− 4 diluted sCJD brain samples developed symptoms; incubation periods were 150 ± 5 days, 193 ± 7 days, 197 ± 6 days, and 243 ± 23 days, respectively (Fig. 1A, B). The Ki-ChM mice inoculated with 10− 5 diluted sCJD brain samples for > 400 days all survived. The incubation time was > 330 days in mice treated with 10− 5 diluted sCJD (Fig. 1A, B). The prion-seeding activity of this sample was also evaluated. As shown in Fig. 1C and D, 100% positive reactions were seen in 5 × 10− 5 and 5 × 10− 6 dilutions; the positive rate dropped to 75% in the 5 × 10− 7 dilution. Following inoculation with the 5 × 10− 8 dilution, there were no signals within 40 h, so the SD50 was calculated as 1010.25/g tissue. The SD50 of other samples was also determined as shown in Fig. 1E. All samples exhibited a similar level of seeding activity (109.17–10.25/g). In all samples from the healthy subjects, the RT-QuIC reaction remained unchanged and maintained a baseline within 48 h.
3.2. Analysis of Extra-Neural Tissues from sCJD Patients the Heathy Subjects Using Endpoint RT-QuIC Assay, Western Blotting, and PrP-Immunohistochemistry
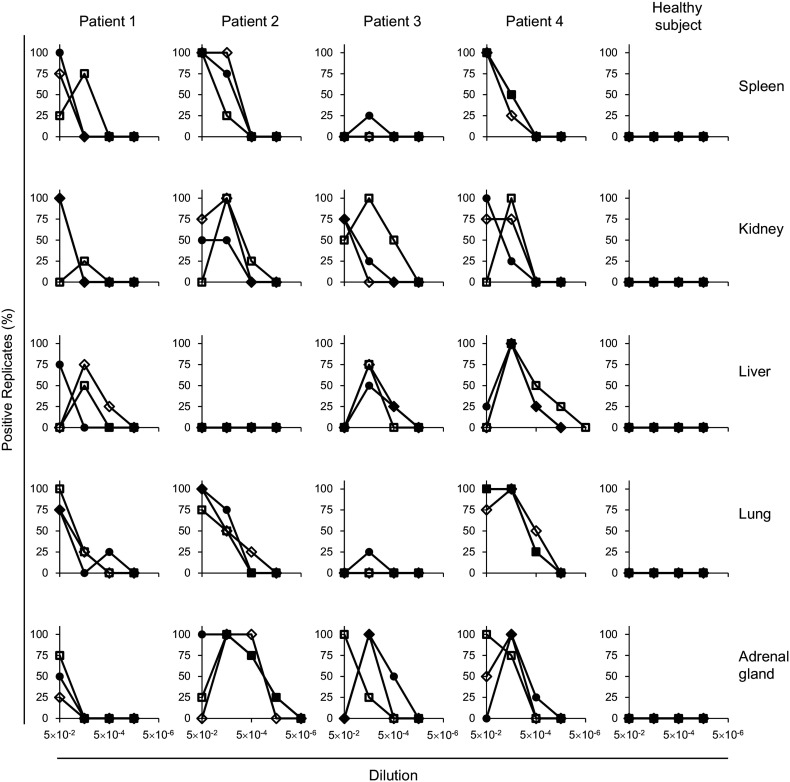
The homogenized samples of five different organs showed positive reactions in RT-QuIC, except for one liver sample from Patient #2. Following inoculation of spleen and lung tissues from Patient #3, positive signals were only detected in one well out of 12, so it was not possible to determine the amount of prion activity in this endpoint assay (Fig. 2). Several positive samples, such as liver and adrenal gland tissues from Patient #1, did not exhibit 100% positivity in repeated tests, so we were not able to determine the SD50. In the remaining 14 samples, 100% of positive replicates were observed at 5 × 10− 2 to 5 × 10− 4 dilutions. And all samples of five different organs in the heathy subjects, the RT-QuIC reaction remained unchanged and maintained a baseline within 48 h. The SD50 values were calculated and are summarized in Table 1.
Fig. 2.
Endpoint RT-QuIC assay of non-neuronal tissues. Homogenates of spleen, kidney, liver, lung, and adrenal gland were diluted and applied to the assay. The experiments were performed in triplicate (three sets of four wells). Each value represents the percentage of positive reactions in four wells.
Table 1.
Clinical features of sporadic CJD patients and endpoint RT-QuIC analysis in tissues. All four female patients fulfilled the WHO diagnostic criteria for CJD and were considered to be definite cases and autopsy cases. The clinical findings and clinical course of disease were consistent with classical types of sporadic CJD. EEG results showed diffuse slowing of background with periodic sharp wave complexes (PSWC). The CSF samples had elevated 14-3-3 protein and t-tau protein, and prion seeding activities were identified by RT-QuIC assay. Western blotting and genetic analysis revealed that these 4 sCJD patients were all MM1 subtype.
N.D.: not detected; N.A.: not available.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at onset (years) | 69 | 70 | 59 | 62 | |||||||||||||
| Period from onset to death (months) | 27 | 18 | 78 | 60 | |||||||||||||
| Period from onset to akinetic mutism (months) | 3 | 2 | 3 | 4 | |||||||||||||
| End-point RT-QUIC (Log SD50/g tissue) | 1st | 2nd | 3rd | mean ± S.D. | 1st | 2nd | 3rd | mean ± S.D. | 1st | 2nd | 3rd | mean ± S.D. | 1st | 2nd | 3rd | mean ± S.D. | |
| Central nervous system | Brain | 10.25 | 10 | 10 | 10.08 ± 0.12 | 9.25 | 9.5 | 9.5 | 9.42 ± 0.12 | 9.75 | 9 | 8.75 | 9.17 ± 0.42 | 9.75 | 10.5 | 9.75 | 10.00 ± 0.35 |
| Non-neuronal tissues | Spleen | 6 | ≤ 5.25 | ≤ 6.25 | ≤ 5.83 | 6.25 | 6.5 | 5.75 | 6.17 ± 0.31 | N.D. | N.D. | N.D. | N.D. | 6 | 5.75 | 6 | 5.92 ± 0.12 |
| Kidney | 5.5 | 5.5 | N.D. | 5.5 | ≤ 5.50 | 6.5 | 6.75 | ≤ 6.25 | ≤ 5.50 | ≤ 4.75 | 7 | ≤ 5.92 | 5.75 | ≤ 6.00 | 6.5 | ≤ 6.08 | |
| Lung | ≤ 5.25 | ≤ 5.50 | 6 | ≤ 5.58 | 6.25 | 6.25 | ≤ 5.75 | ≤ 6.08 | N.D. | N.D. | N.D. | N.D. | 6.75 | 7 | 6.75 | 6.83 ± 0.12 | |
| Liver | ≤ 5.25 | ≤ 6.50 | ≤ 6.00 | ≤ 5.92 | N.D. | N.D. | N.D. | N.D. | ≤ 6.25 | ≤ 6.50 | ≤ 6.25 | ≤ 6.33 | 6.75 | 6.75 | 7.25 | 6.92 ± 0.24 | |
| Adrenal grand | ≤ 5.00 | N.D. | ≤ 5.25 | ≤ 5.13 | 7.25 | 7.5 | 7.5 | 7.42 ± 0.11 | 7 | 6.5 | 5.75 | 6.42 ± 0.51 | 6.75 | 6.5 | 6.25 | 6.5 ± 0.20 | |
N.D. = not detected.
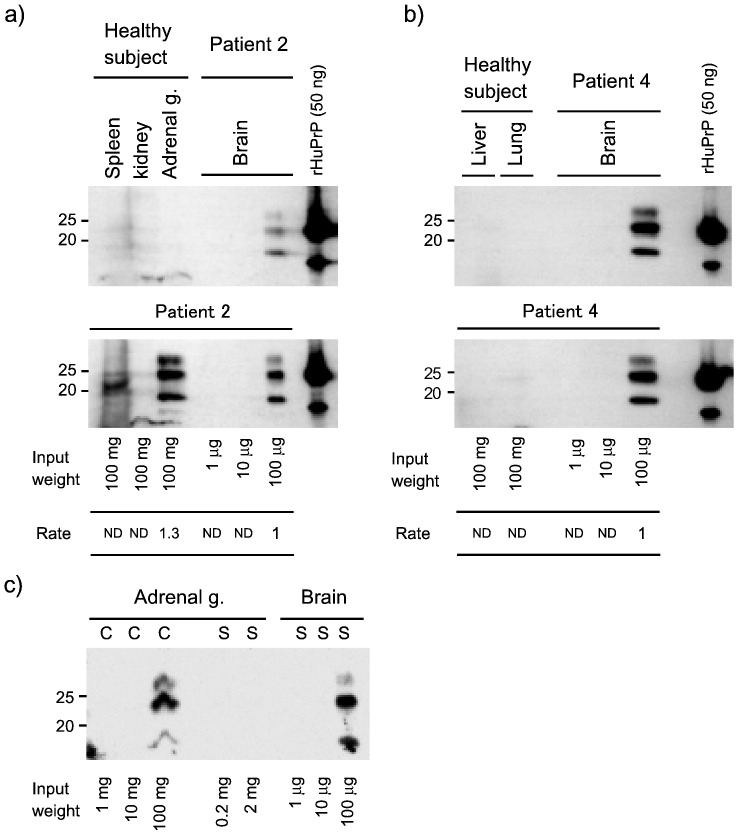
We could not detect PrP-res in tissues of sCJD patients and healthy control subjects by Western blotting or PrP-immunohistochemistry using standard protocols (data not shown), but concentration by centrifugal separation allowed detection of PrP-res in tissues of sCJD patients (Fig. 3). And we were not able to detect the PrP-res in tissues of sCJD patients by PTA concentration (not data shown). This method enabled the concentration of PrP-res to > 103 times that allowed by the standard method.
Fig. 3.
Western blotting analysis of PrP-res in tissue from sporadic CJD patients using the concentration method, which precipitates the sample by centrifugal separation.
The brain (1 mg) or tissue (100 mg of weight) homogenates, such as spleen, kidney, adrenal gland, liver, or lung from normal persons (a–b, upper panel) or sporadic CJD patient 2 (a, lower panel) and 4 (b, lower panel), were digested with PK and samples were prepared for Western blotting (see Materials and Methods). The tissues (100 mg) and brain (1, 10, or 100 μg) samples were loaded on SDS-PAGE, and PrPSc was detected using anti-PrP (3F4) monoclonal antibody and anti-mouse IgG antibody-conjugated HRP. The brain samples were used as a detection limit for Western blotting. The rate indicates measured band intensity, which was compared with brain samples. Recombinant human PrP is used as expose control. c) Detection limits were compared between concentration (C) and standard (S) method. PK-digested samples directly mixed with sample buffer in standard methods, which loaded to indicating weight.
(N.D.: Not detected.)
4. Discussion
Prion-seeding activity in tissues from sCJD patients was evaluated using the endpoint RT-QuIC assay, which revealed an unexpectedly wide distribution of prion activity in all tested patients. The SD50 values reached approximately 106/g in extra-neural organs. With the exception of a single adrenal gland (Patient #2), we were not able to detect PrP-res using the Western blotting assay with PTA. We also did not observe abnormal PrP-immunopositivity on tissue sections. However, this could be due to prion-seeding activities in non-neuronal tissues that were 10,000 times lower than in brain tissues.
The RT-QuIC assay was approximately 104 times more sensitive than the bioassay using knock-in mice expressing a human-mouse chimeric PrP (Fig. 1), although the SD50 was reported to be 100 times greater than the LD50 of 263 K hamster prions (Atarashi et al., 2011). Because the prion-seeding activity in kidney tissues of sCJD patients was 105.5–6.25/g, for example, infectivity (LD50) could be 101.5/g in the organ. This is an extremely low level of infectivity compared with CNS infections. However, it should not be overlooked that prion activity could become detectable in peripheral organs, because human prion disease can develop even after a 30–40-year incubation period (Collinge et al., 2006).
Additionally, the tissue volume was 103–4 greater than the human brain volume, and PrP-res in non-neuronal tissues was 103–4 greater than in the brain. Our method allowed detection of PrP-res in the spleen of Patient #2 (Fig. 3). The SD50 in the Patient #2 brain (SD50: 9.42) was 103–4 greater than the SD50 in the spleen of Patient #2 (SD50: 6.25). Therefore, our method successfully concentrated tissues by approximately 103–4 greater than standard Western blotting.
Expression of physiological PrP in the human body has been well studied. PrPC is expressed in almost all tissues, although mRNA expression levels are highest in the CNS; the spleen and liver are 1/20 of the cortex, the lungs are 1/10, and the kidneys and adrenal glands are 1/5 (The Genotype-Tissue Expression (GTEx) project, 2013; Uhlen et al., 2015). PrP-res has been detected in the spleen and muscles of some sCJD patients by Western blotting analysis when PrP-res in the samples was concentrated by PTA (Glatzel et al., 2003). However, we cannot ignore the possibility that seeding activities detected in peripheral tissues are a result of infectious agents overflowing from the CNS, because results show that kidneys and adrenal glands can be infected and produce abnormal PrP in situ. This study is the first to identify prion-seeding activities in the kidney or liver, and we may have to collect organs from pre-symptomatic CJD patients. The highly sensitive RT-QuIC assay may be useful for providing safer methods and techniques when using human materials.
Author Contributions
Conceived and designed the experiments: HT, KS,TK, RA, and NN. Performed the experiments: HT, TF, TK,TN, and TM. Analyzed the data: HT, KS DI, RA, and NN. Contributed reagents/materials/analysis tools: YT, BM, MT, YI, and MY. Wrote the paper: HT, K·S, RA, and NN.
Conflicts of Interest
None to report.
Acknowledgments
We are grateful to the Japan Prion Disease Surveillance Committee. In particular, we thank Dr. Kitamoto, Tohoku University School of Medicine, for providing the Ki-ChM mice and for the genetic analysis of sCJD patients. This study was supported by Grants-in-Aid from the Research Committee of Surveillance and Infection Control of Prion Disease and from the Research Committee of Prion Disease and Slow Virus Infection of the Ministry of Health, Labour, and Welfare of Japan.
Dr. Satoh received research support from a Grant-in Aid for Scientific Research (C) (24591268) and Scientific Research (B) (Overseas Academic Research) (14507303) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Japan Society for the Promotion of Science.
Dr. Takao received the Ministry of Health, Labour, and Welfare of Japan JSPS KAKENHI, Grant-in-Aid for Scientific Research (C; 26430060) JSPS KAKENHI Grant Number JP 16H06277. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This study was financially supported by grants for Scientific Research from the Ministry of Health, Labour and Welfare of Japan.
References
- Atarashi R., Satoh K., Sano K. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 2011 Feb;17(2):175–178. doi: 10.1038/nm.2294. [DOI] [PubMed] [Google Scholar]
- Bernoulli C., Siegfried J., Baumgartner G. Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet. 1977 Feb 26;1(8009):478–479. doi: 10.1016/s0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- Collinge J., Whitfield J., McKintosh E. Kuru in the 21st century–an acquired human prion disease with very long incubation periods. Lancet. 2006 Jun 24;367(9528):2068–2074. doi: 10.1016/S0140-6736(06)68930-7. [DOI] [PubMed] [Google Scholar]
- Glatzel M., Abela E., Maissen M., Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N. Engl. J. Med. 2003 Nov 6;349(19):1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- Herzog C., Rivière J., Lescoutra-Etchegaray N., Charbonnier A., Leblanc V., Salès N., Deslys J.P., Lasmézas C.I. PrPTSE distribution in a primate model of variant, sporadic, and iatrogenic Creutzfeldt-Jakob disease. J. Virol. 2005 Nov;79(22):14339–14345. doi: 10.1128/JVI.79.22.14339-14345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R., Chang B. Re-assessment of PrP(Sc) distribution in sporadic and variant CJD. PLoS One. 2013 Jul 3;8(7) doi: 10.1371/journal.pone.0066352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi Y., Mohri S., Ironside J.W., Muramoto T., Kitamoto T. Humanized knock-in mice expressing chimeric prion protein showed varied susceptibility to different human prions. Am. J. Pathol. 2003 Dec;163(6):2585–2593. doi: 10.1016/S0002-9440(10)63613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki H., Satoh K., Sano K. Rapid and quantitative assay of amyloid-seeding activity in human brains affected with prion diseases. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0126930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadani V., Penar P.L., Partington J. Creutzfeldt-Jakob disease probably acquired from a cadaveric dura mater graft. Case Rep. J. Neurosurg. 1988;69(5):766–769. doi: 10.3171/jns.1988.69.5.0766. [DOI] [PubMed] [Google Scholar]
- The Genotype-Tissue Expression (GTEx) project Nat. Genet. 2013 Jun;45(6):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M., Fagerberg L., Hallstrom B.M. Proteomics. Tissue-based map of the human proteome. Science. 2015 Jan 23;347(6220):1260419. doi: 10.1126/science.1260419. (New York, NY) [DOI] [PubMed] [Google Scholar]
- Wilham J.M., Orru C.D., Bessen R.A. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010;6(12) doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will R.G., Matthews W.B. Evidence for case-to-case transmission of Creutzfeldt-Jakob disease. J. Neurol. Neurosurg. Psychiatry. 1982 Mar;45(3):235–238. doi: 10.1136/jnnp.45.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]