Abstract
Large bone defect treatment represents a great challenge due to the difficulty of functional and esthetic reconstruction. Tissue-engineered bone grafts created by in vitro manipulation of bioscaffolds, seed cells, and growth factors have been considered potential treatments for bone defect reconstruction. However, a significant gap remains between experimental successes and clinical translation. An emerging strategy for bridging this gap is using the in vivo bioreactor principle and flap prefabrication techniques. This principle focuses on using the body as a bioreactor to cultivate the traditional triad (bioscaffolds, seed cells, and growth factors) and leveraging the body's self-regenerative capacity to regenerate new tissue. Additionally, flap prefabrication techniques allow the regenerated bone grafts to be transferred as prefabricated bone flaps for bone defect reconstruction. Such a strategy has been used successfully for reconstructing critical-sized bone defects in animal models and humans. Here, we highlight this concept and provide some perspective on how to translate current knowledge into clinical practice.
Keywords: Bone regeneration, In vivo bioreactor, In vivo tissue engineering, Flap prefabrication, Bone graft
Highlights
-
•
The in vivo bioreactor principle and flap prefabrication technique is a promising strategy for bone defect reconstruction.
-
•
The in vivo bioreactor principle focuses on using the body’s self-regenerative capacity to regenerate new tissue.
-
•
This strategy has been successfully used to reconstruct critical-sized bone defects in humans.
1. Introduction
1.1. Clinical Aspect
Large volume bony defects resulting from traumatic incidents, congenital abnormalities, infection, or cancer resections represent a great challenge for orthopedic, craniomaxillofacial, and reconstructive surgeons. Ideally, functional reconstruction of bone defects requires the available bone grafts to possess mechanical strength, microstructure, and function as similar to native bone tissue as possible. This allows full integration with the neighboring host bone and, importantly, the performance of the functions of native bone tissue. Thus, an ideal functional bone graft should possess the following characteristics: high osteoinductive and angiogenic potentials, biological safety, low donor-site morbidity, no size restrictions, readily accessible to surgeons, long shelf life, and reasonable cost. Although numerous strategies have been used for bone defect reconstruction, none of the currently available bone substitutes have all of the ideal characteristics.
1.2. Current Approaches for Large Bone Defect Treatment
The currently available approaches for bone defect reconstruction, including bone transport methods, biomaterial implantation, and bone grafting, all have specific indications and limitations. According to the requirements of functional bone defect reconstruction, autologous bone grafting is the gold standard for large bone defect treatment because this graft contains the cell types, matrix, and vasculature necessary for proper bone regrowth in the injured area. However, the difficulties associated with these grafts such as additional host morbidity, donor site shortages, and high infection risk, limit their clinical application. An alternative solution is processed allogenic and xenogenic bone grafts. Although all the living cells are destroyed during graft processing and storage, these grafts remain associated with the risks of immunoreactions, disease transmission, and poor osteoconduction capacity. Other techniques, including distraction osteogenesis, bone marrow aspirate, and growth factors, are commonly used in experimental and clinical conditions for bone defect reconstruction. These methods are associated with several disadvantages, including the limited osseointegration and revascularization of large bone grafts. Therefore, these problems have resulted in increased interest in improving functional bone graft solutions for better patient outcomes.
1.3. Standard Approach and Limitations of Bone Tissue Engineering (BTE)
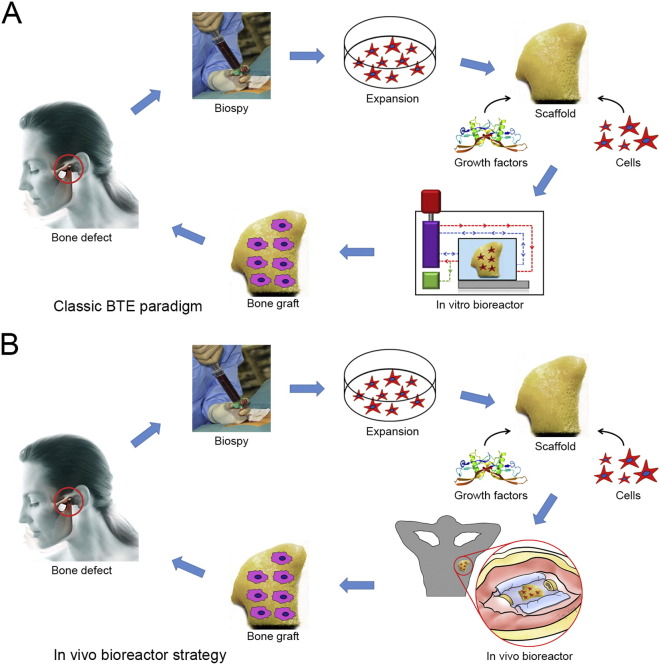
With the progress of new technologies, regeneration of bone tissue following tissue engineering principles now represents another strategy for bone defect reconstruction. BTE aims to regenerate new, cell-driven bony tissue with hierarchical organization and anatomical function similar to naturally occurring bone tissue. This approach requires the collaborative efforts of scientists, engineers, and surgeons. BTE strategies have relied on two approaches: in vitro or in vivo tissue engineering. The in vitro BTE strategy attempts to create functional bone grafts by culturing osteogenic cells on bioactive scaffolds in vitro. In vitro BTE has observed tremendous growth and evolved to a sophisticated level in bioreactor design, scaffold engineering, and long-term tissue construct maintenance (Fig. 1A). However, this methodology does not consider the functional elements of the regenerative environment, including immune, nervous, and hormonal systems, which play crucial roles in tissue regeneration and organ development. Furthermore, diffusion, vascularization, and neurotization challenges are the major obstacles in BTE. Although in vitro bioreactors have been successfully designed to mimic the in vivo microenvironment by precise control of these regeneration-related parameters (Salehi-Nik et al., 2013), recapitulating the true in vivo conditions under ex vivo circumstances is difficult. Therefore, after an in vitro engineered bone graft is transplanted into the body, it lacks its own vascular and nerve networks to support cell survival and matrix synthesis and thus must rely on the ingrowth of neo-vascular structures from its surroundings, resulting in limited long-term outcomes in clinical therapeutic studies.
Fig. 1.
Schematic illustration of the BTE paradigm. (A) Classic in vitro BTE paradigm. (B) In vivo BTE paradigm.
(The photograph of a temporomandibular joint-shaped scaffold is adapted from Grayson et al. (2010).)
An emerging trend to circumvent these problems is following the in vivo bioreactor (IVB) principle, which uses the body as a bioreactor to cultivate the traditional triad (scaffolds, cells, growth factors) and to leverage the body's own self-regenerative capacity to regenerate new tissue (Fig. 1B). A key advantage of the IVB strategy is that the body can offer a constant stream of stem cells to create a regenerative niche and native signals for tissue growth (Fig. 2). Furthermore, the IVB approach often bypasses excessive manipulation of cells and growth factors during ex vivo culture, thus ensuring that the cells retain their functional properties. Recent preclinical and clinical investigations of bone graft prefabrication following the IVB principle have shown promising results and demonstrated efficacy in the reconstruction of large volume bone defects.
Fig. 2.
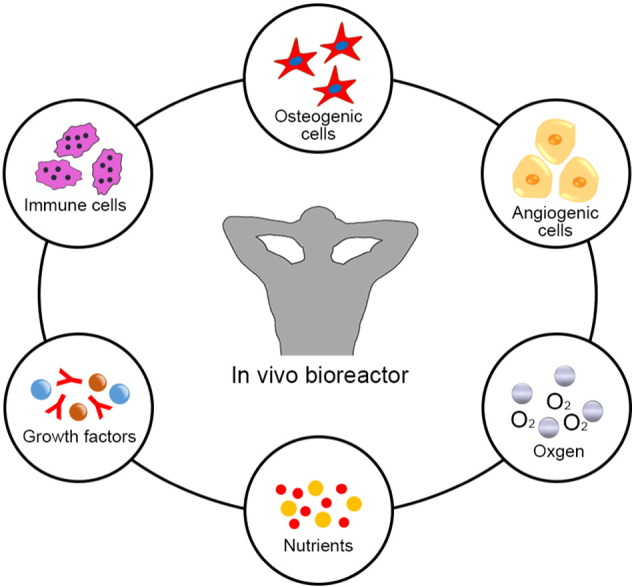
Illustration of the role of an IVB.
A growing body of work and the significant promise of IVB strategies for bone regeneration have motivated the preparation of the current review, in which we introduce the concept of bone graft prefabrication following the IVB principle, discuss different types of IVB strategies, and summarize the current experimental and clinical experiences in bone defect reconstruction using prefabricated bone grafts. Finally, we highlight the bottlenecks of clinical translation of this concept and outline future trends in bone graft prefabrication.
2. Bone Graft Prefabrication Following the IVB Principle: Basic Considerations
2.1. Prefabrication: A Bridge between Reconstructive Surgery and Regenerative Medicine
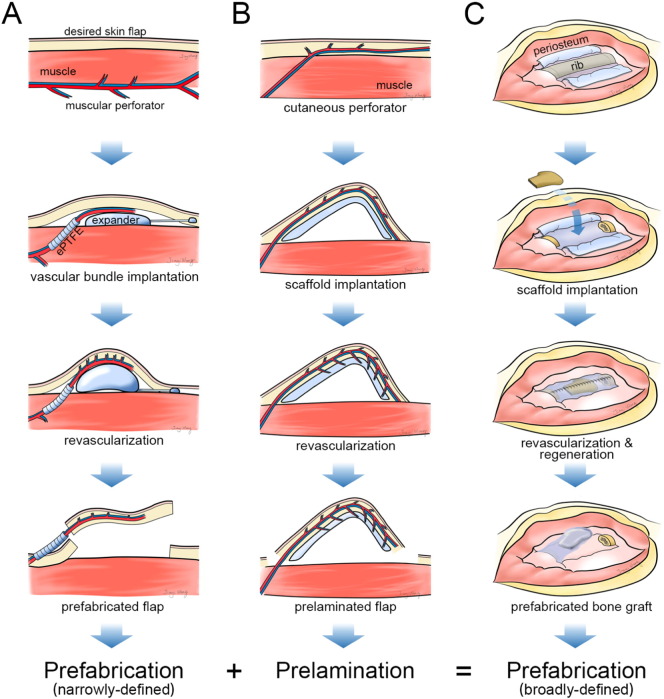
Flap prefabrication is one of the most exciting areas in plastic surgery because of its bridging role between conventional reconstructive surgery and tissue engineering. Prefabrication is a surgical term that was first introduced by Shen (Yao, 1982) and that describes the implantation of a vascular pedicle into a new territory, followed by a neovascularization period and subsequent tissue transfer based on its implanted pedicle (Fig. 3A). Prefabrication allows any defined tissue volume to be transferred to any specified recipient site, greatly expanding the armamentarium of reconstructive options (Xie et al., 2014). Interestingly, this term seems to have “off-label” uses in the literature. For instance, by implantation of an avascular construct, such as a three-dimensional (3D) auricular cartilage construct (von Bomhard et al., 2013) or a tracheal allograft (Vranckx et al., 2014), into a vascular territory for a period, the avascular construct would be revascularized and could be transferred as a composite flap for tissue defect reconstruction (Fig. 3B). This procedure has also been given the surgical term “prelamination”. However, the core concept of prefabrication and prelamination is based on the revascularization phenomenon: from the implanted vascular pedicle to the avascular receipt territory or from the vascular host territory to the implanted avascular construct (Pribaz and Fine, 1994, Yao, 1981). Using the prefabrication or prelamination technique, other tissue, including a vascularized bone flap (Chen et al., 2007), a piece of beating cardiac muscle (Morritt et al., 2007), or a functional neo-endocrine organ (Borud et al., 1996), can be artificially regenerated with the desired size and shape to fit any defect. Gradually, the definition and value of prefabrication have been significantly expanded in clinical practice (Wei and Li, 2013). Thus, the current generalized definition of prefabrication should include not only revascularization and prelamination but also regeneration, which will lead to a new technique for tissue regeneration (Fig. 3C).
Fig. 3.
Schematic concept of flap prefabrication, flap prelamination, and tissue prefabrication. (A) Technological paradigm of traditional flap prefabrication. (B) Technological paradigm of flap prelamination. (C) Technological paradigm of tissue prefabrication following the IVB principle.
2.2. Basic Principles of the IVB Strategy
The term “in vivo bioreactor” was first coined in 2005 by two independent studies (Holt et al., 2005, Stevens et al., 2005) in which the periosteum or vascular pedicle was demonstrated to act as a bioreactor to successfully induce new bone formation. To date, the IVB concept has been applied in a series of experimental and clinical studies to induce the regeneration of various tissues. In these studies, to take advantage of the body's intrinsic self-regenerative capability, the traditional triad elements or a combination thereof are cultivated using the body as a bioreactor at the damage site or within ectopic sites capable of supporting neo-tissue formation. After a period of in vivo cultivation, functional tissues or organs are regenerated and can be used for tissue defect reconstruction. Although the study models and designs entirely differ from each other, the basic principles of an IVB strategy are similar: choose an appropriate anatomical site for providing a regenerative microenvironment, and seek an optimal combination of the traditional triad elements to serve as structural and logistical templates for tissue formation.
The combination of the IVB principle and surgical prefabrication techniques represents a new strategy for tissue regeneration. The IVB principle provides a regenerative niche to create new tissue and surgical prefabrication techniques allow the regenerated tissue to be transferred as prefabricated flap for optimal reconstruction. Bone graft prefabrication following the IVB principle can ideally eliminate the previously described limitations of in vitro BTE. Specifically, the in vitro expansion and seeding of cells can be modified by chemotaxis of pluripotent stem or progenitor cells from the surrounding tissue or blood circulation system, the biochemical molecular signals can be replaced by autologous growth factors as well as other supportive stimuli, and a neurovascular network supplying the regenerated tissue can be recreated in situ, allowing later transplantation of the vascularized neo-tissue or organoid to the desired site. All these components can contribute to the in vivo niche, helping to determine the ultimate functional outcome.
2.3. IVB Types: Basic Characteristics
Anatomically, the IVB is not only a vascular territory but also a regenerative niche for vascularization, regeneration, and remodeling of the prefabricated bone grafts. The most important consideration for an IVB strategy is the tissue type surrounding the bioreactor, which may directly affect interactions with the implanted construct, the recruitment of autologous cells, the reestablishment of a neurovascular network, and the final results of bone graft prefabrication. The location of the IVB is also crucial for the regeneration and clinical application of bone grafts. Orthotopic prefabrication of bone grafts allows direct reconstruction of the bone defect without secondary bone graft transfer surgery. However, in many cases, the bone defect site cannot provide a sufficient regenerative microenvironment because of chronic infection, radiation treatment, or excessive degeneration. Under such conditions, prefabrication of bone grafts at an ectopic site is an alternative option. An adequate ectopic site can provide adequate conditions for cellular colonization, vascularization, physical stimulation, and easy access for surgical operation.
2.3.1. Subcutaneous Pocket
The subcutaneous pocket is the simplest model of all IVB strategies for bone graft prefabrication and has been widely used in ectopic bone formation models (Huang et al., 2014, Huang et al., 2015). Only reports that aimed to regenerate bone grafts following the IVB principle are included in this review. Generally, the subcutaneous pocket is an artificially created space between the superficial and deep subcutaneous fascia, which is a non-osseous environment. This space provides regenerative cues for complex bone graft prefabrication. Using this approach, bone grafts with complex tissue phenotypes and custom-made shapes have been successfully prefabricated in the laboratory (Lee et al., 2009). Surgically, the most important considerations for the subcutaneous pocket strategy are technical. The subcutaneous pocket approach is advantageous as (1) a wide range of anatomical locations can be chosen, (2) implantation in the donor site and transplantation to the recipient site can be performed easily, and (3) donor site morbidity and complications are minimized. However, the current available subcutaneous implantation site for bone formation is a relatively avascular territory without abundant blood flow in comparison to other IVB strategies. Therefore, subcutaneous pockets lack natural osteogenic and angiogenic stem cells, growth factors, and hormones that are required during bone regeneration.
2.3.2. Muscular Pouch or Flap
Muscle tissue has a rich capillary and nerve network and can be used as an IVB for bone graft prefabrication. As a model for the IVB strategy, muscle tissue is usually used to wrap the scaffold in the form of an intramuscular pouch or a pedicled flap to induce ectopic neurovascularization and bone regeneration. This strategy was first reported in 1991 when Khouri et al. placed thigh adductor muscle island flaps and allogenic demineralized bone matrix (DBM) in silicone rubber molds. After a 10-day in vivo cultivation period, the muscular flaps transformed into cancellous bone grafts shaped identically to the mold (Khouri et al., 1991). Since this technique was first reported, it has developed rapidly and been well investigated in subsequent studies. To date, in addition to numerous experimental studies, at least 4 clinical papers have reported promising results for bone graft prefabrication using a muscular pouch or flap as an IVB (Warnke et al., 2004, Heliotis et al., 2006, Mesimäki et al., 2009, Kokemueller et al., 2010).
One of the most important advantages for the muscular strategy is that muscle provides plenty of native skeletal progenitor cells that can be differentiated into bone cells. Therefore, even without the transplantation of exogenous osteoprogenitor/stem cells, regeneration of large volume bone tissue can be achieved in an intramuscular environment (Liu et al., 2014). Another critical advantage of this approach is the presence of bone-forming molecules that spread out from the site of muscle injury and lead to natural upregulation of osteogenic signals, including BMPs, transforming growth factor-beta 1 (TGF-β1), and insulin-like growth factor 1 (IGF-1) (Scott et al., 2012).
2.3.3. Periosteal Flap
Periosteum is a thin but highly vascularized and innervated tissue with a bilayer structure: the outer fibrous layer contains fibroblasts dispersed between collagen fibers and is thought to serve a primarily structural role, whereas the inner cambium layer consists primarily of skeletal progenitor cells and osteoblasts that possess a remarkable capacity to allow appositional bone growth as well as cortical bone modeling and remodeling. In light of these findings, surgeons have harnessed this osteogenic capacity in bone graft prefabrication research to induce bone formation. In the periosteal flap strategy, a periosteal flap is used to wrap the tissue-engineered construct or to cover the chamber containing the tissue-engineered construct. Therefore, the periosteum-construct composite forms an IVB to provide pluripotent cells and bimolecular signals for the process of bone formation, which finally results in a wound-healing response within the space and culminates in neo-bone formation as opposed to a fibrotic scar. Several significant features of the periosteal flap indicate that it is an excellent strategy for bone graft prefabrication. First, the cambium layer of the periosteum provides a reservoir for periosteum-derived mesenchymal stem cells (Chang and Knothe, 2012). Second, the periosteum can synthesize many growth factors, including BMPs, TGF-β1, IGF-1, vascular endothelial growth factor (VEGF), and stromal derived factor 1 (Cuthbert et al., 2013). Third, implants placed in the periosteum envelope are exposed to a highly neurovascular environment and to increased blood/nerve-borne nutrients. Fourth, this strategy of wrapping the implants within a periosteal envelope utilizes the guided bone regeneration treatment concept, which has been proven safe and effective in guiding the bone regeneration process (Dimitriou et al., 2012). However, the periosteal flap strategy also has limitations. One of the most important considerations is the lack of an adequate donor site to offer a large periosteal flap for the prefabrication of large or geometry-customized bone grafts.
2.3.4. Axial Vascular Bundle (AVB)
Unlike the random blood vessel pattern in the above-mentioned subcutaneous pockets and tissue flaps, the AVB strategy is an intrinsic axial osteogenesis and vascularization model for bone graft prefabrication. In this model, an artery and vein are inserted centrally within a scaffold to transport progenitor/stem cells, cytokines, oxygen, and nutrients and to remove waste products from the cells, resulting in extensive vascularization and osteogenesis of the scaffold. The result of the AVB-based prefabrication strategy is an axial pattern bone graft that can be transferred to the bone defect site as a pedicled or free flap. Many reports have described the use of superficial inferior epigastric vessels, saphenous vascular bundles, femoral vascular bundles, and perforating vessels from the thoracodorsal trunk as IVBs to prefabricate bone grafts. These promising results have demonstrated the feasibility of this strategy for use in clinical patients.
Although the AVB supplies progenitor/stem cells from the whole body, due to the small contact surface between the AVB and the scaffold, achieving a well-ossified and well-vascularized bone graft within a certain prefabrication period is difficult. An alternative solution is to envelop the scaffold within an above-mentioned tissue flap, such as a muscular flap or a periosteal flap. The combined use of the AVB and tissue flap to form an IVB is advantageous due to the utilization of two well-established bone graft prefabrication strategies. We previously prefabricated vascularized bone grafts by inserting a saphenous vascular bundle into a beta tricalcium phosphate (β-TCP) scaffold wrapped within a local muscularis membrane (Han and Dai, 2013) or a periosteal flap (Han et al., 2014). The results showed a plentiful capillary network, chondrocytes, and lamella bones in the regenerated grafts. Another challenge in clinical translation of the AVB strategy is the lack of donor AVBs. This strategy requires a long length of artery and vein located at a superficial site for use as a flap pedicle for distant transfer.
2.3.5. Arteriovenous Loop (AVL)
Inadequate adjacent arterial supply and venous outflow due either to the injury or to preexisting vascular disease may preclude the use of an AVB as an IVB. In such a case, the AVL can be employed for bone graft prefabrication. Typically, an AVL is constructed by the direct microsurgical anastomosis of an artery and a vein or by the interposition of a venous graft between an artery and a vein to form an arteriovenous fistula. The AVL is then placed inside a chamber containing a scaffold to create an IVB. A perfused capillary network will sprout from a central vessel into the surroundings and remodel to generate arterioles, post-capillary venules, and venules, finally resulting in accelerated vascularization and new tissue formation (Lokmic et al., 2007). This method was first described by Erol and Spira, who successfully constructed a prefabricated skin graft in rats using an AVL between femoral vessels (Erol and Spira, 1980). Employing the AVL strategy, formation of a significant neovascular network and new tissue, such as cartilage tissue (von Bomhard et al., 2013), cardiac tissue (Morritt et al., 2007), and muscle tissue (Bach et al., 2006), originating from the arteriovenous fistula have been described.
The superiority of the AVL strategy over the AVB strategy in terms of both axial angiogenesis and osteogenesis has been clearly demonstrated. Compared with the AVB strategy, the AVL strategy showed a greater potential for producing capillaries and new tissue and was considered more effective for the development of vascularization and osteogenesis in bone graft prefabrication (Dong et al., 2012a). Theoretically, three potential mechanisms are considered responsible for the accelerated angiogenesis: (1) local inflammation due to the surgical trauma on the vessels promotes the release of inflammatory factors and the development of the capillary network, (2) elevated vascular flow shear stress on the venous wall due to arterialization promotes the growth of collateral vessels and increases the number of microvessels, and (3) gradient hypoxia within the matrix leads to the upregulation of hypoxia-inducible factor 1 and the expression of several angiogenic factors. Subsequently, prevascularization of the scaffolds helps to recruit, proliferate, and differentiate progenitor/stem cells, consequently optimizing bone formation in the scaffold. However, the osteogenic capacity of the AVL also depends on the accompanying bioactive scaffold. A higher extent of vascularization but a lower yield of bone have also been observed for the AVL strategy due to faster β-TCP scaffold degradation (Wu et al., 2015b). Scaffolds with a slower biodegradation rate, such as β-TCP/hydroxyapatite (HA) (Eweida et al., 2014) and processed cancellous bone matrix (Arkudas et al., 2007), continue to be used for bone graft prefabrication.
In addition, the AVL strategy can be created at any surgical site and transferred as a free flap using microsurgery techniques without considering the vascular pedicle length, which greatly expands the clinical application potential of this technique. Furthermore, a modified AVL strategy in which the arteriovenous fistula is placed in a special porous chamber, allowing the connection of extrinsic vessels with intrinsic vessels over time, showed significantly increased angiogenesis and bone regeneration (Weigand et al., 2015). Extrinsic vessels contribute to faster vascularization and finally anastomose with the intrinsic vasculature, reducing the period of bone graft prefabrication and the limitation of operative interventions using only the AVL strategy. However, this method remains highly challenging because most load-bearing bone grafts are not suited to be molded or shaped around the AVL.
3. Literature Review
To analyze the current state of the art, we searched the PubMed Medline databases for articles published between Jan. 1, 1991, and Jan. 1, 2016, using the search terms “in vivo bioreactor”, “in vivo tissue engineering”, “bone tissue engineering”, “bone graft prefabrication”, and “bone regeneration”. We included 110 publications regarding bone graft prefabrication following the IVB principle (Fig. 4). We categorized all the publications into small animal models, large animal models, and clinical studies to evaluate the safety, efficacy, and cost effectiveness of the IVB strategies. The studies published within the last 5 years are summarized in Table 1, Table 2, Table 3.
Fig. 4.
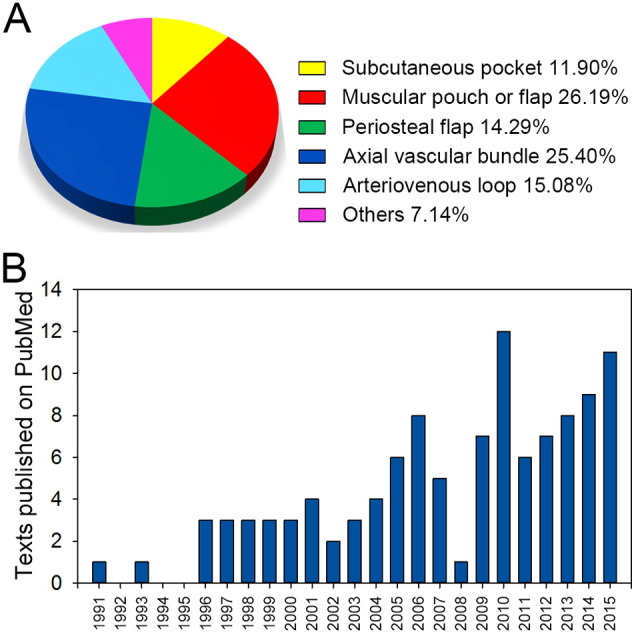
(A) Published articles on bone graft prefabrication following the IVB principle from Jan. 1, 1991, to Jan. 1, 2016, on PubMed. (B) Break-down of the published articles according to IVB strategies.
Table 1.
Recent bone graft prefabrication studies in small animal models.
| Reference/year | Animal model | IVB | Scaffold | Seed cells | Growth factor |
|---|---|---|---|---|---|
| Ersoy et al., 2015 | Rat | Periosteal flap | Bioactive glass or HA within a Gore-Tex pocket | None | None |
| Patel et al., 2015a | Mouse | Subcutaneous pocket | PCL | None | BMP-2 |
| Patel et al., 2015b | Mouse | Subcutaneous pocket | PCL | None | BMP-2 and EPO |
| Buehrer et al., 2015 | Rat | AVL between FVs | HA, β-TCP, and fibrin gel | MSCs | BMP-2 |
| Sathy et al., 2015a, Sathy et al., 2015b | Mouse | Subcutaneous pocket | PCL, HA, and gelatin | MSCs | None |
| Wu et al., 2015a | Mouse | Subcutaneous pocket | β-TCP and CHA | BMSCs | None |
| Han et al., 2014 | Rabbit | Tibial periosteal flap combined with AVB from SVs | β-TCP | BMSCs | None |
| Han and Dai, 2013 | Rabbit | Muscular membrane combined with AVB from SVs | β-TCP | BMP-2-transfected BMSCs | None |
| Nakamura et al., 2013 | Rat | AVB from SVs | Bone allografts | None | BMP-2 |
| Rodrigues et al., 2013 | Rat | AVB from SIEVs | Deep-frozen bone or lyophilized-demineralized bone | None | None |
| Yang et al., 2013 | Rat | AVB from SIEVs | nHA-PA 66 | Endothelial induced ADSCs | None |
| Cai et al., 2013 | Rabbit | AVB from FVs | Fibrin matrix within an inion membrane tube | None | BMP-2 |
| Scotti et al., 2013 | Mouse | Subcutaneous pocket | Type I collagen mesh | Chondrogenic induced MSCs | None |
| Koca et al., 2012 | Rabbit | AVB or subcutaneous pocket | Human bone allografts | None | None |
| Dong et al., 2012c | Rabbit | AVB from SVs | β-TCP within a titanium cage | BMSCs | None |
| Sever et al., 2012 | Rat | AVB from SIEVs | PCHC | BMSCs | VEGF |
| Dong et al., 2012b | Rabbit | AVB from SVs | β-TCP within a titanium cage | BMSCs and PRP | None |
| Dong et al., 2012a | Rabbit | AVL between FVs | Natural coral blocks wrapped with an ePTFE membrane | None | None |
| Arkudas et al., 2012 | Rat | AVL between FVs | HA/β-TCP/fibrin matrix | None | None |
| Rath et al., 2011 | Rat | AVL between facial vessels | PLDLLA-TCP-PCL | Osteoblasts | BMP-2 |
| Kloeters et al., 2011 | Rabbit | AVB from inguinal vessels | PBCB | Osteogenic induced ADSCs | None |
| Farrell et al., 2011 | Mouse | Subcutaneous pocket | Collagen-GAG scaffolds | Osteogenic or chondrogenic induced hMSCs | None |
| Binderman et al., 2011 | Rat | Subcutaneous pocket | DBM | None | None |
| Sever et al., 2010 | Rat | AVB from SIEVs | HA | BMSCs | None |
| Okuda et al., 2010 | Rat | Fascia flap pedicled on SIEVs | β-TCP | Osteogenic induced ADSCs | None |
| Yachouh et al., 2010 | Rabbit | Femur periosteal flap pedicled on the descending artery of the knee | Irradiated bone fragment | None | None |
| Wang et al., 2010 | Rabbit | AVB from FVs | β-TCP | MSCs | None |
| Dong et al., 2010 | Rabbit | AVL between FVs | Natural coral blocks wrapped with an ePTFE membrane | None | None |
| Chang et al., 2010 | Rabbit | AVB from FVs | HA and collagen gel breads wrapped with an ePTFE membrane | MSCs | None |
| Kamei et al., 2010 | Rabbit | Omentum flap wrapped periosteum free graft | None | None | None |
| Arkudas et al., 2010 | Rat | AVL between FVs | HA/β-TCP granula | None | VEGF165 and bFGF |
ADSC, adipose-derived stem cells; AVB, axial vascular bundle; AVL, arteriovenous loop; bFGF, basic fibroblast growth factor; BMP-2, bone morphogenetic protein 2; BMSCs, bone marrow mesenchymal stem cells; β-TCP, beta-tricalcium phosphate; CHA, coralline hydroxyapatite; DBM, demineralized bone matrix; ePTFE, expanded-polytetrafluoroethylene; EPO, erythropoietin; FVs: femoral vessels; HA, hydroxyapatite ceramic; MSCs, mesenchymal stem cells; nHA-PA 66, nano-hydroxyapatite-polyamide 66; PBCB, processed bovine cancellous bone; PCHC, porous calcium hydroxyapatite ceramic; PCL, poly-ε-caprolactone; PLDLLA, poly(l-lactide-co-d,l-lactide); SIEVs: superficial inferior epigastric vessels; SVs: saphenous vessels; VEGF, vascular endothelial growth factor.
Table 2.
Recent bone graft prefabrication studies in large animal models.
| Reference/year | Animal model | IVB | Scaffold | Seed cells | Growth factor |
|---|---|---|---|---|---|
| Spalthoff et al., 2015 | Sheep | Muscular pouch in LDM combined with AVB from thoracodorsal trunk | β-TCP cylinder and MBG | BMA | None |
| Tatara et al., 2015 | Sheep | Cambium layer of rib periosteum | Combination of MBG, HA, and β-TCP within a PMMA chamber | None | None |
| Zhou et al., 2010, Zhou et al., 2015 | Monkey | Muscular pouch in LDM | DFBA or CHA | None | BMP-2 |
| Weigand et al., 2015 | Sheep | AVL between SVs | NanoBone (HA) block | Autologous blood | None |
| Wu et al., 2015b | Dog | AVL between SVs or AVB from SVs | β-TCP cylinder | BMSCs | None |
| Eweida et al., 2011, Eweida et al., 2012, Eweida et al., 2014 | Goat | AVL between facial vessels | 60% HA combined 40% β-TCP | PRP | BMP-2 |
| Liu et al., 2014 | Pig | Muscular pouch in LDM | NBBM or a mixture of NBBM and autogenous bone particles within a titanium cage | None | BMP-7 and VEGF |
| Kokemüller et al., 2014 | Sheep | Muscular pouch in LDM combined with AVB from thoracodorsal trunk | β-TCP and unmodified osteogenic material from the iliac crest within a titanium cage | BMA, NCC, or BMSCs | None |
| Zhi et al., 2014 | Dog | Abdominal cavities or dorsal muscles | HA | None | None |
| Chen et al., 2014 | Dog | Muscular pouch in LDM | DBM | BMSCs or PRP | None |
| Tsao et al., 2014 | Dog | Omentum flap wrapped periosteum-free graft | None | None | ADSCs |
| Boos et al., 2013 | Sheep | AVL between SVs | β-TCP-HA granules | BMSCs | BMP-2 |
| Bigham-Sadegh et al., 2013 | Dog | Omentum flap wrapped periosteum-free graft | None | None | None |
| Beier et al., 2011 | Sheep | AVL between greater SVs | PBCB construct | None | None |
| Kokemueller et al., 2010 | Sheep | Muscular pouch in LDM combined with AVB from thoracodorsal trunk | β-TCP | BMA | None |
| Runyan et al., 2010 | Pig | Periosteal envelope or rectus abdominis muscle with insertion of AVB from SIEVs | Processed allogeneic hemimandible | ADSCs | BMP-2 |
| Beier et al., 2010 | Sheep | AVL between SVs | HA and β-TCP | None | None |
ADSC, adipose-derived stem cells; AVB, axial vascular bundle; AVL, arteriovenous loop; BMA, bone marrow aspirate; BMP-2, bone morphogenetic protein 2; BMP-7, bone morphogenetic protein 7; BMSCs, bone marrow mesenchymal stem cells; β-TCP, beta-tricalcium phosphate; CHA, coralline hydroxyapatite; DBM, demineralized bone matrix; DFBA, demineralized freeze-dried bone allograft; HA, hydroxyapatite ceramic; LDM: latissimus dorsi muscle; MBG, morcellized bone graft; NCC, nucleated cell concentrate; PBCB, processed bovine cancellous bone; PMMA, poly(methyl methacrylate); PRP, platelet-rich plasma; SIEVs: superficial inferior epigastric vessels; SVs: saphenous vessels; VEGF, vascular endothelial growth factor.
Table 3.
Bone graft prefabrication studies in clinical cases.
| Reference/year | Defect treated | No. of patients | IVB | Prefabrication time | Scaffold | Seed cells | Growth factor | Outcome and follow-up time |
|---|---|---|---|---|---|---|---|---|
| Horch et al., 2014 | Tibial defect | 1 | AVL between popliteal vessels | N/A (in situ prefabrication) | Iliac crest autografts | None | None | N/A; 5.5 years |
| Radialis defect | 1 | AVL between radial artery and cephalic vein | β-TCP and HA | BMA | None | N/A; 32 mo | ||
| Kokemueller et al., 2010 | Mandibular defect | 1 | Muscular pouch in LDM combined with AVB from thoracodorsal trunk | Over 6 mo | β-TCP and iliac crest autografts | BMA | None | N/A; 12 mo |
| Mesimäki et al., 2009 | Maxillary defect | 1 | Muscular pouch in rectus abdominis muscle | 8 mo | β-TCP within a titanium cage | ADSCs | BMP-2 | Osseointegrated without adverse events; 12 mo |
| Cheng et al., 2006 | Mandibular defect | 1 | Cambium layer of iliac periosteum | 8 wk | Iliac crest autografts within a PMMA chamber | None | None | Increased mandible height; died of unrelated cancer after 16 mo |
| Heliotis et al., 2006 | Mandibular defect | 1 | Muscular pouch in pectoralis major muscle | 6.5 mo | HA | None | BMP-7 | Infection and necrosis; 5 mo |
| Warnke et al., 2004, Warnke et al., 2006b | Mandibular defect | 1 | Muscular pouch in LDM | 7 wk | BioOsss blocks within a titanium cage | BMA | BMP-7 | Infection and revision; died of cardiac arrest after 15 mo |
| Orringer et al., 1999 | Mandibular defect | 1 | Thin scapular fasciocutaneous flap | 4 mo | Iliac crest autografts within a Dacron-polyurethane cage | None | BMP | N/A; died of recurrence after 2 years |
ADSC, adipose-derived stem cells; AVB, axial vascular bundle; AVL, arteriovenous loop; BMA, bone marrow aspirate; BMP-2, bone morphogenetic protein 2; BMP-7, bone morphogenetic protein 7; β-TCP, beta-tricalcium phosphate; LDM: latissimus dorsi muscle; PMMA, poly(methyl methacrylate).
3.1. Small Animal Models (Table 1)
In this review, small animal models are defined as mice, rats, and rabbits. These studies took advantage of small animals because they are inexpensive to purchase and maintain, because surgeries can easily be performed on them, and because promising results have been observed. The first study of bone graft prefabrication was performed in Lewis rats in 1991 by Khouri et al. Although the concept of bone graft prefabrication following the IVB principle was not clearly elucidated in that paper, the authors described an effective muscular flap strategy for bone graft prefabrication (Khouri et al., 1991). This landmark study proposed the “bone transformation” concept at a clinically available level that matched the current concept of bone graft prefabrication following the IVB principle. Subsequently, bone grafts with geometric shapes and even composite tissue layers were successfully prefabricated in small animal models using different IVB strategies. However, due to the size of small animals, testing the diffusion efficacy and osteogenic and angiogenic capacities of the IVBs is difficult. Furthermore, while validating a new concept or technique in small animal models is feasible, the limitations to the prefabrication of clinically relevant bone grafts with large dimensions and precise shapes in small animal models have always been a challenge to the successful translation of experimental achievements to clinical applications.
3.2. Large Animal Models (Table 2)
After successful results were reported for small animal models, large animal models, including sheep, mini pigs, dogs, and monkeys, were developed to verify the practicability of the IVB strategies closer to real clinical situations. Latissimus dorsi muscle, rib periosteum, and AVL are the most commonly used IVB strategies in these models. Since 1999, Terheyden and his team have reported a series of studies on bone graft prefabrication using the latissimus dorsi muscle as an IVB in mini pigs (Warnke et al., 2006a, Terheyden et al., 1999, Terheyden et al., 2001a, Terheyden et al., 2001b, Terheyden et al., 2004). In light of the promising results in these studies, Warnke et al. translated this strategy into a clinical patient (Warnke et al., 2004, Warnke et al., 2006b). Another extensively studied strategy in large animals is the AVL model, which has been validated in small animal models. By scaling up the AVL model, clinically approved, mechanically stable bone grafts with a significant volume were prefabricated in sheep or goat models, implying the feasibility of clinical translation in future work (Beier et al., 2011, Beier et al., 2010, Boos et al., 2013). Using the AVL strategy, Eweida et al. improved the commonly used ectopic prefabrication approach to the in situ prefabrication approach and tested its clinical translation possibility in goats (Eweida et al., 2011, Eweida et al., 2012, Eweida et al., 2014). The results showed that the AVL strategy induced better vascularization at the central regions and permitted more efficient bone regeneration. This in situ bone graft prefabrication strategy that used the AVL strategy was also successfully translated into clinical patients with critical-sized long bone defects (Horch et al., 2014). Although, large animal studies have shown exciting results in prefabricating vascularized bone grafts with complex geometry and clinically relevant sizes, further efforts are required to optimize the prefabrication time, implantation location, and biomaterial application.
3.3. Clinical Cases (Table 3)
To date, only 7 scientific papers have reported 8 cases of bone defect reconstruction following the IVB principle in humans. The groundbreaking case was performed in 1990 but reported in 1999 by Orringer et al. (1999). A female patient who suffered a subtotal mandibular defect underwent a bone graft prefabrication procedure for mandibular and total lower lip reconstruction. In this case, a mandibular-shaped osteocutaneous flap was successfully prefabricated using a thin scapular fasciocutaneous flap as an IVB and cancellous bone grafts from the iliac crest as scaffold. Although autologous bone grafts were used in this case, magnetic resonance imaging revealed excellent ossification in the flap during the prefabrication phase, indicating the capacity of new bone regeneration in the fasciocutaneous flap. This work provided important inspiration showing that prefabricating a clinically-relevant bone graft following the IVB principle was clinically possible.
The most commonly used strategy in clinical cases is the muscular pouch or flap. In total, 4 of the 7 publications used this strategy and yielded promising results. Following their initial successes using the latissimus dorsi muscle as an IVB in pigs, Warnke et al. reported the growth and transplantation of a vascularized mandible in a patient with an extended mandibular discontinuity defect (Warnke et al., 2004, Warnke et al., 2006b). In this case, an artificially created bone flap was regenerated in the latissimus dorsi muscle by implanting a well-designed scaffold. After 7 weeks, the muscle-bone flap pedicled on the thoracodorsal vessels was harvested and transferred to fill the mandibular defect. Bone scintigraphy showed active bone formation and remodeling during the prefabrication phase and after transplantation, indicating undisturbed vascular perfusion as well as survival of the induced bone cells. This patient gained both functional and esthetic mandibular reconstruction. This landmark case offered an innovative method for designing customized bone grafts and avoiding the creation of secondary bone defects. Inspirited by these promising results, researchers continued to improve the muscle strategy in clinical application. Heliotis et al. successfully transformed a HA/OP-1 composite construct into a vascularized bone graft by implanting this construct into the pectoralis major muscle in a female patient with a hemimandibular defect (Heliotis et al., 2006). This case is notable for the successful prefabrication of a bone flap without the additional use of autologous elements. The next case presented by Mesimäki et al. was a male patient who underwent hemimaxillectomy (Mesimäki et al., 2009). A custom-made bone flap was prefabricated by implanting a preformed titanium cage filled with ADSCs, β-TCP, and BMP-2 into the rectus abdominis muscle. After 8 months, the prefabricated bone flap was placed into the maxillary defect and finally osseointegrated to the host maxillary bone without any adverse events. This clinical case is the first in which ectopic bone formation was produced using good manufacturing practice level ADSCs. Kokemueller et al. improved the muscular strategy by inserting an AVB from the thoracodorsal trunk into the β-TCP cylinders before intramuscular implantation (Kokemueller et al., 2010). Before clinical translation, the AVB-muscle strategy showed excellent angiogenesis, ossification, and ceramic resorption inside the scaffold compared to the muscular pouch strategy in sheep. These results encouraged the authors to forgo the additional use of growth factors and to translate this strategy into patients.
The periosteal flap strategy and AVL strategy have also been applied in clinical cases. To augment mandible height during reconstruction, Cheng et al. fixed a chamber filled with morcellized autografts against the cambium layer of the rib periosteum for 8 weeks (Cheng et al., 2006). Solid bone graft extending from the periosteum was transferred to augment the mandible height. The results revealed well-retained osseointegrated dental implants in the prefabricated bone graft until 16 months later. This case demonstrated the osteogenic and angiogenic capacity of the periosteal flap for clinical bone graft prefabrication. Recently, Horch et al. reported their successful experience with long bone defect reconstruction using the AVL strategy (Horch et al., 2014). For osseous reconstruction, critical-sized bone defects were filled with AVLs and morcellized autografts or β-TCP/HA. Long-term follow-up examinations showed functional osseous reconstruction without any osteosynthetic fixation. The salient results of the study were as follows: 1) this report was the first in situ bone prefabrication study regarding the functional reconstruction of large bone defects of extremities at mechanical loading sites, and 2) this strategy avoided the creation of significant donor-site defects and second operations. However, bone defects caused by tumor sectioning, severe trauma, chronic infection or undergoing radiation treatments are unsuitable for this strategy due to the impaired regenerative microenvironment in the surrounding tissue.
4. Discussion: Future Trends and Challenges
4.1. Is the In Vitro Manipulation of Cells, Growth Factors, and even Scaffolds still Needed?
In the traditional BTE paradigm, seed cells, growth factors, and bioscaffolds are essential elements for bone regeneration. However, in vitro manipulation of these elements limits clinical translation due to the diffusional challenge, insufficient vascularization, and seed cell survival. Following the IVB principle, the seed cells and growth factors can be supplied by the surrounding microenvironment or even the entire body. Lee et al. reported a proof-of-concept study, a TGF-β3 spatially embedded scaffold was used for homing endogenous cells to the scaffold and inducing differentiation of these cells to regenerate articular cartilage (Lee et al., 2010). In addition, several researchers have introduced their successful experiences in new bone regeneration achieved by only a scaffold-based in vivo bioreactor without exogenous cells and growth factors (Stevens et al., 2005, Gao et al., 2016). These studies revealed the possibility of using the human body as a living bioreactor for growth and transplant of complex tissue or organ without cell transplantation and growth factor administration.
To date, the bioscaffold seemed to be an indispensable component of bone graft prefabrication. However, a breakthrough was observed in two promising studies in which bone formation occurred in an IVB consisting of a periosteal-free graft and an omentum flap without bioscaffolds, seed cells, and growth factors (Kamei et al., 2010, Bigham-Sadegh et al., 2013). Furthermore, numerous studies have demonstrated that the periosteum has the capacity to regenerate osseous-like tissue at ectopic sites without the manipulation of these exogenous elements (Chen et al., 2009). Similarly, heterotopic ossification, a pathophysiological phenomenon of ectopic bone formation, actually shows the body's bone regeneration capacity without these elements (Alfieri et al., 2012). Therefore, the regeneration of a large volume of bony tissue in vivo using a scaffold-free strategy may be feasible but requires considerable further development.
Theoretically, an ideal IVB strategy minimizes the use of exogenous scaffolds, seed cells, and growth factors, although many studies still use these elements to enhance bone regeneration (Huang et al., 2016). Current studies have focused on the local microenvironment and surgical techniques, such as searching for an adequate implantation site to maximize the body's own self-regenerative capacity, optimizing the prefabrication time to achieve a fine balance between bone regeneration and remodeling, and stimulating the route of differentiation undertaken by osteogenic cells (Fig. 5).
Fig. 5.
Further trends of bone defect reconstruction using prefabricated bone grafts.
4.2. Is Current Evidence Adequate for Clinical Translation?
Although the experimental evidence is well established and the preliminary results from clinical studies are encouraging, several points still prevent the safe and widespread application of the IVB principle for bone graft prefabrication in humans. Functional reconstruction of bone defects requires the regenerated bone grafts to possess mechanical strength, microstructure, and function as similar to native bone tissue as possible (Guilak and Baaijens, 2014). However, ectopically regenerated bone grafts remain hardly equivalent to the native bone tissue in histological structure. For instance, only 17% of the bone graft prefabricated in Heliotis' clinical case was bone tissue, while the remaining tissue was 37% HA and 46% fibrovascular tissue. Finally, insufficient soft and bony tissue and high HA content in the prefabricated bone graft resulted in necrosis of the bone flap (Heliotis et al., 2006). Furthermore, the healing potential in humans differs from that in animals and occurs with various speeds in different bones. Therefore, the prefabrication time and location of different IVB strategies have not yet been defined in humans. In addition, bone defects in humans may be more complicated in terms of precise geometry and tissue phenotype than that artificially created in animal models. Although anatomically shaped bone grafts with high fidelity and corresponding tissue phenotypes have been artificially regenerated in animals, additional evidence is required regarding continued bone growth and remodeling of the transferred bone graft, osseointegration with the host bone tissue, and long-term follow-up of functional recovery.
Another major issue affecting clinical translation is that the IVB strategies for bone defect reconstruction seem to be more complex than the current clinically adopted approaches. Patients may need multiple operations to achieve predictable outcomes. Furthermore, a perfect surgical schedule, good overall health conditions, and sound cooperation between the patient and surgeons are required. Extensive efforts should be devoted to simplifying the surgical procedure and to improving the cost effectiveness of this method.
4.3. Is it Feasible to Regenerate Complex Tissues, even Whole Organs, Following the IVB Principle?
Recent results in bone graft prefabrication have offered a potential solution to functionally replace complex tissues and whole organs following the IVB principle. This task becomes more complicated when the target tissue is vascularized and composed of a hierarchical organization of heterogeneous tissues. Successful proof of the IVB principle has been shown for complex tissues such as the trachea (Tsao et al., 2014), full-thickness skin (Eriksson and Vranckx, 2004), bladder (Baumert et al., 2007b), esophagus (Badylak et al., 2011), and skeletal muscle (Bitto et al., 2013); and for organs such as the liver (Zhao et al., 2010), heart (Ott et al., 2008), and lung (Wu et al., 2013). However, the unmet clinical demands in these areas and the barriers to clinical translation in existing demonstrations are well known.
Continued challenges for prefabrication of functional complex tissues or organs will focus on solving the following issues: an adequate IVB strategy to provide a correct regenerative microenvironment, and a sophisticated bioactive scaffold for maintaining tissue microarchitecture and providing biological signals. Several studies have introduced IVB strategies for the initial regeneration of complex tissue. Choi et al. used the omentum of adult rats to cultivate a tubular scaffold seeded with full-thickness plugs of intestine (Choi et al., 1998). These organoid units became vascularized and formed a cyst that histologically resembled the small bowel. This model of in vivo maturation using the omentum as an IVB also enabled the generation of a mature neoureter composed of a well-differentiated multilayered urothelium (Baumert et al., 2007a, Baumert et al., 2007b). Additionally, recent advances in the tissue decellularization technique (Arenas-Herrera et al., 2013) have established a foundation for the functional replacement of whole organs with complex tissues and microstructures. The technique removes cells from allogeneic or xenogeneic whole organs but maintains their functional architecture to produce an off-the-shelf 3D organ scaffold. The decellularized organ scaffold then serves as an inductive biological template around which the recipient rebuilds functional tissue through the recruitment of endogenous cells following the IVB principle (Badylak et al., 2012). This impressive feat has been emulated by successful whole large organ reengineering, including the lung (Petersen et al., 2010), liver (Uygun et al., 2010), kidney (Caralt et al., 2015), and limb (Jank et al., 2015). Another key advantage of the decellularized organ is the ready availability of an intact vascular network that can be connected to the recipient's vascular system by a combination of IVB strategies such as AVB and AVL. We believe the current challenges of complex tissue or whole organ prefabrication will be solved in the future by following the IVB principle and employing other technological advances.
5. Conclusion
Comprehensively, these initial results demonstrated that the IVB strategy can be considered a highly promising approach for bone graft prefabrication and subsequent bone defect reconstruction. Although limited success has been observed in clinical cases, this strategy offers a tremendous advantage over the traditional BTE approaches and addresses several significant bottlenecks in clinical translation of BTE. Nevertheless, research in this field is ongoing, with evidence being primarily gained from preclinical studies. Before universal clinical application can be recommended, future research should aim to develop a simplified but effective approach for bone graft prefabrication and translate it to clinical use as rapidly and as safely as possible.
Acknowledgments
This research was supported by grants from the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (Grant No. 2012BA/11B03), the State Key Program of the National Natural Science Foundation of China (Grant No. 81230042), and the National Natural Science Foundation of China (Grant No. 81501679).
References
- Alfieri K.A., Forsberg J.A., Potter B.K. Blast injuries and heterotopic ossification. Bone Joint Res. 2012;1:192–197. doi: 10.1302/2046-3758.18.2000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Herrera J.E., Ko I.K., Atala A., Yoo J.J. Decellularization for whole organ bioengineering. Biomed. Mater. 2013;8:014106. doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- Arkudas A., Beier J.P., Heidner K., Tjiawi J., Polykandriotis E., Srour S., Sturzl M., Horch R.E., Kneser U. Axial prevascularization of porous matrices using an arteriovenous loop promotes survival and differentiation of transplanted autologous osteoblasts. Tissue Eng. 2007;13:1549–1560. doi: 10.1089/ten.2006.0387. [DOI] [PubMed] [Google Scholar]
- Arkudas A., Beier J.P., Pryymachuk G., Hoereth T., Bleiziffer O., Polykandriotis E., Hess A., Gulle H., Horch R.E., Kneser U. Automatic quantitative micro-computed tomography evaluation of angiogenesis in an axially vascularized tissue-engineered bone construct. Tissue Eng. C Methods. 2010;16:1503–1514. doi: 10.1089/ten.tec.2010.0016. [DOI] [PubMed] [Google Scholar]
- Arkudas A., Pryymachuk G., Beier J.P., Weigel L., Körner C., Singer R.F., Bleiziffer O., Polykandriotis E., Horch R.E., Kneser U. Combination of extrinsic and intrinsic pathways significantly accelerates axial vascularization of bioartificial tissues. Plast. Reconstr. Surg. 2012;129:55e–65e. doi: 10.1097/PRS.0b013e3182361f97. [DOI] [PubMed] [Google Scholar]
- Bach A.D., Arkudas A., Tjiawi J., Polykandriotis E., Kneser U., Horch R.E., Beier J.P. A new approach to tissue engineering of vascularized skeletal muscle. J. Cell. Mol. Med. 2006;10:716–726. doi: 10.1111/j.1582-4934.2006.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak S.F., Hoppo T., Nieponice A., Gilbert T.W., Davison J.M., Jobe B.A. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng. A. 2011;17:1643–1650. doi: 10.1089/ten.tea.2010.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak S.F., Weiss D.J., Caplan A., Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- Baumert H., Mansouri D., Fromont G., Hekmati M., Simon P., Massoud W., Molinié V., Malavaud B. Terminal urothelium differentiation of engineered neoureter after in vivo maturation in the “omental bioreactor”. Eur. Urol. 2007;52:1492–1498. doi: 10.1016/j.eururo.2007.04.098. [DOI] [PubMed] [Google Scholar]
- Baumert H., Simon P., Hekmati M., Fromont G., Levy M., Balaton A., Molinié V., Malavaud B. Development of a seeded scaffold in the great omentum: feasibility of an in vivo bioreactor for bladder tissue engineering. Eur. Urol. 2007;52:884–890. doi: 10.1016/j.eururo.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Beier J.P., Horch R.E., Hess A., Arkudas A., Heinrich J., Loew J., Gulle H., Polykandriotis E., Bleiziffer O., Kneser U. Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J. Tissue Eng. Regen. Med. 2010;4:216–223. doi: 10.1002/term.229. [DOI] [PubMed] [Google Scholar]
- Beier J.P., Hess A., Loew J., Heinrich J., Boos A.M., Arkudas A., Polykandriotis E., Bleiziffer O., Horch R.E., Kneser U. De novo generation of an axially vascularized processed bovine cancellous-bone substitute in the sheep arteriovenous-loop model. Eur. Surg. Res. 2011;46:148–155. doi: 10.1159/000324408. [DOI] [PubMed] [Google Scholar]
- Bigham-Sadegh A., Oryan A., Mirshokraei P., Shadkhast M., Basiri E. Bone tissue engineering with periosteal-free graft and pedicle omentum. ANZ J. Surg. 2013;83:255–261. doi: 10.1111/j.1445-2197.2012.06316.x. [DOI] [PubMed] [Google Scholar]
- Binderman I., Yaffe A., Zohar R., Benayahu D., Bahar H. Tissue engineering of bone: an ectopic rat model. Front. Biosci. (Schol. Ed.) 2011;3:61–68. doi: 10.2741/s132. [DOI] [PubMed] [Google Scholar]
- Bitto F.F., Klumpp D., Lange C., Boos A.M., Arkudas A., Bleiziffer O., Horch R.E., Kneser U., Beier J.P. Myogenic differentiation of mesenchymal stem cells in a newly developed neurotised AV-loop model. Biomed. Res. Int. 2013;2013:935046. doi: 10.1155/2013/935046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos A.M., Loew J.S., Weigand A., Deschler G., Klumpp D., Arkudas A., Bleiziffer O., Gulle H., Kneser U., Horch R.E., Beier J.P. Engineering axially vascularized bone in the sheep arteriovenous-loop model. J. Tissue Eng. Regen. Med. 2013;7:654–664. doi: 10.1002/term.1457. [DOI] [PubMed] [Google Scholar]
- Borud L.J., Shaw W.W., Brunicardi F.C., Mullen Y., Passaro E.P., Jr. Prefabrication of a neo-endocrine organ: a rat model. J. Surg. Res. 1996;61:221–226. doi: 10.1006/jsre.1996.0108. [DOI] [PubMed] [Google Scholar]
- Buehrer G., Balzer A., Arnold I., Beier J.P., Koerner C., Bleiziffer O., Brandl A., Weis C., Horch R.E., Kneser U., Arkudas A. Combination of BMP2 and MSCs significantly increases bone formation in the rat arterio-venous loop model. Tissue Eng. A. 2015;21:96–105. doi: 10.1089/ten.tea.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W.X., Zheng L.W., Weber F.E., Li C.L., Ma L., Ehrbar M., Zwahlen R.A. Heterotopic bone formation around vessels: pilot study of a new animal model. Biores. Open Access. 2013;2:266–272. doi: 10.1089/biores.2013.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caralt M., Uzarski J.S., Iacob S., Obergfell K.P., Berg N., Bijonowski B.M., Kiefer K.M., Ward H.H., Wandinger-Ness A., Miller W.M., Zhang Z.J., Abecassis M.M., Wertheim J.A. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am. J. Transplant. 2015;15:64–75. doi: 10.1111/ajt.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H., Knothe T.M.L. Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl. Med. 2012;1:480–491. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.H., Tung K.Y., Wang Y.J., Tsao Y.P., Ni T.S., Liu H.K. Fabrication of vascularized bone grafts of predetermined shape with hydroxyapatite-collagen gel beads and autogenous mesenchymal stem cell composites. Plast. Reconstr. Surg. 2010;125:1393–1402. doi: 10.1097/PRS.0b013e3181d62aab. [DOI] [PubMed] [Google Scholar]
- Chen W.J., Zhang F., Mustain W.C., Tucci M., Hu E.C., Lineaweaver W.C. Prefabrication of vascularized bone flap by demineralized bone matrix. J. Craniofac. Surg. 2007;18:43–48. doi: 10.1097/SCS.0b013e31802ccf54. [DOI] [PubMed] [Google Scholar]
- Chen A.C., Lin S.S., Chan Y.S., Lee M.S., Ueng S.W. Osteogenesis of prefabricated vascularized periosteal graft in rabbits. J. Trauma. 2009;67:165–167. doi: 10.1097/TA.0b013e3181881338. [DOI] [PubMed] [Google Scholar]
- Chen T., Wang Y., Bu L., Li N. Construction of functional tissue-engineered bone using cell sheet technology in a canine model. Exp. Ther. Med. 2014;7:958–962. doi: 10.3892/etm.2014.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M., Brey E.M., Ulusal B.G., Wei F. Mandible augmentation for osseointegrated implants using tissue engineering strategies. Plast. Reconstr. Surg. 2006;118:1e–4e. doi: 10.1097/01.prs.0000221120.11128.1a. [DOI] [PubMed] [Google Scholar]
- Choi R.S., Riegler M., Pothoulakis C., Kim B.S., Mooney D., Vacanti M., Vacanti J.P. Studies of brush border enzymes, basement membrane components, and electrophysiology of tissue-engineered neointestine. J. Pediatr. Surg. 1998;33:991–996. doi: 10.1016/s0022-3468(98)90520-6. Discussion, 996-997. [DOI] [PubMed] [Google Scholar]
- Cuthbert R.J., Churchman S.M., Tan H.B., McGonagle D., Jones E., Giannoudis P.V. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone. 2013;57:484–492. doi: 10.1016/j.bone.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Dimitriou R., Mataliotakis G.I., Calori G.M., Giannoudis P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Med. 2012;10:81. doi: 10.1186/1741-7015-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q.S., Lin C., Shang H.T., Wu W., Chen F.L., Ji X.T., Liu Y.P., Zhang J.R., Mao T.Q. Modified approach to construct a vascularized coral bone in rabbit using an arteriovenous loop. J. Reconstr. Microsurg. 2010;26:95–102. doi: 10.1055/s-0029-1243293. [DOI] [PubMed] [Google Scholar]
- Dong Q.S., Shang H.T., Wu W., Chen F.L., Zhang J.R., Guo J.P., Mao T.Q. Prefabrication of axial vascularized tissue engineering coral bone by an arteriovenous loop: a better model. Mater. Sci. Eng. C Mater. Biol. Appl. 2012;32:1536–1541. doi: 10.1016/j.msec.2012.04.039. [DOI] [PubMed] [Google Scholar]
- Dong Z., Li B., Liu B., Bai S., Li G., Ding A., Zhao J., Liu Y. Platelet-rich plasma promotes angiogenesis of prefabricated vascularized bone graft. J. Oral Maxillofac. Surg. 2012;70:2191–2197. doi: 10.1016/j.joms.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Dong Z., Li B., Zhao J., Ma Q., Bai S., Yang W., Li G., Ma G., Liu Y. Prefabrication of vascularized bone grafts using a combination of bone marrow mesenchymal stem cells and vascular bundles with beta-tricalcium phosphate ceramics. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;114:S153–S159. doi: 10.1016/j.oooo.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Eriksson E., Vranckx J. Wet wound healing: from laboratory to patients to gene therapy. Am. J. Surg. 2004;188:36–41. doi: 10.1016/S0002-9610(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Erol O.O., Spira M. New capillary bed formation with a surgically constructed arteriovenous fistula. Plast. Reconstr. Surg. 1980;66:109–115. doi: 10.1097/00006534-198007000-00021. [DOI] [PubMed] [Google Scholar]
- Ersoy B., Bayramiçli M., Ercan F., Şirinoğlu H., Turan P., Numanoğlu A. Comparison of bone prefabrication with vascularized periosteal flaps, hydroxyapatite, and bioactive glass in rats. J. Reconstr. Microsurg. 2015;31:291–299. doi: 10.1055/s-0034-1396770. [DOI] [PubMed] [Google Scholar]
- Eweida A.M., Nabawi A.S., Marei M.K., Khalil M.R., Elhammady H.A. Mandibular reconstruction using an axially vascularized tissue-engineered construct. Ann. Surg. Innov. Res. 2011;5:2. doi: 10.1186/1750-1164-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eweida A.M., Nabawi A.S., Elhammady H.A., Marei M.K., Khalil M.R., Shawky M.S., Arkudas A., Beier J.P., Unglaub F., Kneser U., Horch R.E. Axially vascularized bone substitutes: a systematic review of literature and presentation of a novel model. Arch. Orthop. Trauma Surg. 2012;132:1353–1362. doi: 10.1007/s00402-012-1550-3. [DOI] [PubMed] [Google Scholar]
- Eweida A.M., Nabawi A.S., Abouarab M., Kayed M., Elhammady H., Etaby A., Khalil M.R., Shawky M.S., Kneser U., Horch R.E., Nagy N., Marei M.K. Enhancing mandibular bone regeneration and perfusion via axial vascularization of scaffolds. Clin. Oral Investig. 2014;18:1671–1678. doi: 10.1007/s00784-013-1143-8. [DOI] [PubMed] [Google Scholar]
- Farrell E., Both S.K., Odörfer K.I., Koevoet W., Kops N., O'Brien F.J., Baatenburg de Jong R.J., Verhaar J.A., Cuijpers V., Jansen J., Erben R.G., van Osch G.J. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet. Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Zhang H., Liu Y., Fan B., Li X., Xiao X., Lan P., Li M., Geng L., Liu D., Yuan Y., Lian Q., Lu J., Guo Z., Wang Z. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci. Rep. 2016;6:23367. doi: 10.1038/srep23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson W.L., Fröhlich M., Yeager K., Bhumiratana S., Chan M.E., Cannizzaro C., Wan L.Q., Liu X.S., Guo X.E., Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F., Baaijens F.P. Functional tissue engineering: ten more years of progress. J. Biomech. 2014;47:1931–1932. doi: 10.1016/j.jbiomech.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Han D., Dai K. Prefabrication of a vascularized bone graft with beta tricalcium phosphate using an in vivo bioreactor. Artif. Organs. 2013;37:884–893. doi: 10.1111/aor.12092. [DOI] [PubMed] [Google Scholar]
- Han D., Guan X., Wang J., Wei J., Li Q. Rabbit tibial periosteum and saphenous arteriovenous vascular bundle as an in vivo bioreactor to construct vascularized tissue-engineered bone: a feasibility study. Artif. Organs. 2014;38:167–174. doi: 10.1111/aor.12124. [DOI] [PubMed] [Google Scholar]
- Heliotis M., Lavery K.M., Ripamonti U., Tsiridis E., di Silvio L. Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest. Int. J. Oral Maxillofac. Surg. 2006;35:265–269. doi: 10.1016/j.ijom.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Holt G.E., Halpern J.L., Dovan T.T., Hamming D., Schwartz H.S. Evolution of an in vivo bioreactor. J. Orthop. Res. 2005;23:916–923. doi: 10.1016/j.orthres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Horch R.E., Beier J.P., Kneser U., Arkudas A. Successful human long-term application of in situ bone tissue engineering. J. Cell. Mol. Med. 2014;18:1478–1485. doi: 10.1111/jcmm.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R.L., Yuan Y., Tu J., Zou G.M., Li Q. Exaggerated inflammatory environment decreases BMP-2/ACS-induced ectopic bone mass in a rat model: implications for clinical use of BMP-2. Osteoarthr. Cartil. 2014;22:1186–1196. doi: 10.1016/j.joca.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Huang R.L., Chen G., Wang W., Herller T., Xie Y., Gu B., Li Q. Synergy between IL-6 and soluble IL-6 receptor enhances bone morphogenetic protein-2/absorbable collagen sponge-induced bone regeneration via regulation of BMPRIA distribution and degradation. Biomaterials. 2015;67:308–322. doi: 10.1016/j.biomaterials.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Huang R.L., Liu K., Li Q. Bone regeneration following the in vivo bioreactor principle: is in vitro manipulation of exogenous elements still needed. Med. Regen. 2016;11:475–481. doi: 10.2217/rme-2016-0021. [DOI] [PubMed] [Google Scholar]
- Jank B.J., Xiong L., Moser P.T., Guyette J.P., Ren X., Cetrulo C.L., Leonard D.A., Fernandez L., Fagan S.P., Ott H.C. Engineered composite tissue as a bioartificial limb graft. Biomaterials. 2015;61:246–256. doi: 10.1016/j.biomaterials.2015.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y., Toriyama K., Takada T., Yagi S. Tissue-engineering bone from omentum. Nagoya J. Med. Sci. 2010;72:111–117. [PMC free article] [PubMed] [Google Scholar]
- Khouri R.K., Koudsi B., Reddi H. Tissue transformation into bone in vivo. A potential practical application. JAMA. 1991;266:1953–1955. [PubMed] [Google Scholar]
- Kloeters O., Berger I., Ryssel H., Megerle K., Leimer U., Germann G. Revitalization of cortical bone allograft by application of vascularized scaffolds seeded with osteogenic induced adipose tissue derived stem cells in a rabbit model. Arch. Orthop. Trauma Surg. 2011;131:1459–1466. doi: 10.1007/s00402-011-1306-5. [DOI] [PubMed] [Google Scholar]
- Koca G., Kankaya Y., Dölen U.C., Uysal A., Sungur N., Uysal Ramadan S., Demirel K., Korkmaz M., Kocer U. Scintigraphic and tomographic evaluation of biological activities of prefabricated free and vascularized bone allografts. J. Craniofac. Surg. 2012;23:732–737. doi: 10.1097/SCS.0b013e31824dbbd1. [DOI] [PubMed] [Google Scholar]
- Kokemueller H., Spalthoff S., Nolff M., Tavassol F., Essig H., Stuehmer C., Bormann K.H., Rücker M., Gellrich N.C. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: experimental pilot study in sheep and first clinical application. Int. J. Oral Maxillofac. Surg. 2010;39:379–387. doi: 10.1016/j.ijom.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Kokemüller H., Jehn P., Spalthoff S., Essig H., Tavassol F., Schumann P., Andreae A., Nolte I., Jagodzinski M., Gellrich N.C. En bloc prefabrication of vascularized bioartificial bone grafts in sheep and complete workflow for custom-made transplants. Int. J. Oral Maxillofac. Surg. 2014;43:163–172. doi: 10.1016/j.ijom.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Marion N.W., Hollister S., Mao J.J. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Eng. A. 2009;15:3923–3930. doi: 10.1089/ten.tea.2008.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Cook J.L., Mendelson A., Moioli E.K., Yao H., Mao J.J. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440–448. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Möller B., Wiltfang J., Warnke P.H., Terheyden H. Tissue engineering of a vascularized bone graft of critical size with an osteogenic and angiogenic factor-based in vivo bioreactor. Tissue Eng. A. 2014;20:3189–3197. doi: 10.1089/ten.TEA.2013.0653. [DOI] [PubMed] [Google Scholar]
- Lokmic Z., Stillaert F., Morrison W.A., Thompson E.W., Mitchell G.M. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21:511–522. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- Mesimäki K., Lindroos B., Törnwall J., Mauno J., Lindqvist C., Kontio R., Miettinen S., Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Morritt A.N., Bortolotto S.K., Dilley R.J., Han X., Kompa A.R., McCombe D., Wright C.E., Itescu S., Angus J.A., Morrison W.A. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- Nakamura O., Kaji Y., Imaizumi Y., Yamagami Y., Yamamoto T. Prefabrication of vascularized bone allograft in a recipient rat using a flow-through vascular pedicle, bone morphogenetic protein, and bisphosphonate. J. Reconstr. Microsurg. 2013;29:241–248. doi: 10.1055/s-0032-1329925. [DOI] [PubMed] [Google Scholar]
- Okuda T., Uysal A.C., Tobita M., Hyakusoku H., Mizuno H. Prefabrication of tissue engineered bone grafts: an experimental study. Ann. Plast. Surg. 2010;64:98–104. doi: 10.1097/SAP.0b013e3181999ec1. [DOI] [PubMed] [Google Scholar]
- Orringer J.S., Shaw W.W., Borud L.J., Freymiller E.G., Wang S.A., Markowitz B.L. Total mandibular and lower lip reconstruction with a prefabricated osteocutaneous free flap. Plast. Reconstr. Surg. 1999;104:793–797. doi: 10.1097/00006534-199909030-00028. [DOI] [PubMed] [Google Scholar]
- Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I., Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Patel J.J., Flanagan C.L., Hollister S.J. Bone morphogenetic protein-2 adsorption onto poly-ɛ-caprolactone better preserves bioactivity in vitro and produces more bone in vivo than conjugation under clinically relevant loading scenarios. Tissue Eng. C Methods. 2015;21:489–498. doi: 10.1089/ten.tec.2014.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.J., Modes J.E., Flanagan C.L., Krebsbach P.H., Edwards S.P., Hollister S.J. Dual delivery of epo and bmp2 from a novel modular poly-ɛ-caprolactone construct to increase the bone formation in prefabricated bone flaps. Tissue Eng. C Methods. 2015;21:889–897. doi: 10.1089/ten.tec.2014.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C., Herzog E., Niklason L.E. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribaz J.J., Fine N.A. Prelamination: defining the prefabricated flap—a case report and review. Microsurgery. 1994;15:618–623. doi: 10.1002/micr.1920150903. [DOI] [PubMed] [Google Scholar]
- Rath S.N., Pryymachuk G., Bleiziffer O.A., Lam C.X., Arkudas A., Ho S.T., Beier J.P., Horch R.E., Hutmacher D.W., Kneser U. Hyaluronan-based heparin-incorporated hydrogels for generation of axially vascularized bioartificial bone tissues: in vitro and in vivo evaluation in a PLDLLA-TCP-PCL-composite system. J. Mater. Sci. Mater. Med. 2011;22:1279–1291. doi: 10.1007/s10856-011-4300-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues L., dos Reis L.M., Denadai R., Raposo-Amaral C.E., Alonso N., Ferreira M.C., Jorgetti V. Prefabricated bone flap: an experimental study comparing deep-frozen and lyophilized-demineralized allogenic bones and tissue expression of transforming growth factor β. J. Craniofac. Surg. 2013;24:1914–1921. doi: 10.1097/SCS.0b013e3182a41be2. [DOI] [PubMed] [Google Scholar]
- Runyan C.M., Jones D.C., Bove K.E., Maercks R.A., Simpson D.S., Taylor J.A. Porcine allograft mandible revitalization using autologous adipose-derived stem cells, bone morphogenetic protein-2, and periosteum. Plast. Reconstr. Surg. 2010;125:1372–1382. doi: 10.1097/PRS.0b013e3181d7032f. [DOI] [PubMed] [Google Scholar]
- Salehi-Nik N., Amoabediny G., Pouran B., Tabesh H., Shokrgozar M.A., Haghighipour N., Khatibi N., Anisi F., Mottaghy K., Zandieh-Doulabi B. Engineering parameters in bioreactor's design: a critical aspect in tissue engineering. Biomed. Res. Int. 2013;2013:762132. doi: 10.1155/2013/762132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathy B.N., Watson B.M., Kinard L.A., Spicer P.P., Dahlin R.L., Mikos A.G., Nair S. Bone tissue engineering with multilayered scaffolds-part II: combining vascularization with bone formation in critical-sized bone defect. Tissue Eng. A. 2015;21:2495–2503. doi: 10.1089/ten.TEA.2015.0099. [DOI] [PubMed] [Google Scholar]
- Sathy B.N., Mony U., Menon D., Baskaran V.K., Mikos A.G., Nair S. Bone tissue engineering with multilayered scaffolds-part I: an approach for vascularizing engineered constructs in vivo. Tissue Eng. A. 2015;21:2480–2494. doi: 10.1089/ten.tea.2015.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M.A., Levi B., Askarinam A., Nguyen A., Rackohn T., Ting K., Soo C., James A.W. Brief review of models of ectopic bone formation. Stem Cells Dev. 2012;21:655–667. doi: 10.1089/scd.2011.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti C., Piccinini E., Takizawa H., Todorov A., Bourgine P., Papadimitropoulos A., Barbero A., Manz M.G., Martin I. Engineering of a functional bone organ through endochondral ossification. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever C., Uygur F., Külahçı Y., Torun Köse G., Urhan M., Küçükodacı Z., Uzun G., Ipçioğlu O., Caycı T. Effect of hyperbaric oxygen therapy on bone prefabrication in rats. Acta Orthop. Traumatol. Turc. 2010;44:403–409. doi: 10.3944/AOTT.2010.2340. [DOI] [PubMed] [Google Scholar]
- Sever C., Uygur F., Kose G.T., Urhan M., Haholu A., Kulahci Y., Sinan O., Cihan S., Omer O. Prefabrication of vascularized bone graft using an interconnected porous calcium hydroxyapatite ceramic in presence of vascular endothelial growth factor and bone marrow mesenchymal stem cells: experimental study in rats. Indian J. Plast. Surg. 2012;45:444–452. doi: 10.4103/0970-0358.105939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalthoff S., Jehn P., Zimmerer R., Möllmann U., Gellrich N.C., Kokemueller H. Heterotopic bone formation in the musculus latissimus dorsi of sheep using beta-tricalcium phosphate scaffolds: evaluation of an extended prefabrication time on bone formation and matrix degeneration. Int. J. Oral Maxillofac. Surg. 2015;44:791–797. doi: 10.1016/j.ijom.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Stevens M.M., Marini R.P., Schaefer D., Aronson J., Langer R., Shastri V.P. In vivo engineering of organs: the bone bioreactor. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatara A.M., Kretlow J.D., Spicer P.P., Lu S., Lam J., Liu W., Cao Y., Liu G., Jackson J.D., Yoo J.J., Atala A., van den Beucken J.J., Jansen J.A., Kasper F.K., Ho T., Demian N., Miller M.J., Wong M.E., Mikos A.G. Autologously generated tissue-engineered bone flaps for reconstruction of large mandibular defects in an ovine model. Tissue Eng. A. 2015;21:1520–1528. doi: 10.1089/ten.tea.2014.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terheyden H., Jepsen S., Rueger D.R. Mandibular reconstruction in miniature pigs with prefabricated vascularized bone grafts using recombinant human osteogenic protein-1: a preliminary study. Int. J. Oral Maxillofac. Surg. 1999;28:461–463. [PubMed] [Google Scholar]
- Terheyden H., Knak C., Jepsen S., Palmie S., Rueger D.R. Mandibular reconstruction with a prefabricated vascularized bone graft using recombinant human osteogenic protein-1: an experimental study in miniature pigs. Part I: prefabrication. Int. J. Oral Maxillofac. Surg. 2001;30:373–379. doi: 10.1054/ijom.2001.0032. [DOI] [PubMed] [Google Scholar]
- Terheyden H., Warnke P., Dunsche A., Jepsen S., Brenner W., Palmie S., Toth C., Rueger D.R. Mandibular reconstruction with prefabricated vascularized bone grafts using recombinant human osteogenic protein-1: an experimental study in miniature pigs. Part II: transplantation. Int. J. Oral Maxillofac. Surg. 2001;30:469–478. doi: 10.1054/ijom.2000.0008. [DOI] [PubMed] [Google Scholar]
- Terheyden H., Menzel C., Wang H., Springer I.N., Rueger D.R., Acil Y. Prefabrication of vascularized bone grafts using recombinant human osteogenic protein-1—part 3: dosage of rhOP-1, the use of external and internal scaffolds. Int. J. Oral Maxillofac. Surg. 2004;33:164–172. doi: 10.1054/ijom.2003.0500. [DOI] [PubMed] [Google Scholar]
- Tsao C.K., Ko C.Y., Yang S.R., Yang C.Y., Brey E.M., Huang S., Chu I.M., Cheng M.H. An ectopic approach for engineering a vascularized tracheal substitute. Biomaterials. 2014;35:1163–1175. doi: 10.1016/j.biomaterials.2013.10.055. [DOI] [PubMed] [Google Scholar]
- Uygun B.E., Soto-Gutierrez A., Yagi H., Izamis M.L., Guzzardi M.A., Shulman C., Milwid J., Kobayashi N., Tilles A., Berthiaume F., Hertl M., Nahmias Y., Yarmush M.L., Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bomhard A., Veit J., Bermueller C., Rotter N., Staudenmaier R., Storck K. Prefabrication of 3D cartilage contructs: towards a tissue engineered auricle—a model tested in rabbits. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranckx J.J., Den Hondt M., Delaere P. Prefabrication and prelamination strategies for the reconstruction of complex defects of trachea and larynx. J. Reconstr. Microsurg. 2014;30:145–152. doi: 10.1055/s-0033-1361928. [DOI] [PubMed] [Google Scholar]
- Wang L., Fan H., Zhang Z.Y., Lou A.J., Pei G.X., Jiang S., Mu T.W., Qin J.J., Chen S.Y., Jin D. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized beta-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials. 2010;31:9452–9461. doi: 10.1016/j.biomaterials.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Warnke P.H., Springer I.N., Wiltfang J., Acil Y., Eufinger H., Wehmöller M., Russo P.A., Bolte H., Sherry E., Behrens E., Terheyden H. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- Warnke P.H., Springer I.N., Acil Y., Julga G., Wiltfang J., Ludwig K., Russo P.A., Sherry E., Sivananthan S., Hedderich J., Terheyden H. The mechanical integrity of in vivo engineered heterotopic bone. Biomaterials. 2006;27:1081–1087. doi: 10.1016/j.biomaterials.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Warnke P., Wiltfang J., Springer I., Acil Y., Bolte H., Kosmahl M., Russo P., Sherry E., Lutzen U., Wolfart S. Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials. 2006;27:3163–3167. doi: 10.1016/j.biomaterials.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Wei X., Li Q. Prefabrication as a term in flap surgery: do we need a broader definition. J. Reconstr. Microsurg. 2013;29:559–560. doi: 10.1055/s-0033-1345433. [DOI] [PubMed] [Google Scholar]
- Weigand A., Beier J.P., Hess A., Gerber T., Arkudas A., Horch R.E., Boos A.M. Acceleration of vascularized bone tissue-engineered constructs in a large animal model combining intrinsic and extrinsic vascularization. Tissue Eng. A. 2015;21:1680–1694. doi: 10.1089/ten.TEA.2014.0568. [DOI] [PubMed] [Google Scholar]
- Wu H., Xu Z.W., Liu X.M., Gong D., Wan J.Y., Xu X.F., Zhou Z.F., Li W.B. An in vivo model of in situ implantation using pulmonary valved conduit in large animals under off-pump condition. Chin. Med. J. 2013;126:4540–4544. [PubMed] [Google Scholar]
- Wu H., Kang N., Wang Q., Dong P., Lv X., Cao Y., Xiao R. The dose-effect relationship between the seeding quantity of human marrow mesenchymal stem cells and in vivo tissue-engineered bone yield. Cell Transplant. 2015;24:1957–1968. doi: 10.3727/096368914X685393. [DOI] [PubMed] [Google Scholar]
- Wu X., Wang Q., Kang N., Wu J., Gu C., Bi J., Lv T., Xie F., Hu J., Liu X., Cao Y. The effects of different vascular carrier patterns on the angiogenesis and osteogenesis of BMSC-TCP-based tissue-engineered bone in beagle dogs. J. Tissue Eng. Regen. Med. 2015 doi: 10.1002/term.2076. [DOI] [PubMed] [Google Scholar]
- Xie F., Zhu H., Gu B., Zan T., Liu K., Li Q. Resurfacing severe facial burn scars: an algorithm based on three different types of prefabricated expanded flaps. J. Reconstr. Microsurg. 2014;30:627–634. doi: 10.1055/s-0034-1372476. [DOI] [PubMed] [Google Scholar]
- Yachouh J., Breton P., Roux J.P., Goudot P. Osteogenic capacity of vascularised periosteum: an experimental study on mandibular irradiated bone in rabbits. J. Plast. Reconstr. Aesthet. Surg. 2010;63:2160–2167. doi: 10.1016/j.bjps.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Yang P., Huang X., Shen J., Wang C., Dang X., Mankin H., Duan Z., Wang K. Development of a new pre-vascularized tissue-engineered construct using pre-differentiated rADSCs, arteriovenous vascular bundle and porous nano-hydroxyapatide-polyamide 66 scaffold. BMC Musculoskelet. Disord. 2013;14:318. doi: 10.1186/1471-2474-14-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S.T. Vascular implantation into skin flap: experimental study and clinical application: a preliminary report. Plast. Reconstr. Surg. 1981;68:404–410. [PubMed] [Google Scholar]
- Yao S.T. Microvascular transplantation of prefabricated free thigh flap. Plast. Reconstr. Surg. 1982;69:568. doi: 10.1097/00006534-198203000-00051. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Xu Y., Zhang B., Wu X., Xu F., Liang W., Du X., Li R. In vivo generation of thick, vascularized hepatic tissue from collagen hydrogel-based hepatic units. Tissue Eng. C Methods. 2010;16:653–659. doi: 10.1089/ten.TEC.2009.0053. [DOI] [PubMed] [Google Scholar]
- Zhi W., Zhang C., Duan K., Li X., Qu S., Wang J., Zhu Z., Huang P., Xia T., Liao G., Weng J. A novel porous bioceramics scaffold by accumulating hydroxyapatite spherulites for large bone tissue engineering in vivo. II. Construct large volume of bone grafts. J. Biomed. Mater. Res. A. 2014;102:2491–2501. doi: 10.1002/jbm.a.34919. [DOI] [PubMed] [Google Scholar]
- Zhou M., Peng X., Mao C., Xu F., Hu M., Yu G.Y. Primate mandibular reconstruction with prefabricated, vascularized tissue-engineered bone flaps and recombinant human bone morphogenetic protein-2 implanted in situ. Biomaterials. 2010;31:4935–4943. doi: 10.1016/j.biomaterials.2010.02.072. [DOI] [PubMed] [Google Scholar]
- Zhou M., Peng X., Mao C., Tian J.H., Zhang S.W., Xu F., Tu J.J., Liu S., Hu M., Yu G.Y. The value of SPECT/CT in monitoring prefabricated tissue-engineered bone and orthotopic rhBMP-2 implants for mandibular reconstruction. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137167. [DOI] [PMC free article] [PubMed] [Google Scholar]