Abstract
Cutaneous abscess infections are difficult to treat with current therapies and alternatives to conventional antibiotics are needed. Understanding the regulatory mechanisms that govern abscess pathology should reveal therapeutic interventions for these recalcitrant infections. Here we demonstrated that the stringent stress response employed by bacteria to cope and adapt to environmental stressors was essential for the formation of lesions, but not bacterial growth, in a methicillin resistant Staphylococcus aureus (MRSA) cutaneous abscess mouse model. To pharmacologically confirm the role of the stringent response in abscess formation, a cationic peptide that causes rapid degradation of the stringent response mediator, guanosine tetraphosphate (ppGpp), was employed. The therapeutic application of this peptide strongly inhibited lesion formation in mice infected with Gram-positive MRSA and Gram-negative Pseudomonas aeruginosa. Overall, we provide insights into the mechanisms governing abscess formation and a paradigm for treating multidrug resistant cutaneous abscesses.
Keywords: Staphylococcus aureus, Pseudomonas aeruginosa, ppGpp, Cationic peptide, DJK-5
Highlights
-
•
Universal stringent stress response mediators drive abscess formation.
-
•
Targeting stress response reduces the severity of cutaneous abscess infections.
-
•
Pharmacological suppression of S. aureus cutaneous toxin production.
-
•
Paradigm for treating Gram-positive and Gram-negative bacterial abscess infections.
1. Introduction
Abscesses are very common. For example, in the United States alone, 3.2 million people were treated in hospital emergency departments for an abscess infection in 2005 (Taira et al., 2009). Cutaneous abscesses, triggered by local bacterial infections, are characterized by an accumulation of fluid/pus within the dermis, which is often associated with severe inflammation and induration and frequently lead to a skin lesion that may present as an open sore (Singer and Talan, 2014). Severe abscesses that display signs of septic infection are not only surgically drained, but also treated with antibiotics to prevent dissemination, although recurrence can occur (Singer and Talan, 2014). An example of a bacterium that often causes abscesses is Methicillin-resistant Staphylococcus aureus (MRSA). In addition to its role as an important nosocomial human pathogen, MRSA infections are now emerging in the community as an important cause of skin and soft-tissue infections, many of which are cutaneous abscesses (Moran et al., 2006). Common antibiotic therapies for MRSA abscesses include trimethoprim-sulfamethoxazole, clindamycin and tetracyclines (Singer and Talan, 2014, Talan et al., 2011). However, conditions within the abscess such as low pH, excessive debris and high bacterial loads, as well as low redox potential, have been shown to limit the penetration and efficacy of antibiotics (Stearne et al., 2001). Thus antibiotics work poorly against abscess infections and the substantial consumption of antibiotics over the past decades has resulted in strains that are resistant to virtually all of the utilized antibiotic treatments (Moran et al., 2006, Singer and Talan, 2014, Talan et al., 2011). Cutaneous abscesses can also be caused by a variety of other Gram-negative (Carpenter, 1990) and Gram-positive bacteria (Maliyil et al., 2011) making broad spectrum treatments more desirable.
Here we hypothesized that abscesses represent a distinct stress-triggered growth state that make them both resistant to treatment and able to cause pathology. In particular, we have considered the involvement of the universal stringent stress response. The stringent response is a conserved stress response employed by various bacteria to respond to and cope with conditions of amino-acid starvation, carbon-source, fatty acid, oxygen or iron limitation, heat shock, antimicrobial challenge, and/or other environmental stressors (Crosse et al., 2000, Potrykus and Cashel, 2008). In most bacteria, the stringent response is signaled by the secondary-messenger guanosine tetraphosphate (ppGpp), which serves as a pleiotropic transcriptional regulator by binding to RNA polymerase (Dalebroux and Swanson, 2012). This leads to the repression of resource-consuming processes (translation, lipid, and cell wall biosynthesis, and to some extent replication and transcription) and diverts resources toward biosynthesis (amino acid biosynthesis and transport, glycolysis) and diverse stress genes to promote survival (Potrykus and Cashel, 2008, Srivatsan and Wang, 2008, Wolz et al., 2010).
Cationic amphipathic peptides are an evolutionarily conserved, multifunctional component of the innate immune system. They are known to have immunomodulatory, direct antimicrobial, and/or anti-biofilm activity (Hancock and Sahl, 2006). Importantly, a distinct subset of cationic peptides have demonstrated broad-spectrum efficacy in targeting recalcitrant biofilm infections by targeting the stringent stress response (de la Fuente-Núñez et al., 2014, de la Fuente-Núñez et al., 2015, Pletzer and Hancock, 2016). Biofilms are a distinct growth state of bacteria on surfaces whereby the bacteria form structured aggregates that are adaptively multi-antibiotic resistant (de la Fuente-Núñez et al., 2014, de la Fuente-Núñez et al., 2015, Overhage et al., 2008). The stringent response and biofilm formation are tightly interconnected processes since ppGpp is required for biofilm initiation and maintenance, such that bacterial mutants defective in the stringent response do not form biofilms (Aberg et al., 2006, de la Fuente-Núñez et al., 2014, He et al., 2012). In S. aureus, upon amino acid starvation, ppGpp (and its precursor pppGpp) production is mediated by the bi-functional synthase/hydrolase enzyme RSH (a RelA/SpoT homolog) (Geiger et al., 2012).
The ppGpp regulon is very complex (Vercruysse et al., 2011). For example, in Escherichia coli, ppGpp mediates the induction of other stress regulators within the universal stress protein (USP) family (Kvint et al., 2003). Likewise, in S. aureus, a homolog, the universal stress protein (designated Usp2) was recently identified and shown to be necessary for persistence under amino acid starvation (Attia et al., 2013) and is positively regulated by ppGpp (Geiger et al., 2012). Despite these findings, the importance of these stringent response regulators in S. aureus pathogenesis remains an understudied topic.
Here we have demonstrated a contribution of the stringent response to S. aureus cutaneous abscess formation (as judged by lesion formation and other altered pathology findings), but not local bacterial growth, and demonstrated that it can be targeted pharmacologically with a peptide. Furthermore, the same pharmacological targeting worked with P. aeruginosa infections in a cutaneous abscess model.
2. Materials and Methods
2.1. Strains
S. aureus wild-type HG001, RSH synthase mutant (rshsyn) and complemented RSH synthase strain were kindly provided by Christiane Wolz (University of Tübingen, Tübingen). S. aureus Newman and Δusp2 were provided by Eric Skaar (Vanderbilt University Medical Center, Nashville, TN). Flow cell analysis was conducted on biofilm forming MRSA strain SAP0017 (clinical isolate kindly provided by Dr. Tony Chow, Vancouver General Hospital). For in vivo peptide studies, bioluminescent S. aureus USA300 was used and kindly provided by Scott Stibitz (Food and Drug Administration, Silver Spring, MD) and P. aeruginosa LESB58 (Liverpool epidemic strain) was from Winstanley et al. (Winstanley et al., 2009). All strains in Table S1 were kindly provided by Michael Otto (National Institute of Health, Bethesda, MD).
2.2. Peptide Synthesis
DJK-5 (VQWRAIRVRVIR-NH2; all D amino acids) was synthesized by CPC Scientific and control peptide IDR-2013 (WQRVRRVKVIRK-NH2) was synthesized by Genscript using solid-phase 9-flurenylmethoxy carbonyl (Fmoc) chemistry and purified to > 95% purity using reverse-phase high-performance liquid chromatography (HPLC). The lyophilized peptide was initially resuspended in endotoxin-free water and used in vitro, or further resuspended in saline and used in vivo.
2.3. Drug Susceptibility Test
The broth microdilution method with minor modifications for cationic peptides (Wiegand et al., 2008) was used for measuring the MIC of peptide DJK-5.
2.4. Flow Cell Analysis
A flow cell system was initially assembled and sterilized as previously described (de la Fuente-Núñez et al., 2014). BM2 biofilm-adjusted medium [62 mM potassium phosphate buffer (pH 7), 7 mM (NH4)2SO4, 2 mM MgSO4, 10 μM FeSO4, 0.4% (wt/vol) glucose] was allowed to pump through the flow cell apparatus for 1 h before the chambers were injected with 1/20 dilutions of overnight culture and bacteria allowed to adhere to the plastic surface of the flow cells for 3 h. To assess the activity of the peptide on pre-formed biofilms, peptide was added to the system two days after the initial bacterial injection and pumped through the system for a subsequent 24 h. Three days following the injection, the flow cells were injected with SYTO-9 and propidium iodide stain [LIVE/DEAD BacLight Bacterial Viability kit (Molecular Probes, Eugene, OR)] to image total and dead cells respectively. Remaining biomass was assessed using a confocal laser scanning microscope (Olympus, Fluoview FV1000) and three-dimensional reconstructions were generated using the Imaris software package (Bitplane AG).
2.5. Mouse Skin Infection Model
Female CD-1 mice (6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA) and were used for the abscess model. Mice were housed together (maximum five/cage) and bedding (shredded paper) and nestlets were provided in each cage. All animal experiments were performed in accordance with The Canadian Council on Animal Care (CCAC) guidelines and were approved by the University of British Columbia Animal Care Committee. The fur on the backs of the mice was removed through shaving and depilatory cream. All S. aureus strains were grown to an optical density at 600 nm (OD600) of 1 in tryptic soy broth (TSB), while P. aeruginosa LESB58 was grown in double yeast tryptone (dYT) medium to an OD600 of 1; subsequently cells were washed twice with sterile PBS, and resuspended to a final concentration of 5 × 107 CFU / 50 μL. For mutant studies, bacteria were injected into the right flank of the back. For peptide intraperitoneal (IP) administration studies, mice were initially given either saline or 6 mg/kg DJK-5 (for MRSA studies) or 4 mg/kg DJK-5 (for P. aeruginosa studies) in saline via IP injection immediately before applying 50 μL of bacteria subcutaneously to the right flank of the back. For intra-abscess studies, mice received 50 μL of bacteria and 1 h later, 3 mg/kg of peptide via intra-abscess injection. Abscess lesion sizes were measured using a caliper every 24 h for a maximum of 5 days. Visible dermonecrosis or white lesions (filled with pus) were considered as part of the abscess lesion. Swelling/inflammation was however disregarded in the measurements. Mice were monitored once daily and no adverse outcomes were reported. To assess the levels of luminescent bacteria in the abscess every 24 h, the in vivo imaging System (IVIS) (Perkin Elmer, Waltham MA) was utilized. Skin abscesses were excised either two, three or five days post-infection, homogenized using a rotor stator for 5 min and serially diluted for CFU quantification. Furthermore, skin explants were fixed in 10% neutral buffered formalin and processed for hematoxylin and eosin and Gram staining using a Wax it kit (University of British Columbia).
2.6. Evaluation of Histological Slides
Histological and Gram stained slides were independently evaluated blindly by veterinarian Dr. Ian Welch at the Centre for Comparative Medicine (Vancouver, Canada) and pathologist Dr. Hamid Masoudi at Vancouver Coastal Health (Vancouver, Canada). All slides used for this independent evaluation are available from the authors upon request with one being presented in Results.
2.7. Measurement of Phenol Soluble Modulins (PSM)
For luminescent reporter studies, the USA300 PSMα luminescence reporter strain (Dastgheyb et al., 2015) was grown in TSB in the presence of 2.5 or 5 μg/mL DJK-5 or water as a control. Luminescence activity was read for up to 8 h using a VICTORX3 Multilabel Plate reader. Alternatively, USA300 was grown in TSB medium for 8 h at 37 °C at 200 rpm in the presence of sub-lethal concentrations of DJK-5 (1 μg/mL) or water as a control. PSM production in culture filtrates was assessed using HPLC/MS as previously described (Joo and Otto, 2014).
2.8. Statistical Evaluation
For bacterial mutant studies, two independent experiments were conducted and data was statistically analyzed using one-way ANOVA or t-test. For peptide studies, three independent experiments were conducted and t-tests were used for statistical evaluation. For all animal experiments, the number of biological replicates is indicated in the figure legend. All other experiments were conducted using three biological replicates and evaluated using t-test.
3. Results
3.1. The stringent response was crucial for cutaneous abscess lesion formation in mice
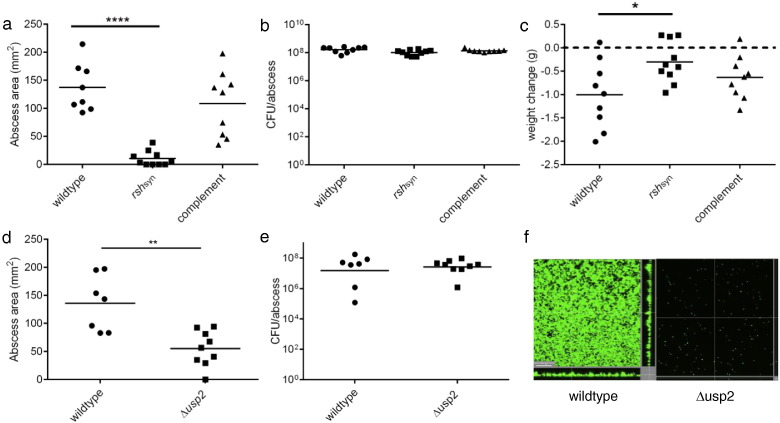
We first hypothesized that bacteria found within a cutaneous abscess are under significant stress due to limited nutrients and oxygen as well as phagocyte-generated oxidative stress. Therefore, we examined the importance of the bacterial stringent response in a murine cutaneous abscess infection model. To study this, we compared in vivo the lesion-forming ability of S. aureus HG001 with that of a mutant defective in RSH synthase (rshsyn) and thus ppGpp production. The RSH-hydrolase domain is essential for preventing toxic accumulation of ppGpp, such that only RSH mutants defective in the synthase domain are viable (Geiger et al., 2010). Our data showed that the rshsyn mutant was severely deficient in abscess lesion formation (13.2 fold smaller lesions compared to the wild type) and genetic complementation of rshsyn restored the ability to form abscess lesions (Fig. 1a). Interestingly, similar viable bacterial counts were recovered from all infected groups (Fig. 1b), indicating that the stringent response was required for the formation of lesions but not local bacterial growth. Moreover, mice injected with the rsh-deficient mutant lost 3.3 fold less weight when compared to the wildtype (Fig. 1c). Mice infected with P. aeruginosa stringent response mutants also formed significantly smaller lesions as compared to wildtype controls (data not shown). Overall, our data indicates that the protective stringent response is critical for cutaneous abscess pathology.
Fig. 1.
The stringent response was critical for S. aureus abscess lesion and biofilm formation. (a) Mice were infected subcutaneously with S. aureus HG001 wildtype, rshsyn and rshsyn complement strains. Lesions were measured 24 h post-infection using a caliper, n = 8–10/group. Statistical significance was determined using one-way ANOVA (****, p < 0.0001) (b) Bacteria were recovered 48 h post-infection from mouse abscesses infected with S. aureus HG001, ∆ rshsyn and complemented-rshsyn and then plated for enumeration, n = 9–10/group. (c) Weight loss/gain of infected mice was assessed by measuring the mouse weights pre-infection and 48 h post-infection, n = 9–10/group (*, p < 0.05). (d) Mice were infected subcutaneously with S. aureus Newman parent strain and its Δusp2 mutant. Lesion formation was measured 24 h post-infection using a caliper, n = 7–9/group. Statistical significance was determined using unpaired t-tests (**, p < 0.01). (e) Bacteria were recovered from Newman- and Δusp2- infected mice abscesses after 48 h and plated for enumeration, n = 7–9/group. (f) Newman and Δusp2 biofilms were grown in flow cells. After 72 h, bacteria were stained with Syto-9 (live bacteria stain) and propidium iodide (dead bacteria stain) prior to confocal imaging. The scale bar represents 30 μm in length and each image shows the xy, yz and xz dimensions. Two independent experiments were conducted for both animal studies and flow cell experiment.
3.2. The universal stress protein (Usp2) was involved in cutaneous lesion and biofilm formation
Based on the interaction of ppGpp with other stress response regulators, we investigated whether Usp2 was also important for cutaneous abscess infections. Mice infected subcutaneously with a usp2 mutant (Δusp2) demonstrated approximately 2.5 fold smaller abscess lesions compared to those infected with wild-type S. aureus Newman (Fig. 1d); a trend that was not attributed to a growth defect (Fig. 1e).
Due to the role of ppGpp in stress adaptation and biofilm formation, we investigated the biofilm-forming capacity of ∆ usp2, which had not been studied to date. Using a flow cell apparatus, imaged by confocal microscopy for live and dead biofilm mass after 3 days of growth, it was determined that ∆ usp2 was severely deficient in biofilm formation (Fig. 1f). These data collectively provided evidence that both tissue injury and biofilm formation involve induction of the universal stress protein Usp2.
3.3. A Stringent-Response Targeted Peptide, DJK-5, Strongly Reduced S. aureus Lesion Formation
Synthetic peptides derived from naturally-occurring antimicrobial peptides target the stringent response alarmone ppGpp as the basis for their antibiofilm activity (de la Fuente-Núñez et al., 2014, de la Fuente-Núñez et al., 2015). In particular, d-enantiomeric peptide, DJK-5, a peptide whose sequence was conceptually based on innate defense regulator (IDR)-1018, impairs bacterial biofilm formation and eradicates preformed biofilms by directly interacting with ppGpp and triggering its degradation (de la Fuente-Núñez et al., 2015). It is worth noting that biofilm and abscess infections are generally considered to be quite distinct, although the above data indicated mechanistic overlap.
DJK-5 exhibited very modest antimicrobial activity toward S. aureus under planktonic growth conditions, with minimal inhibitory concentrations (MICs) against a variety of strains ranging from 16 to 64 μg/mL (Table S1). In contrast, DJK-5 displayed potent antibiofilm activity, eradicating pre-formed MRSA biofilms at only 2.5 μg/mL (Fig. S1).
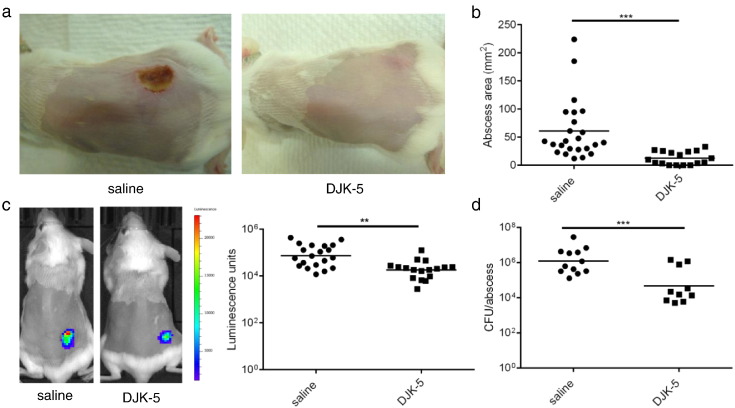
Given the activity of DJK-5 against the stringent response and the observed mechanistic overlap between abscess and biofilm formation, we predicted that pharmacological targeting of the stringent response would impact on lesion formation in vivo. When administered via intraperitoneal (IP) injection, DJK-5 dramatically reduced the severity of abscesses formed by the CA-MRSA strain, USA300, by visibly reducing tissue injury and dermonecrosis (Fig. 2a) as well as significantly reducing lesion size by > 4.4-fold compared to controls (Fig. 2b).
Fig. 2.
Intraperitoneal application of DJK-5 impaired MRSA cutaneous lesion formation. (a) Mice were injected with 6 mg/kg of DJK-5 or saline as control via intraperitoneal injection prior to receiving subcutaneous injection with bioluminescent MRSA USA300. Representative images capturing dermonecrotic abscess lesions were taken 72 h post-infection. (b) Lesion sizes were measured three days post-infection using a caliper, n = 24 saline, n = 17 DJK-5. (c) Bioluminescent bacteria were imaged using In Vivo Imaging System (IVIS) and quantified using Living Image® Software, n = 20 saline, n = 17 DJK-5. (d) Five days post-infection, bacteria were recovered from saline or DJK-5 treated animals and plated for enumeration, n = 12 saline, n = 10 DJK-5. Three independent experiments were conducted and all comparisons were made using unpaired t tests; **, p < 0.01; ***, p < 0.001.
Using bioluminescent bacteria in this model allowed for the daily monitoring of bacteria in vivo. After 3 days post-infection, a drop in photon flux was observed in the DJK-5-treated mice when compared to the untreated, infected controls (Fig. 2c). In previous peptide tracking studies, 2 mg/kg of 3H–radiolabelled peptide injected intraperitoneally reached steady-state concentrations of 2–6 μg of peptide per gram of tissue (equivalent to 2–3 μg/mL in the blood) (Bolouri et al., 2014). Based on this biodistribution data, we predicted that the dose used in this experiment would not reach MIC levels (32 μg/mL) in the tissues or blood. Consistent with this, despite a 10.2-fold reduction in viable bacteria recovered from the infected site in the DJK-5-treated vs. untreated mice (Fig. 2d), there was still a significant bacterial load recovered from DJK-5-treated wounds.
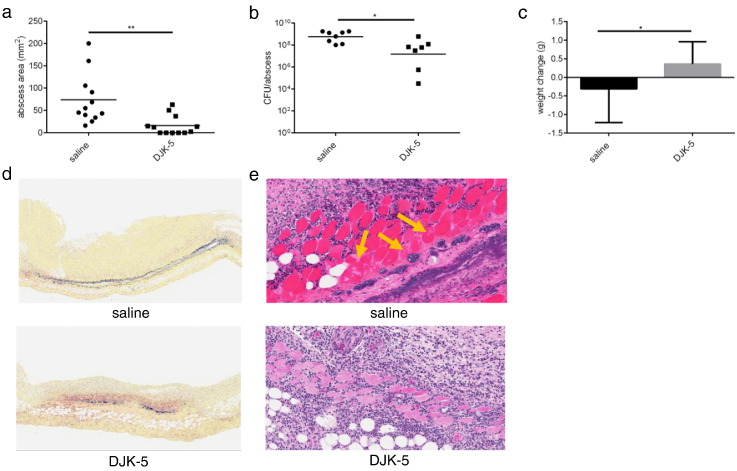
These results motivated us to apply the peptide more directly via intra-abscess injection, since direct application into the abscess reflects a potentially more clinically relevant approach. In mice given DJK-5, there was a substantially lower (4.6-fold smaller) lesion size (Fig. 3a) and 8.4-fold fewer bacteria were recovered from the abscess site (Fig. 3b). In addition, mice receiving saline lost weight (average 0.3 g weight loss) while mice receiving DJK-5 gained weight (Fig. 3c), indicating that DJK-5 improved the overall welfare of the mice. We did not observe an improvement in abscess severity or a reduction in abscess size in mice treated with control peptide IDR-2013, a peptide with no anti-biofilm activity against MRSA (Haney et al., 2015) (Fig. S2). Furthermore, based on independent qualitative observations by two trained pathologists, Gram staining conducted on abscess explants demonstrated that mice treated with DJK-5 exhibited decreased bacterial burden and dissemination within the dermis compared to their saline-treated counterparts (Fig. 3d). Furthermore, Hematoxylin and Eosin (H&E) staining of abscess explants demonstrated greater tissue damage in saline-treated mice (Table S2; cutaneous ulceration and granulation thickness of 0.9 ± 0.3 cm), as compared to peptide-treated mice (0.2 ± 0.07 cm), with damage extending to the deep fascia of skeletal muscle in saline control animals (Fig. 3e and Table S2; pathological findings of panniculus carnosus damage and deep skeletal muscle damage were not observed in peptide treated mice).
Fig. 3.
Intra-abscess application of DJK-5 impaired MRSA cutaneous injury. (a) Mice were injected with 3 mg/kg of DJK-5 or saline (control) via intra-abscess injection after subcutaneous infection with MRSA USA300. Abscess lesion sizes were measured using a caliper after 72 h, n = 12/group. (b) Bacterial enumeration from saline and DJK-5 intra-abscess-treated abscesses three days post-infection, n = 8 saline, n = 7 DJK-5. (c) Weight loss/gain of infected mice was assessed by measuring the mouse weights pre-infection and 72 h post-infection n = 12 saline, n = 11 DJK-5. (d) Representative Gram stain of saline and DJK-5 treated skin biopsies. (e) Hematoxylin and eosin stain of skin explants taken from saline and DJK-5 treated mice. Yellow arrows indicate suppurative myositis.
3.4. DJK-5 Impaired Abscess Lesion Formation by a Gram-Negative Pathogen
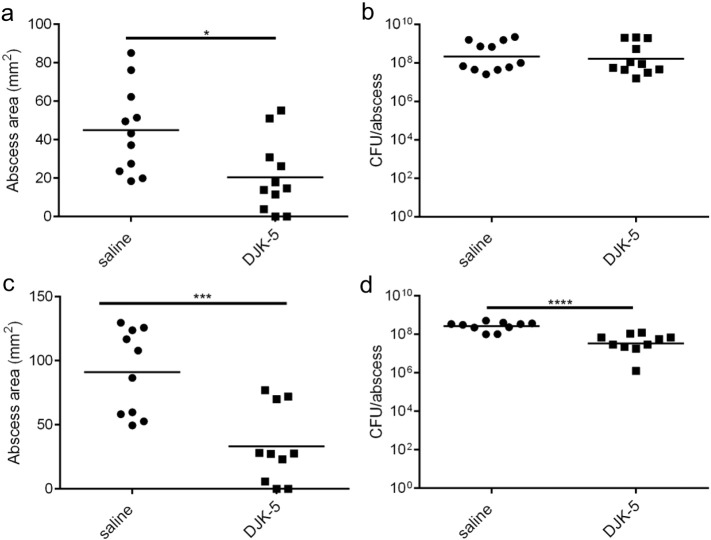
The above results prompted us to ask whether DJK-5 could exert broad-spectrum anti-abscess activity since its target ppGpp, is conserved among Gram-negative and Gram-positive bacteria (de la Fuente-Núñez et al., 2015, Potrykus and Cashel, 2008, Wolz et al., 2010). Therefore, we established a Gram-negative bacterial abscess model in mice infected subcutaneously with the Liverpool epidemic strain of P. aeruginosa LESB58 (Winstanley et al., 2009) and administered 4 mg/kg of DJK-5 or saline as a control via IP injection. Consistent with the results for S. aureus, mice treated with DJK-5 formed significantly (p < 0.05) smaller (2.2-fold) abscess lesions when compared to saline controls (Fig. 4a). There was no significant effect however on recovered viable bacterial counts with peptide treatment (Fig. 4b). When DJK-5 was administered via intra-abscess application, it was observed that lesions formed on mice treated with DJK-5 were significantly smaller by 2.7-fold (Fig. 4c) with 5.7-fold fewer bacteria recovered from DJK-5 treated animals (Fig. 4d). Taken together, these results show the potential for DJK-5 to reduce abscess pathology from both a Gram-positive and a Gram-negative pathogen.
Fig. 4.
DJK-5 suppressed Gram-negative abscess lesion formation. (a) Mice were injected with 4 mg/kg of DJK-5 or saline (control) via intraperitoneal injection prior to subcutaneous infection with P. aeruginosa LESB58. Abscess lesion sizes were measured using a caliper after 72 h, n = 11/group. (b) Bacteria were recovered from saline and DJK-5 IP-treated animals and plated for enumeration three days post-infection, n = 11/group. (c) Mice were infected subcutaneously with P. aeruginosa LESB58 and then treated 2 h later with 4 mg/kg of DJK-5 via intra-abscess injection. Lesions were measured 72 h post-infection with a caliper, n = 10/group. (d) Bacteria were recovered from saline or DJK-5 intra-abscess treated animals and plated for enumeration, n = 10/group. All comparisons were made using unpaired t tests; *, p < 0.05; **, p < 0.01; ***, p < 0.001. All experiments were performed in triplicate.
3.5. DJK-5 Suppressed Major Stringently Regulated Cutaneous Phenol Soluble Modulin Toxins in S. aureus
Strikingly, mice treated with DJK-5 exhibited a significant reduction in tissue damage as well as a 10-fold reduction in bacterial burden within the abscess tissue. Despite the possibility that reduced CFUs could account to some extent for the reduced lesion sizes, since significant bacterial loads remained in DJK5-treated wounds, we hypothesized that the peptide could be suppressing abscess pathology by decreasing the expression of a cutaneous toxin.
The stringently regulated S. aureus phenol soluble modulin (PSM) toxins have been shown to be the major virulence factors contributing to abscess lesion formation and deletion of the psm locus results in the attenuation of virulence in several disease models (Berube et al., 2014). Therefore, using a S. aureus USA300 luminescent reporter strain, we measured the expression of the psmα operon when subjected to DJK-5. In order to ensure bacterial growth was not affecting PSM production, sub-lethal concentrations of DJK-5 were employed (Fig. S3). An increasing reduction in psmα expression was observed with increasing concentrations of DJK-5 (Fig. 5a). We further analyzed PSM production by assessing the culture filtrates of peptide-treated USA300. Using a HPLC/MS-based approach (Joo and Otto, 2014) it was found that the production of PSMα peptides was generally reduced by DJK-5, with values for PSMα 1 and 2 reaching statistical significance (Fig. 5b). These results indicate that by targeting the stress response, DJK-5 can suppress phenol soluble modulins, which are known to be implicated in SSTI virulence.
Fig. 5.
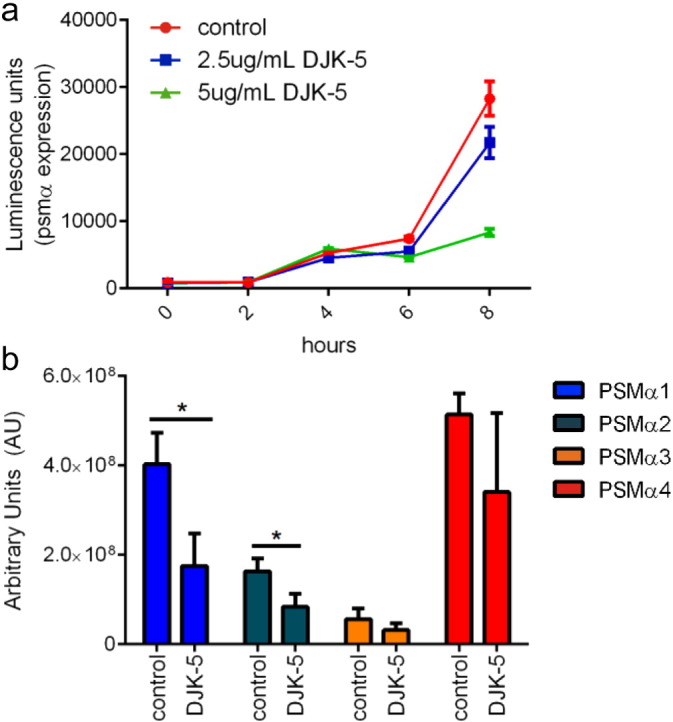
DJK-5 targeted PSM production. (a) MRSA USA300 PSMα luminescence reporter strain was grown in the presence of 2.5 μg/mL, 5 μg/mL DJK-5 or water as a control. Luminescence activity was read using a VICTORX3 Multilabel Plate reader. (b) PSM production in culture filtrates of S. aureus MRSA USA300 treated with 1 μg/mL of DJK-5 or water as a control. All experiments were done in triplicate and all comparisons were done using unpaired t tests; *, p < 0.05.
4. Discussion
Bacteria are highly adaptive, dynamic organisms that use a variety of sensor systems to monitor and adapt to environmental conditions (Potrykus and Cashel, 2008). Here we showed that stringent stress adaption is a critical determinant of S. aureus cutaneous abscess lesion formation. The stringent response has been shown to mediate tolerance of S. aureus to cell wall-active antibiotics (Geiger et al., 2014) and we propose that this adaptation is in part responsible for the limited success of conventional antibiotics against bacteria in abscesses. Specifically, our data showed that mutants deficient in either of the two important stringent response mediators (RSH or Usp2), were impaired in lesion formation. Previous studies were somewhat ambiguous since although an rshsyn mutant formed fewer kidney abscesses in a murine renal abscess model (Geiger et al., 2010), this observation was proposed to reflect the lower viability (recovery of bacteria) of the rshsyn mutant compared to the wild type in the kidneys. In contrast, in the cutaneous abscess model, the very substantial attenuation of abscess lesion formation by the rshsyn mutant occurred despite similar levels of bacteria as the wild type, demonstrating a direct contribution of the stringent response. Consistent with this observation, by targeting the stringent response, DJK-5 was able to suppress tissue injury. Despite the major physiological differences in bacterial abscess and biofilm formation, our studies have revealed a strong mechanistic connection that can be exploited with antibiofilm peptides.
In accordance with these observations, we demonstrated the importance of a newly-identified stress response regulator, Usp2, both in Staphylococcal abscess lesion formation, albeit to a lesser extent than for the rshsyn mutant, and in biofilm formation. This correlates with recent studies that demonstrated the importance of Usp2 in responding to amino acid starvation (Attia et al., 2013). Biofilm formation is a survival strategy for bacteria to adapt to hostile environments and although bacteria in abscesses are largely considered to be loosely associated in pus, recent evidence suggests that bacteria isolated from deep tissue abscesses are embedded in biofilm-like matrices (May et al., 2014). Of great importance, recurring abscesses and biofilms are similarly recalcitrant to antibiotic therapy (Davies, 2003, Stearne et al., 2001). As such, cationic peptides that display antibiofilm activity, can target multidrug resistant bacteria and are synergistic with conventional antibiotics (de la Fuente-Núñez et al., 2014, de la Fuente-Núñez et al., 2015), represent excellent candidates for the adjunctive treatment of recalcitrant cutaneous abscesses.
Our previous studies indicated that DJK-5, a small cationic peptide comprising of D-amino acids, possessed potent antibiofilm activity against a variety of multi-drug resistant Gram-negative species (de la Fuente-Núñez et al., 2015). Furthermore, DJK-5 targets the stress response alarmone ppGpp and enhances survival in two invertebrate P. aeruginosa biofilm infection models (de la Fuente-Núñez et al., 2015). Here we demonstrated that DJK-5 could suppress S. aureus and P. aeruginosa cutaneous injury in mice, despite the very different physiological states of biofilm and abscess infections. Also, the bacterial load recovered after use of the peptide employed at 3 mg/kg IP, was similar to that for the commonly used antibiotic clindamycin at 75 mg/kg (data not shown), highlighting its potential as an improved anti-abscess strategy.
The stringent response triggers a complex cascade of transcriptional events to counteract environmental stresses. Given that high bacterial loads were still recovered from DJK-5-treated mice (Fig. 2d and 3b), we considered that the peptide might be suppressing a toxin and indeed demonstrated here that it suppressed the production of the major cutaneous PSM toxins of S. aureus. PSMs are positively regulated by the stringent response (Geiger et al., 2012) and consistent with this, our studies indicated that DJK-5 suppressed PSMα production, likely through stringent response impairment.
PSMs recruit, activate and subsequently lyse neutrophils, the key effectors and first responders against bacterial infections (Berube et al., 2014). Previous studies have shown that upon uptake of S. aureus in neutrophils, the stringent response is elicited resulting in increased PSM synthesis, which contributes to survival after phagocytosis by mediating neutrophil lysis (Geiger et al., 2012). Thus by suppressing PSM production, DJK-5 could potentially reduce local tissue damage as well as impede a major immune evasion method employed by S. aureus. Given the 10-fold effect on bacterial load, we also considered the potential importance of effects on quorum sensing. Phenol soluble modulins are under the control of the accessory gene regulator (agr) quorum sensing pathway as well as the stringent response. However, studies have shown that production of phenol soluble modulins (especially the virulent α-type which are dysregulated by DJK-5), can be produced in an agr-independent manner (Wang et al., 2007). In fact, during the stringent response, RSH-independent induction of phenol soluble modulins, is not mediated by increased agr expression (Geiger et al., 2012), confirming a limited correlation between quorum sensing and phenol soluble modulin production under stringent conditions.
In conclusion, we have uncovered mechanisms driving bacterial abscess lesion formation and provide a therapeutic approach to minimize the severity of skin infections by both Methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa.
Funding Sources
Research reported in this publication was supported by a grant from the Canadian Institutes for Health Research MOP-74493, the National Institute of Allergy and Infectious Diseases (NIAID) of the U. S. National Institutes of Health under Award Number R33AI098701, and the Intramural Research Program of the NIAID. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.E.W.H. holds a Canada Research Chair in Health and Genomics and a UBC Killam Professorship. S.C. M received the Centre for Blood Research (CBR) graduate student award as well as the Gerhard Henrik Armauer-Hansen Memorial Scholarship. D.P. received a Feodor Lynen postdoctoral fellowship from the Alexander von Humboldt Foundation while C.D.L.F.-N received a scholarship from the Fundación “la Caixa” and Fundación Canadá (Spain). Additionally, C.D.L.F.-N. and R.E.W.H. are co-inventors of a provisional patent application on the use of cationic anti-biofilm peptides (U.S. Patent Application No. 61/870,655).
Competing Interests
R.E.W.H. is an inventor on a patent assigned to his employer The University of British Columbia and covering peptide DJK-5 and its use in treating infections.
Author Contributions
S.C.M. and R.E.W.H. designed the experiments; S.C.M., D.P., P.K. and G.Y.C.C. performed mouse experiments; S.C.M. and H.-S.J. performed HPLC-MS experiments; C.D.L.F.-N., M.O. and R.E.W.H. provided technical advice and assisted with data analysis; M.O. and R.E.W.H. provided materials for all experiments; S.C.M. and R.E.W.H. wrote the paper with the assistance of all other authors.
Acknowledgements
We would like to thank Dr. Ian Welch and Dr. Hamid Masoudi for their help in analyzing histological samples.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.09.015.
Appendix A. Supplementary Data
Supplementary material
References
- Aberg A., Shingler V., Balsalobre C. (p)ppGpp regulates type 1 fimbriation of Escherichia coli by modulating the expression of the site-specific recombinase FimB. Mol. Microbiol. 2006;60:1520–1533. doi: 10.1111/j.1365-2958.2006.05191.x. [DOI] [PubMed] [Google Scholar]
- Attia A.S., Cassat J.E., Aranmolate S.O., Zimmerman L.J., Boyd K.L., Skaar E.P. Analysis of the Staphylococcus aureus abscess proteome identifies antimicrobial host proteins and bacterial stress responses at the host-pathogen interface. Pathog. Dis. 2013;69:36–48. doi: 10.1111/2049-632X.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube B.J., Sampedro G.R., Otto M., Bubeck Wardenburg J. The psmα locus regulates production of Staphylococcus aureus alpha-toxin during infection. Infect. Immun. 2014;82:3350–3358. doi: 10.1128/IAI.00089-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri H., Savman K., Wang W., Thomas A., Maurer N., Dullaghan E., Fjell C.D., Ek C.J., Hagberg H., Hancock R.E.W. Innate defense regulator peptide 1018 protects against perinatal brain injury. Ann. Neurol. 2014;75:395–410. doi: 10.1002/ana.24087. [DOI] [PubMed] [Google Scholar]
- Carpenter J.L. Klebsiella pulmonary infections: occurrence at one medical center and review. Rev. Infect. Dis. 1990;12:672–682. doi: 10.1093/clinids/12.4.672. [DOI] [PubMed] [Google Scholar]
- Crosse A.M., Greenway D.L., England R.R. Accumulation of ppGpp and ppGp in Staphylococcus aureus 8325-4 following nutrient starvation. Lett. Appl. Microbiol. 2000;31:332–337. doi: 10.1046/j.1472-765x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- Dalebroux Z.D., Swanson M.S. ppGpp: magic beyond RNA polymerase. Nat. Rev. Microbiol. 2012;10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- Dastgheyb S.S., Villaruz A.E., Le K.Y., Tan V.Y., Duong A.C., Chatterjee S.S., Cheung G.Y., Joo H.S., Hickok N.J., Otto M. Role of phenol-soluble modulins in formation of Staphylococcus aureus biofilms in synovial fluid. Infect. Immun. 2015;83:2966–2975. doi: 10.1128/IAI.00394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Núñez C., Reffuveille F., Haney E.F., Straus S.K., Hancock R.E.W. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Núñez C., Reffuveille F., Mansour S.C., Reckseidler-Zenteno S.L., Hernandez D., Brackman G., Coenye T., Hancock R.E.W. d-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 2015;22:196–205. doi: 10.1016/j.chembiol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Goerke C., Fritz M., Schafer T., Ohlsen K., Liebeke M., Lalk M., Wolz C. Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect. Immun. 2010;78:1873–1883. doi: 10.1128/IAI.01439-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Francois P., Liebeke M., Fraunholz M., Goerke C., Krismer B., Schrenzel J., Lalk M., Wolz C. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Kastle B., Gratani F.L., Goerke C., Wolz C. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J. Bacteriol. 2014;196:894–902. doi: 10.1128/JB.01201-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R.E.W., Sahl H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Haney E.F., Mansour S.C., Hilchie A.L., de la Fuente-Nunez C., Hancock R.E.W. High throughput screening methods for assessing antibiofilm and immunomodulatory activities of synthetic peptides. Peptides. 2015;71:276–285. doi: 10.1016/j.peptides.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Cooper J.N., Mishra A., Raskin D.M. Stringent response regulation of biofilm formation in Vibrio cholerae. J. Bacteriol. 2012;194:2962–2972. doi: 10.1128/JB.00014-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo H.S., Otto M. The isolation and analysis of phenol-soluble modulins of Staphylococcus epidermidis. Methods Mol. Biol. 2014;1106:93–100. doi: 10.1007/978-1-62703-736-5_7. [DOI] [PubMed] [Google Scholar]
- Kvint K., Nachin L., Diez A., Nystrom T. The bacterial universal stress protein: function and regulation. Curr. Opin. Microbiol. 2003;6:140–145. doi: 10.1016/s1369-5274(03)00025-0. [DOI] [PubMed] [Google Scholar]
- Maliyil J., Caire W., Nair R., Bridges D. Splenic abscess and multiple brain abscesses caused by Streptococcus intermedius in a young healthy man. Proc. (Baylor Univ. Med. Cent.) 2011;24:195–199. doi: 10.1080/08998280.2011.11928714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J.G., Shah P., Sachdeva L., Micale M., Kruper G.J., Sheyn A., Coticchia J.M. Potential role of biofilms in deep cervical abscess. Int. J. Pediatr. Otorhinolaryngol. 2014;78:10–13. doi: 10.1016/j.ijporl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Moran G.J., Krishnadasan A., Gorwitz R.J., Fosheim G.E., McDougal L.K., Carey R.B., Talan D.A., Group, E.M.I.N.S. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- Overhage J., Campisano A., Bains M., Torfs E.C., Rehm B.H., Hancock R.E.W. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008;76:4176–4182. doi: 10.1128/IAI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer D., Hancock R.E.W. Anti-biofilm peptides: potential as broad-spectrum agents. J. Bacteriol. 2016 doi: 10.1128/JB.00017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus K., Cashel M. (p)ppGpp: still magical? Annu. Rev. Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- Singer A.J., Talan D.A. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 2014;370:1039–1047. doi: 10.1056/NEJMra1212788. [DOI] [PubMed] [Google Scholar]
- Srivatsan A., Wang J.D. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr. Opin. Microbiol. 2008;11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Stearne L.E., Gyssens I.C., Goessens W.H., Mouton J.W., Oyen W.J., van der Meer J.W., Verbrugh H.A. In vivo efficacy of trovafloxacin against Bacteroides fragilis in mixed infection with either Escherichia coli or a vancomycin-resistant strain of Enterococcus faecium in an established-abscess murine model. Antimicrob. Agents Chemother. 2001;45:1394–1401. doi: 10.1128/AAC.45.5.1394-1401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira B.R., Singer A.J., Thode H.C., Jr., Lee C.C. National epidemiology of cutaneous abscesses: 1996 to 2005. Am. J. Emerg. Med. 2009;27:289–292. doi: 10.1016/j.ajem.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Talan D.A., Krishnadasan A., Gorwitz R.J., Fosheim G.E., Limbago B., Albrecht V., Moran G.J., Group, E.M.I.N.S Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin. Infect. Dis. 2011;53:144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- Vercruysse M., Fauvart M., Jans A., Beullens S., Braeken K., Cloots L., Engelen K., Marchal K., Michiels J. Stress response regulators identified through genome-wide transcriptome analysis of the (p)ppGpp-dependent response in Rhizobium etli. Genome Biol. 2011;12:R17. doi: 10.1186/gb-2011-12-2-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Braughton K.R., Kretschmer D., Bach T.H., Queck S.Y., Li M., Kennedy A.D., Dorward D.W., Klebanoff S.J., Peschel A. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Winstanley C., Langille M.G., Fothergill J.L., Kukavica-Ibrulj I., Paradis-Bleau C., Sanschagrin F., Thomson N.R., Winsor G.L., Quail M.A., Lennard N. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool epidemic strain of Pseudomonas aeruginosa. Genome Res. 2009;19:12–23. doi: 10.1101/gr.086082.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolz C., Geiger T., Goerke C. The synthesis and function of the alarmone (p)ppGpp in firmicutes. Int. J. Med. Microbiol. 2010;300:142–147. doi: 10.1016/j.ijmm.2009.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material