Abstract
Background
Allogeneic islet transplantation has become a viable option for the treatment of unstable type 1 diabetes. However, the donor shortage and the necessity of the immunosuppressive drugs are two major issues. To solve these issues, we performed islet xenotransplantation using encapsulated neonatal porcine islets without immunosuppressive drugs.
Methods
Two different doses (approximately 5000 IEQ/kg and 10,000 IEQ/kg) of encapsulated neonatal porcine islets were transplanted twice (total approximately 10,000 IEQ/kg and 20,000 IEQ/kg) into four type 1 diabetic patients in each group (total 8 patients).
Findings
In the higher dose group, all four patients improved HbA1c. This was maintained at a level of < 7% for > 600 days with significant reduction of the frequency of unaware hypoglycemic events.
Interpretation
The clinical benefit of islet xenotransplantation with microencapsulation has been shown.
Keywords: Islet transplantation, Xenotransplantation, Encapsulation, Type 1 diabetes, PERV, Porcine islet
Highlights
-
•
Encapsulated porcine neonatal islets were transplanted into 8 type 1 diabetic patients.
-
•
Patients with high dose group could maintain HbA1c < 7% > 600 days with reduced hypoglycemic events.
-
•
There is no PERV infection in all patients
Insulin dependent diabetes mellitus can be successfully treated by human islet cell transplantation. However the shortage of donated human pancreas is the major issue. Islet transplantation using clinical grade porcine pancreas is a promising treatment to alleviate the shortage of donated human pancreas.
In this study, we transplanted encapsulated neonatal porcine islets into 8 insulin dependent diabetic patients. There was no porcine endogenous retrovirus infection. All patients reduced HbA1c levels which indicated glycemic controls were improved.
Encapsulated neonatal porcine islet transplantation appears safe and efficacious to improve glycemic control for insulin dependent diabetic patients.
1. Introduction
Transplantation has been considered one of the medical miracles which can cure incurable diseases (Dalal, 2015). On the other hand, transplantation has been associated with the donor shortage which leads to organ trafficking and organ tourism (Dalal, 2015). The Istanbul declaration, supported by World Health Organization, has emphasized the importance of changing this situation (Dalal, 2015). One of the ultimate solutions for this situation is xenotransplantation. Among the xenotransplantation options, islet xenotransplantation using porcine islets has been considered to be the closest to the clinical reality (Groth et al., 1994). In fact, the initial islet xenotransplantation was conducted in 90s (Groth et al., 1994). Since then several clinical trials of islet xenotransplantation were conducted; however, clear clinical benefits were rarely shown (Matsumoto et al., 2014, Valdes-Gonzalez et al., 2005, Valdes-Gonzalez et al., 2010).
The standard therapy for the type 1 diabetes is intensive insulin therapy which can reduce HbA1c. However the drawback is increasing hypoglycemic episodes (The Diabetes Control and Complications Trial Research Group, 1997). Beta-cell replacement therapy including pancreas and islet transplantation can normalize HbA1c without increasing hypoglycemic episodes; however, the donor shortage and the necessity of immunosuppressant are major issues to apply the treatments widely (Matsumoto, 2010). To alleviate the donor shortage, islet transplantation using non-heart beating human donor was conducted (Matsumoto et al., 2006), and a living donor (Matsumoto et al., 2005), but these approaches can never solve this issue generally. Islet xenotransplantation may be one of the ultimate solutions to solve the shortage of donated organs.
In this study, we conducted islet xenotransplantation using encapsulated neonatal porcine islets without immunosuppressive drugs which resulted in clinical benefit for unstable type 1 diabetic patients. We believe this study provides a prologue for xenotransplantation to solve the shortage of organ donors.
2. Methods
The donor pig herd (Auckland Island pigs; Living Cell Technologies, Manukau, New Zealand, and Diatranz Otsuka Ltd, Auckland New Zealand) was maintained in a designated pathogen-free facility and screened for an extensive panel of infectious agents including porcine endogenous retrovirus (PERV) (Garkavenko et al., 2004). Newborn piglets from the donor herd were shipped to a cell processing facility (Auckland, New Zealand). They were anesthetized and bled. The procured pancreata were then brought into a cell processing room and digested under good manufacturing practices (GMP) as described previously (Hillberg et al., 2013). Digested pancreata were cultured using spinner flasks for 3 days before encapsulation (Hillberg et al., 2013). After counting islet yield, cultured neonatal porcine islets were encapsulated using alginate-poly-l-ornithine-alginate (APA) (Hillberg et al., 2013). APA-encapsulated neonatal porcine islets were cultured for an additional three weeks. Then APA-encapsulated neonatal porcine islets were shipped to Argentine using RPMI culture media at cold temperature.
From August 2011 to July 2012, encapsulated neonatal porcine islets were transplanted into the peritoneal cavity via a laparoscope (Fig. 1) at Hospital Interzonal General de Agudos Eva Peron, San Martin, Provincia de Buenos Aires. The study protocol (ClinicalTrials.gov Identifier NCT01739829) was granted ethics approval in Argentina from the Ministry of Health, Buenos Aires Province and the local institutional ethics committee. All patients provided written consent forms to accept this treatment. Main inclusion criteria for this study are as follows.
-
1.
Adults (males or females) in the age range 18 to 65 years
-
2.
Diagnosis of type 1 diabetes mellitus (DM) (minimum duration of 5 years) in accordance with the American Diabetes Association's criteria. Patients should have been treated continuously with insulin since diagnosis.
-
3.
Patients with established brittle type 1 diabetes mellitus with a well-documented chronic history of metabolic instability who cannot achieve acceptable metabolic control (which may include treatment with the use of a continuous insulin infusion pump) without experiencing multiple episodes of hypoglycemia, often with unawareness.
-
4.
Patients should have a hemoglobin A1c (HbA1c) ≥ 7% and ≤ 15% calculated as the average of the last four consecutive HbA1c readings during the 8-week baseline run-in period.
-
5.
Plasma C-peptide < 0.3 ng/ml following a glucagon stimulation test.
-
6.
In addition, we put “the difference between the highest and lowest of the last four consecutive HbA1C reading should be no more than 1.0%.” as inclusion criteria, however; since patients were brittle type 1 diabetes, this criterion was not practical. Therefore, for this interim analysis, we did not use this as inclusion criteria.
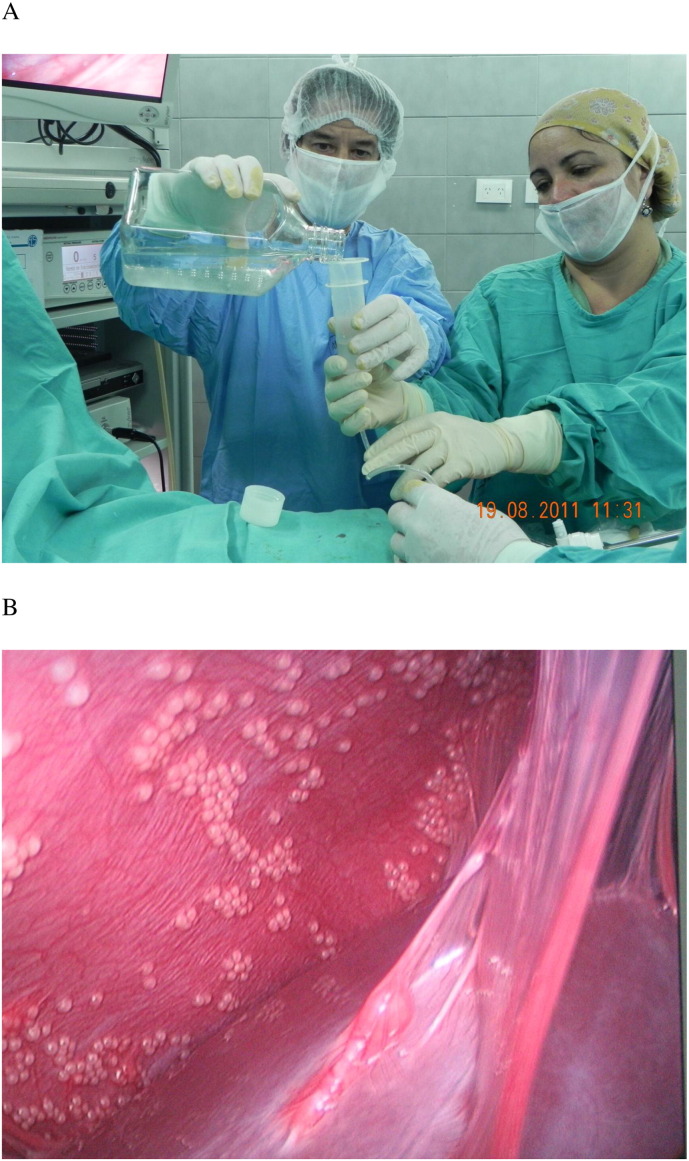
Fig. 1.
Encapsulated neonatal porcine islets were transplanted into peritoneal cavity of type 1 diabetic patient (A). Laparoscopic observation of intra-peritoneal space at the time of second transplantation (B).
There were two groups consisting of patients who received either approximately 5000 islet equivalent (IEQ)/body weight kg twice (total approximately 10,000 IEQ/kg) (group 1, n = 4) or 10,000IEQ/kg twice (total approximately 20,000 IEQ/kg) (group 2, n = 4). The second transplantation was conducted at approximately 3 months after the first transplantation. At the time of second transplantation, abdominal cavity was observed via laparoscopy (Fig. 1B) and samples of encapsulated islets were retrieved for histological analysis.
Outcomes of safety were determined from adverse event reports. PERV provirus and PERV genomic RNA were detected using a PCR-based method (Wynyard et al., 2014). PERV antibodies were tested on patient sera (Wynyard et al., 2014).
Outcomes of efficacy were determined from HbA1c, daily insulin dose (averaged during 2 weeks), and frequency of unaware hypoglycemic events assessed on a regular basis. The regular assessments were conducted 5 times before the first transplantation within approximately 2 months, at the time of transplantation and 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 13, 15, 16, 24, and 29 months after the first transplantation.
To assess the function of transplanted encapsulated neonatal porcine islets, transplant estimated function (TEF) was used (Caumo et al., 2011). TEF was calculated as follows:
The value of HbA1c at the time of the first transplantation was used as HbA1 base. DIR bases were calculated by taking average during two weeks before the first transplantation.
To assess the efficacy of prevention of unaware hypoglycemia, numbers of unaware hypoglycemic events per 4 weeks were assessed using continuous glucose monitoring and diary cards in which any symptoms of hypoglycemia were recorded when patients noticed. For the baseline, unaware hypoglycemic events were counted from week − 4 to week − 1 before transplantation.
To assess the long-term effect, HbA1c, daily insulin dose, TEF and unaware hypoglycemic events were analyzed at > 300 days and 600 days.
Oral glucose tolerance tests (OGTTs) were performed in selected patients (patients 2, 5, 6, 7, and 8) whose fasting blood glucose levels were < 150 mg/dl approximately 15 months after the 1st implantation. Both long-action insulin (NPH) and short acting insulin (insulin aspart) were suspended 24 h and 5 h before OGTT, respectively. Fifty grams of glucose was taken within 10 min and blood glucose levels were measured before OGTT, at 30, 60, 90 and 120 min.
Retrieved encapsulated islets at the time of the second transplantation, were stained with acridine orange/propidium iodide (AOPI) viability staining, and with an Avidin-Biotin method for insulin and glucagon staining.
Of note, this manuscript was created based on the interim data analysis and the final data analysis is not completed yet.
2.1. Statistical Analysis
Differences between two groups and between values of pre-transplantation and post-transplantation were assessed with the use of paired Student t-test. A p value < 0.05 was considered to represent a significant difference. The mean value was described as mean ± standard errors (SE).
3. Results
3.1. Patient Characteristics
Patient characteristics are shown in Table 1. All patients were affected with type 1 DM for at least 5 years. There were no significant differences between groups 1 and 2 in age, duration of type 1 DM and body weight. However, group 2 had significant high body mass index (BMI) compared with group 1 (p < 0.05). Average total amount of transplanted islets were 10,273 ± 278 IEQ/kg in group 1 and 19,099 ± 491 IEQ/kg in group 2.
Table 1.
Patient characteristics.
| Pt | Group | Age (years) | Duration of T1DM (years) | Sex | Body weight (kg) | BMI (kg/m2) | IEQ/kg (1st) | IEQ/kg (2nd) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 40 | 9.0 | F | 59 | 23.2 | 5135 | 4853 |
| 2 | 1 | 19 | 6.0 | M | 62 | 19.9 | 5221 | 5414 |
| 3 | 1 | 38 | 5.1 | M | 64 | 21.5 | 5480 | 4844 |
| 4 | 1 | 22 | 16.1 | M | 55 | 17.3 | 4963 | 5180 |
| 5 | 2 | 64 | 17.4 | F | 50 | 22.9 | 9470 | 10,357 |
| 6 | 2 | 36 | 8.4 | M | 89 | 28.1 | 8866 | 10,086 |
| 7 | 2 | 39 | 29.6 | M | 81 | 27.3 | 8994 | 9849 |
| 8 | 2 | 36 | 21.6 | M | 91 | 30.0 | 8807 | 9965 |
3.2. Safety Data
There was only one serious adverse event possibly related to the procedure which was paralytic ileus. This condition was treated with medication and recovered with no residual effects. There was no severe hypoglycemia which was defined as requiring assistance of another person to administer carbohydrates, glucagon, or take other actions.
For all 8 patients PERV DNA and RNA were assessed at approximately weeks 1, 4 and 12 following the first transplantation and weeks 1, 4, 12, 24, 52 and 104 following the second transplantation (allowing for some deviation due to patient scheduling and availability). No PERV DNA or PERV RNA was found for all time points and all patients (Morozov et al., in press).
PERV antibodies were also tested at approximately weeks − 1, 4 and 12 following the first transplantation and weeks 4, 12, 24 and 52 following the second transplantation (allowing for some deviation due to patient scheduling and availability). None of the sera tested showed reactivity against PERV at any time point. Therefore, no PERV transmission was detected for any of the eight patients (Morozov et al., in press).
3.3. Individual Efficacy Data
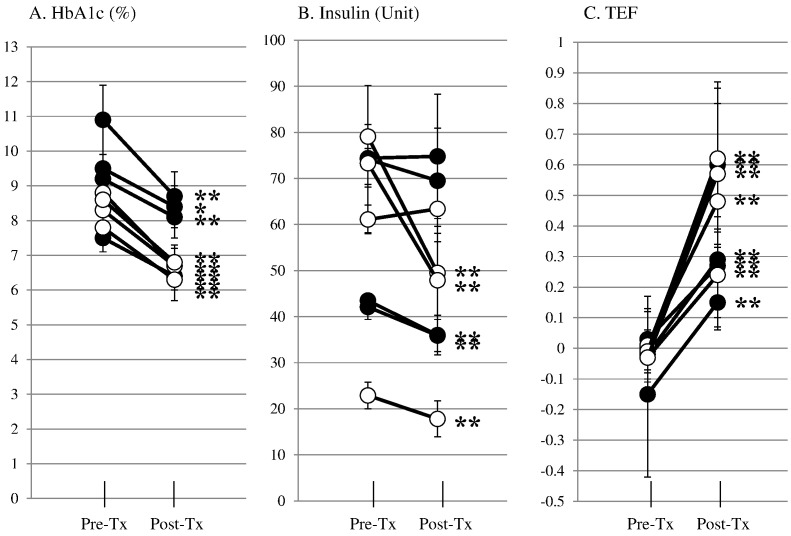
To assess the overall efficacy of islet transplantation, averages of HbA1c, daily insulin dose and TEF were compared before, and after transplantation in each case (Fig. 2). In group 1, all 4 patients significantly improved HbA1c from 9.5 ± 0.5% to 8.4 ± 0.1%, from 7.5 ± 0.2% to 6.4 ± 0.1%, from 10.9 ± 0.4% to 8.7 ± 0.2% and from 9.2 ± 0.2% to 8.1 ± 0.2% in patients 1, 2, 3 and 4 respectively (A), and TEF from − 0.15 ± 0.11 to 0.15 ± 0.02, from − 0.02 ± 0.03 to 0.29 ± 0.03, from 0.00 ± 0.07 to 0.60 ± 0.07 and from 0.03 ± 0.04 to 0.27 ± 0.05 in patients 1, 2, 3 and 4 respectively (C) and two of four patients significantly reduced daily insulin doses from 42.1 ± 1.1 U to 35.9 ± 0.9 U and from 43.5 ± 0.1 U to 36.0 ± 1.1 U in patients 1, and 2 respectively (B). In group 2, all 4 patients significantly improved HbA1c from 8.8 ± 0.2% to 6.7 ± 0.1%, from 8.3 ± 0.1% to 6.7 ± 0.1%, from 7.8 ± 0.2% to 6.2 ± 0.2% and from 8.6 ± 0.1% to 6.8 ± 0.1% in patients 5, 6, 7 and 8 respectively (A), and TEF from 0.01 ± 0.02 to 0.46 ± 0.03, from − 0.01 ± 0.03 to 0.62 ± 0.04, from − 0.03 ± 0.03 to 0.57 ± 0.06 and from − 0.03 ± 0.03 to 0.25 ± 0.03 in patients 5, 6, 7 and 8 respectively (C) and three of four patients significantly reduced daily insulin doses from 22.9 ± 1.2 U to 18.3 ± 1.0 U and from 79.1 ± 1.1 U to 49.1 ± 3.1 U and from 73.3 ± 2.1 U to 48.0 ± 2.9 U in patients 5, 6 and 7 respectively (B). Thus, in both groups encapsulated neonatal porcine islets could provide clinical benefits.
Fig. 2.
Individual averages of pre- (five time points) and post- (16 time points) transplant HbA1c, daily insulin dose and TEF in group 1 (●) and group 2 (○). All cases reduced HbA1c (A) and 5 out of 8 cases reduced insulin doses (B) and all cases increased TEF (C) significantly. *p < 0.05, **p < 0.01.
3.4. Time Course of Each Parameter
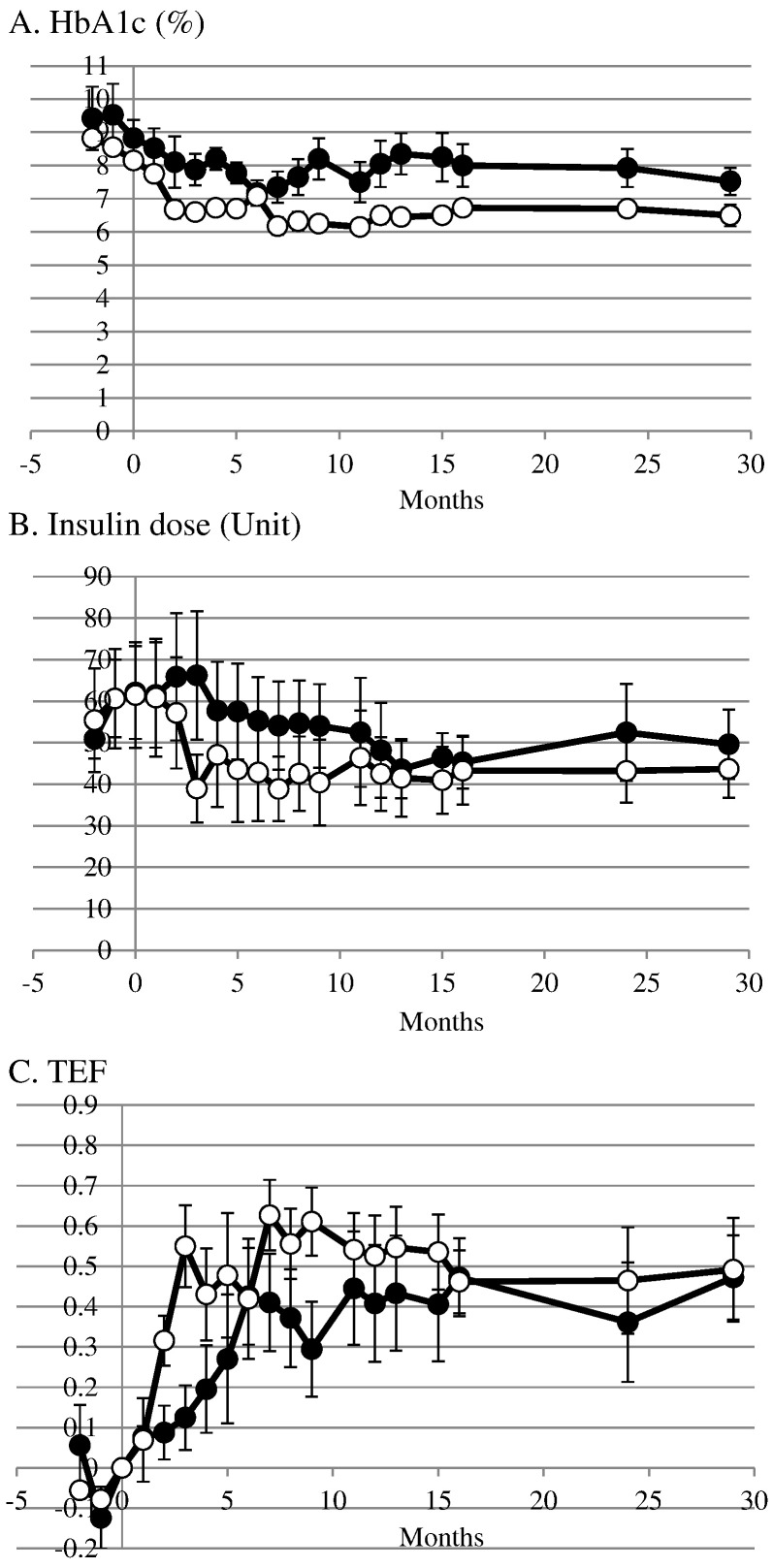
After the first transplantation, HbA1c was gradually decreased from 8.8 ± 0.6% at the time of transplantation to 7.9 ± 0.5% at three months in group 1 and from 8.2 ± 0.2% at the time of transplantation to 6.6 ± 0.2% at three months in group 2 (Fig. 3A). After the second transplantation, the levels of HbA1c maintained and the values were 7.5 ± 0.4% in group 1 and 6.5 ± 0.3 in group 2 at 29 months.
Fig. 3.
Time course of HbA1c (A), daily insulin dose (B) and TEF (C) in group 1 (●) and group 2 (○) after encapsulated neonatal porcine islet transplantation twice. The second transplantation was performed after 3 months of the first transplantation.
After the first transplantation, insulin doses were increased transiently from 62 ± 11 U at the time of transplantation to 66 ± 15 U at three months in group 1 but decreased from 62 ± 13 U at the time of transplantation to 39 ± 8 U at three months in group 2 (Fig. 3B). Then insulin doses were decreased to 48 ± 11 U at 12 months and 50 ± 8 U at 29 months in group 1. The decreased insulin doses were maintained at 43 ± 9 U at 12 months and 44 ± 7 U at 29 months in group 2.
Before transplantation, TEF were around zero in both groups (Fig. 3C). After the first transplantation TEF increased to 0.12 ± 0.08 in group 1 and 0.55 ± 0.10 in group 2 at 3 months. After the second transplantation TEF maintained high levels and the values were 0.47 ± 0.10 in group 1 and 0.49 ± 0.12 in group 2 at 29 months.
3.5. Long-term Functional Efficacy
To assess the long-term effect, averages of HbA1c, daily insulin dose and TEF were analyzed at > 300 days (Table 2A) and > 600 days (Table 2B) in both groups. When analyzed, data at > 300 days (7 time points in each patient) (Table 2A), both groups had significantly improved TEF compared with base-line values (6 time points in each patient). In addition, group 2 had significantly improved HbA1c. When analyzed data at > 600 days (2 time points in each patient) (Table 2B), both groups had still significantly improved TEF. In addition, group 2 had still significantly improved HbA1c. Thus, encapsulated neonatal porcine islets could function > 600 days.
Table 2.
Long-term efficacies.
| A. Long-term efficacy > 300 days | ||||
|---|---|---|---|---|
| Group | Time | HbA1c (%) | Insulin dose (U) | TEF |
| 1 | Pre-transplant | 9.3 ± 1.4 | 58.6 ± 18.2 | − 0.03 ± 0.08 |
| > 300 days | 8.0 ± 1.2 | 47.3 ± 16.4 | 0.43 ± 0.23⁎ | |
| 2 | Pre-transplant | 8.4 ± 0.4 | 59.1 ± 25.2 | − 0.01 ± 0.02 |
| > 300 days | 6.5 ± 0.2⁎⁎ | 43.1 ± 17.1 | 0.51 ± 0.19⁎ | |
| B. Long-term efficacy > 600 days | ||||
| Group | Time | HbA1c (%) | Insulin dose (U) | TEF |
| 1 | Pre-transplant | 9.3 ± 1.4 | 58.6 ± 18.2 | − 0.03 ± 0.08 |
| > 600 days | 7.7 ± 0.9 | 51.0 ± 18.3 | 0.42 ± 0.21⁎ | |
| 2 | Pre-transplant | 8.4 ± 0.4 | 59.1 ± 25.2 | − 0.01 ± 0.02 |
| > 600 days | 6.6 ± 0.5⁎ | 43.4 ± 14.5 | 0.48 ± 0.25⁎ | |
p < 0.001 compared with pre-transplant value.
p < 0.01 compared with pre-transplant value.
3.6. Prevention of Unaware Hypoglycemic Events
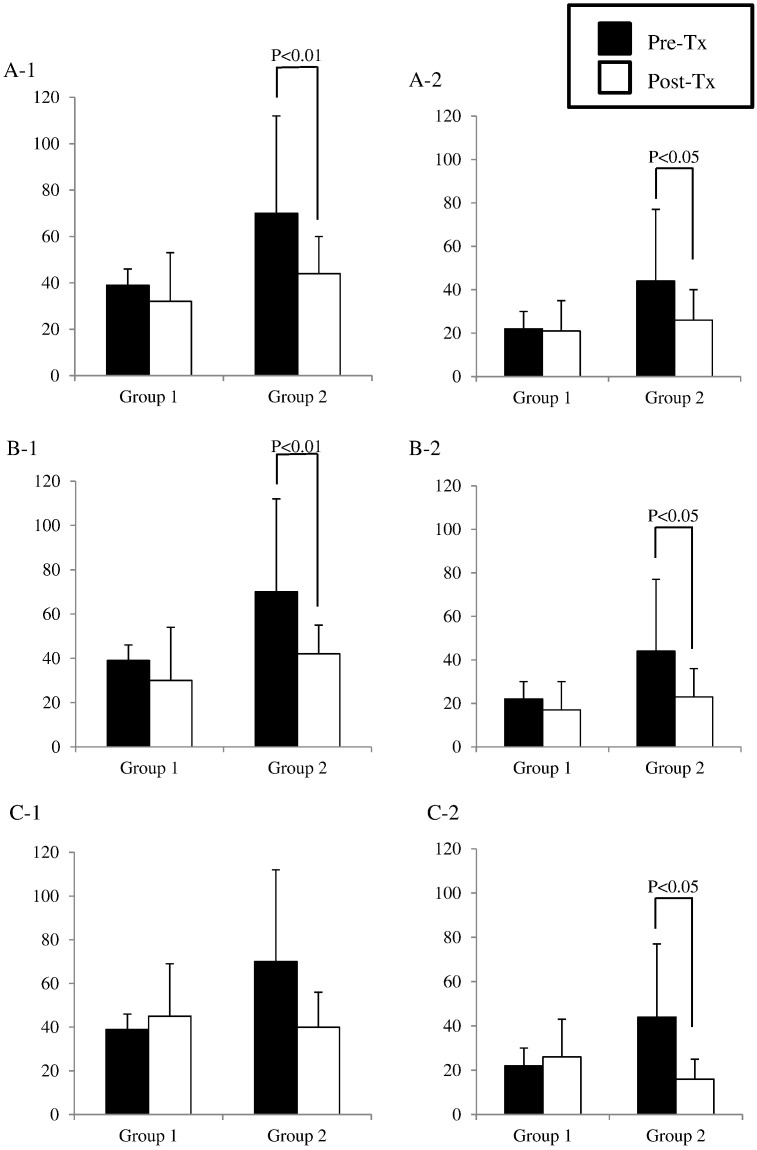
The numbers of mild (< 4 mmol/L) (Fig. 4, A-1) and serious (< 3 mmol/L) (Fig. 4, A-2) unaware hypoglycemic events were compared between pre-transplant base line and after transplantation. In both groups, both mild and serious hypoglycemic events were decreased, however; only group 2 showed significant reductions. Approximately 40% reduction of the number of both mild (pre-transplant: 69.5 ± 21.2/month to post-transplant: 43.6 ± 2.1/month) and serious (pre-transplant: 43.5 ± 16.7/month to post-transplant: 26.2 ± 1.8/month) hypoglycemic events was observed in group 2.
Fig. 4.
Averages of mild (< 4 mmol/L) (A-1) and serious (< 3 mmol/L) (A-2) unaware hypoglycemic events before transplantation (each patient has 1 time point) (■) and after transplantation (each patient has 19 time points) (□). Averages of mild (< 4 mmol/L) (B-1) and serious (< 3 mmol/L) (B-2) unaware hypoglycemic events before transplantation (■) and after > 300 days of transplantation (each patient has 8 time points) (□). Averages of mild (< 4 mmol/L) (C-1) and serious (< 3 mmol/L) (C-2) unaware hypoglycemic events before transplantation (■) and after > 600 days of transplantation (each patient has 2 time points) (□).
3.7. Long Term Effects on Number of Unaware Hypoglycemic Events
To assess the long-term effect on the number of unaware hypoglycemic events, the number at > 300 days (Fig. 4, B-1, 2) and 600 days (Fig. 4, C-1, 2) were analyzed. At > 300 days, group 2 significantly reduced the number of both mild and serious hypoglycemic events (mild: pre-transplant: 69.5 ± 21.2/month to post-transplant: 42.0 ± 2.3/month, serious: pre-transplant: 43.5 ± 16.7/month to post-transplant: 24.1 ± 2.3/month) (Fig. 4, B-1, 2). At > 600 days, none of group significantly reduced the number of mild hypo glycemic events (Fig. 4, C-1), however; group 2 significantly reduced the number of serious hypoglycemic events (pre-transplant: 43.5 ± 16.7/month to post-transplant: 16.4 ± 3.2/month) (Fig. 4, C-2). An approximately 50% reduction of the number serious hypoglycemic events was observed in group 2 at both > 300 and 600 days.
3.8. Oral Glucose Tolerance Test
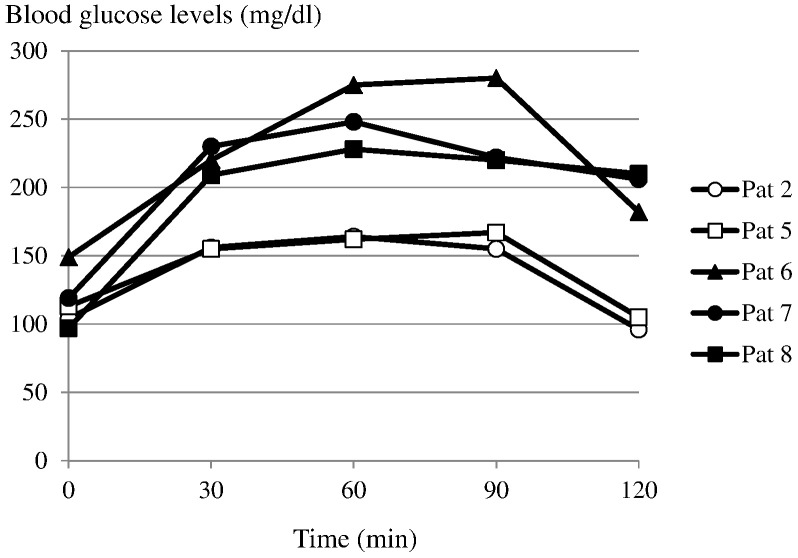
Fasting blood glucose levels were 104, 113, 149, 119 and 97 mg/dl in patients 2, 5, 6, 7 and 8 respectively (Fig. 5). The peaks blood glucose levels were 164, 167, 280, 248, and 228 mg/dl at 60, 90, 90, 60 and 60 min in patients 2, 5, 6, 7 and 8, respectively. The blood glucose levels at 120 min were 96, 105, 182, 206 and 210 mg/dl in patients 2, 5, 6, 7 and 8, respectively. Patient 2 showed a normal pattern and patient 5 showed a marginal pattern. Patients 6, 7 and 8 showed diabetic patterns.
Fig. 5.
Glucose profile of oral glucose tolerance tests in patients 2, 5, 6, 7, and 8 at approximately 1 year after the second transplantation.
3.9. Histological Study
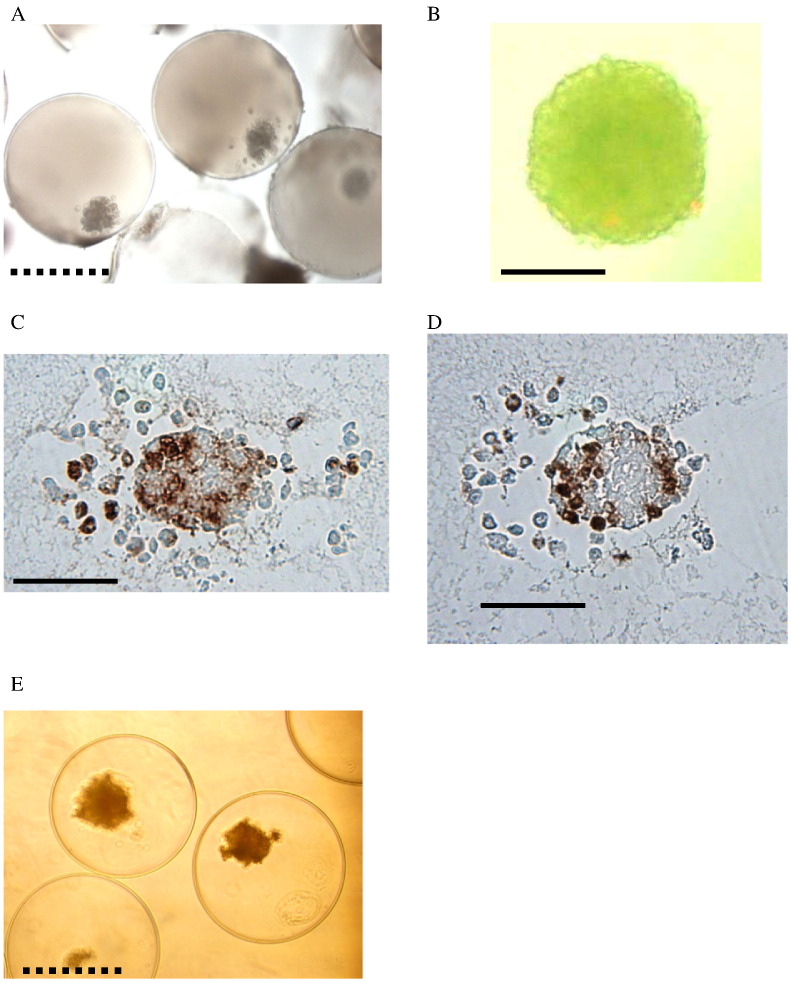
Representative appearances of the capsules retrieved three months after the first transplant are shown in Fig. 6A–D. Retrieved encapsulated islets were intact (Fig. 6A) and viable (Fig. 6B). The islets contained insulin (Fig. 6C) and glucagon (Fig. 6D). For comparison encapsulated porcine islets before transplantation is also shown (Fig. 6E). Comparing pre- and post-transplanted encapsulated islets, transplanted capsule suffered some fibrosis which might be one of the reasons for not completely reversing diabetes.
Fig. 6.
Retrieved encapsulated islets were observed under inverted microscope (A: patient 8) (original magnification × 40) (scale bar: ---- 500 μm), stained with AO/PI (B: patient 8) (original magnification × 200) (scale bar: — 100 μm), and stained for insulin (C: patient 7) (original magnification × 200) (scale bar: — 100 μm) and glucagon (D: patient 7) (original magnification × 200) (scale bar: — 100 μm). Encapsulated islets before transplantation were shown for comparison (E) (original magnification × 40) (scale bar: ---- 500 μm).
4. Discussion
In this study we assessed safety and efficacy of islet xenotransplantation using encapsulated neonatal porcine islets without immunosuppression.
In terms of safety, there was only one procedure related serious adverse event and it was resolved without residual effects. For the observed period PERV infection was not seen for any of the patients tested (Morozov et al., in press) and is consistent with an earlier study using the same technology (Matsumoto et al., 2014, Wynyard et al., 2014).
In terms of efficacy, group 2 (10,000 IEQ/kg × 2 transplantation group) could maintain HbA1c < 7.0% > 600 days with significant reduction of serious unaware hypoglycemia. Hence this study was able to show the clinical benefit by encapsulated porcine islet xenotransplantation without immunosuppression.
On the other hand, HbA1c could be reduced by meticulous blood glucose control. To reduce the HbA1c by meticulous blood glucose control, the doses of insulin need to be increased. However, in our study, both HbA1c and daily insulin doses were decreased after transplantation. Increasing TEF after transplantation also reflects that the effect of reducing the HbA1c is theoretically due to implanted islets. In addition, we put 8 weeks run-in period before transplantation and during the run-in period the TEF was approximately 0. Therefore meticulous blood glucose levels cannot explain the effect of both reducing HbA1c and daily insulin dose after transplantation. Oral glucose tolerance test in two recipients (2 and 5) at 15 months after the first transplantation also suggested transplanted islets maintained their function.
It has been demonstrated that measurements of C-peptide levels in peripheral blood is difficult when islets were transplanted into peritoneal cavity (Matsumoto et al., 2014, Tuch et al., 2009, Jacobs-Tulleneers-Thevissen et al., 2013). Therefore we applied TEF to assess the function of transplanted islets because the TEF does not require C-peptide levels. TEF was proved to be correlated with other islet function assessment methods including beta-score, C-peptide/glucose ratio, acute insulin response stimulated by Arginine, and SUITO index (Caumo et al., 2011). Among the assessment methods of islet function, SUITO index was well evaluated (Takita and Matsumoto, 2012). In cases of allogeneic islet transplantation, it was demonstrated that high SUITO index (> 26), which usually needs multiple islet transplantations, was associated with achieving insulin independence, improving IVGTT profile, reducing hypoglycemic events, and improving quality of life (QOL) (Takita and Matsumoto, 2012). Middle SUITO index (10–25), which usually requires only one islet transplantation, was associated with improving IVGTT profile, reducing hypoglycemic events and improving QOL even without achieving insulin independence (Takita and Matsumoto, 2012). SUITO index 26 and 10 are equivalent to TEF 0.5 and 0.3 respectively (Caumo et al., 2011). Interestingly, in group 2, after the first transplantation TEF exceeded 0.3 and after the second transplantation the TEF exceeded 0.5 which were similar to the allogeneic islet transplantation.
Previously we transplanted encapsulated porcine islets into type 1 diabetic patients with 4 different doses (5000 IEQ/kg, 10,000 IEQ/kg, 15,000 IEQ/kg and 20,000 IEQ/kg) (Matsumoto et al., 2014). TEF was the highest when transplanted 5000 IEQ/kg but the average of the value was only 0.17 (Matsumoto et al., 2014). We speculated that too many islet transplantation into abdominal cavity at once caused oxygen insufficiency for each islet which led to cell death. To avoid this, we transplanted either 5000 IEQ/kg or 10,000 IEQ/kg first followed by a second transplantation after 3 months in this study. Multiple small dose transplantation might contribute to improve the efficacy.
This study demonstrated significant improvement of HbA1c, reduction of unaware hypoglycemic events with improved TEF; however, the reduction of insulin doses was marginal. In cases of allogeneic islet transplantation, it was demonstrated that single islet transplantation could improve glycemic control but marginally reduced insulin doses due to necessity of bolus insulin injection associated with meals (Sassa et al., 2006). In this study, transplanted encapsulated neonatal porcine islets might not sufficiently respond to the meal. Considering high TEF, which suggests sufficient insulin secretary ability of islets, this insufficiency might be due to intra-peritoneal transplant site for encapsulated islets. In addition, even HbA1c was significantly reduced after transplantation; HbA1c did not reach the normal levels. This might be also due to intra-peritoneal transplant site because there might be a lag time between sensing blood glucose levels and delivering insulin via ascites. Better transplant sites, for example vascularized areas under skin or omentum pouch might improve this issue.
Another possible cause of marginal effects of transplanted encapsulated islets was fibrosis of capsule after transplantation as indicated in the histology study. Though, the histology study was not performed for quantitative analysis. It was demonstrated that microcapsule could prevent direct contact of neonatal porcine islets by host immune cells, however, shed xeno-antigens might escape from the capsule (Rayat et al., 2000, Kobayashi et al., 2006). This shed xeno-antigen might activate indirect pathway resulted in recruitment of CD4 + T cells and macrophages around the microcapsule. The recruited CD4 + T cells might produce interferon-gamma and interleukin-10 which induced inflammatory reaction. This inflammatory reaction could cause the fibrosis of microcapsule. Prevention of the indirect pathway might improve this issue.
In addition, maturation of neonatal islets is one of the important factors for proper insulin secretory ability (Kobayashi et al., 2008). In this study, we did not assess the maturity of neonatal islets before transplantation, however; we plan to assess the maturity of neonatal islets before transplantation for the next clinical trial.
Several clinical studies have been reported about encapsulated human allogeneic islet transplantations (Tuch et al., 2009, Jacobs-Tulleneers-Thevissen et al., 2013, Basta et al., 2011), however, insulin independence or significant reduction of insulin doses were rare. The best clinical outcomes were demonstrated by Basta et al. (2011) which showed 4 patients maintained HbA1c < 8% at 12 months and 24 months. In our study, the values of HbA1c in group 2 were < 7% > 600 days. This suggests that healthy porcine islets could provide better function compared with human islets which are usually recovered from deceased donors.
In conclusion, encapsulated neonatal porcine islet xenotransplantation could maintain HbA1c < 7% with significant reduced hypoglycemic events without immunosuppression > 600 days. We believe this study is the prologue for the clinical xenotransplantation to solve the issue of donor shortage.
Authors' Contributions
Shinichi Matsumoto: literature search, figures, study design, data collection, data analysis, data interpretation, writing manuscript.
Adrian Abalovich: literature search, figures, data collection, data analysis, data interpretation, editing manuscript.
Carlos Wechsler: literature search, data collection, data analysis, data interpretation, editing manuscript.
Shaun Wynyard: literature search, data collection, data analysis, data interpretation, editing manuscript.
Robert B Elliott: literature search, study design, data analysis, data interpretation, editing manuscript.
Conflict of Interest Statements
Shinichi Matsumoto is an employee of Otsuka Pharmaceutical Factory Inc.
Adrian Abalovich has no conflict of interests.
Carlos Wechsler has no conflict of interests.
Shaun Wynyard is an employee of Diatranz Otsuka Ltd.
Robert B Elliott is an employee of Diatranz Otsuka Ltd.
Role of Funding Source
Entire researches have been funded by Living Cell Technologies and Diatranz Otsuka Ltd.
Ethics Committee Approval
The study protocol (ClinicalTrials.gov Identifier NCT01739829) was granted ethics approval in Argentina from the Ministry of Health, Buenos Aires Province.
References
- Basta G., Montanucci P., Luca G. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: four cases. Diabetes Care. 2011;34:2406–2409. doi: 10.2337/dc11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caumo A., Maffi P., Nano R. Comparative evaluation of simple indices of graft function after islet transplantation. Transplantation. 2011;92:815–821. doi: 10.1097/TP.0b013e31822ca79b. [DOI] [PubMed] [Google Scholar]
- Dalal A.R. Philosophy of organ donation: review of ethical facets. World. J. Transplant. 2015;5:44–51. doi: 10.5500/wjt.v5.i2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garkavenko O., Croxon M.C., Irgang M., Karlas A., Denner J., Elliott R.B. Monitoring for presence of potentially xenotic viruses in recipients of pig islet xenotransplantation. J. Clin. Microbiol. 2004;42:5353–5356. doi: 10.1128/JCM.42.11.5353-5356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth C.G., Korsgren O., Tibell A. Transplantation of porcine fetal pancreatic to diabetic patients. Lancet. 1994;344:1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Hillberg A.L., Kathirgamanathan K., Lam J.B., Law L.Y., Garkavenko O., Elliott R.B. Improving alginate-poly-l ornithine-alginate capsule biocompatibility through genipin crosslinking. J. Biomed. Mater. Res. Part B. 2013;101B:258–268. doi: 10.1002/jbm.b.32835. [DOI] [PubMed] [Google Scholar]
- Jacobs-Tulleneers-Thevissen D., Chintinne M., Ling Z. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to pilot study in a type 1 diabetic patient. Diabetologia. 2013;56:1605–1614. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Harb G., Rajotte R.V. Immune mechanisms associated with the rejection of encapsulated neonatal porcine islet xenografts. Xenotransplantation. 2006;13:547–559. doi: 10.1111/j.1399-3089.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Arefanian H., Harb G. Prolonged survival of microencapsulated neonatal porcine islet xenografts in immune-competent mice without antirejection therapy. Cell Transplant. 2008;17:1243–1256. doi: 10.3727/096368908787236602. [DOI] [PubMed] [Google Scholar]
- Matsumoto S. Islet cell transplantation for type 1 diabetes. J. Diabetes. 2010;2:16–22. doi: 10.1111/j.1753-0407.2009.00048.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Okitsu T., Iwanaga Y. Insulin independence after living-donor distal pancreatectomy and islet allotransplantation. Lancet. 2005;365:1642–1644. doi: 10.1016/s0140-6736(05)66383-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Okitsu T., Iwanaga Y. Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method. Transplantation. 2006;27:460–465. doi: 10.1097/01.tp.0000231710.37981.64. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Tan P., Baker J. Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant. Proc. 2014;46:1992–1995. doi: 10.1016/j.transproceed.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Morozov V.A., Wynyard S., Matsumoto S., Abalovich A., Denner J., Elliott R. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res. 2016 doi: 10.1016/j.virusres.2016.08.012. (in press) [DOI] [PubMed] [Google Scholar]
- Rayat G.R., Rajotte R.V., Ao Z., Korbutt G.S. Microencapsulation of neonatal porcine islets: protection from human antibody-complement-mediated cytolysis in vitro and long-term reversal of diabetes in nude mice. Transplantation. 2000;69:1084-69. doi: 10.1097/00007890-200003270-00011. [DOI] [PubMed] [Google Scholar]
- Sassa M., Fukuda K., Fujimoto S. A single transplantation of the islets can produce glycemic stability and reduction of basal insulin requirements. Diabetes Res. Clin. Pract. 2006;73:235–240. doi: 10.1016/j.diabres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Takita M., Matsumoto S. SUITO index for evaluation of clinical islet transplantation. Cell Transplant. 2012;21:1341–1347. doi: 10.3727/096368912X636885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- Tuch B.E., Keogh G.W., Williams L.J. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32:1887–1889. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Gonzalez R.A., Morantes L.M., Garibay G.N. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur. J. Endocrinol. 2005;153:419–427. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- Valdes-Gonzalez R., Rodriguez-Ventura A.L., White D.J.G. Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets. Clin. Exp. Immunol. 2010;162:537–542. doi: 10.1111/j.1365-2249.2010.04273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynyard S., Nathu D., Garkavenko O., Denner J., Elliot R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21:309–323. doi: 10.1111/xen.12102. [DOI] [PubMed] [Google Scholar]