Abstract
Emerging evidence suggests that Helicobacter pylori infection is associated with insulin resistance (IR) yet the underlying mechanisms are still obscure. The vital role of gut microbiota in triggering IR has been increasingly reported, however, no study has explored the correlation of gut microbiota and H. pylori-associated IR. Using H. pylori-infected mice model fed different diet structures, we demonstrated that H. pylori infection significantly aggravated high-fat diet (HFD)-induced metabolic disorders at the early stage, the extent of which was close to the effect of long-term HFD. Interestingly, we observed dynamic alterations in gut microbiota that were consistent with the changes in the metabolic phenotype induced by H. pylori and HFD. There may be an interaction among H. pylori, diet and gut microbiota, which dysregulates the host metabolic homeostasis, and treatment of H. pylori may be beneficial to the patients with impaired glucose tolerance in addition to diet control.
Keywords: Helicobacter pylori, High-fat diet, Insulin resistance, Gut microbiota
Highlights
-
•
H. pylori infection aggravates high-fat diet induced metabolic disorders at the early stage in C57BL/6 mice.
-
•
H. pylori infection in high-fat diet induces dynamic alterations of gut microbiota consistent with the metabolic phynotype.
H. pylori is one of the most common human bacterial pathogens which causes gastric disorders. Epidemiological studies show that its infection is associated with insulin resistance although the mechanism is obscure. Our study demonstrates that H. pylori infection significantly aggravates high-fat diet induced metabolic disorders at the early stage, accompanied by dramatic alterations of gut microbiota. Moreover, the changes of gut microbiota are consistent with the metabolic phynotype, indicating an interaction among H. pylori, diet and gut microbiota. Thus, the treatment of H. pylori may be beneficial to the patients with impaired glucose tolerance in addition to diet control.
1. Introduction
It has been established that Helicobacter pylori infection can lead to the development of many gastroduodenal diseases (Goh et al., 2011). Accumulated evidence has suggested that this infection is associated with metabolic diseases, such as metabolic syndrome (Chen et al., 2015a, Chen et al., 2015b), type 2 diabetes mellitus (T2DM) (He et al., 2014b) and atherosclerotic diseases (He et al., 2014a), yet the underlying mechanism is obscure. Insulin resistance (IR) and subsequent hyperinsulinemia are the key pathogenic factors contributing to these metabolic abnormalities. Thus, investigating the impact of H. pylori infection on insulin sensitivity may elucidate the association between H. pylori and the metabolic diseases.
Increasing evidence has shown that the gut microbiota play a fundamental role in the pathogenesis of metabolic disorders like obesity and T2DM, possibly through modulating energy balance, glucose metabolism and chronic inflammatory response (Cani et al., 2008, Ley et al., 2006, Musso et al., 2010). Thus far, the interaction of gastric microbiota with H. pylori in human and animal models has been investigated, and several of these microbes are supposed to contribute to H. pylori-associated diseases such as gastric cancer (He et al., 2016). However, studies regarding the impact of H. pylori infection on gut microbiota are limited. In this study, we investigated the effect of H. pylori infection on high-fat diet (HFD) induced IR and the associated alterations of gut microbiota in C57BL/6 mice. We also assessed the levels of metabolic hormones and cytokines in response to H. pylori colonization with or without a HFD.
2. Material and Methods
2.1. Animals and H. pylori Strains
Six- to eight-week-old male C57BL/6 mice (Hunan Slac Jingda Laboratory Animal Company, Ltd., Changsha, China) and two mouse-adapted H. pylori strains including the Sydney strain (SS1) and NCTC11637 were used in this study. The H. pylori strains were inoculated on Campylobacter agar plates containing 10% sheep blood and incubated at 37 °C under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) for 24-48 h and then subcultured in Brucella broth supplemented with 10% FBS at 37 °C under the microaerophilic conditions for 16-18 h. Bacterial density was estimated spectrophotometrically at 600 nm (OD600), and viable cells were determined as colony-forming units (CFU)/ml (1 OD600 = 109 CFU/ml). Animals were housed in a controlled environment (12 h daylight cycle) with food and water ad libitum. After 2 weeks of acclimation on a normal chow diet (Beijing KeAoXieLi Company, Ltd., Beijing, China), the mice were randomly divided into six groups (Supplement Fig. 1). Four groups were received gavage in a dosage of 1 × 109 CFU of H. pylori SS1 or NCTC11637 five times at 2 day intervals, and concurrently fed either a chow diet or HFD containing 45.37% lipids, 18.21% proteins and 36.42% carbohydrates, whereas the other two groups received the sterile Brucella broth and were fed the corresponding diet. Body weight, food intake and waist circumference were assessed twice a month. The waist circumference was measured at the midpoint between the lower ribs and iliac crest with a measuring tape by two investigators who were blinded as to which group the animal has been assigned (Knigge et al., 2015, Zhang et al., 2007). All procedures were approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University.
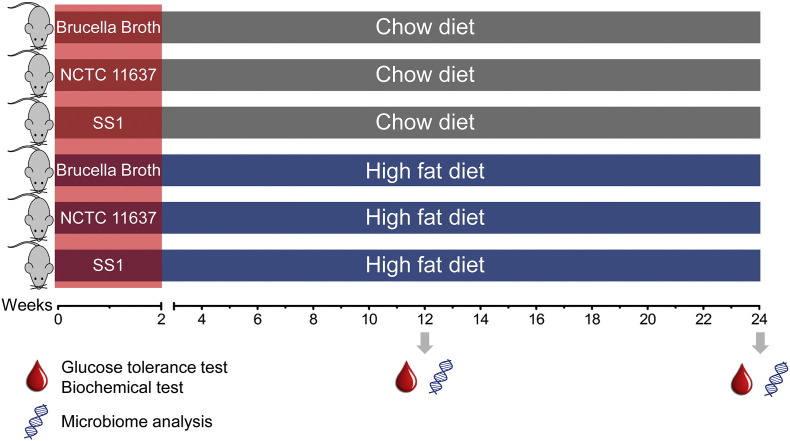
Supplement Fig. 1.
Experimental scheme. 6- to 8-week old male C57BL/6 mice were intragastrically inoculated H. pylori (SS1 or NCTC11637) five times with a 2-day interval or sterile Brucella broth as control, and concurrently fed either chow diet (CD) or high-fat diet (HFD). Glucose tolerance tests and microbiome analysis were performed on the indicated time points.
2.2. Glucose Homeostasis
After 12 and 24 weeks on different diets, mice were fasted overnight (12 h) and the glucose tolerance test (IPGTT) was performed after the mice were intraperitoneally injected with glucose (2 g/kg). Blood glucose concentrations were measured using a handheld glucometer (OneTouch Ultra Easy, LifeScan) via tail bleed before (0 min) and after (15, 30, 60, 120 min) the glucose injection. For the intraperitoneal insulin tolerance test (IPITT), mice were fasted for 6 h and the glucose levels were measured before (0 min) and after (15, 30, 60, 120 min) insulin (0.75 U/kg) administration.
2.3. Blood Serum Analysis
At week 12 and 24, mice were fasted overnight and sacrificed for subsequent analysis. Blood was collected into microfuge tubes and allowed to clot for 30 min. Then samples were centrifuged at 3000 rpm for 20 min and serum was collected and stored at − 80 °C until analysis. Serum insulin (CrystalChem Inc.) was quantified by ELISA. The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated based on the following formula: fasting insulinemia (μUI/ml) × fasting glycaemia (mM)/22.5. The concentrations of inflammatory cytokines IL-1β, IL-6 and TNF-α were quantified using the Mouse Cytokine/Chemokine Magnetic Bead Panel (Millipore). The Milliplex MAP Kit for Mouse Metabolic Magnetic Bead Panel (Millipore) was used to quantify hormones including resistin, gastric inhibitory polypeptide (GIP) and leptin.
2.4. 16S rRNA Gene Sequencing and Analysis
Fresh faeces were collected at different time points and total genomic DNA from each sample was extracted using the QIAamp DNA Stool Mini Kit (QIAGEN) according to the manufacturer's instructions. DNA concentrations and integrity were assessed using a fluorometer and agarose gel electrophoresis. Then, the qualified DNA samples were stored at − 80 °C prior to 16S ribosomal RNA (rRNA) gene amplicon sequencing. The 16S rRNA gene amplicon sequencing was performed on the Illumina MiSeq platform (BGI Tech Solutions Co., Ltd., Shenzhen, China) according to protocols described by Caporaso et al. (2012)). The sequences of the primers targeting the V4 hyper-variable region of the bacterial 16S rRNA genes were as follows: 515F GTGCCAGCMGCCGCGGTAA; 806R GGACTACHVGGGTWTCTAAT. The raw data were filtered to obtain clean reads by eliminating the adapter pollution and low quality sequences (Fadrosh et al., 2014). High quality paired-end reads were combined to tags with an average read length of 252 bp using FLASH (Fast Length Adjustment of Short reads, v 1.2.11) (Magoc and Salzberg, 2011). The tags were then clustered as OTU (Operational Taxonomic Unit) by scripts of USEARCH (v 7.0.1090) software with a 97% similarity threshold (Edgar, 2013). The representative OTU sequences were taxonomically classified using Ribosomal Database Project (RDP) Classifier v.2.2 trained on the Greengenes database. Finally, an OTU table and a phylogenetic tree were generated for diversity analysis. Principal coordinate analysis (PCoA) was conducted according to the distance matrices created by QIIME (v 1.80) (Caporaso et al., 2010). Linear discrimination analysis coupled with effect size (LEfSe) was performed to identify the bacterial taxa differentially represented between groups at the genus or higher taxonomy levels (Segata et al., 2011).
2.5. Western Blotting
10 mg of liver and jejunum tissue were homogenized using the Mixer Mill MM 400 (Retsch) in lysis buffer (50 mM Tris-HCl, pH 7.8, 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 50 mM β-glycerol-phosphate, 1 mM DTT, 0.5% TritonX-100, 10 mM NaF and protease inhibitors). Protein concentrations were determined using a Coomassie brilliant blue staining method. An equal amount of protein (20-25 μg) was separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes (Whatman GmbH, Dassel, Germany). After the membranes were blocked with 5% nonfat milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST) at room temperature for 1 h, they were incubated overnight with the primary antibody at 4 °C. Subsequently, the membranes were washed and incubated with HRP-conjugated secondary antibody. Immuno-reactive proteins were detected using an ECL kit (Bio-Rad, USA). Antibodies against protein kinase B (Akt) and p-Akt (Ser473) were purchased from Cell Signaling Technology, insulin receptor substrate 1 (IRS1) and p-IRS1 (Tyr896) and occludin from Abcam, ZO-1 from Invitrogen and claudin-1 from Life Technologies.
2.6. Statistical Analysis
Statistical analysis was performed using the SPSS 13.0 software. Data are expressed as the means ± standard error of means (SEM). Groups were compared by one-way ANOVA and LSD's multiple range test with a value of p < 0.05 as the cut-off for statistical significance.
3. Results
3.1. H. pylori Infection Accelerates Diet-induced Central Obesity
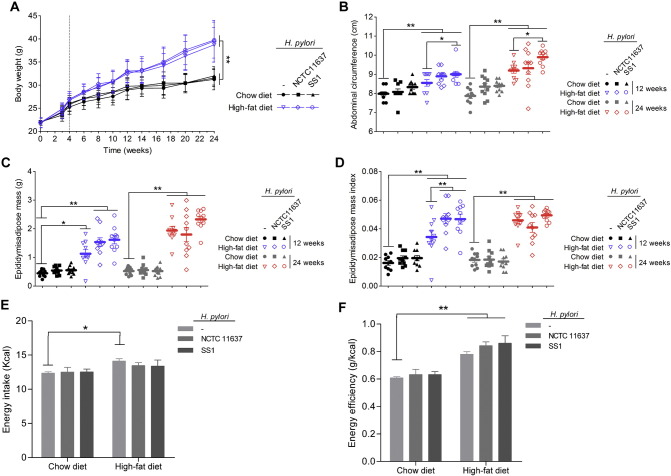
The C57BL/6 mice were successfully infected with H. pylori after 12 and 24 weeks, which was confirmed by Giemsa staining while none of the mice administered Brucella broth alone was positive for H. pylori (Supplement Fig. 2). As shown in Fig. 1A and B, both the body weight and the waist circumference were significantly increased early after HFD feeding. Interestingly, HFD-fed mice infected with H. pylori SS1 had a larger waist circumference without increased body weight compared to that of the HFD controls. Moreover, the visceral fat, especially the epididymal adipose mass, was much heavier and the relative epididymal fat pad weight was significantly greater in mice with H. pylori infection after 12 weeks of HFD, the degree of which was almost comparable to the level of 24 weeks HFD controls (Fig. 1C and D). Additionally, in contrast to the HFD control group, H. pylori-infected mice feeding HFD showed no difference of energy intake in comparison with the controls but exhibited a significant increase in energy efficiency at 12 weeks (Fig. 1E and F). Together, these data suggest that H. pylori infection accelerates HFD-induced fat redistribution and the emergence of central obesity which may be related to the enhanced energy harvest.
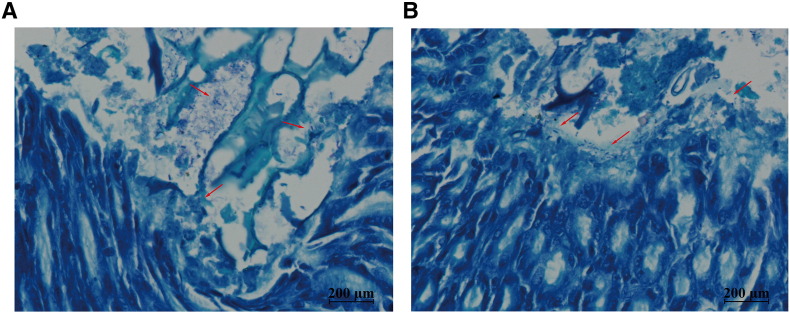
Supplement Fig. 2.
Giemsa staining of C57BL/6 mice gastric mucosa at different time points. After 12 (A) and 24 weeks (B) intragastric administration of H. pylori all gastric tissues were harvested from the gastric antrum of the mice. Red arrows indicate colonization by H. pylori infection (Magnification: × 200).
Fig. 1.
Effects of H. pylori infection and different dietary regiments on body composition. Mice were fed either a chow or a high-fat diet (HFD) for 12 weeks and 24 weeks. H. pylori-infected groups were intragastrically administered either SS1 strain or NCTC11637 strain with a 2 day interval for a total of five infusions. Chow and HFD fed control groups were gavaged with vehicle (Brucella broth). (A) Body weight curves; (B) abdominal circumference; (C) epididymisadipose mass; (D) epididymisadipose mass index (ratio between epididymisadipose mass and body weight); (E) energy intake; (F) energy efficiency (ratio between body weight gain and energy intake). The body weight was significantly increased by four weeks after HFD. n = 8–10. Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01.
3.2. H. pylori Infection Aggravates Early Diet-induced IR
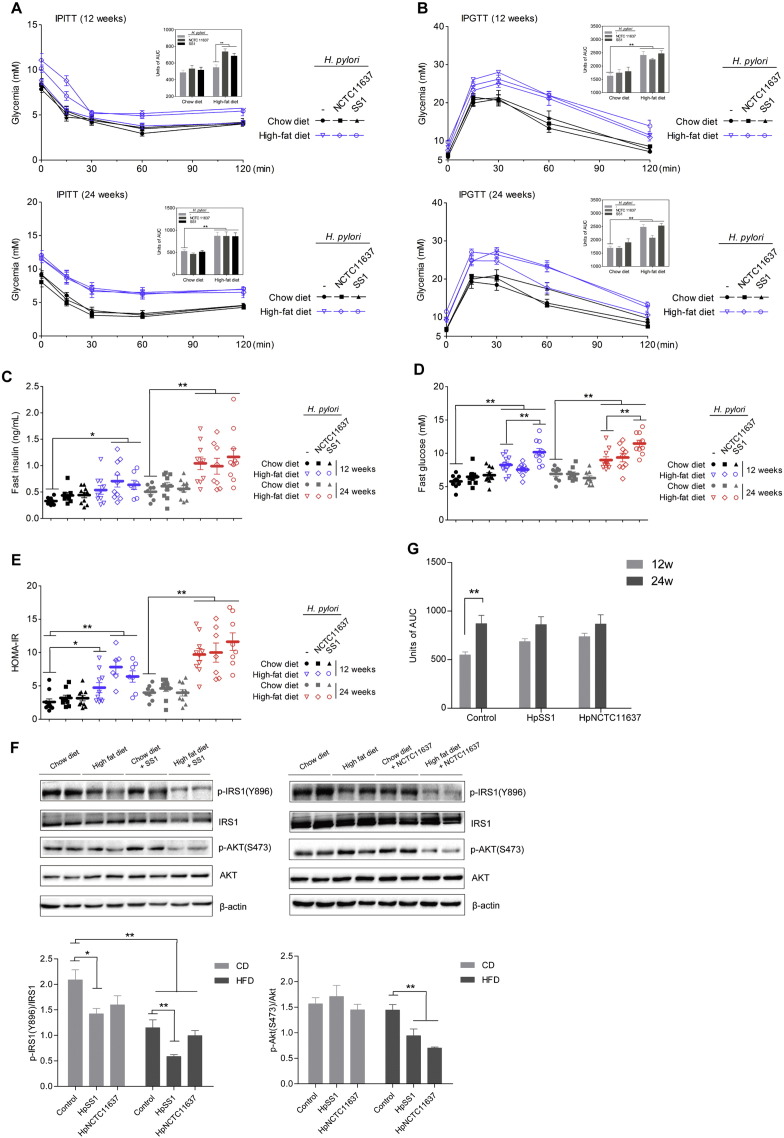
We next examined the impact of H. pylori infection on glucose homeostasis and insulin sensitivity in mice feeding a normal diet or HFD after 12 and 24 weeks. We found that H. pylori-infected HFD-fed mice displayed more severe IR compared to HFD controls at 12 weeks as revealed by intraperitoneal insulin tolerance tests (IPITTs) (Fig. 2A). Although H. pylori infection did not significantly influence HFD-induced glucose intolerance (Fig. 2B), the higher fasting hyperinsulinemia and homeostasis model assessment of insulin resistance (HOMA-IR) index values further supported that H. pylori infection aggravated IR after 12 weeks of HFD consumption (Fig. 2C and E). Moreover, the fasting blood glucose was higher in HFD mice infected with H. pylori SS1 than uninfected HFD controls (Fig. 2D), suggesting that H. pylori infection had a synergistic effect on the dysregulation of glucose homeostasis. We then measured the expression of insulin signaling proteins in liver and found a substantial reduction in the phosphorylation of IRS1 (Tyr896) and Akt (Ser473) in H. pylori-infected mice administered a HFD (Fig. 2F), further confirming the deleterious effects of H. pylori infection combined with HFD on IR. However, after long-term HFD consumption, the diet itself had a strong effect on glucose dysregulation that seems greater than H. pylori infection, as that the insulin sensitivity was lower and the fasting insulin and HOMA-IR were higher in HFD groups than the chow diet (CD) controls at 24 weeks, with no statistical difference between H. pylori-infected and non-infected groups (Fig. 2A, C and E). We also found that the IR of HFD controls increased strikingly at 24 weeks compared with 12 weeks as revealed by IPITTs, whereas the H. pylori-infected HFD groups only increased slightly, which supported the crucial role of diet after long-term treatment (Fig. 2G).
Fig. 2.
H. pylori infection promotes diet-induced IR and hyperinsulinaemia at the early stage. The intraperitoneal insulin and glucose tolerance tests were performed at different time points during the treatment (A, B). Mice were fasted overnight for plasma insulin (C), blood glucose (D) determination and the HOMA-IR index was calculated (E). Immunoblots of phosphorylated insulin receptor substrate 1 (p-IRS1) (Tyr896) and phosphorylated Akt (p-Akt) (Ser473) in the chow or HFD groups with or without H. pylori infection (F). Area under the curve for insulin tolerance tests after 12 and 24 weeks of HFD consumption in H. pylori-infected and non-infected groups (G). Data are expressed as the mean ± SEM. ⁎p < 0.05, **p < 0.01.
3.3. H. pylori Infection Influences the Circulating Metabolic Hormones Instead of Inflammatory Cytokines
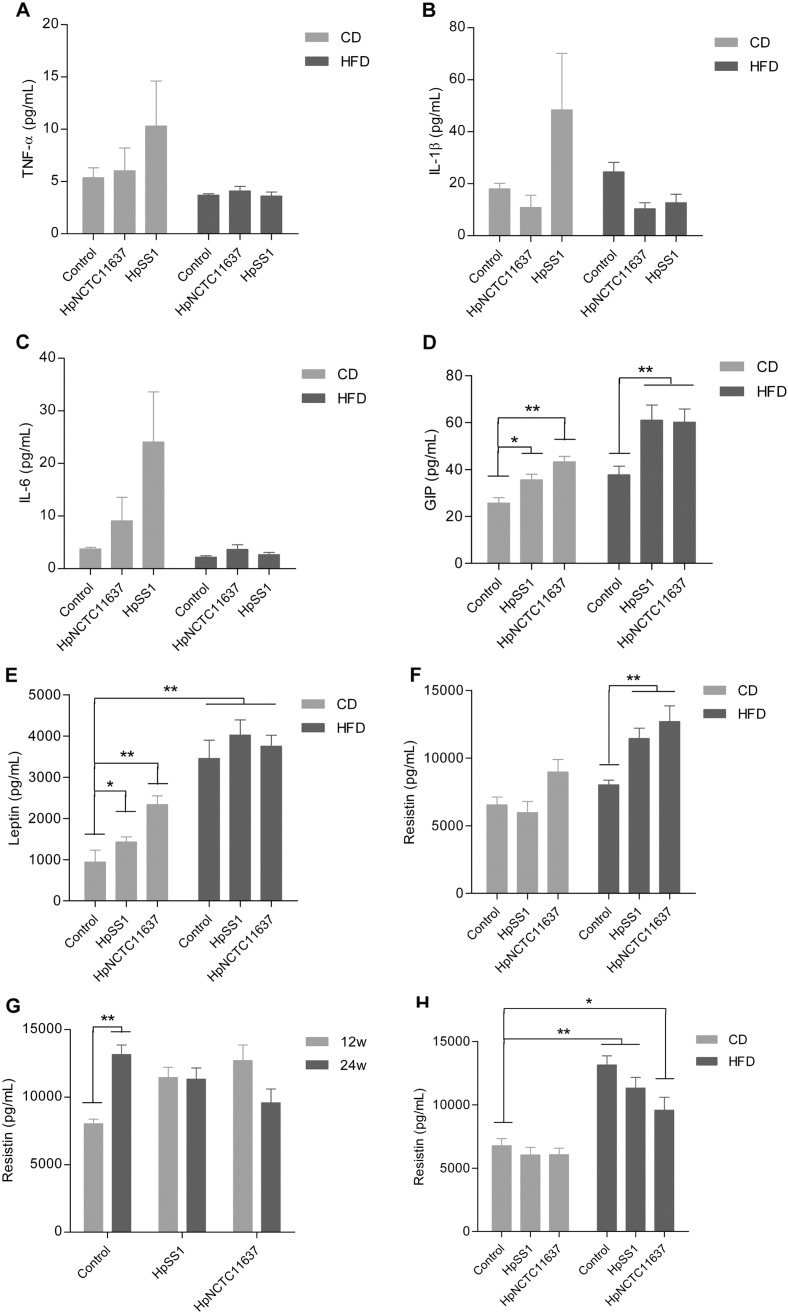
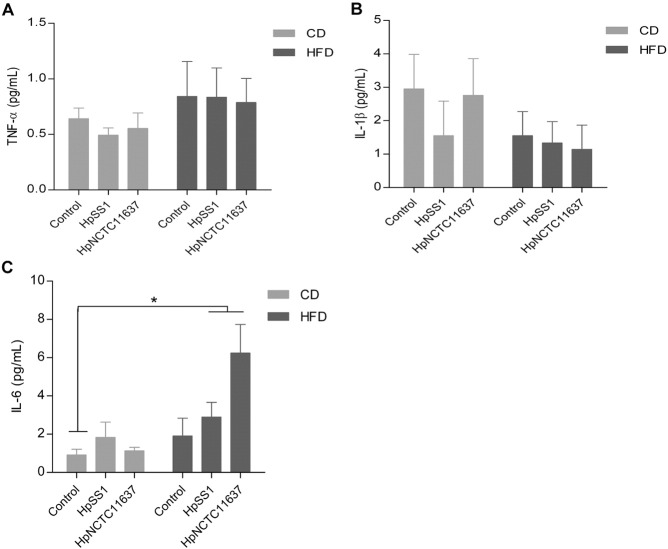
Chronic inflammation and alterations in metabolic hormones are believed to be responsible for IR. In our study, the serum levels of TNF-α, IL-1β and IL-6 trended to be higher in animals infected with H. pylori SS1 on a normal diet at 12 weeks, while no difference was observed between H. pylori-infected and non-infected groups in HFD (Fig. 3A-C). Similarly, after 24 weeks the levels of these cytokines in HFD-fed H. pylori-infected groups were not different from the HFD controls except IL-6 (Supplement Fig. 3A–C). H. pylori has been reported to influence physiological regulation of metabolic hormones involved in food intake, energy expenditure and body mass (Khosravi et al., 2015). We found that the serum level of gastric inhibitory polypeptide (GIP) and leptin were higher in animals infected with H. pylori when given a normal diet at 12 weeks compared to the non-infected controls, and the level of GIP was further increased in H. pylori-infected group fed HFD compared with HFD controls (Fig. 3D and E). Moreover, H. pylori infection apparently elevated the circulating level of resistin which was identified to play a significant role in the onset of IR after 12 weeks HFD (Fig. 3F). At 24 weeks the level of resistin increased rapidly in the HFD controls compared with 12 weeks and no significant difference in resistin was observed in HFD groups regardless of H. pylori infection status (Fig. 3G and H). These results suggest that H. pylori infection influences the secretion of metabolic hormones which associates with the aggravation of IR at an early stage of HFD feeding although the diet itself has a much stronger effect after long-term administration of HFD.
Fig. 3.
Effects of H. pylori infection with different diets on circulating inflammatory cytokines and metabolic hormones. Serum levels of TNF-α (A), IL-1β (B) and IL-6 (C) were not significantly different between H. pylori-infected and non-infected mice regardless of the diet structure at 12 weeks. Animals with H. pylori infection, especially those fed a HFD, showed significant increases in serum levels of metabolic hormones including GIP (D), leptin (E) and resistin (F) compared to their vehicle-treated controls at 12 weeks. The level of resistin increased rapidly in the HFD control group after 24 weeks compared with 12 weeks (G) and thus come up with H. pylori-infected groups fed HFD at 24 weeks (H). Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01.
Supplement Fig. 3.
Effects of H. pylori infection with different diet consumption on the inflammatory cytokines after 24 weeks. Serum levels of TNF-α (A) and IL-1β (B) were not significant different between H. pylori-infected and non-infected mice regardless of the diet structure. The levels of IL-6 were higher in HFD-fed H. pylori-infected groups than the chow diet controls. Data are expressed as the mean ± SEM. *p < 0.05 versus chow controls.
3.4. The Alteration of Gut Microbiota is Correlated with H. pylori-associated IR
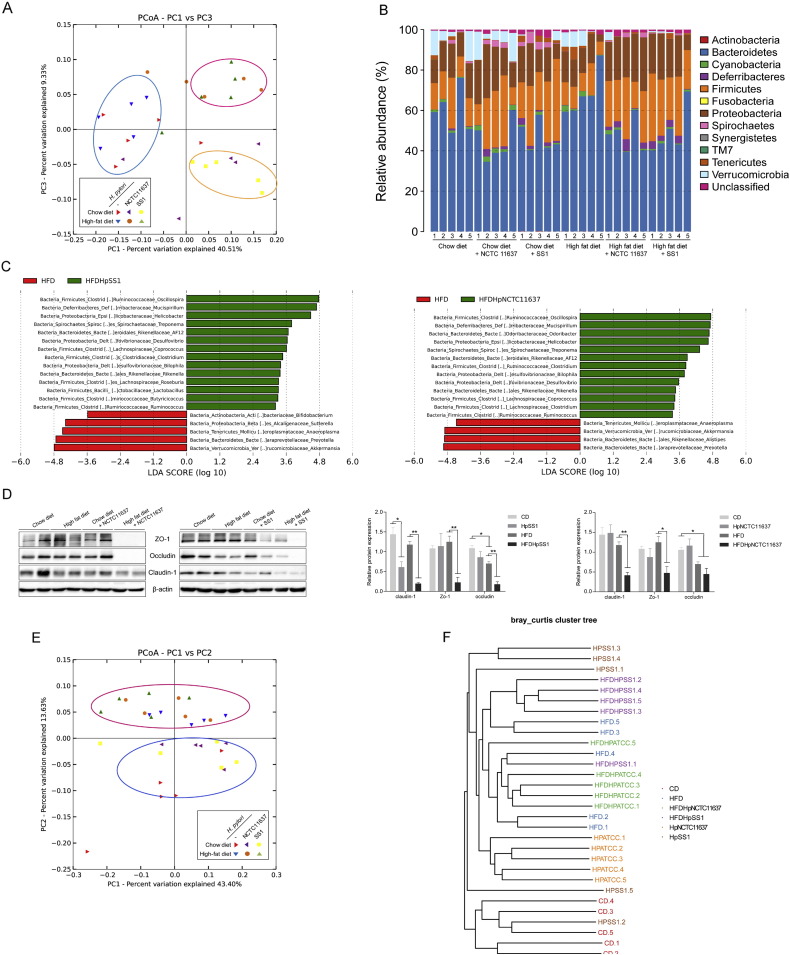
Accumulating evidence suggests that dysbiosis of gut microbiota plays a vital role in triggering obesity, IR and other metabolic disorders (Owyang and Wu, 2014). Recent reports have highlighted that there is an ongoing crosstalk between H. pylori and the gut microbiota which is associated with the gut inflammation (Khosravi et al., 2015). Thus, we investigated whether the aggravating effect of H. pylori on HFD-induced IR is associated with perturbation of the gut microbiota. The 16S rRNA gene sequencing of fecal samples at 12 weeks revealed that H. pylori-infected mice feeding HFD had a distinct microbiota community that clustered separately from both the HFD controls and the chow diet groups (Fig. 4A). Compared to the HFD controls at the phylum level, the H. pylori-infected group feeding HFD had a significantly increased proportion of sequences assigned to Firmicutes and Proteobacteria, whereas reads assigned to Bacteroidetes and Verrucomicrobia were relatively reduced (Fig. 4B). To identify bacterial taxa that were significantly differentiated between H. pylori-infected and non-infected groups feeding HFD, a metagenomic biomarker discovery approach (LEfSe) was used. We found that Desulfovibrionaceae and Mucispirillum spp. sequences were significantly enriched in the HFD-fed H. pylori infected group, as were sequences from the families Helicobacter, Lachnospiraceae, and Ruminococcaceae, while Akkermansia spp., Prevotella spp., and Bifidobacterium spp. were more abundant in HFD controls (Fig. 4C). Given that intestinal dysbiosis in HFD-fed animals may affect gut permeability and subsequently lead to systematic inflammation and IR(Tran et al., 2015), we next examined the expression of the tight junction components including occludin, zonula occludens-1 (ZO-1) and claudin-1 in the intestine. Expression of these proteins was significantly reduced in H. pylori-infected HFD groups, indicating an aberration of gut barrier function (Fig. 4D). However, at 24 weeks the principal coordinate analysis (PCoA) scores clearly separated the HFD groups from the normal diet groups regardless of H. pylori infection status, indicating the dominant role of HFD in modulating gut microbiota after long-term treatment (Fig. 4E and F). Consistent with the alterations in the gut microbiota, the insulin sensitivity was comparable between H. pylori-infected mice and non-infected HFD-fed mice at 24 weeks but was significantly different from the chow diet groups. These results suggest that H. pylori-associated IR after early HFD is closely correlated with the specific modifications of gut microbiota.
Fig. 4.
The interaction of H. pylori infection and HFD induces the alteration of gut microbiota which is associated with IR. Microbiota composition was analyzed in faeces of H. pylori-infected and non-infected mice fed either chow or HFD (n = 5 for each group). (A) Plots shown were generated using the weighted version of the UniFrac-based Principal coordinate analysis (PCoA) after 12 weeks. Samples from H. pylori-infected mice fed HFD included in the red circle clustered distinctly from those of HFD-fed controls embraced in the blue circle. (B) Bacterial taxonomic profiling at the phylum level of gut bacteria from different groups after 12 weeks. (C) Linear discriminant analysis (LDA) coupled with effect size measurements identifies the most differentially abundant taxa between HFD-fed mice with H. pylori infection and HFD controls after 12 weeks. HFD controls-enriched taxa are indicated with a negative LDA score (red), and taxa enriched in H. pylori-infected HFD group have a positive score (green). Only taxa meeting an LDA significant threshold of > 2 are shown. (D) The jejunum tissues of mice were analyzed for the expression of intestinal tight junction proteins including ZO-1, occludin and claudin-1 after 12 weeks. The PCoA (E) and unweighted pair group method with arithmetic mean (UPGMA) (F) analysis showed the alterations of microbial community after 24 weeks. Samples from the HFD group regardless of H. pylori infection status clustered together in the red circle which are different from those given chow diet in the blue circle.
4. Discussion
After the first prospective cohort study demonstrated that H. pylori infection resulted in an increased rate of incident diabetes, following studies also showed that H. pylori infection was significantly associated with higher levels of HbA1c which is a valid and reliable biomarker for long-term blood glucose level in humans (Chen and Blaser, 2012, Hsieh et al., 2013, Jeon et al., 2012). Moreover, a recent randomized double-blind placebo-controlled trial demonstrated that H. pylori eradication improves glucose homeostasis in patients with type 2 diabetes by decreasing HOMA-IR and fasting insulin levels (Bonfigli et al., 2016). In a previous study, we also found in Mongolian gerbils that long-term H. pylori infection induced increased levels of HbA1c, but its effect on glucose tolerance and the underlying mechanism remains unclear (Yang et al., 2015). Despite the positive association between H. pylori and insulin resistance reported in some researches, there are opposite perspectives denying their relationship (Upala and Sanguankeo, 2016). Thus more investigation is needed to determine whether there are some other factors involving in the association between H. pylori and insulin resistance which could account for the discrepancies.
Given the increasing intake of high-fat and high-calorie diets in the post-industrialization era and the high prevalence of H. pylori infection at a global level, we therefore investigated the impact of H. pylori infection in combination with HFD on body insulin sensitivity and subsequent predisposition to metabolic disorders. Our findings of increased visceral fat and larger waist circumference in H. pylori-infected mice after 12 weeks HFD, which almost reached the level of 24 weeks of HFD, suggest that H. pylori infection may accelerate HFD-induced fat redistribution and the emergence of central obesity. We also demonstrated that H. pylori infection aggravated HFD-induced IR, and this effect occurred at the early stage. These results are consistent with previous observation in humans indicating that the role of H. pylori in impaired glucose tolerance may be potentiated by higher body mass index level (Chen and Blaser, 2012). In the present study, we showed that the expressions of p-IRS1 (Tyr896) and the downstream protein p-Akt (Ser473) in the liver of mice with H. pylori infection were decreased after early HFD administration. The changes in phosphorylation of insulin signaling proteins could directly lead to IR(Xu et al., 2014). After 24 weeks of HFD, we found that the IR of the control group was much higher than that of 12 weeks and almost come up with the H. pylori-infected groups. These results suggest that diet itself exerts a strong effect on glucose dysregulation that seems greater than H. pylori infection after long-term HFD.
Recently, the gut microbiota has emerged as an essential modulator that influences host metabolism and has been suggested to play a vital role in metabolic diseases. H. pylori, an ancient gastric pathogen, has been reported to interact with other gastric microbiota whereas the literature regarding its association with gut microbiota is rare (He et al., 2016). Our study demonstrated that there is an interaction of H. pylori, diet and gut microbiota, and the adverse effect of H. pylori on IR is associated with gut microbiota. Using 16S rRNA gene sequence analysis, we showed that H. pylori induced a dramatic shift in the gut microbiota of mice feeding HFD by increasing the proportion of Firmicutes and decreasing the proportion of Bacteroidetes. This phylum-wide pattern of compositional reshape in the microbial community has been thought to be a typical characteristic of obesity-driven dysbiosis as shown in both overweight humans and animals (Cotillard et al., 2013, Mozes et al., 2008). Additionally, H. pylori infection with HFD induced a decreased percentage of Verrucomicrobia, which was mostly contributed by a significant reduction of Akkermansia spp. Akkermansia within the mucus layer has been implicated in regulating the gut barrier function and protecting the host from obesity-linked metabolic syndrome (Everard et al., 2013, Roopchand et al., 2015). Oral administration of this bacterium (i.e., as a probiotic) has been reported to reverse HFD-induced metabolic disorders and also mimic the antidiabetic effects of metformin in diabetic mice (Everard et al., 2013, Shin et al., 2014). In contrast, several bacterial families like Desulfovibrionaceae, Ruminococcaceae and Lachnospiraceae were enriched, which is consistent with prior reports that similar alterations of the microbial communities are positively correlated with the development of metabolic diseases (Kameyama and Itoh, 2014, Kim et al., 2012, Zhang et al., 2010). Identified as the potentially important endotoxin producers, members in Desulfovibrionaceae are capable of reducing sulphate to H2S and damaging the gut barrier (Zhang et al., 2010). Our results showed that H. pylori infection in combination with HFD decreased the expression of tight junction proteins in the intestine, and the impairment of intestinal integrity may in turn facilitate the release of endotoxin into the bloodstream and therefore promote metabolic inflammation and IR (Cani et al., 2008). After 24 weeks, the microbiota community from HFD-fed mice with H. pylori infection clustered together with the HFD controls, both of which were separated from the normal diet groups. Thus, we speculate that there is a close correlation between gut microbiota and metabolic phenotype and changes in several key microbiota populations after H. pylori infection play a critical role in the aggravation of IR.
Although the serum levels of some inflammatory cytokines such as TNF-α were not elevated in H. pylori-infected HFD mice, the increased abundance of Desulfovibrionaceae which can produce endotoxins and the compromised intestinal barrier cast some doubt on the role of inflammation. In addition to inflammation hypothesis, increasing evidence indicates that H. pylori infection can influence the production of metabolic hormones involved in energy homeostasis both in human and animal studies, which is another potential mechanism for H. pylori-associated insulin resistance (Boltin and Niv, 2012, Khosravi et al., 2015, Roper et al., 2008). In our study, we also found remarkable changes of metabolic hormones after H. pylori infection given HFD. Both GIP and resistin which have been reported to play a cardinal role in IR and associated metabolic disturbances were observed to increase significantly in H. pylori-infected mice with HFD at the early stage (Gault et al., 2005, Muse et al., 2004). Moreover, the HFD itself induced dramatic elevation of circulating resistin after long-term treatment, and thus no statistical difference was observed between H. pylori-infected and non-infected groups. The changes of resistin are consistent with the alteration in body IR, which indicates the role of metabolic hormones in H. pylori-associated IR.
Both H. pylori strains used in this study are observed to promote HFD-induced metabolic disorders, although the degree of their pathogenicity is not identical. The discrepancies in the association of H. pylori and IR in epidemiological studies are likely due to the infection of different strains as well as diet consumption. Further experiments using germ-free animals are highly warranted to elucidate the causal relationship between gut microbiota and H. pylori-associated IR in the presence of HFD.
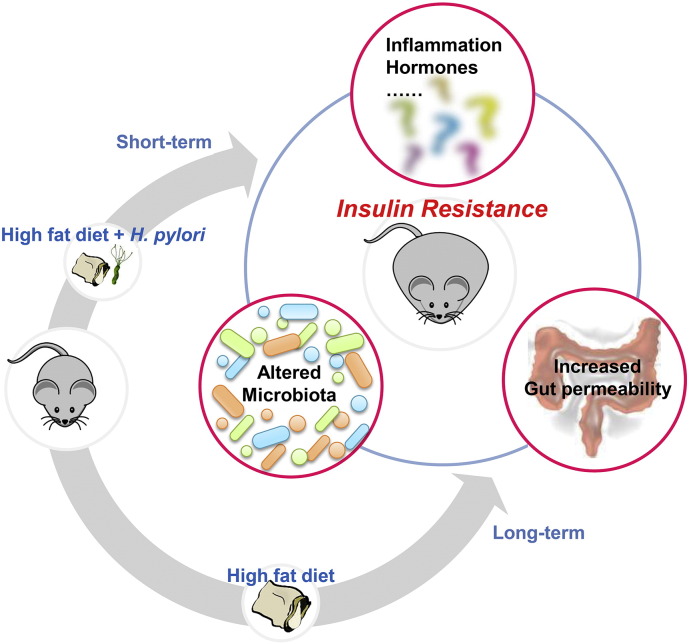
In conclusion, we found that H. pylori infection promoted HFD-induced central obesity and IR at the early stage, the extent of which is close to the effect of long-term HFD. Meanwhile, we observed a dynamic alteration in the gut microbiota that is consistent with the changes of metabolic phenotype (Supplement Fig. 4). Thus, there may be an interaction among H. pylori, diet and gut microbiota, which dysregulates host metabolic homeostasis and the treatment of H. pylori may be beneficial to the patients with impaired glucose tolerance in addition to diet control.
Supplement Fig. 4.
H. pylori infection aggravates HFD-induced IR in association with the gut microbiota. H. pylori-infected mice after short-term HFD displayed more severe metabolic syndrome compared to the HFD controls, the extent of which was comparable to the effect of long-term HFD. Meanwhile, there is a dynamic alteration of gut microbiota that is consistent with the overall changes of metabolic phenotype. We speculate that there may be an interaction of H. pylori, diet and gut microbiota which accelerates the development of IR and the possible mechanisms include impaired intestinal integrity, metabolic inflammation and the abnormal secretion of metabolic hormones.
The following are the supplementary data related to this article.
Funding Sources
This work was supported in part by grants from the National Natural Science Foundation of China (81470832) and the Graduate Innovation Fund of Jiangxi Province (YC2014-B021).
Conflicts of Interest
No author had any financial or personal relationships that could inappropriately influence or bias this work.
Author Contributions
Cong He and Zhen Yang were involved in all aspects of the study. Dandan Cheng, Chuan Xie and Yin Zhu were involved in acquisition, analysis and interpretation of data. Zhongming Ge and Zhijun Luo were involved in revision of the manuscript. Nonghua Lu was involved in study concept, design and supervision.
Acknowledgments
We thank Prof. JZ Zhang at the National Institute for Communicable Diseases and Prevention of Chinese Center for Disease Control and Prevention for providing the H. pylori type strains SS1 and NCTC11637.
References
- Boltin D., Niv Y. Ghrelin, Helicobacter pylori and body mass: is there an association? Isr. Med. Assoc. J. 2012;14:130–132. [PubMed] [Google Scholar]
- Bonfigli A.R., Boemi M., Festa R., Bonazzi P., Brandoni G., Spazzafumo L., Olivieri F., Ceriello A., Genovese S., Testa R. Randomized, double-blind, placebo-controlled trial to evaluate the effect of Helicobacter pylori eradication on glucose homeostasis in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2016 doi: 10.1016/j.numecd.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in HIGh-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Huntley J., Fierer N., Owens S.M., Betley J., Fraser L., Bauer M. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Blaser M.J. Association between gastric helicobacter pylori colonization and Glycated hemoglobin levels. J. Infect. Dis. 2012;205:1195–1202. doi: 10.1093/infdis/jis106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.W., Chien C.Y., Yang K.J., Kuo S.F., Chen C.H., Chien R.N. Helicobacter pylori infection increases insulin resistance and metabolic syndrome in residents younger than 50 years old: a community-based study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.P., Hung H.F., Chen M.K., Lai H.H., Hsu W.F., Huang K.C., Yang K.C. Helicobacter pylori infection is positively associated with metabolic syndrome in Taiwanese adults: a cross-sectional study. Helicobacter. 2015;20:184–191. doi: 10.1111/hel.12190. [DOI] [PubMed] [Google Scholar]
- Cotillard A., Kennedy S.P., Kong L.C., Prifti E., Pons N., Le Chatelier E., Almeida M., Quinquis B., Levenez F., Galleron N. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadrosh D.W., Ma B., Gajer P., Sengamalay N., Ott S., Brotman R.M., Ravel J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2:6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault V.A., Irwin N., Green B.D., McCluskey J.T., Greer B., Bailey C.J., Harriott P., O'Harte F.P., Flatt P.R. Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes. 2005;54:2436–2446. doi: 10.2337/diabetes.54.8.2436. [DOI] [PubMed] [Google Scholar]
- Goh K.L., Chan W.K., Shiota S., Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16(Suppl 1):1–9. doi: 10.1111/j.1523-5378.2011.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Yang Z., Lu N.H. Helicobacter pylori-an infectious risk factor for atherosclerosis? J. Atheroscler. Thromb. 2014;21:1229–1242. doi: 10.5551/jat.25775. [DOI] [PubMed] [Google Scholar]
- He C., Yang Z., Lu N.H. Helicobacter pylori infection and diabetes: is it a myth or fact? World J. Gastroenterol. 2014;20:4607–4617. doi: 10.3748/wjg.v20.i16.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Yang Z., Lu N. Imbalance of Gastrointestinal Microbiota in the Pathogenesis of Helicobacter pylori-Associated Diseases. Helicobacter. 2016 doi: 10.1111/hel.12297. [DOI] [PubMed] [Google Scholar]
- Hsieh M.C., Wang S.S., Hsieh Y.T., Kuo F.C., Soon M.S., Wu D.C. Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur. J. Clin. Investig. 2013;43:949–956. doi: 10.1111/eci.12124. [DOI] [PubMed] [Google Scholar]
- Jeon C.Y., Haan M.N., Cheng C., Clayton E.R., Mayeda E.R., Miller J.W., Aiello A.E. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520–525. doi: 10.2337/dc11-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K., Itoh K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes and environments/JSME. 2014;29:427–430. doi: 10.1264/jsme2.ME14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi Y., Seow S.W., Amoyo A.A., Chiow K.H., Tan T.L., Wong W.Y., Poh Q.H., Sentosa I.M., Bunte R.M., Pettersson S. Helicobacter pylori infection can affect energy modulating hormones and body weight in germ free mice. Sci. Rep. 2015;5:8731. doi: 10.1038/srep08731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.A., Gu W., Lee I.A., Joh E.H., Kim D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knigge A., Kloting N., Schon M.R., Dietrich A., Fasshauer M., Gartner D., Lohmann T., Dressler M., Stumvoll M., Kovacs P. ADCY5 gene expression in adipose tissue is related to obesity in men and mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Magoc T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes S., Bujnakova D., Sefcikova Z., Kmet V. Developmental changes of gut microflora and enzyme activity in rat pups exposed to fat-rich diet. Obesity. 2008;16:2610–2615. doi: 10.1038/oby.2008.435. [DOI] [PubMed] [Google Scholar]
- Muse E.D., Obici S., Bhanot S., Monia B.P., McKay R.A., Rajala M.W., Scherer P.E., Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J. Clin. Invest. 2004;114:232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G., Gambino R., Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33:2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owyang C., Wu G.D. The gut microbiome in health and disease. Gastroenterology. 2014;146:1433–1436. doi: 10.1053/j.gastro.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Roopchand D.E., Carmody R.N., Kuhn P., Moskal K., Rojas-Silva P., Turnbaugh P.J., Raskin I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high fat diet-induced metabolic syndrome. Diabetes. 2015 doi: 10.2337/db14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper J., Francois F., Shue P.L., Mourad M.S., Pei Z., Olivares de Perez A.Z., Perez-Perez G.I., Tseng C.H., Blaser M.J. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. J. Clin. Endocrinol. Metab. 2008;93:2350–2357. doi: 10.1210/jc.2007-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N.R., Lee J.C., Lee H.Y., Kim M.S., Whon T.W., Lee M.S., Bae J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- Tran C.D., Grice D.M., Wade B., Kerr C.A., Bauer D.C., Li D., Hannan G.N. Gut permeability, its interaction with gut microflora and effects on metabolic health are mediated by the lymphatics system, liver and bile acid. Future Microbiol. 2015;10:1339–1353. doi: 10.2217/FMB.15.54. [DOI] [PubMed] [Google Scholar]
- Upala S., Sanguankeo A. Association between Helicobacter pylori infection and insulin resistance: a meta-analysis. Diabetes Metab. Res. Rev. 2016;32:176–177. doi: 10.1002/dmrr.2733. [DOI] [PubMed] [Google Scholar]
- Xu E., Schwab M., Marette A. Role of protein tyrosine phosphatases in the modulation of insulin signaling and their implication in the pathogenesis of obesity-linked insulin resistance. Rev. Endocr. Metab. Disord. 2014;15:79–97. doi: 10.1007/s11154-013-9282-4. [DOI] [PubMed] [Google Scholar]
- Yang Z., Li W., He C., Xie C., Zhu Y., Lu N.H. Potential effect of chronic helicobacter pylori infection on glucose metabolism of Mongolian gerbils. World J. Gastroenterol. 2015;21:12593–12604. doi: 10.3748/wjg.v21.i44.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.L., Yan Liu D., Ma L.Q., Luo Z.D., Cao T.B., Zhong J., Yan Z.C., Wang L.J., Zhao Z.G., Zhu S.J. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhang M., Wang S., Han R., Cao Y., Hua W., Mao Y., Zhang X., Pang X., Wei C. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]