Abstract
The quality of clinical biobank samples is crucial to their value for life sciences research. A number of factors related to the collection and storage of samples may affect the biomolecular composition. We have studied the effect of long-time freezer storage, chronological age at sampling, season and month of the year and on the abundance levels of 108 proteins in 380 plasma samples collected from 106 Swedish women. Storage time affected 18 proteins and explained 4.8–34.9% of the observed variance. Chronological age at sample collection after adjustment for storage-time affected 70 proteins and explained 1.1–33.5% of the variance. Seasonal variation had an effect on 15 proteins and month (number of sun hours) affected 36 proteins and explained up to 4.5% of the variance after adjustment for storage-time and age. The results show that freezer storage time and collection date (month and season) exerted similar effect sizes as age on the protein abundance levels. This implies that information on the sample handling history, in particular storage time, should be regarded as equally prominent covariates as age or gender and need to be included in epidemiological studies involving protein levels.
Keywords: Plasma proteins, Biobank, Covariate, Storage time, Sampling month, Proximity extension assay
Highlights
-
•
Storage time explains up to 35 % of plasma protein concentration variation in frozen biobank samples from healthy women.
-
•
Storage time exert similar effect sizes as individual age and should be included as a covariate in epidemiological studies.
One basic requirement of life science research is the quality of samples. Proper handling and rigorous biobanking of clinical samples is crucial for collection of samples for rare diseases, for monitoring individual variation in longitudinal studies and for prospective studies of biomarkers and risk of developing for instance cardiovascular disease. We have studied the effect of long-time storage, individual age and sampling month and conclude that storage-time has similar impact on protein levels as age. The results emphasize the need to include sample parameters as covariates in future epidemiological studies, which may facilitate future discoveries of novel biomarkers for disease.
1. Introduction
The stability of biomolecules in clinical biobanks is essential to many applications in life sciences. The short-term stability is important in order to handle batch effects in the laboratory analyses, while the long-term stability is essential for collection of samples for rare-diseases, for monitoring individual variance in longitudinal studies over extended time periods, and for retrospective studies with samples stored over different time-spans or storage methods. Biobanks should strive to maintain the biomolecular composition of the sample at the time of collection, in order to allow for unbiased comparisons of samples obtained using different methods, collected at different time points, stored over different time-spans, and subjected to different number of freeze-thaw cycles. In previous studies of solid tissue or plasma samples that have been stored frozen, parameters such as sample handling and short-term effects of storage regimes on blood samples before freezing have been examined (Skogstrand et al., 2008). Plasma and serum cytokines concentrations have been found to increase with storage time before separation and freezing. It has also been shown that concentrations of three of the investigated proteins in sets of brain tissue were significantly affected by freezer storage time (Harish et al., 2011). The number of freeze-thaw cycles can have an effect on different proteins (Lee et al., 2015). In addition, other factors, such as time of day, weekday and general intra-individual variation have also been described (Stenemo et al., 2016). However, there have been no examinations of a large set of proteins for these parameters.
Sample collection for a cohort is often carried out at the time of diagnosis, time of treatment or time of screening of healthy individuals, and not confined to a certain time of the year. This implies that environmental factors, such as pollen concentration in the spring, may cause downstream effects on proteins in immune response pathways. For instance, plasma levels of soluble intercellular adhesion molecule-1 (sICAM-1) and soluble E-selectin have been shown to be increased in inhalation allergic children compared to healthy controls (Reich et al., 2003). Also, seasonal differences in variables such as sunlight and ultra violet (UV)-radiation could affect the abundance levels of circulating proteins. This is particularly relevant in parts of the world where there are large differences in amount of solar radiation between different parts of the year. UV-radiation causes inflammatory responses in the skin and several cytokines are affected by UV-radiation. Specifically, production of C-X-C motif chemokine ligand 5 (CXCL5) have been shown to be up-regulated in human skin (Reichert et al., 2015) in response to UV-radiation and changes in plasma levels of several interleukins have been shown in vivo in mouse models (Vostalova et al., 2013), suggesting that seasonal variation is likely to be measurable in human plasma. In order to investigate the effect of storage time in freezer, chronologic age at sampling, sampling month, and season (sunlight), we determined the abundance level of 122 plasma proteins using the Protein Extension Assay (PEA) in 106 women sampled at 380 occasions spanning from 1988 to 2014.
2. Materials and Methods
2.1. Samples
380 female plasma samples from the Västerbotten intervention program (Hallmans et al., 2003) (VIP, N = 208) and Mammography Screening Project (MA, N = 172) were obtained from the Umeå Biobank in Sweden. The plasma samples were drawn between 1988 and 2014 and donated by 106 women. The plasma was treated with ethylenediaminetetraacetic acid (EDTA) separated and frozen within 1 h after sampling, followed by long time storage in − 80 °C. In the VIP cohort almost all samples has been taken after overnight fasting. The samples analyzed had not previously been thawed.
2.2. Protein Abundance Measurements
Plasma protein abundance levels were quantified using the Proximity Extension Assay (PEA) (Assarsson et al., 2014) multiplex assays from Olink Proteomics AB, Uppsala, Sweden. In brief, for each observed protein, a pair of oligonucleotide-labeled antibodies probes bind to the targeted protein, and if the two probes are in close proximity, a PCR target sequence is formed by a proximity-dependent DNA polymerization event and the resulting sequence is subsequently detected and quantified using standard real-time PCR. Data is then normalized and transformed using internal extension controls and inter-plate controls, to adjust for intra- and inter-run variation. The final assay read-out is given in Normalized Protein eXpression (NPX), which is an arbitrary unit on log2-scale where a high value corresponds to a higher protein expression. Each protein has specific lower limit of quantification (LLOQ) and an upper limit of quantification (ULOQ) between which a 1-unit increase in NPX correspond to a two-fold increase in protein concentration. Internal controls specify a run-specific lower limit of detection (LOD) as 3 times the standard deviation over background. The manufacturers webpage (www.olink.com) contains information on protein-specific detection limits and records of technical validations of intra- and inter-variability (in terms of coefficients of variation (CV) of each analyzed protein. Samples were analyzed for the complete Proseek Multiplex Inflammation panel I and 20 proteins each from the Proseek Multiplex CVD I panel and Proseek Multiplex Oncology II panels (http://www.olink.com/proseek-multiplex/). The plasma samples were analyzed, quality-controlled and pre-processed into NPX at Olink Proteomics AB, Uppsala, Sweden. In total, 122 unique proteins were quantified out of which 10 were overlapping between the Inflammation panel and either of the other panels.
2.3. Statistical Analysis
All statistical analyses were carried out in R (R Development Core Team, 2014). Effect of storage time on protein levels was examined by fitting a linear model with protein abundance levels as response and whole years since original sampling date as variable. Significance was evaluated by ANOVA-analysis. Proteins where storage time was found to be a significant influence was adjusted by removing the contribution of storage time using the beta (β)-coefficient from the linear models as described above. Contribution of individual age to protein abundance levels was analyzed in the same way using storage-time adjusted protein levels as input. Monthly and seasonal variation in protein levels was examined using storage time and age-adjusted protein levels using a two-sided Wilcoxon ranked-sum test. Contribution of monthly sun hours to protein levels were examined by fitting a linear model with protein levels as response and sun hours as variable. This analysis was repeated with a 1–11 month shift in sun hours, allowing for a possible delay in protein levels depending on sun activity. Significance was evaluated by ANOVA analysis. Calculations of the variance explained by a variable to the response, were carried out using the ‘summary’-function applied to the fitted linear models.
2.4. Ethical Considerations
The study has been approved by the Regional Ethics Board in Umeå, Dnr 2013-314-32M and 2012-229-31M.
3. Results
3.1. Study Design
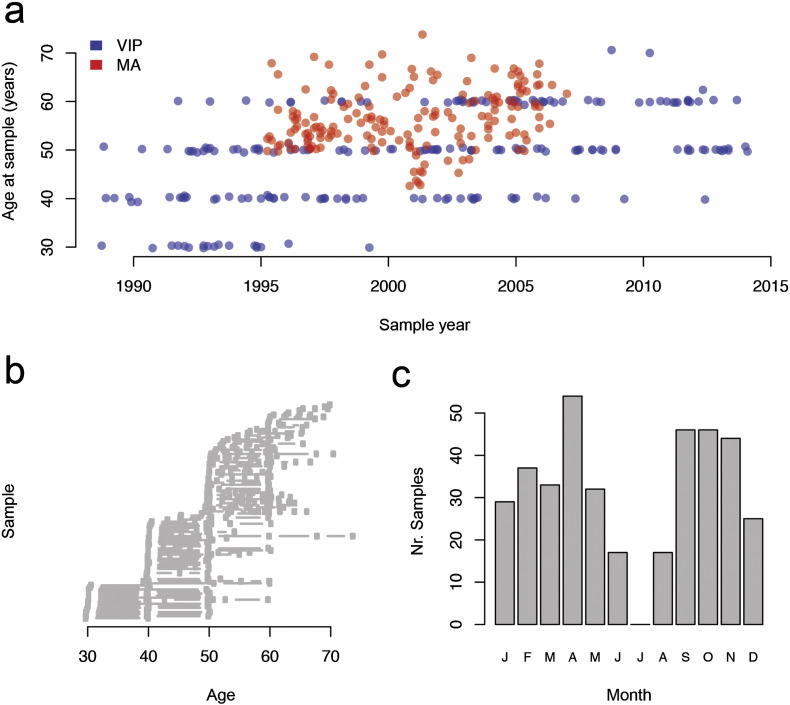
The Västerbotten Intervention Program (Hallmans et al., 2003) invites participants at 10-year intervals starting from the age of 30 or 40, while women conduct mammography check-ups at intervals normally spaced 18–24 months apart. Because of the different sampling-strategies in the VIP and the MA-cohorts, the VIP-samples allows for study of the impact of storage time independent of the actual age of the individual, by restricting the analysis to a given age-span. The samples from the MA-cohort have a more uniform distribution of ages and, when corrected for storage time, are suited for analysis of the contribution of chronological age at sampling to protein levels. Among the samples used here, the oldest was collected in 1988 and the most recent in 2014 (Fig. 1A). Out of the 106 women, 96 had > 1 sample, and the maximum age-range for a single woman was 33.5 years (first sample at age 40.3 and latest at 73.8 years) (Fig. 1B). Ages of participants in the VIP ranged from 29 to 70 and in MA from 42 to 73. Overall the samples were collected over the entire year, with the exception of July with 0 samples (Fig. 1C). July is traditionally the time of summer vacation in Sweden and no samples have been collected during this month.
Fig. 1.
Study design. (a) Distribution of samples over time and individual age at time of sampling. Blue dots correspond to samples from the Västerbotten Intervention Program (VIP) and red dots to samples from the Mammography Screening Program (MA). (b) The VIP-study has been collecting samples since 1985 and the vast majority of the individuals in this study have donated samples at multiple occasions, some over a period of over 3 decades. (c) With the exception of July, the samples have been collected throughout the whole year.
3.2. Protein Abundance Levels
Relative abundance levels for 132 proteins were quantified using the Proximity Extension Assay (PEA) in 380 plasma samples from 106 Swedish women in the Umeå-area from the VIP and MA-cohorts. Each of the 132 assays had a lower limit of detection specified by the manufacturer and here, 14 of the 132 (10.6%) assayed proteins had > 80% of the individual measurements below the detection limit. This is similar to the pattern seen previously when analyzing protein levels from these panels in non-disease cohorts (Enroth et al., 2015a, Enroth et al., 2014). These 14 proteins (IL2RB, IL1a, IL2, TSLP, RA1, PD-L1, IL24, ARTN, TNF, IL20, IL33, IL4, LIF and NRTN) were excluded from further statistical analysis. Moreover, 10 of the 118 remaining proteins were replicated over two different panels (Material and Methods). Each pair of replicated assays was significantly correlated (p < 8.0 × 10− 53, Spearman's Rho), with a mean correlation coefficient (R2) of 0.78. The highest correlation was found for C-X-C motif chemokine ligand 11 (CXCL11) with R2 = 0.93 (p < 2.2 × 10− 209), while the lowest was found for the Cluster of differentiation 40 protein (CD40) with R2 = 0.62 (p < 1.4 × 10− 77) (Supplementary Fig. 1).
3.3. Correlation with Storage Time
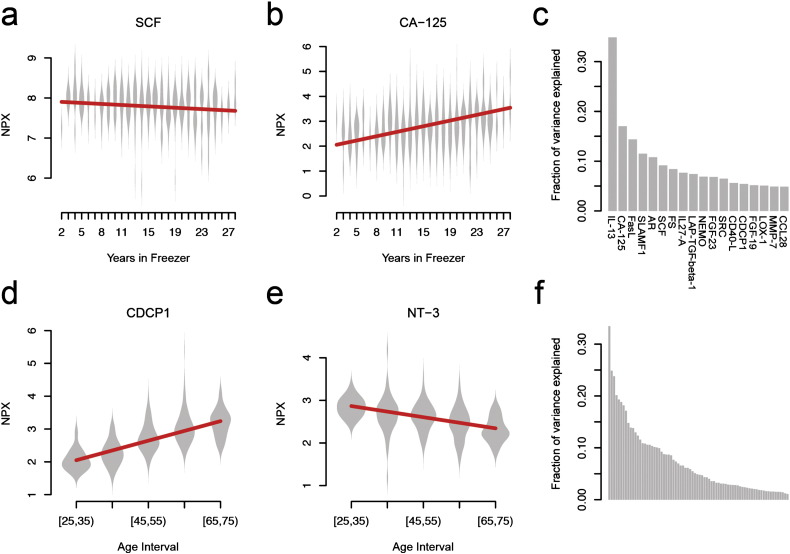
Since the majority of samples in our data have been sampled on multiple occasions, it is difficult to separate the effect of the ageing of the individuals from the storage time in the freezer. Therefore, we restricted the analysis of storage time to 92 individuals' aged 50 ± 0.5 years. Due to the sampling strategy of the VIP-study, this age-interval contains the highest number of samples (Fig. 1A). The storage time distribution among these individuals does not differ from the rest of the samples (p > 0.4, Wilcoxon ranked sum test), and is thus representative of the complete material. Analysis of coefficients of variance (CV) for all proteins for these samples in bins of storage time (Supplementary Fig. 2A) did not indicate any clear trends of changes in CV with increased storage-time. We found that 18 of the 108 proteins investigated were influenced by storage time (Supplementary Table 1, nominally significant, p < 0.05), and one protein (Cancer antigen 125; CA-125 also known as Mucin 16) remained statistically significant after correction for testing of multiple hypotheses (Bonferroni, p < 0.05/108 = 4.6 × 10− 4). Storage time induces either an increase or a decrease in levels for different proteins (Supplementary Table 1, Fig. 2A, B). Among the 18 proteins, storage time alone explained between 4.9 and 34.9% of the observed variance (Fig. 2C). Using the 18 protein-specific linear models built from the 92 observations with fixed ages, all observations were adjusted for the storage time, removing the contribution of storage time from the measurements.
Fig. 2.
Associations and variance explained by different variables. (a) Decreasing protein abundance levels (NPX) with longer storage time, (b) Increasing abundance levels (NPX) with longer storage time. (c) Fraction of variance explained by storage time (all proteins with nominally significant associations, Supplementary Table 1). (d) Increasing protein abundance levels (NPX) for CUB domain-containing protein 1 (CDCP1) with age after storage-time component removed (linear model). (e) Same as (d), but decreasing with increased age of individual for Neurotrophin-3 (NT-3). (f) Fraction of variance explained by age in the residuals after correcting for storage time (all proteins with nominally significant associations, Supplementary Table 2).
3.4. Correlation with Age
Using the storage time adjusted observations, we proceeded to investigate if there was any effect of age using all 380 samples (Material and Methods). As above, analysis of CV for all proteins for these samples in bins of age at sample collection (Supplementary Fig. 2B) did not indicate any clear trends of changes in CV with increased age of the individual. We found 70 out of 108 proteins to be significantly influenced by age (Fig. 2DE, Supplementary Table 2, nominal p-value < 0.05). Out of these, 45 proteins remained significant when adjusting for multiple testing (Bonferroni, p < 0.05 / 108 = 4.6 × 10− 4). Age explained between 1.1 and 33.5% of the variance seen in the residuals after correcting for storage time (Fig. 2F). 16 of the 70 proteins were among the 18 previously corrected for storage time. Using multiply sampled individuals from the VIP-cohort, the effect of age on protein levels (after correction for storage time) can be easily visualized. For example, levels of Interleukin 27 subunit alpha (IL-27A) increased with age (Supplementary Fig. 3A), while for the Kit ligand (SCF) levels decreased with age (Supplementary Fig. 3B).
3.5. Seasonal Differences and Correlations With Sampling Month
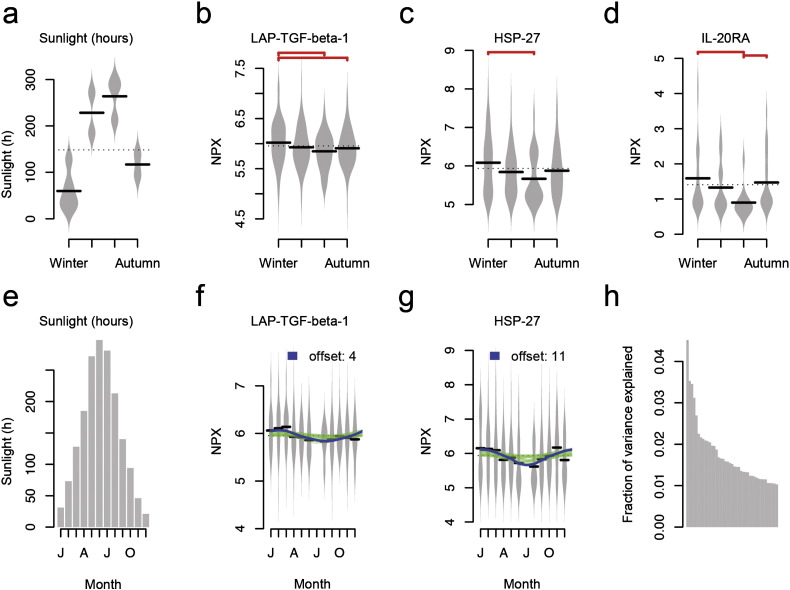
We grouped all 380 measurements according to the season (Winter, Spring, Summer or Autumn) the samples were originally collected. Using meteorological data for the Umeå-region obtained from the Swedish Meteorological and Hydrological Institute (Swedish Meteorological and Hydrological Institute, 2016) (SMHI) we investigated any difference between season and protein abundance levels, adjusted for storage time and age. 15 of the 108 proteins showed nominally significant differences between any two seasons (Supplementary Table 3). Six tests were performed per protein (each season versus the others) and no differences remained significant after adjusting the significance threshold for multiple hypotheses testing (p < 0.06 / 6 / 108 = 7.7 × 10− 5. Out of the 15 nominal associations, 12 involved summer or winter compared to any other season. The town of Umeå is located at 63 degrees North and receives an average of 60 sunlight hours per month during the winter months (November to March) compared to over 260 h per month during summer (June to August) (Fig. 3A). Fig. 3 B–D shows three examples where the observed protein levels are lower during the summer than the other seasons.
Fig. 3.
Seasonal and monthly variation in protein abundance levels. (a) Mean sun hours per season (Winter, Spring, Summer, Autumn) (b–d) proteins abundance levels (NPX) over seasons for Latency-associated peptide transforming growth factor beta-1 (LAP-TGF-beta-1), Heat shock 27 kDa protein (HSP 27) and Interleukin-20 receptor subunit alpha (IL-20RA). Red connections indicate nominally significant (p < 0.05, two-sided Wilcox test) differences between pair of seasons. (e) Sun hours per month. (f–g) Monthly differences in protein abundance levels for two proteins (LAP-TGF-beta-1 and HSP-27). Solid lines represent responses from linear models fitted with 0–11 months offset between sunlight and protein abundance levels (NPX). Blue lines indicate nominally significant contribution of sunlight hours to protein abundance levels for a specific offset. The best model in (f) was found for an offset of 8 months delay, and for (g) with a 1 month delay. (h) Fraction of variance explained by sunlight (as a proxy variable for a number of possible factors) on protein abundance levels after adjusting for storage time and age of the individual.
With the exception of July, the samples used were collected throughout the whole year. Using the protein abundance levels corrected for storage time and age, the majority of the analyzed proteins showed statistically different distributions between at least one pair of months, with February and November being the months most often displaying alternate distributions to another month. This corresponds well with the seasonal analysis. We therefore reasoned that increased or decreased sunlight hours could explain some of the variance seen on the plasma protein abundance levels. We also reasoned that there could be a delay in effect on protein levels and therefore repeated the variation analysis allowing the sun hours to be shifted 0 to 11 months compared to the protein abundance levels.
We found nominally significant correlations between protein levels and sun hours for 36 proteins, most commonly offset:ed 3–5 or 9–11 months (Supplementary Table 4 and Fig. 3F,G). After correction for multiple hypotheses testing (Bonferroni) for all offsets (12) and proteins (108), none of these associations remained significant. The correlation between sun hours and protein levels was found to be highest in a particular month, and flanked by lower correlations in the adjacent months with either positive of negative sign due to the 0 to 11 month shifts, where an 11 months shift in sunlight corresponds to a 1 month delay in effect on the protein abundance levels. The contribution of sun hours on actual protein levels was, however, small (Fig. 3H), and explained at most 4.5% (Interleukin 10 receptor subunit alpha; IL10RA) of the variation seen among the proteins. In the linear modeling, the coefficient for IL10RA was positive, meaning that an increase in sunlight also increases the observed levels of IL10RA.
4. Discussion
Biobanking of clinical samples is central to the advancement of life sciences research. A number of factors related to both collection and storage of samples may affect the biomolecular composition, and subsequently the results obtained from analyzing the samples. Large efforts have been made in collecting carefully matched case and control cohorts, adjusting for non-disease related factors such as age and gender, or risk factors such as body mass index (BMI), weight and smoking. Few studies have had the possibility to investigate the contribution of factors related to collection and long-term sample storage separately from e.g. chronological age at sample collection and gender. Here we have taken advantage of the sampling strategy of the Västerbotten Intervention Program and Mammography screening program in northern Sweden to disentangle the effects of freezer storage time and individual age. Individual age has been shown to affect the plasma protein abundance levels in many studies, and specifically in three studies employing the same protein detection assay as used here, the proximity extension assay (Enroth et al., 2015a, Enroth et al., 2014, Larsson et al., 2015). In a previous study we showed that the plasma protein profile alone explain over 85% of the variation seen in age (Enroth et al., 2015b). Age alone can account for over 27% of the variation seen in a single protein (Enroth et al., 2014). In the present study, among individuals aged 50 and whose samples had been stored over the course of up to 30 years, we found storage time alone to account for up to 35% of the variation seen in a single protein. This demonstrates the large variance introduced by storage time, and underscores the inclusion of storage time as an important variable in future epidemiological studies.
To the best of our knowledge, no other studies have investigated the effect of storage time on the plasma protein levels and we therefore cannot compare the effects found here to others' results. We studied the reproducibility of our age effects by comparison to Larsson et al. (Larsson et al., 2015), that examined the influence of age and gender on 63 cytokines in 33 individuals and employing the same assay as here. In total, 19 of the 20 nominally significant reported associations with age were replicated here using nominally significant p-values (11 of 15 after multiple hypothesis testing). The only association that was not replicated was for MCP-3, which had a calculated significance level with age at p = 0.38 in our material. This discrepancy could be due to several reasons; we have female samples only (N = 380) whereas Larsson and colleagues had both male (N = 16) and female samples (N = 17) and both sample size and gender distribution could influence the analysis. Larsson et al. (Larsson et al., 2015), however, found no significant gender difference for MCP-3 and we found no significant storage effects, so the underlying reasons have to be sought elsewhere.
We also noticed that there are statistically significant differences in protein abundance levels depending on the season, or month, the samples were collected. After adjusting our protein abundance levels for storage time and individual age, we found only nominally significant associations with monthly sun hours, explaining up to 4.6% of the variation seen in protein abundance levels. The lack of significant associations with monthly sun hours could be attributed to a number of factors. Although UV-radiation have previously been shown to directly affect the circulating levels of plasma proteins (Vostalova et al., 2013, Reichert et al., 2015), the sun hours are likely to be viewed as a proxy variable to other seasonal changes such as blooming or pollen levels which in turn triggers the immune system, or various forms of changes in lifestyle such as seasonal intakes of food and changes in habits of physical activity. The number of sun-hours per month available from the SMIHI is also an average over the past 30 years and the actual sun-hours at the individual sampling dates could differ from the used number. Taken together, there are several sources of additional noise not accounted for in our analysis of sun-hours compared to protein levels. Our study was further limited by analyzing only female samples from the north of Sweden and the effects seen here may therefore not necessarily be directly applicable to a different population. We also cannot rule out that there are effects of general changes in lifestyle of individuals aged 50 during the course of three decades in terms of dietary habits, exercise patterns and direct or secondary inhalation from smoking at e.g. work places that would contribute to the storage time variation. Daily smoking among women aged 16–84 in Sweden have for instance gone down from 29% in the early 80s to 13% in 2010 (Centralförbundet för alkohol-och narkotikaupplysning (CAN), 2012). The smoking history of the currently investigated cohort is however not known. We, and others, have previously shown that smoking affects circulating proteins such as interleukin 12 (IL-12) (Kroening et al., 2008, Enroth et al., 2015b). Here, we did not find any effects of storage time on IL-12 but further studies are warranted to elucidate effects due to common lifestyle changes over the past decades in a general population to investigate such mechanisms.
In conclusion, we have shown that storage time has similar effect sizes on protein levels in biobanked plasma samples as individual age. We also show that there is significant seasonal variation that can be partially explained by environmental factors such as variations in sunlight. The results emphasize the need to include sample key parameters as natural covariates in epidemiological studies.
Funding Sources
This study was supported by the Swedish Cancer Foundation (Grant no. CF-2017) and the Swedish Foundation for Strategic Research (Grant no. SSF-SB12-0086). The funding sources had no role in the study design, the data collection and analysis, decision to publish the results, or the preparation of the manuscript.
Conflicts of Interest
Ulf Gyllensten and Stefan Enroth are authors on a patent application entitled ‘Determination and analysis of Biomarkers in clinical samples’, United Kingdom Patent Application UK nos. 1410956.5 and 1414913.2 (2014, Pending). The remaining authors declare no competing financial interests.
Author Contributions
Conceived of the study (SE, UG), designed analyses (SE), analyzed data (SE), produced figures (SE), wrote the paper (SE, UG), provided samples (GH, KG), data collection (UG). All authors read, corrected, and approved the final manuscript.
Acknowledgements
We thank the Umeå Biobank (Biobank North) for providing samples and Åsa Ågren and Kerstin Enquist at Biobank North for assistance with the sample extraction. The PEA measurements were performed by Olink Proteomics AB, Uppsala, Sweden.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.08.038.
Appendix A. Supplementary Data
Supplementary materials.
References
- Assarsson E., Lundberg M., Holmquist G., Bjorkesten J., Thorsen S.B., Ekman D., Eriksson A., Rennel Dickens E., Ohlsson S., Edfeldt G., Andersson A.C., Lindstedt P., Stenvang J., Gullberg M., Fredriksson S. Homogenous 96-plex pea immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centralförbundet För Alkohol-Och Narkotikaupplysning (Can) Drogutvecklingen i Sverige 2011. In: LEIFMAN H.K., editor. Stockholm. 2012. [Google Scholar]
- Enroth S., Bosdotter Enroth S., Johansson A., Gyllensten U. Effect of genetic and environmental factors on protein biomarkers for common non-communicable disease and use of personally normalized plasma protein profiles (PNPPP) Biomarkers. 2015;20(6-7):355–364. doi: 10.3109/1354750X.2015.1093546. [DOI] [PubMed] [Google Scholar]
- Enroth S., Enroth S.B., Johansson A., Gyllensten U. Protein profiling reveals consequences of lifestyle choices on predicted biological aging. Sci. Rep. 2015;5:17282. doi: 10.1038/srep17282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth S., Johansson A., Enroth S.B., Gyllensten U. Strong effects of genetic and lifestyle factors on biomarker variation and use of personalized cutoffs. Nat. Commun. 2014;5:4684. doi: 10.1038/ncomms5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmans G., Agren A., Johansson G., Johansson A., Stegmayr B., Jansson J.H., Lindahl B., Rolandsson O., Soderberg S., Nilsson M., Johansson I., Weinehall L. Cardiovascular disease and diabetes in the Northern Sweden health and disease study cohort — evaluation of risk factors and their interactions. Scand. J. Public Health Suppl. 2003;61:18–24. doi: 10.1080/14034950310001432. [DOI] [PubMed] [Google Scholar]
- Harish G., Venkateshappa C., Mahadevan A., Pruthi N., Bharath M.M., Shankar S.K. Effect of storage time, postmortem interval, agonal state, and gender on the postmortem preservation of glial fibrillary acidic protein and oxidatively damaged proteins in human brains. Biopreserv. Biobanking. 2011;9:379–387. doi: 10.1089/bio.2011.0033. [DOI] [PubMed] [Google Scholar]
- Kroening P.R., Barnes T.W., Pease L., Limper A., Kita H., Vassallo R. Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J. Immunol. 2008;181:1536–1547. doi: 10.4049/jimmunol.181.2.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A., Carlsson L., Gordh T., Lind A.L., Thulin M., Kamali-Moghaddam M. The effects of age and gender on plasma levels of 63 cytokines. J. Immunol. Methods. 2015 doi: 10.1016/j.jim.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Kim S.Y., Shin S.Y. Effect of repeated freezing and thawing on biomarker stability in plasma and serum samples. Osong Public Health Res. Perspect. 2015;6:357–362. doi: 10.1016/j.phrp.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Develpment Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Reich M., Niess J.H., Bar C., Zwacka G., Markert U.R. Elevated nonspecific plasma proteins in allergic patients. J. Investig. Allergol. Clin. Immunol. 2003;13:60–65. [PubMed] [Google Scholar]
- Reichert O., Kolbe L., Terstegen L., Staeb F., Wenck H., Schmelz M., Genth H., Kaever V., Roggenkamp D., Neufang G. UV radiation induces CXCL5 expression in human skin. Exp. Dermatol. 2015;24:309–312. doi: 10.1111/exd.12652. [DOI] [PubMed] [Google Scholar]
- Skogstrand K., Ekelund C.K., Thorsen P., Vogel I., Jacobsson B., Norgaard-Pedersen B., Hougaard D.M. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J. Immunol. Methods. 2008;336:78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Stenemo M., Teleman J., Sjostrom M., Grubb G., Malmstrom E., Malmstrom J., Nimeus E. Cancer associated proteins in blood plasma: determining normal variation. Proteomics. Jul 2016;16(13):1928–1937. doi: 10.1002/pmic.201500204. Epub 2016 Jun 2. [DOI] [PubMed] [Google Scholar]
- Swedish Meteorological and Hydrological Institute. 2016. Swedish Meteorological and Hydrological Institute [Online]. (Available) http://www.smhi.se2016
- Vostalova J., Rajnochova Svobodova A., Galandakova A., Sianska J., Dolezal D., Ulrichova J. Differential modulation of inflammatory markers in plasma and skin after single exposures to UVA or UVB radiation in vivo. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2013;157:137–145. doi: 10.5507/bp.2013.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.