Abstract
Dedifferentiation of follicular cells is a central event in resistance to radioactive iodine and patient mortality in papillary thyroid carcinoma (PTC). We reveal that platelet derived growth factor receptor alpha (PDGFRα) specifically drives dedifferentiation in PTC by disrupting the transcriptional activity of thyroid transcription factor-1 (TTF1). PDGFRα activation dephosphorylates TTF1 consequently shifting the localization of this transcription factor from the nucleus to the cytoplasm. TTF1 is required for follicular cell development and disrupting its function abrogates thyroglobulin production and sodium iodide transport. PDGFRα also promotes a more invasive and migratory cell phenotype with a dramatic increase in xenograft tumor formation. In patient tumors we confirm that nuclear TTF1 expression is inversely proportional to PDGFRα levels. Patients exhibiting PDGFRα at time of diagnosis are three times more likely to exhibit nodal metastases and are 18 times more likely to recur within 5 years than those patients lacking PDGFRα expression. Moreover, high levels of PDGFRα and low levels of nuclear TTF1 predict resistance to radioactive iodine therapy. We demonstrate in SCID xenografts that focused PDGFRα blockade restores iodide transport and decreases tumor burden by > 50%. Focused PDGFRα inhibitors, combined with radioactive iodine, represent an additional avenue for treating patients with aggressive variants of PTC.
Keywords: Platelet derived growth factor receptor, Metastases, Papillary thyroid cancer, TTF1(Nkx2-1)
Graphical Abstract
Highlights
-
•
PDGFRα induces dedifferentiation of papillary thyroid cancer cells.
-
•
This depends on decreased phosphorylation and decreased nuclear targeting of TTF1.
-
•
Loss of nuclear TTF1 decreases thyroglobulin production and NaI transport.
-
•
PDGFRα expression is prognostic of PTC recurrence and treatment resistance.
-
•
Blocking PDGFRα activation is a potential therapeutic target.
Treatment of papillary thyroid cancer historically relied upon a combination of surgery and radioactive iodine ablation with few alternatives if the disease progresses. We found that platelet derived growth factor receptor alpha (PDGFRα) is a key driver of metastatic disease and resistance to radioactive iodine therapy. PDGFRα expression can be tested in tumor specimens to predict aggressive disease. In addition, we show that targeting PDGFRα could restore sensitivity to radioactive iodine treatment that might slow disease growth and spread.
1. Introduction
Deaths from papillary thyroid cancer, in excess of 30,000/year worldwide, are typically preceded by dedifferentiation and resistance to radioactive iodine treatment (Ferlay et al., 2013). Thyroid development and follicular cell function is defined by co-expression of the transcription factors, TTF1 (Nkx2-1) and Pax8, but how these transcription factors are regulated in thyroid malignant disease is unclear (Antonica et al., 2012). Follicular cell dedifferentiation, with disrupted thyroglobulin synthesis and NaI symporter (NIS) function, is considered central to poor outcomes in PTC (Grogan et al., 2013, Lundgren et al., 2006, Xing, 2013, Ke et al., 2013). Patients with aggressive PTC variants often require multiple doses of radioactive iodine or repeated surgeries to address metastatic disease (Dadu and Cabanillas, 2013, Schneider et al., 2013, Haugen et al., 2016, Randolph et al., 2012). The Cancer Genome Atlas project defines PTC as an ERK-driven cancer, but the differentiation status of tumors is complex and the regulation of TTF1 and Pax8 defies individual assessments of BRAF or RAS gene mutations (Cancer Genome Atlas Research Network, 2014). Treatments including resveratrol, rapamycin, and retinoic acid have been examined for their ability to slow tumor growth or induce differentiation (Liu et al., 2007, Kogai et al., 2008, Vivaldi et al., 2009, Fernandez et al., 2009, Hou et al., 2010, Zhang et al., 2011, Oh et al., 2011, Malehmir et al., 2012, Coelho et al., 2011, Sherman et al., 2013, Yu et al., 2013, Giuliani et al., 2014, Plantinga et al., 2014). As yet, the benchtop results are conflicting and selective changes in NIS protein expression and iodide uptake in many of these studies have failed to translate into clinically relevant and durable responses in radioactive iodine therapy.
The ongoing efforts to identify targeted therapy for dedifferentiated, metastatic PTC has led to empirically driven clinical trials of different tyrosine kinase receptor inhibitors that disrupt MEK, VEGFR, FGFR and other signaling pathways. These therapies were intended to slow disease progression, and/or upregulate sodium iodide symporter (NIS) expression and restore radioactive iodine sensitivity (Gupta-Abramson et al., 2008, Carr et al., 2010, Bible et al., 2010, Schneider et al., 2012, Ho et al., 2013, Schlumberger et al., 2015). The most recent and largest studies, including those with selumetinib, lenvatinib, and sorafenib, demonstrated varying objective response rates and exhibited significant toxicity (Ferrari et al., 2015). The mixed outcomes in clinical trials, combined with significant side-effect profiles and transient effectiveness, has limited the widespread application of these and other tyrosine kinase receptor inhibitors in the treatment of advanced disease (Gild et al., 2011, Klein Hesselink et al., 2015).
To identify factors that drive thyroid dedifferentiation, we assessed PTC primary tumors, metastatic specimens, primary cell cultures and cell lines as a function of TTF1 and Pax8 expression. We discovered that disrupted nuclear TTF1 targeting is characteristic of dedifferentiated PTC and that PDGFRα is central regulator of thyroid follicular cell dedifferentiation and disease progression in PTC. PDGFRα, but not its isoform PDGFRβ, specifically downregulates TTF1 nuclear expression disrupting iodide transport and thyroglobulin production in follicular cells as well as potentiating tumor growth in vivo. Clinically, PDGFRα expression is strongly associated with metastatic disease and is a marker for disease recurrence as well as resistance to radioactive iodine therapy in PTC. These results provide a strong rationale for the use of PDGFRα blockade as a therapy to disrupt metastatic PTC tumor growth as well as to restore differentiation and sensitivity to radioactive iodine. This focused approach for patients with aggressive variants of PTC may provide equal or better outcomes with minimal toxicity compared to current trials using multi-kinase inhibitors.
2. Materials and Methods
Additional details are in the Supplementary Materials and Methods.
2.1. Patient Specimens
Ethics approval was obtained through the University of Alberta Heath Research Ethics Board ID Pro00018758 (Supplementary Materials and Methods). A total of 287 patient specimens were selected with thyroid tumors of which 181 are papillary thyroid carcinomas (113 without and 68 with lymphatic metastases), 57 are benign follicular neoplasms and there are 36 normal thyroid tissue specimens and 13 section of metastatic lymph nodes. Clinical data was obtained from a prospective database maintained at the Cross Cancer Institute tracking disease from 2002 onwards. Recurrence is defined as an increase in unstimulated thyroglobulin levels of > 0.4 ng/mL, stimulated thyroglobulin levels > 2 ng/mL, and/or pathologic evidence of recurrence based on ultrasound-guided fine needle aspiration biopsy. Radiation dose records obtained directly from the radioisotope records maintained at the Cross Cancer Institute.
2.2. Isolation of Primary Thyroid Cancer Cell
Primary thyroid cancer cells were obtained using the Cancer Cell Isolation Kit (Panomics, Inc., Fremont, CA, USA). Tissue was minced to small pieces under aseptic conditions, digested for 2 h with gentle mixing at 37 °C and cancer cells were purified following manufacturer's protocol. Isolated primary cancer cells were cultured in DMEM/F12 medium supplemented with 10% FBS and 6H (10 mU/mL TSH, 0.01 mg/mL insulin, 10 nM hydrocortisone, 5 μg/mL transferring, 10 ng/mL somatostatin and 10 ng/mL glycyl-l-histidyl-l-lysine).
2.3. Short Hairpin (shRNA) Stable Transductions
To selectively and stably knock down the expression of PDGFRα in the TPC1 and 8305C cell lines we used the HuSH-29 shRNA Vector system (HuSH-29 shRNA Retroviral Vector Systems; OriGene Technologies, Inc.). Briefly, PTC cells were transduced with the pRS shRNA retrovirus system (Puro +) followed by selection in puromycin (2.5 μg/mL). Resistant cells were assessed by western blot to select the sequences that produced the highest levels of protein expression knock-down. To stably knock down the PDGFRβ receptor, cells were transduced with the pGFP-BR-S shRNA retrovirus system (BSD +) followed by selection in blasticidin (500 μg/mL). Resistant cells were again assessed by western blot to select the sequences that produced the highest levels of protein expression knock-down.
2.4. Gene Transfer
To express human PDGFRα complementary DNA, we used a doxycycline-inducible retrovirus system (Lenti-X Lentiviral Expression Systems; Clontech Laboratories, Inc., Mountain View, CA, USA). Briefly, PTC cells were first transduced with the LVX-Tet-On advanced lentivirus (Neo +) followed by selection in G418 (1.0 mg/mL). Resistant cells were then transduced with the LVX-Tight-Puro (Puro +) vector or sequence-verified derivatives expressing wild-type human PDGFRα complementary DNA, followed by selection in puromycin (2.5 μg/mL). Complementary DNA expression was induced by addition of doxycycline (2 μg/mL). To express PDGFRα in the rat cell line FRTL5, the human cDNA sequence was inserted into pLenti-C-mTagGFP (SgfI/MluI) following transduction with lentiviral particles cells were sorted by flow cytometry and the GFP positive population was cultured.
2.5. Wound Healing, Clonogenic, Transwell Invasion and Proliferation Assays
Cytoselect™ 24-well cell invasion basement membrane assay kit (Cell Biolabs, San Diego, CA, USA) was used to measure the invasive properties of the cells. Briefly, the stable TPC1, 8305C and BCPAP cell lines were seeded at a density of 3 × 105 cells/well and cultured for 48 h. Invasive cells passed through the basement membrane layer, dissociated using detachment buffer and then quantified by means of CyQuant GR fluorescent dye. Adherent colony formation assays were performed as described. Fifty or 100 cells per well were plated in six-well plates, fed 5% FBS supplemented growth medium and allowed to form colonies for 20 days. Colonies were stained with 0.5% crystal violet solution in 25% methanol and counted. For the wound healing assay, cells were plated in 6 well plates at 80–90% confluence. A wound was created by manually scratching the cell monolayer with a p1000 or p200 pipet tip. Cellular debris was removed by washing the monolayer with PBS and the cells were fed with complete growth medium or serum-free medium. Images and measurements were acquired at times 0, 20 and 44 h after wound creation. To document the effect of PDGFRα or β on proliferation, cultures were incubated in regular or serum-free-medium and enumerated daily for 5 days with an electronic cell counter (Coulter Model Zf). The MTS assay (Promega, Madison, WI, USA) was also performed in 8–16 replicates after 48 and 72 h of growth.
2.6. Mouse Xenograft Models
All experiments were conducted in accordance with the guidelines of the University of Alberta Animal Care and Use Committee (ACUC) and the Canadian Council of Animal Care. BCPAP cells (1 × 106) expressing PDGFRα protein or empty vector (mock) were inoculated subcutaneously (1:1 v/v matrigel–PBS) on the left and right flanks (respectively) of beige SCID mice that received a 0.5 mg slow release doxycycline pellet, subcutaneously 48 h prior to cell inoculation. The stable TPC1 and 8305C cells (1 × 106) were inoculated subcutaneously as described above. Tumor growth was followed and documented and animals were sacrificed once the tumors reached a 1 cm3 size. Crenolanib was purchased from Selleckchem.
2.7. Sodium Iodide Uptake
Ex vivo measurements of sodium iodide transport in normal thyroid tissue as well as papillary thyroid carcinomas were performed both as direct measurement of radioactive iodide uptake and using a colorimetric iodide assay (Waltz et al., 2010, Weiss et al., 1984). Briefly, 50,000 cells/well were seeded on poly-l-Lys or collagen-coated 96-well plates and allowed to attach overnight. The rat cell line FRTL5 was used as positive control for all experiments. Cells were washed twice in iodide uptake buffer (10 mM HEPES/HBSS). After the final wash, 80 μL of uptake buffer was added to all wells and further supplemented with 10 μL of 100 μM NaI solution (uptake wells), 10 μL of 100 μM NaI/450 μM NaClO4 solution (uptake inhibition wells) or 10 μL of uptake buffer (background control wells). Plates were incubated for 1 h at 37 °C, 5% CO2 atmosphere then the solution was completely removed from wells and plates allowed to dry by blotting on paper towel. Water (100 μL) was added to all wells followed by 100 μL of 10.5 mM Ammonium Cerium(IV) Sulfate and 100 μL of 24 mM Sodium Arsenite(III) solution and plates were incubated in the dark for 30–90 min at RT, absorbance readings (420 nm) were taken at 30 min intervals.
2.8. Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections of 4 μm thickness were deparaffinized and rehydrated. Evaluation of immunostaining was performed without knowledge of the clinical outcome and all specimens had representative sections confirming that > 90% of the specimen consisted of papillary thyroid carcinoma. Sample cores on the tissue array that were fragmented or incomplete were not scored. As described in multiple reports, the cytoplasmic staining of PDGFRα and PDGFRβ was assessed for each case, in triplicate, as 3 +, (strong, diffuse), 2 + (strong, focal), 1 + (weak staining), or 0 (minimal staining) (Zhang et al., 2012, Gonzalez-Campora et al., 2011, Barreca et al., 2011).
2.9. Statistics
Data were expressed as the mean ± standard error of mean from a minimum of three independent experiments. Statistical analyses were performed using the two-tailed Student's t-test for unpaired samples, with equal variance. The correlations between protein expression and metastatic status were assessed using Fisher's exact test for tables and Spearman rank correlation for continuous variables. Statistical tests are two-tailed with a P value < 0.05 considered to be statistically significant. Descriptive statistics were used to present the study variables. Mean and standard deviation were reported for the continuous data variables, frequency and percentages were reported for categorical variables. Recurrence free survival (RFS) was calculated from the date of treatment to date of recurrence and the patients who did not recur were considered censored for the analysis. Kaplan-Meier methods were used to time to event data, and the median survival and the corresponding 95% confidence interval were reported. When the median survival was not reached, then the survival probabilities were reported. Log rank tests were used to compare the two survival curves. All statistical analysis was conducted in SPSS version 15. A P-value < 0.05 was used for statistical significance.
3. Results
3.1. Expression of TTF1, but not Pax8, Is Downregulated by PDGFRα in Dedifferentiated, Metastatic PTC
Both TTF1 and Pax8 are necessary and sufficient to generate a differentiated thyroid follicular cell that is capable of thyroglobulin production and sodium iodide transport (Antonica et al., 2012, Mu et al., 2012, Zhang et al., 2006). We assessed validated thyroid cancer cell lines for differentiation as defined by TTF1 and Pax8 and correlated each transcription factor with the expression of tyrosine kinase receptors linked to aggressive thyroid malignancy (Gild et al., 2011, Schweppe et al., 2008). We revealed a strong and specific association of PDGFRα with dedifferentiation and loss of TTF1 expression in these thyroid cancer cell lines (Fig. 1a). Conversely, all the cancer cell lines expressed Pax8, whereas TTF1 was found in only BCPAP and KTC1 cells in which PDGFRα was lacking (Fig. 1a). We confirmed the reciprocal expression of TTF1 and PDGFRα through qPCR analysis. (Supplementary Fig. 1, a and b). Examples of PDGFRβ and TTF1 co-expression were identified, notably in the BCPAP cell line and in primary cultures of normal human thyroid cells. Pax8 protein expression was not influenced by the presence or absence of either the α- or β-subunits of PDGFR. Expression levels of other tyrosine kinase receptors linked to PTC, including FGFR, VEGFR, IGFR, and EGFR, did not reveal variations in thyroid cell differentiation status as defined by TTF1 or Pax8 expression (Supplementary Fig. 1c).
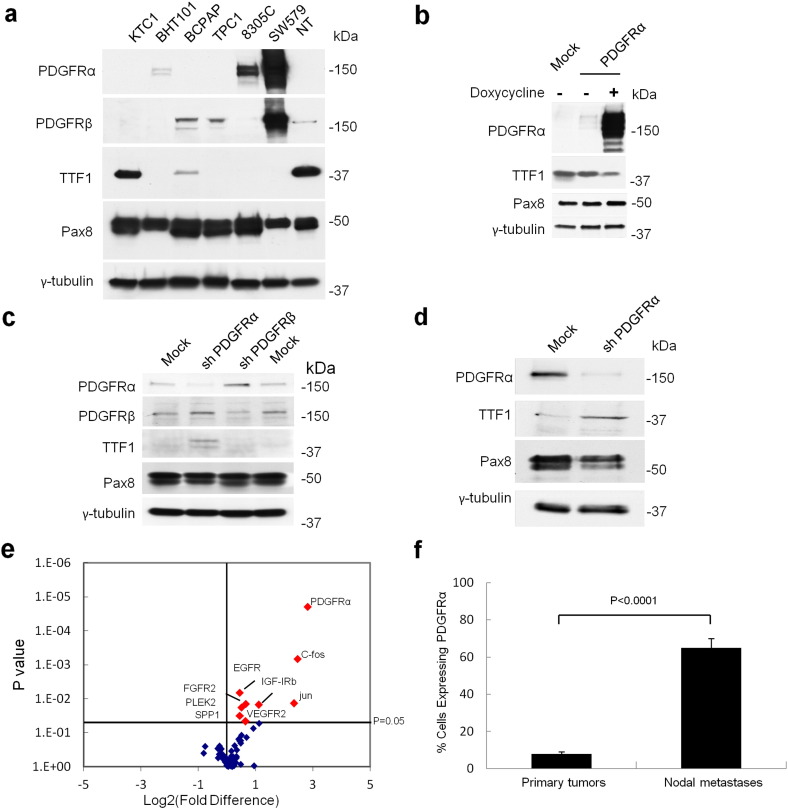
Fig. 1.
PDGFRα expression induces dedifferentiation in thyroid cancer cells by disrupting nuclear TTF1 protein expression. (a) Western blot of thyroid cancer cell lines KTC1, BHT101, BCPAP, TPC1, 8305C, and SW579 demonstrate an inverse relationship between PDGFRα and TTF1 expression. NT: normal thyroid primary culture control. (b) Western blot of BCPAP cells with inducible expression of PDGFRα under a doxycycline promoter disrupts TTF1 expression. BCPAP cells transduced with empty vector as a control (mock). (c) Western blot demonstrating that expression, or selective knockdown, of PDGFRα in TPC1 cells disrupts or restores, TTF1 expression. This effect is not altered by the presence of PDGFRβ. Cells transduced with non-effective shRNA cassette as control (mock). (d) Selective knockdown of PDGFRα in dedifferentiated thyroid cancer cell line 8305C restores TTF1 expression. (e) mRNA screen comparing patient matched primary tumors and metastatic disease. (f) Quantitative analysis using flow cytometry of freshly isolated primary tumors comparing PDGFRα positive cells in primary tumors lacking metastases (n = 4) with proven metastatic disease (n = 6), results are means ± SEM.
To test our hypothesis that expression of PDGFRα disrupts the expression of TTF1, we generated pooled populations of stable homo- and heterodimers of PDGFRα and PDGFRβ subunits using verified PTC cell lines BCPAP (native PDGFRβ), TPC1 (native PDGFRα and PDGFRβ) and 8305C (native PDGFRα). All thyroid cancer cell lines express significant levels of PDGF ligands AA, BB, and DD providing for endogenous signal activation of the receptors (Zhang et al., 2012). Fig. 1, b–d show representative Western blots from the cell lines BCPAP, TPC1, and 8305C in which we demonstrate that PDGFRα protein expression reduced TTF1 protein levels (BCPAP cells). Conversely, disruption of PDGFRα expression increases TTF1 protein levels (TPC1 and 8305C cells). Notably, the presence or forced absence of PDGFRβ (BCPAP and TPC1 cells respectively) had no impact on the expression of TTF1 in these cell lines. Strikingly, the forced expression of PDGFRα in primary cultures derived from benign neoplastic thyroid tissue also markedly decreased TTF1 expression, even with only small amounts of protein (Supplementary Fig. 1d). There is also no relationship between the expression of Pax8 and the level of PDGFRα or PDGFRβ in immortalized cell lines or patient derived primary cultures (see Fig. 1, b–e).
We then determined TTF1, Pax8 and PDGFRα expression in fresh tumor isolates from patients since freezing or fixation of thyroid tumor specimens can induce dramatic variations in nuclear protein isolation (Supplementary Fig. 1e and Supplementary Fig. 2a). Primary tumors lacking clinical evidence of metastases appear to commonly exhibit TTF1, but lack PDGFRα (Supplementary Fig. 1e). By contrast, isolates from metastatic specimens expressed high levels of PDGFRα but minimal TTF1 in almost all cases (P = 0.005) (Supplementary Fig. 1e). These results are consistent with previous studies indicating that PDGFRα gene transcript levels are 30–40 times lower than PDGFRβ in normal thyroid tissue (GTEx Consortium, 2013). Moreover, our own mRNA screen comparing patient matched primary tumors and metastatic disease specimens revealed a dramatic upregulation of PDGFRα at levels much higher than other tyrosine kinase receptors (Fig. 1e). Pax8 protein expression does not appear to vary based on the presence of metastatic disease. Lastly, we reveal using flow cytometry that primary thyroid carcinomas lacking metastases exhibited low levels of PDGFRα but that metastatic specimens sectioned from lymph nodes revealed much higher levels of PDGFRα on the cell surface (P < 0.0001) (Fig. 1f). Overall, PDGFRα is a prominent feature of metastatic disease and it is inversely related to TTF1 expression in both cell lines and clinical specimens.
3.2. PDGFRα Expression Creates a Dedifferentiated Phenotype by Disrupting Nuclear Localization of TTF1
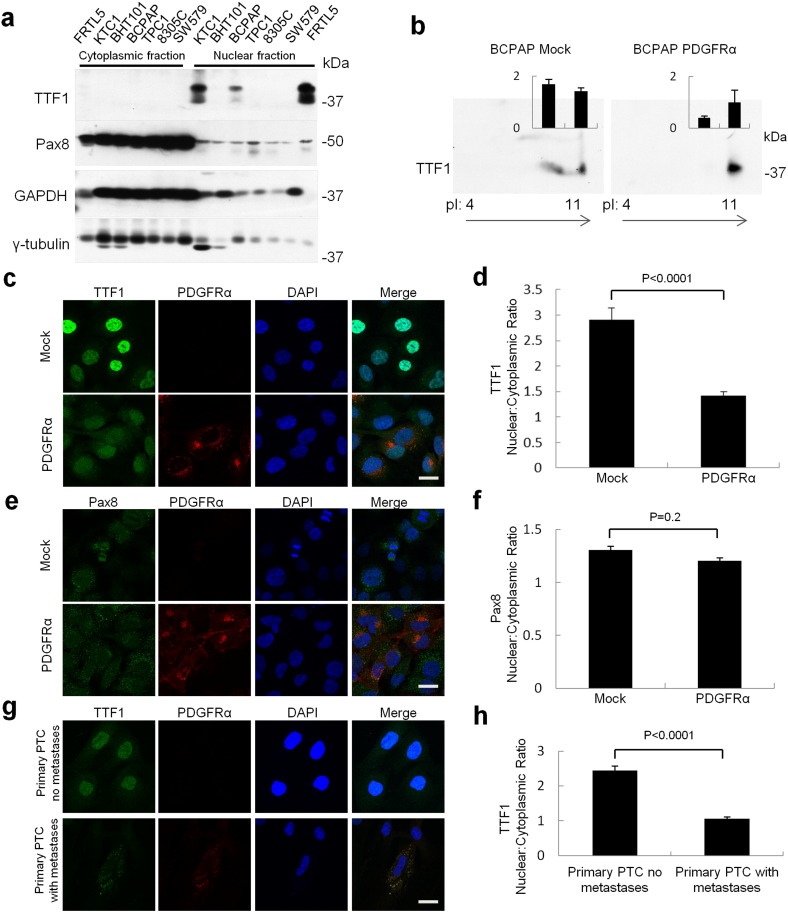
TTF1 protein levels are clearly diminished in the presence of PDGFRα. However, TTF1 mRNA levels decreased only marginally when assessed with, and without, exogenous ligand to maximize PDGFRα activation and under varying growth conditions (Supplementary Fig. 2b). The same is true for Pax8 mRNA levels (Supplementary Fig. 2c). Given that nuclear localization of TTF1 and Pax8 is required for function, we examined cytosolic and nuclear cell fractions for the abundance of each transcription factor (Antonica et al., 2012, Zannini et al., 1996). Pax8 was found in both the cytoplasm and nucleus in the thyroid cancer cell lines whereas TTF1, when expressed, was almost exclusively localized to the nuclear cellular fractions (Fig. 2a). Given the similar mRNA levels of TTF1 with and without PDGFRα expression, we then examined the phosphorylation status of TTF1 in BCPAP cells. We hypothesized that nuclear targeting of TTF1 may be altered by variations in phosphorylation since previous studies indicated that TTF1 transcriptional activity varied with phosphorylation but not DNA-binding activity (Zannini et al., 1996, Missero et al., 2000). Using 2D electrophoresis we consistently observed two populations of TTF1 in the absence of PDGFRα (Fig. 2b). With expression of PDGFRα we observed > 80% reduction of the second, more acidic population consistent with decreased TTF1 phosphorylation. The PDGFRα-induced dephosphorylation of TTF1 was possibly driving decreased nuclear targeting and functional impairment of TTF1 so we quantified the nuclear:cytoplasmic ratios of TTF1 and Pax8 in the BCPAP cell line using confocal microscopy. There was a significant cytoplasmic shift in TTF1 protein localization with PDGFRα expression (Fig. 2, c and d). The expression of PDGFRα did not significantly impact the localization or relative expression levels of Pax8 in the BCPAP cell line (Fig. 2, e and f). We also confirmed that there was a shift in TTF1 from the nucleus to the cytoplasm with the expression of PDGFRα in primary cultures of clinical specimens of metastatic PTC (Fig. 2, g and h). Lastly, we were able to increase nuclear targeting of TTF1 in both mock and PDGFRα + BCPAP cells using phosphatase inhibitors, as shown in Supplementary Fig. 2d. Our results are consistent with recent work outlining the key role of nuclear TTF1 in folliculogenesis (Silberschmidt et al., 2011) and we demonstrate the role of phosphorylation in subcellular targeting of TTF1.
Fig. 2.
PDGFRα expression decreases TTF1, but not Pax8, expression and nuclear localization. (a) Western blot of cytoplasmic and nuclear protein extracts of the papillary thyroid carcinoma cell lines examined in this study. (b) 2D electrophoresis pattern of TTF1 as measured in BCPAP mock and PDGFRα expressing cells. The spot intensity for the two most prominent protein spots (relative to the single spot to the far left in PDGFRα expressing cells) is shown as mean ± SEM of four independent runs. (c) Confocal microscopy reveals cytoplasmic shift of TTF1 localization through comparison of BCPAP mock and BCPAP transduced with inducible PDGFRα. Scale bar 25 μm. (d) Confocal microscopy was used to quantify the change in the nuclear:cytoplasmic ratio of TTF1 localization through comparison of BCPAP mock and BCPAP transduced with inducible PDGFRα. (e) Expression of PDGFRα protein does not have a significant impact on Pax8 localization as shown by confocal microscopy. Scale bar 25 μm. (f) Confocal microscopy reveals no change in the nuclear:cytoplasmic ratio of Pax8 comparing BCPAP mock and BCPAP PDGFRα cells. (g) PTC primary cultures with and without metastases demonstrate the cytoplasmic localization of TTF1 with PDGFRα expression and this is quantified in (h). Scale bar 25 μm. Data are presented as mean ± SEM, n = 10–25 individual cells.
3.3. PDGFRα Drives Profound Changes in Follicular Cell Morphology, Colony Formation, Migration and Invasive Potential
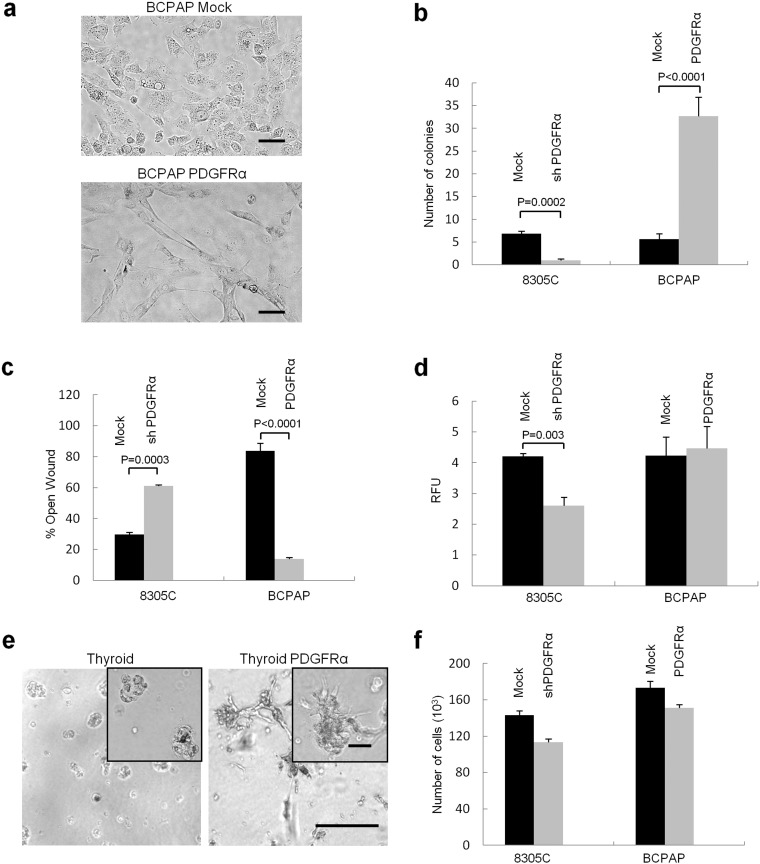
We next assessed surrogate markers of tumorigenic potential including proliferation, colony formation, migration and invasive potential in the cell lines and primary cultures that were manipulated in different ways to selectively express, or repress, PDGFRα. Expression of PDGFRα in the PTC cell line produced dramatic changes in the morphology of the cells grown in two-dimensional culture. In particular, we saw a cellular morphology consistent with dendritic-projections and increased cell surface area as shown for BCPAP (Fig. 3a). These changes in cellular morphology were quantified by cell area (Supplementary Fig. 3a). Expression of PDGFRα increased colony formation nearly 6-fold in BCPAP cells, while colony formation in 8305C cells was inhibited > 5-fold with PDGFRα knock-down (Fig. 3b and Supplementary Fig. 3b). Wound closure rates for BCPAP and 8305C cells were also significantly faster in cells incubated in nutrient rich environment (10% FBS) when PDGFRα was expressed (Fig. 3c). The invasion assay generally confirmed our other functional assessments where selective blockade or disruption of PDGFRα expression generally leads to a less invasive phenotype (Fig. 3d). We also assessed the ability of thyroid cancer tissue isolates, with and without PDGFRα, to generate thyroid spheres as another surrogate of tumorigenic potential. In thyroid follicular cells lacking PDGFRα, we saw small thyrospheres that plateaued in growth after approximately 14 days. However, primary thyroid cultures transfected with PDGFRα demonstrated a growth pattern consistent with invadopodia-like structures as was seen with 3D-culture (Fig. 3e). There were minimal differences in cell growth rates when we altered the expression of the alpha subunit in PTC cell lines (Fig. 3f). The results for TPC1 cells were qualitatively similar to that described above, with or without PDGFRα, as shown in Supplementary Fig. 3, c–e. The PDGFRα subunit also had no effect on the length of the cell cycle phases whether expressed alone or with PDGFRβ (Supplementary Fig. 3f).
Fig. 3.
PDGFRα expression induces a dramatic phenotypic change in PTC cell lines. (a) Two-dimensional culture micrographs demonstrate the significant change in cellular morphology with insertion of the PDGFRα gene into BCPAP cells. Scale bar 50 μm. (b) Colony formation in the cell lines with different PDGFR subunit compositions. n = 6. (c) The wound healing assay with closure of the wound examined at 44 h in three independent experiments. Results were qualitatively and quantitatively similar with (shown) and without mitomycin C to inhibit cell division. n = 8. (d) Invasive potential was studied using the basement membrane cell invasion assay kit. After 48 h incubation, invasive cells were dissociated, lysed, and quantified by CyQuant GR Dye. RFU: relative fluorescence units. n = 8. (e) 3D culture assessment of varying growth patterns between PDGFRα-positive and negative thyroid primary cultures. Scale bars 200 μm, inset 50 μm. (f) Cellular proliferation as quantified by cell count in the PTC cell lines did not reveal significant differences in proliferative potential with the insertion of the PDGFRα subunit. n = 8. Results in B, C, D and F are means ± SEM.
3.4. PDGFRα Expression Decreases Thyroglobulin Production and Sodium Iodide Transport in Tumorigenic and Non-tumorigenic Thyroid Follicular Cells
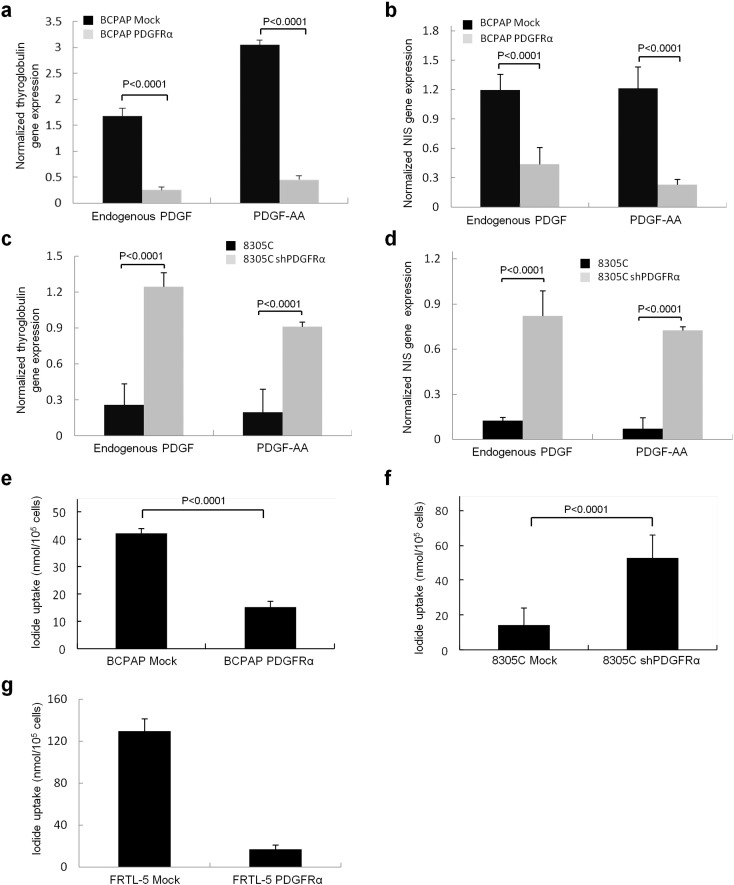
Functional differentiation in thyroid cancer cell lines is typically measured by thyroglobulin production and iodide transport. There was minimum or no detectable mRNA for the NIS or thyroglobulin in the thyroid cancer cell lines BHT101, 8305C, TPC1, SW579, consistent with previous reports (Pilli et al., 2009). However, native BCPAP cells exhibit both TTF1 and Pax8 and there was a correspondingly modest mRNA expression for thyroglobulin and NIS (Fig. 4, a and b). These results are consistent Pax8 and TTF1 being necessary for differentiated thyroid cell function (Antonica et al., 2012). Expressing PDGFRα in BCPAP cells caused a dramatic decrease in mRNA levels for both thyroglobulin and NIS consistent with loss of TTF1 functionality (Fig. 4, a and b). Note that PDGFRα-driven changes in differentiation were not qualitatively altered by the growth conditions or different growth factors as documented by thyroglobulin levels (Supplementary Fig. 4). Conversely, when we knocked down PDGFRα (restoring TTF1 expression) we were able to restore thyroglobulin (Fig. 4c) and NIS (Fig. 4d) mRNA levels in 8305C cells. As expected, corresponding changes in iodide transport were seen with decreased NaI transport in PDGFRα + BCPAP cells (Fig. 4e), whereas PDGFRα knockdown facilitated limited iodide transport in 8305C cells (Fig. 4f). We show that the PDGFRα-mediated disruption of NaI transport is dramatic and virtually complete even in non-tumorigenic cell lines such as in rat FRTL5 cells, which represent the model system by which NaI transport has been defined (Fig. 4g).
Fig. 4.
Follicular cell function is disrupted by PDGFRα protein expression. The effects of PDGFRα expression on the relative mRNA levels in BCPAP cells are shown for (a) thyroglobulin and (b) sodium iodide symporter (NIS). (c) and (d) show relative thyroglobulin and NIS mRNA expression, respectively, in 8305C cells. (e) Dedifferentiation and the consequential effect on sodium iodide transport in BCPAP cells with and without PDGFRα expression. (f) Dedifferentiation and the consequential effect on sodium iodide transport in 8305C cells (mock and shPDGFRα). Results are means ± SEM, n = 6 of three independent experiments. (g) Effect on sodium iodide transport in FRTL5 cells expressing PDGFRα. Results are means ± SEM, n = 8 of 5 independent experiments. Gene expression was normalized to GAPDH expression levels in all experiments.
3.5. PDGFRα Drives Tumorigenesis in SCID Mice Models of PTC
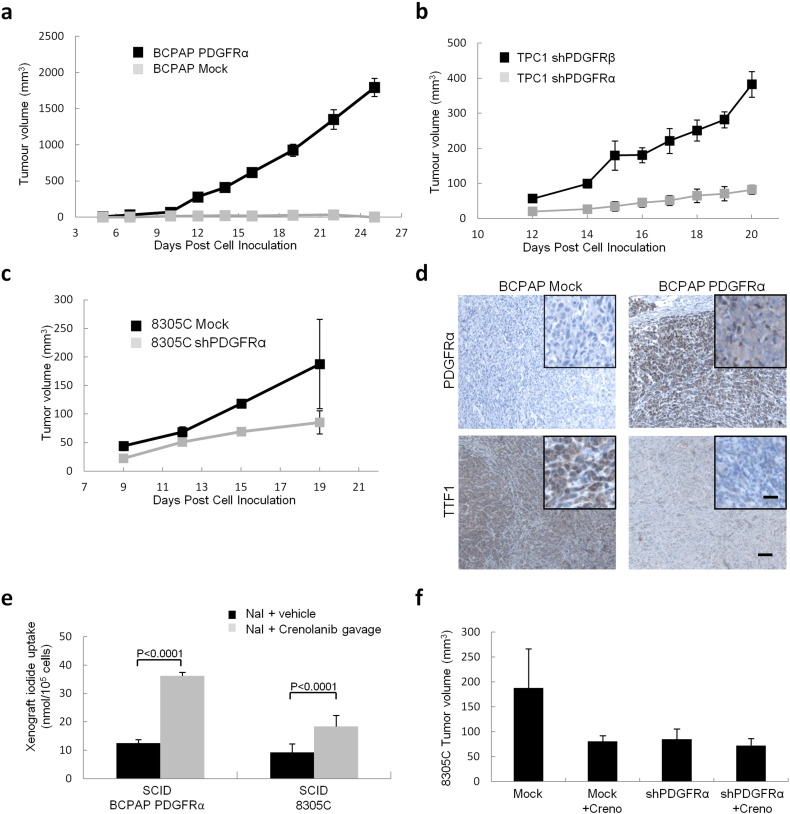
The tumorigenic potential of PDGFRα was assessed by implanting BCPAP cells, with and without PDGFRα expression, into SCID mice. PDGFRα expression was associated with a nearly 10-fold increase in tumor growth (Fig. 5a). The same pattern was seen with 8305C and TPC1 cells where the selective disruption of PDGFRα expression lead to a dramatic decrease in tumorigenic potential but blockade of PDGFRβ did not decrease tumorigenic potential (Fig. 5, b and c). Representative H&E sections for the BCPAP mock cell line tumors and those with PDGFRα cell line are shown in Supplementary Fig. 5a. As predicted the expression of TTF1, but not Pax8, in the tumors is decreased by PDGFRα as shown by Western blot (Supplementary Fig. 5b). Immunohistochemistry confirms the inverse relationship between PDGFRα and TTF1 protein expression in BCPAP mouse xenografts (Fig. 5d). We also demonstrated that iodide transport in BCPAP xenograft cells was significantly decreased when the PDGFRα subunit was expressed in the xenografted cells (Fig. 5e). Moreover, as a model for dedifferentiated tumors, we used native 8305C cells to demonstrate that knocking down PDGFRα or blocking PDGFRα activation with crenolanib significantly decreased tumor burden (Fig. 5f).
Fig. 5.
PDGFRα drives tumorigenesis in vivo. (a) BCPAP-derived tumors, with and without PDGFRα in SCID mice. Five mice per group were inoculated with BCPAP mock or BCPAP PDGFRα derived cell lines in Matrigel, results are means ± SEM. (b) Knockdown of the alpha or beta subunits of PDGFR in TPC1 reveal that blockade of the alpha subunit produces the most dramatic decrease in tumor growth. The average tumor volume is shown until sacrifice at day 20. Results are mean ± SEM. (c) 8305C-derived tumors in SCID mice. Five mice per group were inoculated with 8305C mock or shPDGFRα derived cell lines in Matrigel. Results are means ± SEM. (d) Reciprocal relationship between the pattern of PDGFRα and TTF1 staining in tumor xenografts obtained by injecting BCPAP mock and BCPAP PDGFRα cells into SCID mice. Scale bars 400 μm and 50 μm (inset). (e) Effect on sodium iodide transport of crenolanib treatment (0.8 mg/mL) in the SCID xenograft of BCPAP expressing PDGFRα and 8305C cells. Results are means ± SEM, n = 3 independent ex-vivo isolations (f) 8305C-derived tumors in SCID mice. Five mice per group. Animals were treated daily with vehicle (peanut oil) or crenolanib (8 mg/kg), administered daily at 0.8 mg/mL starting 24 h post inoculation until termination (day 19). Results are means ± SEM, n = 3 vehicle, n = 5 crenolanib.
3.6. PDGFRα Expression Is Associated With Cytoplasmic TTF1 Expression and Nodal Metastases
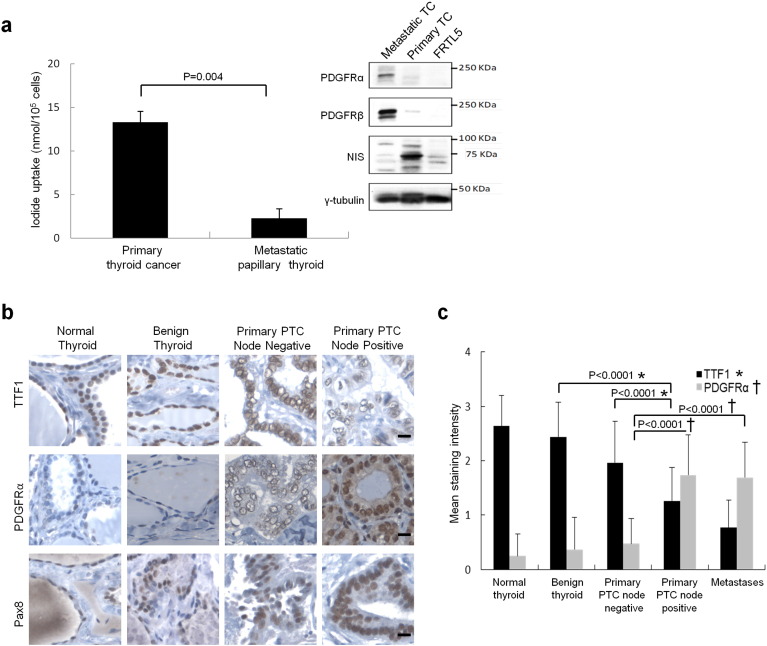
Resistance to radioactive iodine therapy is a central feature of disease recurrence and poor outcomes in thyroid cancer. Assessments of individual freshly isolated PTC specimens revealed that PDGFRα expression by Western blot in metastatic disease was associated with decreased NIS levels and loss of sodium iodide transport (Fig. 6a). This relationship was further explored with tissue arrays comprised of normal thyroid tissue, benign neoplasms, PTC primary tumors with and without lymph node metastases with clinical follow-up data for an average of 7.9 years (range 2.4 years to 11.1 years). Patient age, sex, tumor size and lymph node yields are outlined for each group in Supplementary Table 1.
Fig. 6.
High PDGFRα expression in human metastatic PTC is associated with decreased sodium iodide symporter transport function and follicular cell differentiation. (a) Primary thyroid cultures that were prepared from freshly isolated cells from patients show the inverse relation between iodide transport and PGFRα expression in primary thyroid cancer versus metastatic PTC. Inset: Western blots of corresponding primary thyroid cultures revealing predicted changes in sodium iodide symporter (NIS) protein levels due to expression of PDGFRα, rat FRTL5 thyroid cells are shown for comparison. Results are means ± SEM, n = 6. (b) Representative immunohistochemical staining for TTF1 (top), PDGFRα (middle) and Pax8 (bottom) in normal thyroid, benign neoplasms and primary tumors with, and without, nodal metastases. Scale bars 50 μm. (c) Mean staining intensities for TTF1 and PDGFRα proteins in arrayed thyroid clinical specimens including normal tissue, benign neoplasms, primary PTC and metastatic PTC. Results are means ± standard errors. N as described in Supplementary Table 1.
The substantial cross-reactivity of commercially available PDGFRα and β antibodies required an antibody screening process using a series of T47D breast cancer cell lines that we generated to selectively express human PDGFRα and PDGFRβ (see Supplementary Fig. 5c). PDGFRα immunohistochemical staining in patient tumor samples was linked strongly to a shift in TTF1 staining from the nucleus to cytoplasm and this was especially pronounced in metastatic disease. Node negative PTC specimens exhibited greater nuclear than cytoplasmic staining, whereas we consistently observed strong and specific nuclear staining for TTF1 in histologically normal or benign follicular disease (Fig. 6b, top panel). The alpha subunit of PDGFR was found at much higher levels in PTC specimens with nodal metastases compared to primary tumors lacking evidence of metastases (Fig. 6b, center panel). It should be noted that Pax8 exhibits primarily a nuclear localization in all samples (Fig. 6b, bottom panel). The scoring summary for the immunohistochemistry is shown in Supplementary Table 2 and the quantification of the mean nuclear staining intensities of TTF1 and PDGFRα as a function of tissue type is shown in Fig. 6c.
3.7. PDGFRα and CYTOPLASMIC TTF1 Levels Are Prognostic Markers of Recurrence, Lymph Node Involvement and Radioactive Iodine Resistance in PTC
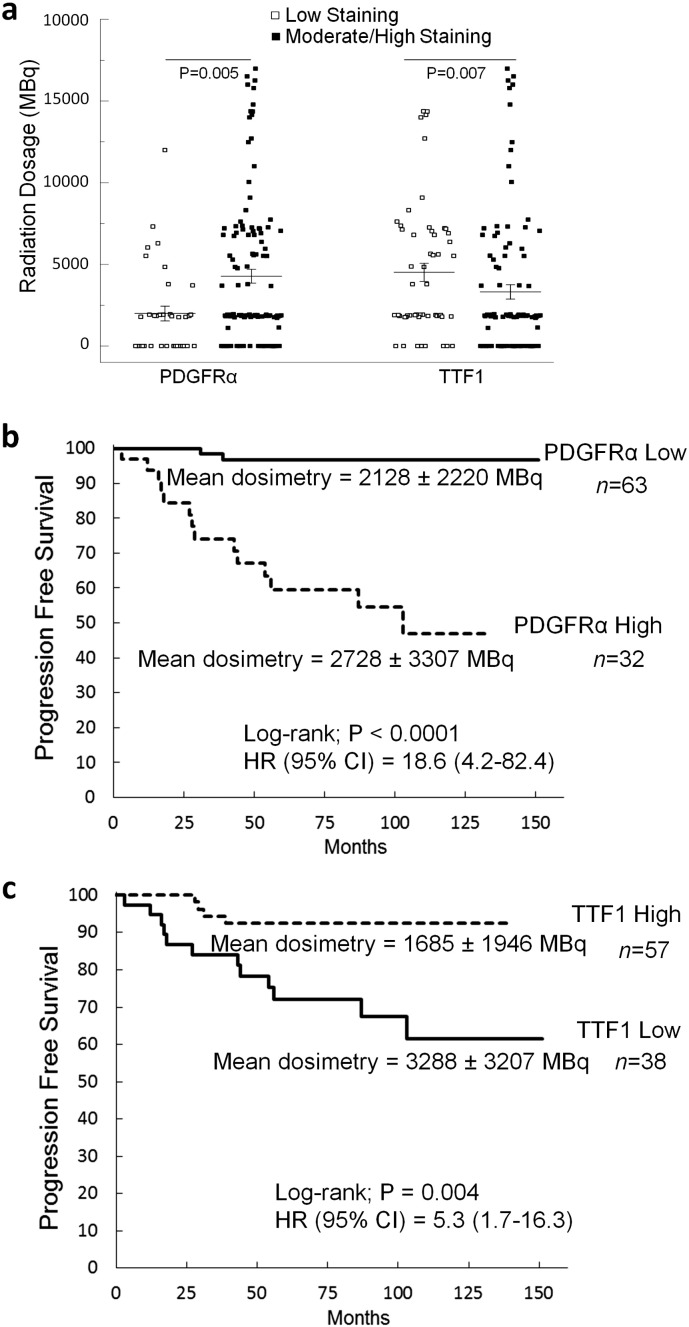
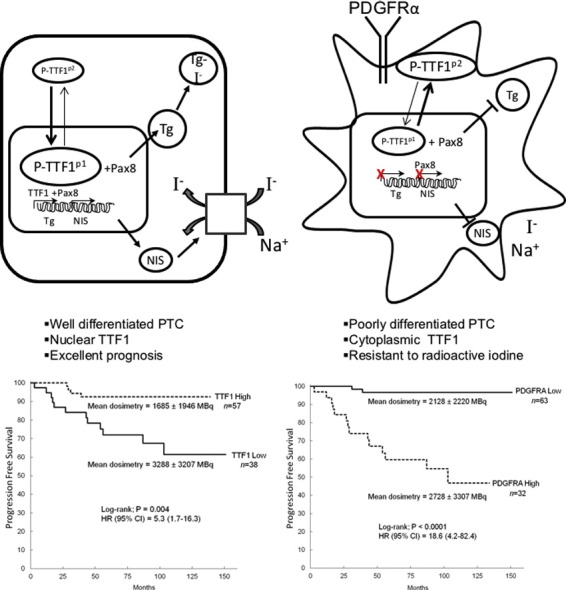
PDGFRα positive tumor specimens at the time of diagnosis were more than three times more likely to exhibit nodal metastases (P < 0.0001), and were larger (P = 0.03) than tumors with minimal PDGFRα staining. TTF1 exhibited the inverse relationship with low nuclear staining levels associated with larger tumors (P = 0.0001) and nodal metastases (P = 0.0008). Radiation data reveal that patients exhibiting PDGFRα were given significantly higher therapeutic doses of radioactive iodine (P = 0.005) compared to patients with low TTF1 levels (P = 0.007) (Fig. 7a). We also examined a cohort of patients initially lacking ultrasound or biochemical (thyroglobulin (Tg) < 0.4 ng/mL) evidence of nodal metastases that were followed routinely for at least 48 months. Patients were assessed for recurrent disease as defined by positive ultrasound scans, an unstimulated Tg level of > 0.4 ng/mL or a stimulated Tg of > 2.0 ng/mL. PDGFRα-positive tumors were > 18 times more likely to recur (95% CI: 4.2–82.4, P < 0.0001) than those cases lacking PDGFRα even when matched for the dose of radiation supplied to the patients (P = 0.28) (Fig. 7b).
Fig. 7.
Radiation dose distribution as a function of PDGFRα and TTF1 staining. (a) Scatter plot of radiation doses for patients stratified by high or low levels of PDGFRα and TTF1 on immunohistochemical stains. Results are unpaired t-tests for 152 patients. (b) Kaplan-Meier plots of disease recurrence as a function of time based on PDGFRα immunohistochemical staining. (c) Kaplan-Meier plots of disease recurrence as a function of time based on TTF1 immunohistochemical staining.
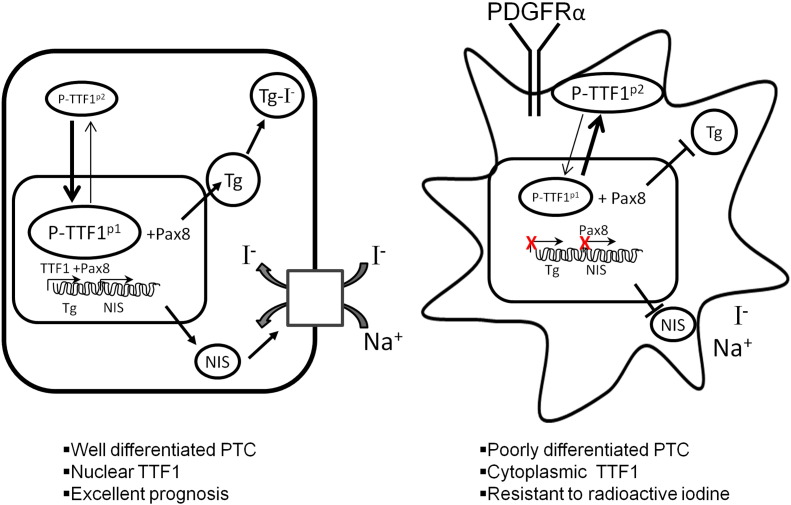
Low levels of TTF1 nuclear expression correlated strongly with recurrent disease (HR = 5.3 95% CI: 1.7–16.3, P = 0.004) even when they were subjected to higher doses of radiation (P = 0.003) (Fig. 7c). These results did not vary for patients above or below 45 years of age as shown in Supplementary Fig. 6, a and b. Given the inverse relationship between TTF1 and PDGFRα, the overall expression of TTF1 and the nuclear/cytoplasmic ratio may be very useful surrogates to identify both increased metastatic potential and resistance to radioactive iodine and a potential for treatment with PDGFRα blockade. In our current model, it appears that targeted blockade of PDGFRα may be an essential to restore differentiation in the treatment of aggressive PTC variants with metastases resistant to radioactive iodine (Fig. 8).
Fig. 8.
PDGFRα-mediated dedifferentiation of thyroid follicular cells. PDGFRα promotes dedifferentiation by decreasing TTF1 nuclear localization, which decreases iodide transport and thyroglobulin production in thyroid follicular cells.
4. Discussion
We demonstrated that PDGFRα, but not PDGFRβ, can induce thyroid follicular cell dedifferentiation by excluding TTF1 from the nucleus. PDGFRα promotes an aggressive disease phenotype in PTC via two mechanisms. First, PDGFRα disruption of TTF1 transcriptional activity abrogates NIS function and iodide transport. Second, this receptor also drives an increase in the invasive potential of thyroid follicular cell lines and promotes tumor formation in xenografts. Patients exhibiting PDGFRα were three times more likely to present with nodal metastases and they received nearly twice the dose of radioactive iodine compared to patients lacking expression of PDGFRα in their tumors. Patients lacking clinical evidence of metastatic disease at diagnosis, but with PDGFRα-positive tumors, were much more likely to have recurrent disease than patients lacking PDGFRα. We ensured that this was true when patients received similar doses of radiation and also when matched for patient age. Differences in survival were not observed in this cohort. However, previous studies examining outcomes over decades showed that recurrent PTC is predictive of increased mortality (Grogan et al., 2013). It is also well-documented that increased use of radioactive iodine and repeated surgeries increase morbidity including lifelong hypoparathyroidism and sialoadenitis (Fard-Esfahani et al., 2014). Since PDGFRα predicts resistance to radioactive iodine and increased risk for nodal metastases, this could be used to inform decisions regarding prophylactic node surgery in PTC. It can also define those patients at low risk of recurrence who may not benefit from the use of radioactive iodine. Lastly, inhibition of PDGFRα in vitro and in vivo generated a differentiated disease phenotype with slower tumor growth and greater avidity for radioactive iodine uptake.
Dedifferentiation through loss of TTF1 transcriptional activity is central to the mechanism of PDGFRα action in PTC. We demonstrated that PDGFRα-induced changes in TTF1 phosphorylation transformed benign thyroid follicular cells, as well as immortalized thyroid cancer cell lines of varying derivations, into more invasive phenotypes lacking expression of thyroglobulin and sodium iodide transport. Our cell lines exhibit BRAF V600E (BCPAP) or RET/PTC (TPC1) mutations, or represent a poorly differentiated form (p53 mutation) of PTC (8305C). Thus, the inverse relationship between PDGFRα and TTF1 expression is not a reflection of a single cell line variant or activation of a single pathway. Regulation of TTF1 is not well understood in thyroid neoplastic disease, but its role in defining patient outcomes and response to therapy has been studied extensively in acute lymphoblastic leukemia and lung cancer (Yamaguchi et al., 2013). Previous studies revealed a complex picture of TTF1 regulation that could not be accounted by individual assessments of Ras, ERK, PI3K, protein kinase A, and cyclic AMP contributions to TTF1 transcriptional activity (Missero et al., 2000, Silberschmidt et al., 2011, Yan and Whitsett, 1997, Feliciello et al., 2000). Thyroid-specific expression of thyroglobulin and other genes relies on the phosphorylation of three to seven serines on TTF1 (Zannini et al., 1996). However, these same investigators found that DNA-binding of TTF1 is phosphorylation-independent (Zannini et al., 1996). In the current work it is clear that subcellular nuclear targeting of TTF1 is the gateway to thyroid follicular cell function. The dephosphorylation of TTF1 moves it from the nucleus and into the cytoplasm ultimately disrupting TTF1-mediated transcription. This accounts for the results of Zannini et al. (1996) and explains why phosphorylation influences transcriptional activity without altering DNA binding activity. We also observe that overall levels of TTF1 protein diminish with dephosphorylation and cytoplasmic localization, consistent with that shown for thyroid (Missero et al., 2000) and lung cell lines (Kumar et al., 2000). We further show that TTF1 in clinical specimens is increasingly localized to the cytoplasm as expected with more aggressive disease. Zhang et al., 2012 previously outline that phospho-PDGFRα activates the Akt and ERK pathways with both pathways contributing to the invasive potential of thyroid cancer cell lines. We continue to explore how altered TTF1 function and increased downstream signaling of PDGFRα pathways (i.e. Akt, ERK, STAT3) work together to promote aggressive variants of PTC.
A central challenge to advancing therapy for progressive PTC is to improve the responses to tyrosine kinase receptor therapy, while minimizing toxicity. So far, the response rates are typically 20% (range 0–45%) and there was substantial toxicity documented from trials using tyrosine kinase receptor inhibitors such as sorafenib, selumetinib, and lenvatinib (Gild et al., 2011, Klein Hesselink et al., 2015). The mechanism of action of these drugs is complex, possibly reflecting an impact on PDGFRα (in the mM range) as well as other downstream pathways including ERK that may influence differentiation. Targeted therapies such as sorafenib clearly inhibit multiple signaling pathways in thyroid carcinoma and can induce cell cycle arrest and initiation of apoptosis. No previous study has documented changes in TTF1 or Pax8 expression or transcription. Here we establish a link between the tyrosine kinase receptor PDGFRα, TTF1 expression, dedifferentiation, and radioactive iodine transport in PTC. We are exploring if focused disruption of PDGFRα by small molecules or monoclonal antibody therapy could be sufficient to induce clinically relevant responses in tumor growth, differentiation, and iodide transport. While it is clear that BRAF and RAS mutations may have a role in tumorigenesis, the subset of thyroid cancers most likely to generate metastases and poor outcomes are dedifferentiated tumors. We believe that targeted therapy of PDGFRα may decrease tumor progression while also inducing differentiation without the side-effects that limit the use of multi-kinase agents to only patients with the most advanced disease.
We demonstrate in cell lines, SCID xenografts and human tumor specimens that PDGFRα promotes dedifferentiation in PTC by decreasing TTF1 expression in the nucleus, which decreases iodide transport and thyroglobulin production in thyroid follicular cells. Our study provides a proof of principle that selectively blocking PDGFRα signaling could significantly improve radioactive iodine treatment and decrease metastases in PTC. This selective therapy could improve outcomes while minimizing side effects compared to drugs that block multiple tyrosine kinase receptors.
Funding Sources
The authors would like to acknowledge the funding support of the Canadian Institutes of Health Research (61710), the Edmonton Civic Employees Charitable Fund (16263), and Alberta Innovates Health Solutions (201311) (TPWM).
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Author Contributions
TPWM, ALC and RL conceived the studies. ALC and EE designed and performed the majority of experiments. RL, AT performed the tissue immunohistochemical analysis. MKB and YMK assisted with the in vivo analysis. PW, DB, and JD assisted with the radioactive iodine assays and 3D culture models. TM and DCW maintained the patient database. KC, LV, DCW, and SG provided statistical analysis. TPWM, ALC, LV, and DB wrote the paper with input from all authors. TPWM, DCW and ALC are inventors on the patent describing PDGFRα blockade to restore radioactive iodine sensitivity.
Acknowledgements
The assistance of the Alberta Research Tumour Bank is greatly appreciated.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.09.007.
Appendix A. Supplementary data
Supplementary Materials and Methods and Supplementary Figures.
References
- Antonica F., Kasprzyk D.F., Opitz R., Iacovino M., Liao X.H., Dumitrescu A.M., Refetoff S., Peremans K., Manto M., Kyba M., Costagliola S. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca A., Fornari A., Bonello L., Tondat F., Chiusa L., Lista P., Pich A. KIT and PDGFRA mutations and PDGFRA immunostaining in gastrointestinal stromal tumors. Mol. Med. Rep. 2011;4:3–8. doi: 10.3892/mmr.2010.399. [DOI] [PubMed] [Google Scholar]
- Bible K.C., Suman V.J., Molina J.R., Smallridge R.C., Maples W.J., Menefee M.E., Rubin J., Sideras K., Morris J.C., 3rd, McIver B., Burton J.K., Webster K.P., Bieber C., Traynor A.M., Flynn P.J., Goh B.C., Tang H., Ivy S.P., Erlichman C. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L.L., Mankoff D.A., Goulart B.H., Eaton K.D., Capell P.T., Kell E.M., Bauman J.E., Martins R.G. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin. Cancer Res. 2010;16:5260–5268. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho S.M., Vaisman F., Buescu A., Mello R.C., Carvalho D.P., Vaisman M. Follow-up of patients treated with retinoic acid for the control of radioiodine non-responsive advanced thyroid carcinoma. Braz. J. Med. Biol. Res. 2011;44:73–77. doi: 10.1590/s0100-879x2010007500120. [DOI] [PubMed] [Google Scholar]
- Dadu R., Cabanillas M.E. Optimizing therapy for radioactive iodine-refractory differentiated thyroid cancer: current state of the art and future directions. Thyroid. 2013;23:593–599. [PMC free article] [PubMed] [Google Scholar]
- Fard-Esfahani A., Emami-Ardekani A., Fallahi B., Fard-Esfahani P., Beiki D., Hassanzadeh-Rad A., Eftekhari M. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl. Med. Commun. 2014;35:808–817. doi: 10.1097/MNM.0000000000000132. [DOI] [PubMed] [Google Scholar]
- Feliciello A., Allevato G., Musti A.M., De Brasi D., Gallo A., Avvedimento V.E., Gottesman M.E. Thyroid transcription factor 1 phosphorylation is not required for protein kinase A-dependent transcription of the thyroglobulin promoter. Cell Growth Differ. 2000;11:649–654. [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. International Agency for Research on Cancer; Lyon, France: 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] [Google Scholar]
- Fernandez C.A., Puig-Domingo M., Lomeña F., Estorch M., Camacho M.V., Bittini A.L., Marazuela M., Santamaría J., Castro J., Martínez de Icaya P., Moraga I., Martín T., Megía A., Porta M., Mauricio D., Halperin I. Effectiveness of retinoic acid treatment for redifferentiation of thyroid cancer in relation to recovery of radioiodine uptake. J. Endocrinol. Investig. 2009;32:228–233. doi: 10.1007/BF03346457. [DOI] [PubMed] [Google Scholar]
- Ferrari S.M., Fallahi P., Politti U., Materazzi G., Baldini E., Ulisse S., Miccoli P., Antonelli A. Molecular targeted therapies of aggressive thyroid cancer. Front. Endocrinol. 2015;20:176. doi: 10.3389/fendo.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gild M.L., Bullock M., Robinson B.G., Clifton-Bligh R. Multikinase inhibitors: a new option for the treatment of thyroid cancer. Nat. Rev. Endocrinol. 2011;7:617–624. doi: 10.1038/nrendo.2011.141. [DOI] [PubMed] [Google Scholar]
- Giuliani C., Bucci I., Di Santo S., Rossi C., Grassadonia A., Mariotti M., Piantelli M., Monaco F., Napolitano G. Resveratrol inhibits sodium/iodide symporter gene expression and function in rat thyroid cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Campora R., Delgado M.D., Amate A.H., Gallardo S.P., Leon M.S., Beltran A.L. Old and new immunohistochemical markers for the diagnosis of gastrointestinal stromal tumors. Anal. Quant. Cytol. Histol. 2011;33:1–11. [PubMed] [Google Scholar]
- Grogan R.H., Kaplan S.P., Cao H., Weiss R.E., Degroot L.J., Simon C.A., Embia O.M., Angelos P., Kaplan E.L., Schechter R.B. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013;154:1436–1446. doi: 10.1016/j.surg.2013.07.008. [DOI] [PubMed] [Google Scholar]
- GTEx Consortium The genotype-tissue expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta-Abramson V., Troxel A.B., Nellore A., Puttaswamy K., Redlinger M., Ransone K., Mandel S.J., Flaherty K.T., Loevner L.A., O'Dwyer P.J., Brose M.S. Phase II trial of sorafenib in advanced thyroid cancer. J. Clin. Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., Schuff K.G., Sherman S.I., Sosa J.A., Steward D.L., Tuttle R.M., Wartofsky L. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.L., Grewal R.K., Leboeuf R., Sherman E.J., Pfister D.G., Deandreis D., Pentlow K.S., Zanzonico P.B., Haque S., Gavane S., Ghossein R.A., Ricarte-Filho J.C., Domínguez J.M., Shen R., Tuttle R.M., Larson S.M., Fagin J.A. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P., Bojdan I.E., Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J. Clin. Endocrinol. Metab. 2010;95:820–828. doi: 10.1210/jc.2009-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke C.C., Liu R.S., Yang A.H., Liu C.S., Chi C.W., Tseng L.M., Tsai Y.F., Ho J.H., Lee C.H., Lee O.K. CD133-expressing thyroid cancer cells are undifferentiated, radioresistant and survive radioiodide therapy. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:61–71. doi: 10.1007/s00259-012-2242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Hesselink E.N., Steenvoorden D., Kapiteijn E., Corssmit E.P., van der Horst-Schrivers A.N., Lefrandt J.D., Links T.P., Dekkers O.M. Therapy of endocrine disease: response and toxicity of small-molecule tyrosine kinase inhibitors in patients with thyroid carcinoma: a systematic review and meta-analysis. Eur. J. Endocrinol. 2015;172:215–225. doi: 10.1530/EJE-14-0788. [DOI] [PubMed] [Google Scholar]
- Kogai T., Sajid-Crockett S., Newmarch L.S., Liu Y.Y., Brent G.A. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J. Endocrinol. 2008;199:243–252. doi: 10.1677/JOE-08-0333. [DOI] [PubMed] [Google Scholar]
- Kumar A.S., Gonzales L.W., Ballard P.L. Transforming growth factor-beta(1) regulation of surfactant protein B gene expression is mediated by protein kinase-dependent intracellular translocation of thyroid transcription factor-1 and hepatocyte nuclear factor 3. Biochim. Biophys. Acta. 2000;1492:45–55. doi: 10.1016/s0167-4781(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Liu D., Hu S., Hou P., Jiang D., Condouris S., Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin. Cancer Res. 2007;13:1341–1349. doi: 10.1158/1078-0432.CCR-06-1753. [DOI] [PubMed] [Google Scholar]
- Lundgren C.I., Hall P., Dickman P.W., Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106:524–531. doi: 10.1002/cncr.21653. [DOI] [PubMed] [Google Scholar]
- Malehmir M., Haghpanah V., Larijani B., Ahmadian S., Alimoghaddam K., Heshmat R., Ghavamzadeh A., Adabi K., Ghaffari S.H. Multifaceted suppression of aggressive behavior of thyroid carcinoma by all-trans retinoic acid induced re-differentiation. Mol. Cell. Endocrinol. 2012;348:260–269. doi: 10.1016/j.mce.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Missero C., Pirro M.T., Di Lauro R. Multiple ras downstream pathways mediate functional repression of the homeobox gene product TTF-1. Mol. Cell. Biol. 2000;20:2783–2793. doi: 10.1128/mcb.20.8.2783-2793.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D., Huang R., Li S., Ma X., Lou C., Kuang A. Combining transfer of TTF-1 and Pax-8 gene: a potential strategy to promote radioiodine therapy of thyroid carcinoma. Cancer Gene Ther. 2012;19:402–411. doi: 10.1038/cgt.2012.13. [DOI] [PubMed] [Google Scholar]
- Oh S.W., Moon S.H., Park d.J., Cho B.Y., Jung K.C., Lee D.S., Chung J.K. Combined therapy with 131I and retinoic acid in Korean patients with radioiodine-refractory papillary thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:1798–1805. doi: 10.1007/s00259-011-1849-2. [DOI] [PubMed] [Google Scholar]
- Pilli T., Prasad K.V., Jayarama S., Pacini F., Prabhakar B.S. Potential utility and limitations of thyroid cancer cell lines as models for studying thyroid cancer. Thyroid. 2009;19:1333–1342. doi: 10.1089/thy.2009.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga T.S., Heinhuis B., Gerrits D., Netea M.G., Joosten L.A., Hermus A.R., Oyen W.J., Schweppe R.E., Haugen B.R., Boerman O.C., Smit J.W., Netea-Maier R.T. mTOR inhibition promotes TTF1-dependent redifferentiation and restores iodine uptake in thyroid carcinoma cell lines. J. Clin. Endocrinol. Metab. 2014;99:1368–1375. doi: 10.1210/jc.2014-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph G.W., Duh Q.Y., Heller K.S., LiVolsi V.A., Mandel S.J., Steward D.L., Tufano R.P., Tuttle R.M. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22:1144–1152. doi: 10.1089/thy.2012.0043. [DOI] [PubMed] [Google Scholar]
- Schlumberger M., Tahara M., Wirth L.J., Robinson B., Brose M.S., Elisei R., Habra M.A., Newbold K., Shah M.H., Hoff A.O., Gianoukakis A.G., Kiyota N., Taylor M.H., Kim S.B., Krzyzanowska M.K., Dutcus C.E., de las Heras B., Zhu J., Sherman S.I. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- Schneider T.C., Abdulrahman R.M., Corssmit E.P., Morreau H., Smit J.W., Kapiteijn E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur. J. Endocrinol. 2012;167:643–650. doi: 10.1530/EJE-12-0405. [DOI] [PubMed] [Google Scholar]
- Schneider D.F., Chen H., Sippel R.S. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann. Surg. Oncol. 2013;20:1906–1911. doi: 10.1245/s10434-012-2802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweppe R., Klopper J.P., Korch C., Pugazhenthi U., Benezra M., Knauf J.A., Fagin J.A., Marlow L.A., Copland J.A., Smallridge R.C., Haugen B.R. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J. Clin. Endocrinol. Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman E.J., Su Y.B., Lyall A., Schöder H., Fury M.G., Ghossein R.A., Haque S., Lisa D., Shaha A.R., Tuttle R.M., Pfister D.G. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid. 2013;23:593–599. doi: 10.1089/thy.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberschmidt D., Rodriguez-Mallon A., Mithboakar P., Cali G., Amendola E., Sanges R., Zannini M., Scarfò M., De Luca P., Nitsch L., Di Lauro R., De Felice M. In vivo role of different domains and of phosphorylation in the transcription factor Nkx2-1. BMC Dev. Biol. 2011;11:9. doi: 10.1186/1471-213X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaldi A., Miasaki F.Y., Ciampi R., Agate L., Collecchi P., Capodanno A., Pinchera A., Elisei R. Re-differentiation of thyroid carcinoma cell lines treated with 5-aza-2′-deoxycytidine and retinoic acid. Mol. Cell. Endocrinol. 2009;307:142–148. doi: 10.1016/j.mce.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Waltz F., Pillette L., Ambroise Y. A nonradioactive iodide uptake assay for sodium iodide symporter function. Anal. Biochem. 2010;396:91–95. doi: 10.1016/j.ab.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Weiss S.J., Philp N.J., Grollman E.F. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology. 1984;114:1090–1098. doi: 10.1210/endo-114-4-1090. [DOI] [PubMed] [Google Scholar]
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Hosono Y., Yanagisawa K., Takahashi T. NKX2-1/TTF-1: an enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression. Cancer Cell. 2013;10:718–723. doi: 10.1016/j.ccr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Yan C., Whitsett J.A. Protein kinase A activation of the surfactant protein B gene is mediated by phosphorylation of thyroid transcription factor 1. J. Biol. Chem. 1997;272:17327–17332. doi: 10.1074/jbc.272.28.17327. [DOI] [PubMed] [Google Scholar]
- Yu X.M., Jaskula-Sztul R., Ahmed K., Harrison A.D., Kunnimalaiyaan M., Chen H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol. Cancer Ther. 2013;12:1276–1287. doi: 10.1158/1535-7163.MCT-12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannini M., Acebron A., De Felice M., Arnone M.I., Martin-Pérez J., Santisteban P., Di Lauro R. Mapping and functional role of phosphorylation sites in the thyroid transcription factor-1 (TTF-1) J. Biol. Chem. 1996;271:2249–2254. doi: 10.1074/jbc.271.4.2249. [DOI] [PubMed] [Google Scholar]
- Zhang P., Zuo H., Nakamura Y., Nakamura M., Wakasa T., Kakudo K. Immunohistochemical analysis of thyroid-specific transcription factors in thyroid tumors. Pathol. Int. 2006;56:240–245. doi: 10.1111/j.1440-1827.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- Zhang M., Guo R., Xu H., Zhang M., Li B. Retinoic acid and tributyrin induce in vitro radioiodine uptake and inhibition of cell proliferation in a poorly differentiated follicular thyroid carcinoma. Nucl. Med. Commun. 2011;32:605–610. doi: 10.1097/MNM.0b013e3283463027. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang P., Dykstra M., Gelebart P., Williams D., Ingham R., Adewuyi E.E., Lai R., McMullen T. Platelet-derived growth factor receptor-α promotes lymphatic metastases in papillary thyroid cancer. J. Pathol. 2012;228:241–250. doi: 10.1002/path.4069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods and Supplementary Figures.