Abstract
Background
Population-level monitoring of hepatitis C virus (HCV) infected people across the cascade of care identifies gaps in access and engagement in care and treatment. We characterized a population-level cascade of care for HCV in British Columbia (BC), Canada and identified factors associated with leakage at each stage.
Methods
The BC Hepatitis Testers Cohort (BC-HTC) includes 1.5 million individuals tested for HCV, HIV, reported cases of hepatitis B, and active tuberculosis in BC from 1990 to 2013 linked to medical visits, hospitalizations, cancers, prescription drugs and mortality data. We defined six HCV cascade of care stages: 1) estimated population prevalence; 2) HCV diagnosed; 3) HCV RNA tested; 4) genotyped; 5) initiated treatment; and 6) achieved sustained virologic response (SVR).
Results
We estimated that 73,203 people were HCV antibody positive in BC in 2012 (undiagnosed: 18,301, 25%; diagnosed: 54,902, 75%). Of these, 56%(40,656) had HCV RNA testing; 34%(26,300) were genotyped; 12%( 8532 ) had received interferon-based therapy and 7%(5197) had SVR. Males, older birth cohorts, and HBV coinfected were less likely to undergo HCV RNA testing. Among those with chronic HCV infection, 32% had received liver-related care. Retention in liver care was more likely in those with HIV, cirrhosis, and drug/alcohol use and less likely in males and HBV coinfected.
Conclusions
Although there are gaps in HCV RNA testing and genotyping after HCV diagnosis, the major gap in the cascade of care was low treatment initiation. People with comorbidities progressed through the cascade of testing and care but few received treatment.
Keywords: Cascade of care, Hepatitis C, Linkage with care, Testing, Engagement with care, Prevalence, Treatment
Highlights
-
•
Integration of various data sources enables HCV monitoring across the care cascade to assess program effectiveness.
-
•
The majority of anti-HCV positive individuals were tested for RNA and genotyping.
-
•
Very small proportion of HCV infected individuals received treatment.
-
•
People with HIV coinfection and drug use despite being in liver care were less likely to receive treatment.
We have assembled data on all individuals testing for hepatitis C in British Columbia to establish a system to monitor infection and care. The majority of the individuals testing positive for anti- HCV antibodies were tested for hepatitis C RNA and subsequently genotyping, both needed for treatment. However, very small percentage received interferon-based hepatitis C treatment and it was successful in about half of them. People with HIV co-infection and drug use were more likely to receive liver care but they were less likely to receive treatment. Changes at laboratory level could overcome remaining gaps in testing while highly tolerable and effective new drugs could reduce treatment gaps.
1. Introduction
Hepatitis C virus (HCV) is a major global public health problem. Although HCV incidence is declining, morbidity and mortality related to chronic HCV infection are increasing (Aspinall et al., 2015, Kuo et al., 2015). In many countries worldwide, the majority of infected individuals acquired the HCV decades ago, and are now increasingly presenting with serious liver-related illnesses including decompensated cirrhosis and hepatocellular carcinoma (Janjua et al., 2015). Potentially curative interferon based treatments have been available for > 15 years, but < 15% of those infected had been treated. HCV cure is associated with reduced morbidity and mortality (Simmons et al., 2015, Singal et al., 2010).
Availability of well-tolerated, short-course (8–12 weeks), interferon-free, direct-acting antiviral (DAA) drugs with cure rates approaching 95% is expected to be a game changer in preventing progressive liver disease (Smith et al., 2015, Smith and Lim, 2015). However, for these drugs to have major population-level impact on morbidity and mortality, screening efforts must reach undiagnosed individuals, diagnosed individuals must be linked with care and people remain engaged with care to be assessed for and receive treatment.
The cascade of care has been used to monitor the progress of HIV programs aimed at reducing the epidemic impact on individuals and populations (Nosyk et al., 2014). Monitoring the HCV affected population across stages of a cascade of care (diagnosis, linkage with care, treatment, and cure) at a broader population level provides a measure of program effectiveness and identifies service and access gaps. Population-level program progress and effectiveness data is critical to policy makers for forecasting budgetary impacts of treatment with very expensive drugs. The HCV cascade of care has been presented for specific population groups such as US veterans or small community programs for people who inject drugs (PWID) but not for an overall population (Maier et al., 2016, Viner et al., 2015, Wade et al., 2015).
In British Columbia (BC), DAAs became available in 2014 and are publicly-funded for people with advanced liver disease (≥ F2 METAVIR or equivalent). The data presented in this paper based on all tested and diagnosed individuals in BC characterized the population-level HCV cascade of care in BC, Canada, and identified factors associated with leakage at each stage. This provides a population-based benchmark for monitoring the progress of hepatitis care programming to guide policy in British Columbia which can also be used as a framework for other jurisdictions internationally.
2. Methods
2.1. Setting
The study presents data from the province of BC, Canada. All residents of BC are registered in publicly funded Medical Services Plan (MSP) that act as a single payer system and covers services provided by fee for service practitioners including general practices, private laboratories and other fee for service providers. HCV laboratory testing for the entire province is centralized at BC Public Health Laboratory (BCPL) except for < 5% of tests performed at a regional laboratory which sends positive tests to BCPHL for confirmation. Prescription drugs are either covered by public program for eligible patients or by their private extended health insurance which is usually provided by employers. All dispensed prescriptions are recorded in central system called PharmaNet. Interferon-based combination therapies (Interferon/Ribavirin) for HCV treatment were first publicly funded in 2000 and the more efficacious Pegylated interferon/Ribavirin therapy became available in May 2003.
2.2. The Cohort
We used data from the BC Hepatitis Testers Cohort (BC-HTC). The BC-HTC construction and data linkage has been described elsewhere (Janjua et al., 2016a, Janjua et al., 2016b). Briefly, the BC-HTC includes all individuals tested for HCV or HIV, or reported as a case of HBV, HCV, HIV or active tuberculosis (TB) in BC between 1990 and 2013 linked with data on medical visits, hospitalizations, cancers, prescription drugs, and deaths (BC Vital Statistics Agency, 2014, British Columbia Ministry of Health, 2014a, British Columbia Ministry of Health, 2014b, British Columbia Ministry of Health, 2014c, British Columbia Ministry of Health, 2014d, British Columbia Ministry of Health, 2014e). For this evaluation of the HCV cascade of care, individuals had to be alive on December 31st, 2012 and HCV diagnosed on or before December 31st, 2012. This study was approved by the University of British Columbia Research Ethics Board (H14-01649).
2.3. Cascade of Care
We used the 2012 BC population estimate (4,542,508 persons) as the denominator for prevalence calculations (Stats, 2012). Table 1 provides operational definitions for six stages of the HCV cascade of care: 1) estimated population prevalence; 2) HCV diagnosed; 3) HCV RNA tested; 4) genotyped; 5) initiated antiviral treatment; and 6) sustained virologic response (SVR). Genotyping was used as proxy for linkage with HCV care, though this may change with streamlined testing cascade. We applied these definitions to the data to estimate the number and proportion of individuals in each stage.
Table 1.
HCV Cascade of Care Definitions.
| 1. HCV Prevalence 2012 BC population: 4,542,508 The literature reports a range of 20–44% HCV undiagnosed. Among linked cohort members alive on Dec. 31, 2012, there were 54,902 HCV Diagnosed (1.2% of the 2012 BC population) From this number, we added 25% undiagnosed, or 73,203 anti-HCV positive persons living in BC in 2012 (1.6% of the 2012 BC population) |
| 2. HCV Diagnoseda On or before Dec. 31, 2012:
|
| 3. HCV RNA Tested At least one HCV RNA (PCR) test on record on or before Dec. 31, 2012 |
| 3a. HCV RNA Negative Among HCV Diagnosed individuals with no dispensation of interferon-based antivirals (untreated), the last HCV RNA test on record on or before Dec. 31, 2012 is negative. These individuals are considered not actively infected/non-viraemic |
| 4. HCV Genotyped At least one valid genotype test result on record on or before Dec. 31, 2012 |
| 5. Initiated HCV Antiviral Treatment Dispensation, on or before Dec. 31, 2012, of interferon-based antivirals specific to HCV treatmentb |
| 6. Estimated SVRc Among treated individuals:
|
NB: 1361(16.0%) treated individuals lacked adequate HCV RNA data in the timeframe of interest to be assessed for SVR.
Earliest available laboratory testing data (1992), reportable disease data (1990), and drug dispensation data (2000).
Drug Information Number/Product Identification Number (DINPIN): 2254603, 2254638, 2254646, 2371448, 2371456, 2371464, 2371472, 2371553, 2370816, 2241159, 2239730, 2246030, 2246028, 2246029, 2246027, 2246026, 2253410, 2253429, 2254581.
HCV RNA data up to Dec. 31st, 2013 was included to allow assessment of SVR for 2012 treatment starts.
2.4. Estimate of Viraemia
The estimate of actively infected persons in BC in 2012 was based on: 1) the number of untreated individuals in which the last HCV RNA test on record is positive; 2) 75% of those who were positive by antibody testing and had no HCV RNA or genotype testing done as about 25% of antibody positive individuals clear infection spontaneously; 3) 75% of the untested and undiagnosed estimate; 4) those treated individuals determined not to have achieved SVR.
2.5. Characteristics of HCV Diagnosed and those with and without RNA Testing or Genotyping
We described the demographic characteristics and comorbidity profile of HCV diagnosed including: individuals with and without RNA testing; and RNA positive individuals with and without HCV genotyping. Demographic characteristics included sex, age in 2012, birth cohort (< 1945, 1945–1964, 1965–1974, ≥ 1974), and social and material deprivation quintiles (Pampalon et al., 2009). Comorbidity indicators were derived from MSP data containing physician fee-for-service billing and diagnostic codes, and hospitalization data for mental health diagnoses, problematic alcohol and drug use, recent hospitalization, Elixhauser comorbidity index, cirrhosis, and decompensated cirrhosis (Supplement-Table 1) (Kramer et al., 2008, Lo Re et al., 2011, McDonald et al., 2010, Nehra et al., 2013). We assessed factors associated with not having received RNA testing and HCV genotyping using multivariable logistic regression modeling.
2.6. Engagement with Care
Engagement in liver related care was defined as at least one medical visit to a gastroenterologist, infectious disease specialist, internal medicine specialist, or general/family practitioner for a liver-related diagnostic code or fee item(including tests for monitoring liver function); or a liver-related hospitalization; or a liver biopsy; or HCV genotyping or current HCV treatment (Supplement-Table 2).
Table 2.
Characteristics associated with lack of HCV RNA testing among HCV Diagnosed individuals in BC, Canada, 1992–2012.
| All HCV Diagnosed |
HCV RNA Tested |
Not HCV RNA Tested |
Unadjusted OR (95% CI) |
Adjusteda OR (95% CI) |
|
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| n (row %)b | 54,902 (100) | 40,656 (74.1) | 14,246 (25.9) | ||
| Sex | |||||
| Male | 35,430 (64.5) | 25,692 (63.0) | 9801 (68.8) | 1.29 (1.24,1.35) | 1.23 (1.18,1.28) |
| Female | 19,472 (35.5) | 15,027 (37.0) | 4445 (31.2) | ref | ref |
| Birth Cohort | |||||
| < 1945 | 3252 (5.9) | 2181 (5.4) | 1071 (7.5) | 1.94 (1.76,2.13) | 1.88 (1.70,2.07) |
| 1945–1964 | 33,738 (61.5) | 25,035 (61.6) | 8703 (61.1) | 1.37 (1.28,1.47) | 1.34 (1.25,1.44) |
| 1965–1974 | 11,659 (21.2) | 8451 (20.8) | 3208 (22.5) | 1.50 (1.39,1.61) | 1.49 (1.38,1.61) |
| ≥ 1975 | 6253 (11.4) | 4989 (12.3) | 1264 (8.9) | ref | ref |
| HIV/AIDS Coinfection | |||||
| Yes | 3178 (5.8) | 2605 (6.4) | 573 (4.0) | 0.61 (0.56,0.67) | 0.63 (0.57,0.69) |
| No/Unknown | 51,724 (94.2) | 38,051 (93.6) | 13,673 (96.0) | ref | ref |
| HBV Coinfection | |||||
| Yes | 2104 (3.8) | 1346 (3.3) | 758 (5.3) | 1.64 (1.50,1.80) | 1.67 (1.52,1.83) |
| No/Unknown | 52,798 (96.2) | 39,310 (96.7) | 13,488 (94.7) | ref | ref |
| Active TB | |||||
| Yes | 292 (0.5) | 232 (0.6) | 60 (0.4) | 0.84 (0.64,1.10) | 0.99 (0.75, 1.30) |
| No/Unknown | 54,610 (99.5) | 40,424 (99.4) | 14,186 (99.6) | ref | ref |
| Major Mental Health Diagnosis | |||||
| Yes | 16,550 (30.1) | 13,135 (32.3) | 3415 (24.0) | 0.66 (0.63,0.69) | 0.70 (0.67,0.73) |
| No/Unknown | 38,352 (69.9) | 27,521 (67.7) | 10,831 (76.0) | ref | ref |
| Problematic Alcohol Use | |||||
| Yes | 19,624 (35.7) | 14,895 (36.6) | 4729 (33.2) | 0.86 (0.83,0.90) | 0.96 (0.92,1.01) |
| No/Unknown | 35,278 (64.3) | 25,761 (63.4) | 9517 (66.8) | ref | ref |
| Illicit Drug Use | |||||
| Yes | 28,243 (51.4) | 21,269 (52.3) | 6974 (49.0) | 0.87 (0.84,0.91) | 1.08 (1.03,1.13) |
| No/Unknown | 26,659 (48.6) | 19,387 (47.7) | 7272 (51.0) | ref | ref |
| Material Deprivation Quintilec | |||||
| Q1 (most privileged) | 7006 (12.8) | 5425 (13.3) | 1581 (11.1) | ref | ref |
| Q2 | 8347 (15.2) | 6268 (15.4) | 2079 (14.6) | 1.01 (0.94,1.08) | 1.00 (0.94,1.08) |
| Q3 | 9557 (17.4) | 7213 (17.7) | 2344 (16.5) | 1.01 (0.95,1.09) | 1.01 (0.94,1.08) |
| Q4 | 12,086 (22.0) | 9133 (22.5) | 2953 (20.7) | 1.11 (1.04,1.19) | 1.10 (1.02,1.17) |
| Q5 (most deprived) | 15,665 (28.5) | 11,591 (28.5) | 4074 (28.6) | 0.98 (0.91,1.05) | 0.99 (0.92,1.06) |
| Unknown | 2241 (4.1) | 1026 (2.5) | 1215 (8.5) | 2.52 (2.30,2.76) | 2.46 (2.24,2.69) |
Adjusted for all covariates listed in multivariable logistic regression.
All percentages displayed are column percent, except for the first row, which is row percent
At the time of diagnosis.
To assess 2012 levels of healthcare engagement among known positives, we excluded subjects known to be HCV RNA negative at the last HCV RNA test on record and those with inadequate follow-up time (< 12 months) during the most recent two years of data, 2011–2012, or diagnosis on or after January 1st, 2012. To better distinguish those not attending primary care from those who may have out-migrated from the province, we excluded individuals who had no record in the MSP client roster and no medical visits or hospitalization from 2007 to 2012. Those remaining are most likely to be viraemic and still requiring assessment for treatment. We then assessed medical visit and hospitalization records in 2011–2012 to determine and categorize individuals as: 1) retained in liver-related care; 2) retained in primary healthcare but not liver-related care; or 3) not retained in primary healthcare. We compared characteristics of those retained in primary care, and those not retained in care with those retained in liver-related care in 2011–12 by multinomial logistic regression modeling.
2.7. Role of the Funding Source
The BC Centre for Disease Control supported construction of the BC-HTC to inform policy and program related to HCV in British Columbia. The funders of the study had no role in study design, data analysis, data interpretation, or writing of the article. NJ has full access to all the data in the study and made decision to submit the paper for publication.
3. Results
3.1. Estimated HCV Population Distribution across Cascade of Care
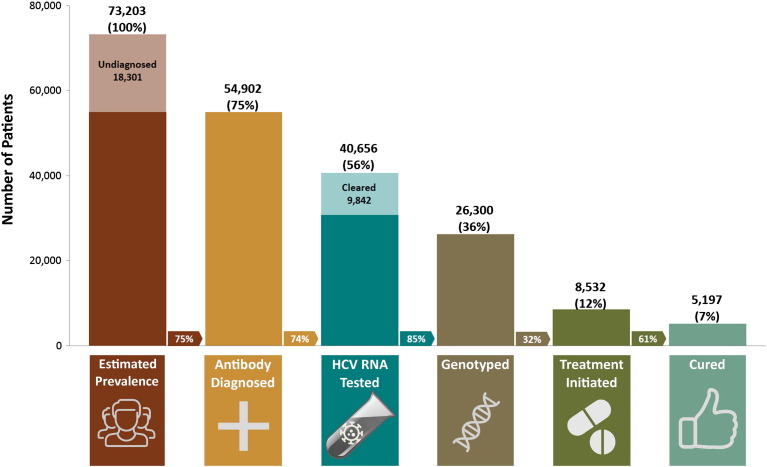
There were 54,902 HCV diagnosed individuals alive by the end of 2012, 1.2% of the 2012 BC population (Table 1). Although literature and national modeling estimates suggest that 20–44% of HCV infections remain undiagnosed in Canada (Trubnikov et al., 2014), because of high HCV testing rates in BC and declining positivity among baby boomers (born 1945–64), (Janjua et al., 2016b, Consolacion et al., 2015) we used an undiagnosed estimate of 25%, bringing the anti-HCV prevalence in BC in 2012 to 73,203(1.6% of the population). Of these, 56% (40,656) had at least one HCV RNA test; 36% (26,300) had genotype testing; 12% (8532) had been dispensed treatment; and 7% (5197) were estimated to have achieved SVR (Fig. 1).
Fig. 1.
HCV Cascade of Care as of 31-Dec-2012, British Columbia, Canada.
Arrows between bars represents the proportion of patients in each step of the cascade from the patients in the preceding step. E.g., 32% of those genotyped initiated HCV treatment.
3.2. Diagnosed and Tested Subjects across the Cascade of Care
Of 54,902 HCV diagnosed individuals alive at the end of 2012, 40,656 (74%) had at least one HCV RNA test on record. Among these, 9842 (24%) were negative at the last available HCV RNA test and had received no treatment, and 30,814 (76%) had a positive HCV RNA test. Among HCV RNA positive individuals, 26,300 (85%) had been genotyped. Of the 8532 individuals who initiated treatment, 5197(61%) had a SVR; 1974 (23%) failed treatment; and 1461(17%) lacked available HCV RNA testing records to assess treatment outcome (Fig. 1).
3.3. Estimated Population Prevalence of Active Infection
We estimated 50,027 persons were actively infected in 2012: 1) 22,282 untreated individuals with HCV RNA testing on record, in which the last HCV RNA test on record remained positive; 2) 75% (10,684) of 14,246, who were positive by antibody testing only (no HCV RNA on record); 3) 75% (13,726) of the 18,301 undiagnosed estimate; and 4) 3335 treated individuals who did not achieved SVR.
3.4. Characteristics of HCV Diagnosed
Almost two-thirds of the 54,902 HCV diagnosed individuals were male. Sixty-two percent were born 1945–1964 with an additional 6% born before 1945, thus, two-thirds are currently 50 years of age or older (Table 2). Prevalence of HIV, HBV and active TB coinfection was 5.8%, 3.8%, and 0.6%, respectively. The proportion with a history of a major mental health diagnosis was high (30%), as was problematic alcohol (36%) and drug use (51%).
Three-quarters of HCV diagnosed had at least one HCV RNA test on record, and males had higher odds than females of not being HCV RNA tested (adjusted odds ratio (AdjOR) = 1.23; 95%CI:1.18,1.28) (Table 2). As compared to the youngest birth cohort(born ≥ 1975), older birth cohorts were less likely to be HCV RNA tested and those born before 1945 were least likely to have RNA testing. Those with HIV coinfection were more likely to be tested for RNA (AdjOR = 0.63, 95%CI: 0.57,0.69) while those with HBV coinfection were more likely to lack RNA testing (AdjOR = 1.67; 95%CI:1.52,1.83).
Among the 30,841 HCV RNA positive people, 85% underwent genotyping (Supplement Table 3). Those born 1945–64 were the most likely to be genotyped. HIV coinfection remained associated with moving forward in the testing continuum while those with HBV coinfection were slightly less likely to be genotyped. Drug use history was associated with a higher odds of not being genotyped (AdjOR = 1.17; 95%CI:1.09, 1.22) as were those in the lowest socioeconomic quintile.
Table 3.
Retention in Healthcare and Liver-Related Care among HCV positive individuals, BC, Canada, in 2011–2012.
| Individuals Requiring Ongoing Liver Monitoring and Care |
Ever Dispensed HCV Treatmenta |
||||
|---|---|---|---|---|---|
| Eligible for Evaluation | Retained in Liver Care | Retained in Primary Care | Not Retained in any Care | ||
| n (row %)b | 31,511 (100) | 10,083(32.0) | 17,358(55.1) | 4070 (12.9) | 8532 (100) |
| Sex | |||||
| Female | 10,757 (34.1) | 3437 (34.1) | 6147 (35.4) | 1173 (28.8) | 2762 (32.4) |
| Male | 20,754 (65.9) | 6646 (65.9) | 11,211 (64.6) | 2897 (71.2) | 5770 (67.6) |
| Age in 2012 | |||||
| < 15 | 72 (0.2) | 19 (0.2) | 36 (0.2) | 17 (0.4) | 2 (0.02) |
| 15–24 | 252 (0.8) | 84 (0.8) | 136 (0.8) | 32 (6.7) | 22 (0.3) |
| 25–34 | 1906 (6.1) | 819 (8.1) | 815 (4.7) | 272(19.0) | 327 (4.1) |
| 35–44 | 5189 (16.5) | 1808 (17.9) | 2606 (15.0) | 775 (36.7) | 1006 (11.8) |
| 45–54 | 10,312 (32.7) | 3169 (31.4) | 5649 (32.5) | 1494 (36.7) | 2393 (28.1) |
| ≥ 55 | 13,780 (43.7) | 4184 (41.5) | 8116 (46.8) | 1480 (36.4) | 4782 (56.1) |
| Birth Cohort | |||||
| < 1945 | 1828 (5.8) | 450 (4.5) | 1174 (6.8) | 204 (5.0) | 501 (5.9) |
| 1945–1964 | 19,744 (62.7) | 6109 (60.6) | 11,275(65.0) | 2360 (58.0) | 6184 (72.5) |
| 1965–1974 | 6624 (21.0) | 2211 (21.9) | 3394 (19.6) | 1019 (25.0) | 1275 (14.9) |
| ≥ 1975 | 3315 (10.5) | 1313 (13.0) | 1515 (8.7) | 487 (12.0) | 572 (6.7) |
| HIV/AIDS Coinfection | 1882 (6.0) | 957 (9.5) | 778 (4.5) | 147 (3.6) | 408 (4.8) |
| HBV Coinfection | 1074 (3.4) | 300 (3.0) | 631 (3.6) | 143 (3.5) | 236 (2.8) |
| Active TB | 170 (0.5) | 69 (0.7) | 90 (0.5) | 11 (0.3) | 27 (0.3) |
| Major Mental Health Diagnosis | |||||
| Ever | 10,217 (32.4) | 3652 (36.2) | 5722 (33.0) | 843 (20.7) | 2557 (30.0) |
| Recent (2011 − 2012) | 3488 (11.1)a | 1667 (16.5) | 1821 (10.5) | unable to evaluate | unable to evaluate |
| Problematic Alcohol Use | |||||
| Ever | 12,342 (39.2) | 4281 (42.5) | 6869 (39.6) | 1192 (29.3) | 2476 (29.0) |
| Recent (2011–2012) | 2293 (7.3)a | 996 (9.9) | 1297 (7.5) | unable to evaluate | unable to evaluate |
| Illicit Drug Use | |||||
| Ever | 17,486 (55.5) | 6072 (60.2) | 9535 (54.9) | 1879 (46.2) | 3468 (40.7) |
| Recent (2011–2012) | 7371 (23.4)a | 3299 (32.7) | 4072 (23.5) | unable to evaluate | unable to evaluate |
| Recent Hospitalization (2011–2012) | 10,116 (32.1)a | 4419 (43.8) | 5697 (32.8) | unable to evaluate | unable to evaluate |
| Elixhauser Comorbidity Index (2008–2012) | |||||
| 0 | 20,883 (66.3) | 5701 (56.5) | 11,581(66.7) | 3601 (88.5) | n/a |
| 1–2 | 7077 (22.5) | 2762 (27.4) | 3995 (23.0) | 320 (7.9) | n/a |
| 3–4 | 2435 (7.7) | 1110 (10.9) | 1222 (7.0) | 113 (4.6) | n/a |
| 4 + | 1116 (3.5) | 520 (5.2) | 560 (3.2) | 36 (0.9) | n/a |
| Cirrhosis (Ever) | 1753 (5.6) | 850 (8.4) | 759 (4.4) | 144 (3.5) | 861 (10.1) |
| Decompensated Cirrhosis (Ever) | 951 (3.0) | 473 (4.7) | 388 (2.2) | 90 (2.2) | 429 (5.0) |
| 2012 Material Deprivation Quintile | |||||
| Q1 (most privileged) | 4068 (12.9) | 1380 (13.7) | 2184 (12.6) | 504 (12.4) | 1234 (14.5) |
| Q2 | 4861 (15.4) | 1498 (14.9) | 2750 (15.8) | 613 (15.1) | 1413 (16.6) |
| Q3 | 5699 (18.1) | 1722 (17.1) | 3246 (18.7) | 731 (18.0) | 1647 (19.3) |
| Q4 | 7223 (22.9) | 2321 (23.0) | 3947 (22.7) | 955 (23.5) | 1950 (22.9) |
| Q5 (most deprived) | 9144 (29.0) | 2973 (29.5) | 5020 (28.9) | 1151 (28.3) | 2193 (25.7) |
| Unknown | 516 (1.6) | 189 (1.9) | 211 (1.2) | 116 (2.9) | 95 (1.1) |
| 2012 Social Deprivation Quintile | |||||
| Q1 (most privileged) | 3226 (10.2) | 1015 (10.1) | 1799 (10.4) | 412 (10.1) | 1209 (14.2) |
| Q2 | 3922 (12.5) | 1244 (12.3) | 2197 (12.7) | 481 (11.8) | 1340 (15.7) |
| Q3 | 5274 (16.7) | 1654 (16.4) | 2973 (17.1) | 647 (15.9) | 1574 (18.5) |
| Q4 | 6686 (21.2) | 2090 (20.7) | 3792(21.9) | 804 (19.8) | 1754 (20.6) |
| Q5 (most deprived) | 11,887 (37.7) | 3891 (38.6) | 6383 (36.8) | 1610 (39.6) | 2560 (30.0 |
| Unknown | 516 (1.6) | 189 (1.9) | 211 (1.2) | 116 (2.9) | 95 (1.1) |
Treated subjects were alive (no death record) as of 31-Dec.-2012 and had a least one dispensation record for an eligible HCV pharmacotherapy drug prior to 2013.
Note that treated subjects are appended for comparison but most were not eligible for the retention evaluation and some measures cannot be compared.
All percentages displayed are column percent, except for the first row, which is row percent.
3.5. Retention in Healthcare and HCV-Related Care
31,511 individuals, known to be HCV positive at the last test on record and eligible for the analysis based on adequate follow-up time were assessed for retention in the healthcare system and liver-related care during the 2011–2012 (Table 3). Of these, 10,083(32%) were found be retained in liver-related care; 17,358(55%) were found to be retained in primary healthcare but not in liver-related care; and 4070(13%) had no physician or hospital visits in the two year period, suggesting lack of retention in any care. The 31,511 individuals assessed were predominantly older and male, with over half falling in the lowest two material deprivation quintiles. As expected due to shorter follow-up time, recent mental health and substance use indicators were lower than lifetime history, particularly for alcohol. However, 32% of this group experienced a hospitalization in 2011–2012 and one-third had a least one comorbidity.
In multivariable model (Table 4), compared to those retained in liver-related care, those retained in primary care only were more likely to be female, older, and HBV coinfected. Those with HIV, cirrhosis, or drug use were more likely to be in liver care. Compared to those in liver care, those not retained in any care in 2011–12 had higher odds of being male, HBV co-infected and born in 1965–1974 (Table 4). People with HIV, cirrhosis, mental illness, alcohol, and drug use were less likely to be not in any care.
Table 4.
Multivariable multinomial logistic regression model of factors associated with retention in Primary Care or No Care compared to those retained in liver-related care among eligiblea HCV Positive individuals, BC, Canada, in 2011–2012.
| Primary Care n = 17,358 (55.1% ) |
No Care n = 4070 (12.9%) |
|
|---|---|---|
| Adj OR (95%CI) | Adj OR (95%CI) | |
| Sex | ||
| Male vs. Female | 0.93(0.89–0.99) | 1.27(1.17–1.38) |
| Birth Cohort (ref ≥ 1975) | ||
| < 1945 | 2.21(1.93–2.53) | 0.92(0.76–1.13) |
| 1945–1964 | 1.62(1.49–1.76) | 0.94(0.84–1.06) |
| 1965–1974 | 1.37(1.25–1.50) | 1.30(1.15–1.49) |
| HIV/AIDS Coinfection | ||
| Yes vs. No/Unknown | 0.48(0.44–0.53) | 0.43(0.35–0.51) |
| HBV Coinfection | ||
| Yes vs. No/Unknown | 1.30(1.13–1.50) | 1.31(1.07–1.61) |
| Illicit Drug Use | ||
| Yes vs. No/Unknown | 0.93(0.87–0.98) | 0.71(0.65–0.77) |
| Cirrhosis | ||
| Yes vs. No/Unknown | 0.45(0.40–0.50) | 0.43(0.36–0.51) |
| Material Deprivation Index (ref Q1/most privileged) | ||
| Q2 | 1.15(1.05–1.27) | 1.13(0.98–1.30) |
| Q3 | 1.19(1.08–1.30) | 1.18(1.03–1.35) |
| Q4 | 1.09(1.00–1.19) | 1.16(1.02–1.32) |
| Q5 | 1.10(1.01–1.19) | 1.13(0.99–1.27) |
| Unknown | 0.73(0.59–0.90) | 1.59(1.23–2.06) |
| Major Mental Health Diagnosis | ||
| Yes vs. No/Unknown | 0.95 (0.89–1.00) | 0.58(0.53–0.64) |
| Problematic Alcohol Use | ||
| Yes vs. No/Unknown | 1.02(0.96–1.08) | 0.77(0.71–0.84) |
As per Table 3, there were 31,511 persons eligible for evaluation of retention in care. They were categorized as retained in liver-related monitoring or care; primary care; no care. The referent group is those retained in liver monitoring or care (n = 10,083) (32%).
In those with a least one treatment dispensation (n = 8532), most (73%) were born in 1945–1964 (Table 3). Small proportion of HIV (4.8%) and HBV (2.8%) coinfected individuals were ever dispensed treatment. While 10% of those in liver-related monitoring or care are HIV coinfected, this was 5% in the dispensed treatment group. Of 2094 HCV RNA positive individuals who were also HIV positive, 408(20%) were ever dispensed HCV treatment. Finally, the treatment group had a low proportion of individuals with a history illicit drug use.
4. Discussion
This population-based study characterized the cascade of HCV care in British Columbia, Canada. A large proportion of antibody positive persons received HCV RNA testing (75%) and subsequent genotyping (80%). In recent years, HCV RNA testing has increased to > 80% of those diagnosed. However, only a small proportion initiated HCV treatment and were subsequently cured. Males, older birth cohorts, and those with HBV coinfection were less likely to undergo HCV RNA testing while those with a history of illicit drug use were less likely to be genotyped. People with HIV, cirrhosis, problem drug or alcohol use were more likely to be in liver care while males and those with HBV coinfection were less likely to be retained in care. In summary, most people moved along the testing continuum, but retention in care and treatment initiation were major gaps. People with major comorbidities have been more engaged in the testing continuum, but few had initiated treatment in the interferon era. These findings have important implications for HCV prevention, care and treatment programs.
We demonstrated that linked administrative datasets that integrate data from various sources similar to the BC-HTC could be used to monitor the progress of HCV infected individuals across the cascade of care to assess program effectiveness in providing services at various stages of the cascade. We identified characteristics (demographic, comorbidities, co-infections, socioeconomic and geographic disparities) of HCV patients who did not progress to the next stage of care that could be used to realign services and programs to target individuals not progressing and falling behind along the cascade. The cascade was presented at the provincial level but is replicable for regions within BC to assess local program progress to inform interventions for improvements. In summary, the cascade serves as an instrument to guide the policy to achieve the World Health Organization's goal of HCV elimination (World Health Organization, 2016).
Although, HCV RNA and genotype testing rates have improved in recent years and are similar to those reported for HCV positive Veterans and Kaiser Permanente members in the United States (95%) and higher than for primary care in Canada and United States (47.0% to 60.9%), there is a further need to reduce the gap between those antibody diagnosed and those undergoing further testing (Maier et al., 2016, Jonas et al., 2016, Spradling et al., 2014, Viner et al., 2015). We demonstrated that people with high risk activities progress to RNA testing while men and older cohorts, especially those born in 1945–64 lacked RNA testing (Spradling et al., 2014, Cachay et al., 2014). A major factor for lack of genotyping among those RNA tested was history of drug use. Data from Canada and other countries suggest that lack of knowledge among primary care physicians, especially those not involved in providing care to those with known risk activities, regarding next steps in the cascade and poor knowledge among patients are associated with the gap from HCV diagnosis to RNA testing (Butt et al., 2013, Grebely et al., 2013, Guirgis et al., 2012). Although guidelines and education for primary care physicians are important instruments to bridge this gap, a single blood sample test strategy in which those testing positive for antibody are also tested for RNA (reflex testing) and possibly for genotype could be an effective structural intervention to bridge this gap. Reflex testing for RNA has been implemented in the VA system and recently in Kaiser Permanente system, and was associated with higher RNA testing rates (Maier et al., 2016, Jonas et al., 2016).
Despite a robust testing continuum for those accessing screening, BC remains similar to the US, Australia and other countries in initiating treatment (Maier et al., 2016, Viner et al., 2015, Lazarus et al., 2014, Hajarizadeh et al., 2016). We found that a small proportion (15%) of HCV diagnosed individuals initiated HCV treatment by 2012. Those who did not progress to treatment, even while in liver care included those with HIV coinfection and people currently engaged in illicit drug use. Factors related to poor tolerability and efficacy of interferon based treatments along with other factors at the patient (poor knowledge, unemployment, competing priorities, stigma, lack insurance and provider relationship), provider (perception of poor adherence, ongoing substance use, psychiatric illness, potential re-infection) and, system (lack of consensus on screening and treatment and coverage of HCV treatments) levels have been reported to be associated with lower treatment rates in these populations in Canada and other settings (Grebely et al., 2013, Aspinall et al., 2013). DAAs are expected to overcome some of these barriers, though high drug costs may affect reimbursement coverage.
Those with HBV coinfection were less likely to have HCV RNA testing and thus be aware of their HCV infection status; and be retained in any liver-related monitoring or care. The inability to move forward in the initial testing stages of the HCV cascade of care precludes being offered treatment, as also seen in the low numbers of HBV positive persons dispensed treatment. Chronic HBV is more common in immigrants from HBV endemic countries. Further characterization of HCV-HBV coinfection is needed, including evaluation by ethnicity, to clarify factors related to lack of RNA testing and progression in the HCV cascade of care. Immigrants from HCV endemic countries have a high burden of HCV but data on the current burden in BC is limited. Characterization of burden and assessment of progression across the cascade of care among immigrants is needed.
Despite comprehensive data available to us for characterization of the cascade of care, there are several limitations that impact each stage. The estimate of prevalent HCV cases is based on the number of HCV undiagnosed cases derived in a national model based on reported cases and survey data (Trubnikov et al., 2014). Historically, BC has tested more individuals for HCV(by December 2014, 33% of BC's population has been tested at least once) than other provinces and in recent years testing volumes have increase substantially, suggesting our undiagnosed proportion may be lower than the national average (Consolacion et al., 2015). We have also seen decline in HCV positivity among baby boomers from 13% in 2001 to 2% in 2013, suggesting declining pool of undiagnosed individuals in BC (Janjua et al., 2016b). All these data suggest lower percentage of undiagnosed individuals in BC than national average. In our evaluation of those requiring ongoing monitoring and care, we excluded those with patterns suggesting outmigration. While unlikely, a small proportion of these people may still be in the province but not accessing care. We used diagnostic codes in administrative datasets to assess history of mental illness and substance use. This raises multiple issues: bias towards underestimating prevalence in those less engaged in healthcare, and potential misclassification related to sensitivity and specificity of these measures. Potentially lower linkage rates in vulnerable groups would result in less representation of homeless, street-involved, and incarcerated individuals (Janjua et al., 2016a).
We have demonstrated the utility of the BC-HTC integrating data from various sources to monitor the progress of HCV infected individuals across the cascade of care, which will inform refinement in programs and policies in British Columbia to optimize HCV response within the context of a changed treatment landscape. Similar systems could be established in other jurisdictions to monitor program progress. In BC, HCV infected individuals; especially those with high risk conditions, progressed well through the testing continuum and also had liver monitoring, though some gaps remain. The major gap in the HCV cascade of care remains treatment initiation. Efforts are needed to estimate the number of undiagnosed individuals, and to assess cost effectiveness of various screening options to inform the optimal screening strategy. Implementation of reflex RNA testing would bridge the gap between HCV diagnosis and confirmation of active infection. There is also a need to link older populations with multiple comorbidities and a second group of younger, less engaged persons into care. BC needs to work across multiple levels of the healthcare system to define the supports required to link into care those who remain unassessed as well as to expand capacity to assess and treat in a variety of settings.
Authors Contributions
NJ, MKu, MKr conceived the analysis presented in this paper. NJ, MKu, AY, MA, MKr, MT, JW participated in the study design. NJ guided the statistical analysis performed by MK in support from AY and SW. MKu wrote first draft along with NJ and NJ incorporated revisions. All authors contributed in the interpretation of results, manuscript preparation and revisions. All authors read and approved the final manuscript.
Declaration of Interests
MKr has received grant funding via his institution from Roche Molecular Systems, Boehringer Ingelheim, Merck, Siemens Healthcare Diagnostics and Hologic Inc. JG received grants from Abbvie, BMS, grants and personal fees from Gilead, and Merck/MSD unrelated to the current paper. AR has served as an investigator and/or consultant for AbbVie, Bristol-Myers Squibb, Gilead, Merck, Janssen, and Novartis and has received speaker's grants from AbbVie, Gilead, Merck, and Janssen.
Acknowledgements
This work was supported by BC Centre for Disease Control and the Canadian Institutes of Health Research [Grant # 201503NHC-348216-NHC-ADWY-62134 and 201410PHE-337680-PHE-CAAA-179547].
We acknowledge the assistance of BCCDC, PHSA Performance measurement and reporting, Information Analysts, Ministry of Health Data Access, Research and Stewardship, & MSP, DAD and Medical Beneficiary and Pharmaceutical Services programme areas, BC Ministry of Health, and BC Cancer Agency and their staff involved in data access and procurement, and data management.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.08.035.
Appendix A. Supplementary data
Supplementary tables.
References
- Aspinall E.J., Corson S., Doyle J.S. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin. Infect. Dis. 2013;57(Suppl 2):S80–S89. doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]
- Aspinall E.J., Hutchinson S.J., Janjua N.Z. Trends in mortality after diagnosis of hepatitis C virus infection: an international comparison and implications for monitoring the population impact of treatment. J. Hepatol. 2015;62(2):269–277. doi: 10.1016/j.jhep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- BC Vital Statistics Agency Vital Statistics Deaths. BC Vital Statistics Agency. Data Extract. BC Vital Statistics Agency (2014) 2014 [Google Scholar]
- British Columbia Ministry of Health Client Roster (Client Registry System/Enterprise Master Patient Index) British Columbia Ministry of Health. Data Extract. MOH (2013) 2014 http://www.health.gov.bc.ca/data/ [Google Scholar]
- British Columbia Ministry of Health Discharge Abstract Database (Hospital Separations) British Columbia Ministry of Health. Data Extract. MOH (2013) 2014 http://www.health.gov.bc.ca/data/ [Google Scholar]
- British Columbia Ministry of Health Medical Services Plan (MSP) Payment Information File. British Columbia Ministry of Health. Data Extract. MOH (2013) 2014 http://www.health.gov.bc.ca/data/ [Google Scholar]
- British Columbia Ministry of Health PharmaCare. British Columbia Ministry of Health. Data Extract. MOH (2013) 2014 http://www.health.gov.bc.ca/data/ [Google Scholar]
- British Columbia Ministry of Health PharmaNet. British Columbia Ministry of Health. Data Extract. MOH (2013) 2014 http://www.health.gov.bc.ca/data/ [Google Scholar]
- Butt G., McGuinness L., Buller-Taylor T., Mitchell S. Reasons for nonattendance across the hepatitis C disease course. ISRN Nurs. 2013;2013:579529. doi: 10.1155/2013/579529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachay E.R., Hill L., Wyles D. The hepatitis C cascade of care among HIV infected patients: a call to address ongoing barriers to care. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolacion T.B., Yu A., Janjua N.Z. 2015. Impact of STOP HIV/AIDS Program on HIV, Hepatitis C and Syphilis Testing Volumes in British Columbia. IAS 2015. Vancouver. [Google Scholar]
- Grebely J., Oser M., Taylor L.E., Dore G.J. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. The Journal of infectious diseases. 2013;207(Suppl 1):S19–S25. doi: 10.1093/infdis/jis928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirgis M., Yan K., Bu Y.M., Zekry A. General practitioners' knowledge and management of viral hepatitis in the migrant population. Intern. Med. J. 2012;42(5):497–504. doi: 10.1111/j.1445-5994.2011.02440.x. [DOI] [PubMed] [Google Scholar]
- Hajarizadeh B., Grebely J., McManus H. Chronic hepatitis C burden and care cascade in Australia in the era of interferon-based treatment. J. Gastroenterol. Hepatol. 2016 doi: 10.1111/jgh.13453. [DOI] [PubMed] [Google Scholar]
- Janjua N.Z., Kuo M., Chong M. Assessing hepatitis C burden and treatment effectiveness through the British Columbia Hepatitis Testers Cohort (BC-HTC): design and characteristics of linked and unlinked participants. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjua N.Z., Yu A., Kuo M., Alvarez M., Krajden M. 2015 CAG CDDW/CASL Meeting. Vol. 2015. 2015. Hepatitis C epidemiology in British Columbia to inform screening and treatment response; p. A235. Banff, AB. [Google Scholar]
- Janjua N.Z., Yu A., Kuo M. Twin epidemics of new and prevalent hepatitis C infections in Canada: BC hepatitis testers cohort. BMC Infect. Dis. 2016;16:334. doi: 10.1186/s12879-016-1683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas M.C., Rodriguez C.V., Redd J., Sloane D.A., Winston B.J., Loftus B.C. Streamlining screening to treatment: the hepatitis C cascade of care at Kaiser Permanente Mid-Atlantic States. Clin. Infect. Dis. 2016;62(10):1290–1296. doi: 10.1093/cid/ciw086. [DOI] [PubMed] [Google Scholar]
- Kramer J.R., Davila J.A., Miller E.D., Richardson P., Giordano T.P., El-Serag H.B. The validity of viral hepatitis and chronic liver disease diagnoses in veterans affairs administrative databases. Aliment. Pharmacol. Ther. 2008;27(3):274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- Kuo M., Janjua N.Z., Burchell A.N., Buxton J.A., Krajden M., Gilbert M. Decreasing Hepatitis C incidence among a population with repeated tests: British Columbia, Canada, 1993–2011. Am. J. Public Health. 2015;105(8):1604–1610. doi: 10.2105/AJPH.2015.302591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus J.V., Sperle I., Maticic M., Wiessing L. A systematic review of Hepatitis C virus treatment uptake among people who inject drugs in the European Region. BMC Infect. Dis. 2014;14(Suppl 6):S16. doi: 10.1186/1471-2334-14-S6-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Re V., 3rd, Lim J.K., Goetz M.B. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol. Drug Saf. 2011;20(7):689–699. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier M.M., Ross D.B., Chartier M., Belperio P.S., Backus L.I. Cascade of care for hepatitis C virus infection within the US Veterans Health Administration. Am. J. Public Health. 2016;106(2):353–358. doi: 10.2105/AJPH.2015.302927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S.A., Hutchinson S.J., Bird S.M. Hospitalization of hepatitis C-diagnosed individuals in Scotland for decompensated cirrhosis: a population-based record-linkage study. Eur. J. Gastroenterol. Hepatol. 2010;22(1):49–57. doi: 10.1097/MEG.0b013e32832ff35d. [DOI] [PubMed] [Google Scholar]
- Nehra M.S., Ma Y., Clark C., Amarasingham R., Rockey D.C., Singal A.G. Use of administrative claims data for identifying patients with cirrhosis. J. Clin. Gastroenterol. 2013;47(5):e50–e54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B., Montaner J.S., Colley G. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect. Dis. 2014;14(1):40–49. doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampalon R., Gamache P., Hamel D. A deprivation index for health planning in Canada. Chronic Dis. Can. 2009;29(4) [PubMed] [Google Scholar]
- Simmons B., Saleem J., Heath K., Cooke G.S., Hill A. Long-term treatment outcomes of patients infected with Hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin. Infect. Dis. 2015;61(5):730–740. doi: 10.1093/cid/civ396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal A.G., Volk M.L., Jensen D., Di Bisceglie A.M., Schoenfeld P.S. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin. Gastroenterol. Hepatol. 2010;8(3):280–288. doi: 10.1016/j.cgh.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Smith M.A., Lim A. Profile of paritaprevir/ritonavir/ombitasvir plus dasabuvir in the treatment of chronic hepatitis C virus genotype 1 infection. Drug Des. Devel. Ther. 2015;9:6083–6094. doi: 10.2147/DDDT.S80226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.A., Chan J., Mohammad R.A. Ledipasvir-sofosbuvir: interferon −/ribavirin-free regimen for chronic hepatitis C virus infection. Ann. Pharmacother. 2015;49(3):343–350. doi: 10.1177/1060028014563952. [DOI] [PubMed] [Google Scholar]
- Spradling P.R., Tong X., Rupp L.B. Trends in HCV RNA testing among HCV antibody-positive persons in care, 2003-2010. Clin. Infect. Dis. 2014;59(7):976–981. doi: 10.1093/cid/ciu509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stats B.C. Population Estimates. 2012. http://www.bcstats.gov.bc.ca/StatisticsBySubject/Demography/PopulationEstimates.aspx2015
- Trubnikov M., Yan P., Archibald C. Estimated prevalence of hepatitis C virus infection in Canada, 2011. Can. Commun. Dis. Rep. 2014;40(19):429–442. doi: 10.14745/ccdr.v40i19a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner K., Kuncio D., Newbern E.C., Johnson C.C. The continuum of hepatitis C testing and care. Hepatology. 2015;61(3):783–789. doi: 10.1002/hep.27584. [DOI] [PubMed] [Google Scholar]
- Wade A.J., Macdonald D.M., Doyle J.S. The cascade of care for an Australian community-based hepatitis C treatment service. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. 2016 http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1 Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.