Abstract
Aging-associated cardiovascular diseases (CVDs) have some risk factors that are closely related to oxidative stress. Salvia miltiorrhiza (SM) has been used commonly to treat CVDs for hundreds of years in the Chinese community. We aimed to explore the effects of SM on oxidative stress in aging-associated CVDs. Through literature searches using Medicine, PubMed, EMBASE, Cochrane library, CINAHL, and Scopus databases, we found that SM not only possesses antioxidant, antiapoptotic, and anti-inflammatory effects but also exerts angiogenic and cardioprotective activities. SM may reduce the production of reactive oxygen species by inhibiting oxidases, reducing the production of superoxide, inhibiting the oxidative modification of low-density lipoproteins, and ameliorating mitochondrial oxidative stress. SM also increases the activities of catalase, manganese superoxide dismutase, glutathione peroxidase, and coupled endothelial nitric oxide synthase. In addition, SM reduces the impact of ischemia/reperfusion injury, prevents cardiac fibrosis after myocardial infarction, preserves cardiac function in coronary disease, maintains the integrity of the blood-brain barrier, and promotes self-renewal and proliferation of neural stem/progenitor cells in stroke. However, future clinical well-designed and randomized control trials will be necessary to confirm the efficacy of SM in aging-associated CVDs.
1. Introduction
Cardiovascular diseases (CVDs) are a group of disorders related to the heart or blood vessels. Major CVDs include stroke, ischemic heart disease, cardiomyopathy, rheumatic heart disease, hypertensive heart disease, endocarditis, atrial fibrillation, aortic aneurysm, and peripheral arterial disease [1]. Global life expectancy increased from 65.3 years in 1990 to 71.5 years in 2013. At the same time, the numbers of deaths from noncommunicable diseases increased steadily [2]. CVDs are the leading form of noncommunicable diseases [2]. In 2012 and 2013, 17.3 million deaths worldwide resulted from CVDs [3]. Among these deaths, coronary artery disease and stroke contributed most to the total global burden of CVDs [1]. It is estimated that 90% of CVDs are preventable [4]. The Framingham and World Health Organization MONICA studies found several risk factors for CVDs (e.g., age, smoking, physical inactivity, unhealthy diet, obesity, family history, hypertension, diabetes mellitus, and hyperlipidemia) [5–10]. Some of these risk factors are immutable; however, many important risk factors are modifiable. When relevant risk factors decrease, the incidence and mortality of CVDs improved.
Most CVD risk factors are related to oxidative stress. Reactive oxygen species (ROS) are the main cause of oxidative stress and are highly reactive with proteins, lipids, and DNA, damaging these cellular components [11]. Under normal conditions, the production of ROS during aerobic metabolism and the scavenging of ROS by tissue antioxidant systems are in balance [12]. This balance is shifted in favor of oxidative stress in the presence of cardiovascular risk factors [5, 13, 14].
The most important forms of ROS are nitric oxide (NO), superoxide, hydrogen peroxide, and peroxynitrite (Figure 1). NO is produced in normal physiologic conditions from L-arginine by coupled endothelial nitric oxide synthase (eNOS) that is activated via protein kinase A- or Akt-dependent phosphorylation [15]. NO is a crucial mediator of blood vessel homeostasis by inhibiting vascular smooth muscle contraction and growth, platelet aggregation, and leukocyte adhesion to the endothelium. Under some circumstances, such as hypertension, hyperglycemia, and hypercholesterolemia, eNOS becomes uncoupled and superoxide is synthesized rather than NO [16–20]. When normal NO production is impaired, CVDs may occur [21].
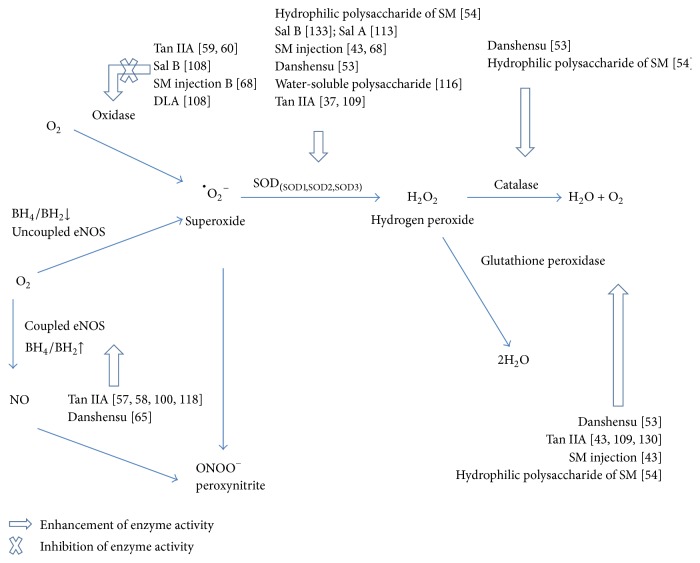
Figure 1.
Vascular reactive oxygen species production. Oxidases convert oxygen to superoxide, which is then dismutated to H2O2 by superoxide dismutase (SOD). H2O2 can be converted to H2O by catalase or glutathione peroxidase. In addition, coupled endothelial NO synthase (eNOS) catalyzes the formation of nitric oxide (NO). When tetrahydrobiopterin (BH4) generation is reduced, the uncoupled eNOS produces superoxide instead of NO. The superoxide can react rapidly with NO to form peroxynitrite (ONOO−), a powerful oxidant and nitrating agent. Reference numbers are inside the parentheses. DLA: 3,4-dihydroxyphenyl lactic acid; SM: Salvia miltiorrhiza; Sal A: salvianolic acid A; Sal B: salvianolic acid B; Tan IIA: tanshinone IIA.
In aged vessels, endothelial dysfunction occurs owing to eNOS uncoupling by reducing the tetrahydrobiopterin to dihydrobiopterin ratio [22]. Superoxide is the product of a univalent reduction of oxygen by various oxidases [23]. The important oxidases include xanthine oxidase, uncoupled NO synthases, cytochrome P450 enzymes, and mitochondrial and NADPH oxidases [24–26]. Superoxide is toxic and possesses three main effects that contribute to the pathogenesis of CVDs [27]: (1) rapid inactivation and reaction with NO to form the more highly reactive peroxynitrite; (2) mediation of aberrant redox signaling in the vasculature to induce alterations of vascular function, and (3) direct oxidative damage to cell components [28].
Peroxynitrite is a powerful oxidant and nitrating agent. Peroxynitrite can damage a wide array of cellular molecules, including carbonate, proteins, low-density lipoproteins (LDL), and DNA to form nitration and nitrosation products that are involved in the pathogenesis of cardiovascular complications [29, 30].
The superoxide dismutases (SODs) are the major antioxidant enzymes that degrade superoxide. There are three isoforms of SOD in mammals: cytoplasmic Cu/ZnSOD (SOD1), mitochondrial MnSOD (SOD2), and extracellular Cu/ZnSOD (SOD3) [31]. All forms of SOD rapidly dismutate superoxide to the more stable ROS, hydrogen peroxide, that is then converted to water and oxygen by either catalase or glutathione peroxidase [32].
Salvia miltiorrhiza (SM) belongs to the family of Labiatae and its dried root, referred to as “Danshen” in traditional Chinese medicine, has been commonly used for hundreds of years in the treatment of CVDs [33]. Our previous population-based studies demonstrated that SM is the most common herbal drug used to treat ischemic heart disease [34] and ischemic stroke [35]. In traditional Chinese medicine, Danshen is regarded as an important herb for “activating circulation and dispersing stasis or sludging of blood.” SM exhibits strong antioxidant activity by scavenging ROS [36]. SM also modulates endothelial cell permeability, inhibits platelet aggregation [37], and protects human umbilical vein endothelial cells against homocysteine-induced endothelial dysfunction [38] or vascular smooth muscle cells proliferation [39].
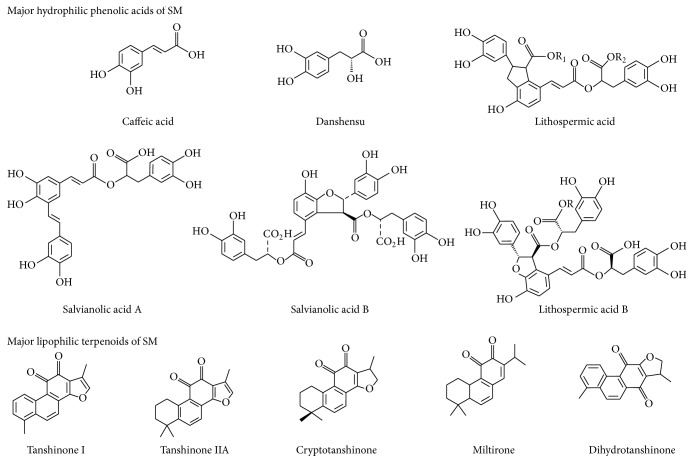
There are many active constituents found in alcohol and water extracts of SM (Figure 2). At least 49 diterpenoid quinones, more than 36 hydrophilic phenolic acids, and 23 essential oil constituents have been isolated and identified from SM [40]. Among the chemical components, tanshinone IIA (Tan IIA) and salvianolic acid B (Sal B) are usually chosen as markers to assess the quality of SM [41].
Figure 2.
The chemical structure of major hydrophilic phenolic acids and lipophilic terpenoids of Salvia miltiorrhiza.
Terpenoids are the most important lipophilic components of SM. Most diterpenoids are present as tanshinones and their analogs, which are a class of abietane diterpene compounds found exclusively in the Salvia genus [42]. Tanshinone I, Tan IIA, and cryptotanshinone are the major constituents of the tanshinones and are studied mainly for their biological activity [43]. SM also contains some triterpenoids [44]. These terpenoids possess a wide range of biological activities including antioxidant [45], antibacterial [46], anti-inflammatory [47], antiatherogenic [36], neuroprotective [48], antitumor [49], and antidiabetic [50] effects.
Phenolic acids are the main type of hydrophilic component from SM. Most of the phenolic acids in SM are condensation derivatives of caffeic acid in different linkage forms and numbers [51]. The most prominent effects of the phenolic acids in SM are antioxidant, anticoagulant, and antithrombotic activities and protection of cells from ischemia-reperfusion (I/R) injury [33, 52, 53]. Moreover, over the past few years, there are several studies showing that the polysaccharides extracted from SM also have antioxidant and antitumor activities [54, 55].
Unfavorable changes in CVD risk factors are seen in aging individuals and will likely be reflected in worsening morbidity and mortality [56]. Thus, the minimization of risk factors is a key point for decreasing the incidence of aging-related CVD.
Many researchers have demonstrated that SM can reduce the impact of CVD risk factors and the injury caused by oxidative stress. This article provides an overview of SM in terms of its ability to reduce oxidative stress-induced injury and aging-related risk factors associated with CVD. Studies of the effects of SM on the two most frequent CVDs, coronary artery disease and stroke, are also reviewed.
2. Materials and Methods
The current review focuses on the role of oxidative stress and SM (Danshen) in aging-associated CVDs. Literature searches were done using the Medicine, PubMed, EMBASE, Cochrane library, CINAHL, and Scopus databases, and the contents of the identified articles were summarized.
3. Results and Discussion
3.1. Hypertension
Hypertension is the most readily modifiable risk factor for CVDs [57]. Oxidative stress, an aberrant vascular redox system, and endothelial dysfunction can contribute to hypertension [13, 28]. In traditional Chinese medicine, Danshen is the most frequently prescribed single herb for hypertension [58]. Tan IIA has a vasodilatory effect through restoring eNOS coupling by increasing the ratio of tetrahydrobiopterin to dihydrobiopterin and reducing the production of superoxide by inhibiting the expression of NOX4, a member of the NADPH oxidase family [59, 60]. In addition to reducing ROS, Tan IIA protects against endothelial cell damage by decreasing the Bax/Bcl-2 ratio and inhibiting caspase-3 activation [61]. The water extract of SM that contains lithospermic acid B (also named tanshinoate B) and Sal B exhibits an antihypertensive effect through the inhibition of angiotensin-converting enzyme or the renin angiotensin system [62–65]. Sal B and danshensu, the major hydrophilic constituents of SM, can regulate vascular tone and reduce blood pressure by activating of the calcium-activated big potassium (BKCA) channel [66, 67]. In addition, SM injection can decrease plasma levels of endothelin-1 and thromboxane B2 [68].
3.2. Smoking
Cigarette smoking is an important and reversible risk factor for CVDs but is ranked lower than hypertension because of the widespread implementation of smoke-free legislation [69]. However, in some countries, smoking remains the third leading risk factor for CVDs, behind dietary risks and hypertension. Smokers return to the risk level of never-smokers after cessation of smoking for at least 10 years [69].
Smoking may enhance oxidative stress not only through the production of ROS but also through weakening of the antioxidant defense systems [70]. Smoking-associated CVDs include abdominal aortic aneurysm, peripheral artery disease, unheralded coronary death, and subarachnoid hemorrhage [71]. SalA attenuates the formation of aortic aneurysms in apolipoprotein E-deficient mice by selectively inhibiting matrix metalloproteinase-9 (MMP-9) to maintain the integrity of blood vessels [72]. A crude extract of SM dilates isolated rat femoral arteries by opening tetraethylammonium-sensitive potassium channels in smooth muscle cells [73] and produces a vasorelaxant effect in the rat knee joint through the release of calcitonin gene-related peptide and the endothelium-derived relaxant factors NO and prostaglandins [74]. One recent study revealed that the injection of Danshen root could suppress cigarette smoking-induced lung inflammation by decreasing the levels of interleukin- (IL-) 8, IL-6, and tumor necrosis factor- (TNF-) α in Sprague-Dawley rats [75].
3.3. Hyperglycemia
Hyperglycemia and diabetes mellitus are strong, significant, and independent risk factors for CVDs [76, 77]. Hyperglycemia induces oxidative stress in diabetic patients, and the overproduction of ROS contributes to the development of CVDs [78, 79]. Peroxynitrite plays an important role in the pathogenesis of diabetic CVD complications through oxidative and nitrosative stress [29]. In the presence of hyperglycemia, vascular remodeling is augmented by uncoupled eNOS [80], increases in endothelial superoxide levels that inhibit vascular smooth muscle Na-K-ATPase activity [81], and downregulation of transient receptor potential cation channel subfamily V member 4 that regulates vascular function [82]. The hydrophilic extract of SM clearly ameliorates oxidative stress stimulated by hyperglycemia in diabetic patients with coronary heart disease [83]. The induction of vascular endothelial growth factor (VEGF) expression by high glucose levels is also reversed by the SM hydrophilic extract through amelioration of mitochondrial oxidative stress [84].
Sal B is the main bioactive component in the SM hydrophilic extract [85]. The tanshinones are insulin sensitizers that enhance the activity of insulin on tyrosine phosphorylation through the activation of Akt and extracellular signal-regulated kinase (ERK)1/2 and by glycogen synthase kinase (GSK)3β and glucose transporter (GLUT)4 translocation [86]. Furthermore, the hydrophilic polysaccharide of SM protects against the development of type 2 diabetes by attenuating insulin resistance through increases in the activities of catalase, MnSOD, and glutathione peroxidase in rats [54].
3.4. Hyperlipidemia
Hyperlipidemia is the most important risk factor for atherosclerosis and a major cause of CVDs [87, 88]. Increased transcytosis of lipoproteins is the initial event in atherogenesis. ROS generated by activated inflammatory cells and the production of oxidized lipoproteins are key points for atherosclerotic plaque erosion and rupture [89]. We showed that Tan IIA exhibits a strong antiatherosclerotic effect associated with reduced vascular cell adhesion molecule- (VCAM-) 1, intercellular adhesion molecule- (ICAM-) 1, and CX3CL1 expression through inhibition of the NF-κB signaling pathway in human vascular endothelial cells [90]. Sal B inhibits LDL oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolemic rabbits through inhibition of ROS production [91]. Magnesium tanshinoate B, an important aqueous component of SM, can also inhibit oxidative modification of LDLs, prevent the uptake of LDLs by macrophages [92], and protect endothelial cells against oxidized lipoprotein-induced apoptosis [93].
3.5. Overweight and Obesity
Obesity has become a global epidemic. The 2013 National Health and Nutrition Examination Survey (NHANES) guidelines recommended 64.5% of American adults for weight loss treatment [94]. Obesity is an independent risk factor for CVDs [95]. Adipose tissue is a significant source of TNF-α, IL-6, resistin, leptin, angiotensinogen, and adiponectin [96]. The production of these proinflammatory cytokines may contribute to the low-level systemic inflammation seen in obesity-associated chronic pathologies [97].
Endothelial dysfunction is present in obese individuals due to decreased NO and increased oxidative stress [98]. Cryptotanshinone inhibits phosphorylation of STAT3 during early adipogenesis and then downregulates the expression of the early transcription factors C/EBPβ and PPARγ to suppress preadipocyte differentiation [99]. Sal B can also suppress the expression of PPARγ and C/EBPα and increase the expression of GATA binding proteins 2 and 3 to prevent the differentiation of preadipocytes and weight gain in obese mice [100]. There is also a study in a rodent model demonstrating that a Chinese herbal extract (SK0506) containing SM possesses a favorable impact on the metabolic syndrome through suppression of visceral fat accumulation and regulation of lipid metabolism [101].
3.6. Coronary Artery and Ischemic Heart Diseases
3.6.1. Angina
Angina pectoris and coronary artery spasm are the most common coronary artery diseases. Tan IIA elicits a strong vasodilatory effect in rat and porcine coronary arterioles through the BKCA channel and increased NO and cytochrome P450 metabolites [102, 103]. SalB also can relax the rat coronary artery by inhibiting calcium channels in vascular smooth muscle cells [104]. A systematic review of 60 eligible randomized controlled trials indicates that the Danshen dripping pill, in which SM is the main component, is more effective than isosorbide dinitrate in treating angina pectoris [105].
3.6.2. Myocardial Infarction (MI)
Tan IIA prevents platelet activation by inhibiting the mitogen-activated protein kinase (MAPK) pathway, such as Erk-2 phosphorylation [106]. After MI, reperfusion of ischemic tissue provides oxygen and substrates that are necessary for tissue recovery. However, reperfusion may also induce I/R injury, including excessive production of ROS, enhanced biosynthesis of adhesion molecules, activation of leukocytes, and involvement of cytokines and other inflammatory mediators that cause target and remote organ damage [107]. Both the hydrophilic and lipophilic constituents of SM appear to improve the I/R-induced vascular damage multifactorially and synergistically [108]. The protective function of Tan IIA on myocardial I/R injury may be through inhibiting ROS production and attenuating the expression of high mobility group box B1 protein that results in the activation of proinflammatory pathways [109]. Tan IIA can also reduce monocyte chemoattractant protein-1 expression and macrophage infiltration.
The expression of transforming growth factor-β1 in cardiac fibroblasts is inhibited by Tan IIA via the NF-κB signaling pathway [110]. The water-soluble fraction of an SM root extract possesses antioxidant activity, and the hydrophilic components of SM, including protocatechuic aldehyde and Sal B, inhibit the TNF-α-induced expression of ICAM-1 and VCAM-1 and the NF-κB and activator protein-1 DNA binding activities in human umbilical vein endothelial cells [111].
Danshensu, the major water-soluble component of SM, protects isolated heart tissue against I/R injury through activation of Akt/ERK1/2/Nrf2 signaling [53]. Recent research shows that Sal A has antiapoptotic effects via activating ERK1/2 and downregulating c-Jun N-terminal kinase (JNK), with increased Bcl-2 and reduced Bax protein expression [112, 113]. A combination of SalB and ginsenoside Rg1 increases the viability of cardiac myocytes and reduces infarct size, thereby improving the functional parameters of the heart against I/R injury in rats [114]. The injection of SM containing water-soluble components such as Sal A shows a cardioprotective effect after infarction by inhibiting L-type Ca2+ channels and decreasing the contractility of adult rat cardiac myocytes [115]. Moreover, even the polysaccharide from SM possesses cardiac protective properties [116].
3.6.3. Cardiac Remodeling
Cardiac remodeling is an important aspect of the progression to heart failure observed after MI [117]. Patients with reverse remodeling during treatment have better outcomes and lower mortality than those without such remodeling [118]. Tanshinone VI protects the myocardium against I/R injury and attenuates the progression of myocardial remodeling in vitro [119]. Tan IIA attenuates the expression of angiotensin II-induced collagen type I, ROS formation, and the proliferation of cardiac fibroblasts [120, 121]. Recent research also demonstrates that Tan IIA inhibits extracellular matrix remodeling induced by angiotensin II in human cardiac fibroblasts through inhibition of Smad signaling and MMP-9 expression via nuclear localization of NF-κB [122]. Salvianolic acids, including SalA and Sal B, suppress ROS at the early stage of acute MI and then inhibit the subsequent transcription and posttranslational activation of MMP-9 [123, 124]. Sal B functions as a competitive inhibitor of MMP-9 and inhibits the migration, proliferation, collagen synthesis, and cytokine secretion of cardiac fibroblasts [125, 126].
3.7. Stroke
Stroke is the second leading global cause of death behind heart disease [3]. A nationwide population-based study surveyed the usage of traditional Chinese medicine for stroke patients in Taiwan. This study revealed that about 15% of stroke patients used traditional Chinese medicine and that SM was the most used single herb [127]. Disruption of the blood-brain barrier (BBB), inflammatory processes, and nerve cell apoptosis occur after stroke. Tan IIA decreases BBB permeability and suppresses the expression of ICAM-1 and MMP-9 significantly to reduce the infarct area [128]. Another study found that the protective effect of Tan IIA on I/R-induced nerve cells apoptosis involves suppression of excess activation of glial cells, inhibiting the activities of caspase-3 and caspase-8, central regulators of apoptosis [129].
Tan IIA protects the integrity of the BBB through the increased expression of critical endothelial tight junction proteins [130]. Tan IIA is neuroprotective against ischemic stroke, but its short half-life and poor permeability across the BBB limits its effectiveness. There are reports demonstrating that albumin-conjugated PEGylated Tan IIA possesses better brain delivery efficacy and displays remarkable neuroprotective effects through modulation of the MAPK signal pathways and inflammatory cascades [131, 132]. SalB exhibits its neuroprotective effect through antioxidant and antiapoptotic activities by reducing the Bax/Bcl-2 ratio in hippocampal CA1 neurons in mice with I/R injury [133]. The expression of silent information regulator 1, a nicotinamide adenine dinucleotide-dependent deacetylase, is also upregulated by Sal B yielding an antiapoptotic effect after ischemia [134].
A novel derivative of Sal B exhibits a neuroprotective effect against cerebral ischemic injury through angiogenesis and nerve function recovery via the JAK2/STAT3 and VEGF/Flk-1 pathways [135]. Danshensu also possesses a neuroprotective effect against I/R injury by inhibiting apoptosis through activating the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway [136]. Rehabilitation can facilitate some recovery of neurological function after a stroke with evidence of neurogenesis [137]. Sal B promotes neural stem/progenitor cell self-renewal and proliferation through the PI3K/Akt signaling pathway, resulting in improved cognitive function after stroke in rats [138]. These results suggest that SM may act as a potential drug in the treatment of brain injury or neurodegenerative diseases.
3.8. Future Prospects
Figures 1 and 2 illustrate the chemical structure and effects of SM on ROS. Table 1 lists the main antiapoptotic and anti-inflammatory mechanisms of SM. In addition, there are several clinical trials related to SM underway in the United States, including two phase III clinical trials. One is the “Phase III Trial of Dantonic® (T89) Capsule to Prevent and Treat Stable Angina.” The other trial is the “Phase III Study of Compound Danshen Dripping Pills to Treat Diabetic Retinopathy.” Both trials focus on oxidative stress and CVDs. The results will provide important new information about the clinical utility of SM.
Table 1.
The main antiapoptotic and anti-inflammatory mechanisms of SM.
| Mechanism | References | |
|---|---|---|
| Antiapoptosis | ||
| Salvianolic acid A | MAPK signaling pathway ↑ERK1/2; ↓JNK with ↓Bax/Bcl-2 |
[112, 113] |
| Salvianolic acid B | ↑SIRT1; ↓Ac-FOXO1 with ↓Bax/Bcl-2 | [134] |
| Magnesium tanshinoate B | ↓JNK, ↓cytochrome c release, ↓caspase-3 | [93] |
| Danshensu | ↑PI3K/Akt signal pathway; ↑p-GSK-3β levels | [136] |
| Tanshinone IIA | ↓Bax; ↓caspase-3, and ↓Bax/Bcl-2 ratio | [37] |
| Anti-inflammation | ||
| Danshen root | ↓IL 8; ↓IL-6 and ↓TNFα | [75] |
| Salvianolic acid B | ↑SIRT1; ↓TNF-α; ↓IL-1β | [134] |
| Protocatechuic aldehyde | ↓NF-κB; ↓AP-1; ↓VCAM-1; ↓ICAM-1 | [111] |
| Tanshinone IIA | ↓HMGB1; ↓NF-κB; ↓MCP-1; ↓TNF-α; ↓TGF-β1; ↓CX3CL1; ↓ICAM- 1; ↓VCAM-1 | [90, 109, 110] |
| Albumin-conjugated PEGylated Tan IIA | ↓p38 MAPK; ↓ERK1/2; ↓JNK; iNOS; ↓MPO; ↓TNF-α; ↓IL-1β; ↓IL-6; ↓TNF-α; ↓IL-8; ↓GFAP; ↓MMP-9; ↓COX-2; ↑PPARγ; ↑IL-10; ↑TGF-β1 |
[131, 132] |
↑ means upregulation; ↓ means downregulation.
COX-2, cyclooxygenase-2; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; IL, interleukin; GFAP, glial fibrillary acidic protein; GSK-3β, glycogen synthase kinase-3β; interleukin; MPO, myeloperoxidase; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-alpha; TGF-β1, transforming growth factor-β1; SIRT1, silent information regulator 1; PI3K, phosphoinositide 3-kinase; VCAM-1, vascular cell adhesion molecule; ICAM-1, intercellular adhesion molecule; MMP-9, matrix metalloproteinase-9; PPARγ, peroxisome proliferator activated receptor γ.
The tanshinones are functionally active components in SM. However, they are poorly water-soluble with a low dissolution rate that results in low oral bioavailability. Many studies have focused on improving the drug delivery systems for tanshinones including the use of liposomes [139], nanoparticles [131, 140], and solid dispersions [141]. Further research in this area is needed to assure optimal delivery of SM products.
4. Conclusion
SM exhibits antioxidant, antiapoptotic, and anti-inflammatory effects. SM reduces ROS production through inhibiting oxidases, reducing the production of superoxide, inhibiting oxidative modification of LDLs, and ameliorating mitochondrial oxidative stress. It also increases the activities of catalase, MnSOD, glutathione peroxidase, and coupled eNOS. Moreover, in coronary artery disease and stroke, SM not only reduces the impact of I/R injury but also prevents cardiac fibrosis after MI, preserves cardiac function in coronary disease, and maintains the integrity of the BBB, thereby promoting neural stem/progenitor cell self-renewal and proliferation following a stroke. Therefore, SM can be an effective agent for the prevention and treatment of CVDs. However, in accordance with in vitro and in vivo laboratory evidence, well-designed clinical studies are necessary to confirm the efficacy of SM in the treatment of CVDs.
Acknowledgments
This work was supported partly by National Science Research Grant of Taiwan (NSC-96-2320-B-182-023-MY2) and Chang Gung Memorial Hospital (CMRPG83011; CMRPD32027).
Competing Interests
The authors declare no competing interests.
Authors' Contributions
Cheng-Chieh Chang and Yu-Chun Chang contributed equally to this work.
References
- 1.Moran A. E., Roth G. A., Narula J., Mensah G. A. 1990–2010 Global cardiovascular disease atlas. Global Heart. 2014;9(1):3–16. doi: 10.1016/j.gheart.2014.03.1220. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth G. A., Huffman M. D., Moran A. E., et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132(17):1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 4.McGill H. C., Jr., McMahan C. A., Gidding S. S. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation. 2008;117(9):1216–1227. doi: 10.1161/circulationaha.107.717033. [DOI] [PubMed] [Google Scholar]
- 5.Margolis J. R., Gillum R. F., Feinleib M., Brasch R. C., Fabsitz R. R. Community surveillance for coronary heart disease: the framingham cardiovascular disease survey: methods and preliminary results. American Journal of Epidemiology. 1974;100(6):425–436. doi: 10.1093/oxfordjournals.aje.a112054. [DOI] [PubMed] [Google Scholar]
- 6.Gillum R. F., Feinleib M., Margolis J. R., Fabsitz R. R., Brasch R. C. Community surveillance for cardiovascular disease: the Framingham cardiovascular disease survey. Some methodological problems in the community study of cardiovascular disease. Journal of Chronic Diseases. 1976;29(5):289–299. doi: 10.1016/S0002-8703(76)80402-4. [DOI] [PubMed] [Google Scholar]
- 7.Kannel W. B., McGee D., Gordon T. A general cardiovascular risk profile: the Framingham study. The American Journal of Cardiology. 1976;38(1):46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 8.Gillum R. F., Fabsitz R. R., Feinleib M., Wolf P. A., Margolis J. R., Brasch R. C. Community surveillance for cerebrovascular disease: the Framingham Cardiovascular Disease Survey. Public Health Reports. 1978;93(5):438–442. [PMC free article] [PubMed] [Google Scholar]
- 9.Keil U. The worldwide WHO MONICA project: results and perspectives. Gesundheitswesen. 2005;67(supplement 1):S38–S45. doi: 10.1055/s-2005-858240. [DOI] [PubMed] [Google Scholar]
- 10.Tsao C. W., Vasan R. S. Cohort Profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. International Journal of Epidemiology. 2015;44(6):1800–1813. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papaharalambus C. A., Griendling K. K. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends in Cardiovascular Medicine. 2007;17(2):48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. The American Journal of Medicine. 1994;97(3):5S–13S. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 13.Dinh Q. N., Drummond G. R., Sobey C. G., Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/406960.406960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tousoulis D., Briasoulis A., Papageorgiou N., et al. Oxidative stress and endothelial function: therapeutic interventions. Recent Patents on Cardiovascular Drug Discovery. 2011;6(2):103–114. doi: 10.2174/157489011795933819. [DOI] [PubMed] [Google Scholar]
- 15.Khazaei M., Moien-afshari F., Laher I. Vascular endothelial function in health and diseases. Pathophysiology. 2008;15(1):49–67. doi: 10.1016/j.pathophys.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Bouloumié A., Bauersachs J., Linz W., et al. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension. 1997;30(4):934–941. doi: 10.1161/01.HYP.30.4.934. [DOI] [PubMed] [Google Scholar]
- 17.Cosentino F., Hishikawa K., Katusic Z. S., Lüscher T. F. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96(1):25–28. doi: 10.1161/01.CIR.96.1.25. [DOI] [PubMed] [Google Scholar]
- 18.Landmesser U., Dikalov S., Price S. R., et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. The Journal of Clinical Investigation. 2003;111(8):1201–1209. doi: 10.1172/jci200314172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawashima S., Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(6):998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard K. A., Jr., Groszek L., Smalley D. M., et al. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circulation Research. 1995;77(3):510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 21.Chen K., Pittman R. N., Popel A. S. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxidants and Redox Signaling. 2008;10(7):1185–1198. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y.-M., Huang A., Kaley G., Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. American Journal of Physiology—Heart and Circulatory Physiology. 2009;297(5):H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridovich I. Superoxide anion radical (), superoxide dismutases, and related matters. The Journal of Biological Chemistry. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 24.Terada L. S., Willingham I. R., Rosandich M. E., Leff J. A., Kindt G. W., Repine J. E. Generation of superoxide anion by brain endothelial cell xanthine oxidase. Journal of Cellular Physiology. 1991;148(2):191–196. doi: 10.1002/jcp.1041480202. [DOI] [PubMed] [Google Scholar]
- 25.Clancy R. M., Leszczynska-Piziak J., Abramson S. B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. The Journal of Clinical Investigation. 1992;90(3):1116–1121. doi: 10.1172/jci115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J.-M., Shah A. M. Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovascular Research. 2001;52(3):477–486. doi: 10.1016/s0008-6363(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 27.Li J.-M., Shah A. M. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2004;287(5):R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 28.Lee M. Y., Griendling K. K. Redox signaling, vascular function, and hypertension. Antioxidants & Redox Signaling. 2008;10(6):1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacher P., Szabó C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Current Opinion in Pharmacology. 2006;6(2):136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Z., Li Y. Potent inhibition of peroxynitrite-induced DNA strand breakage by ethanol: possible implications for ethanol-mediated cardiovascular protection. Pharmacological Research. 2004;50(1):13–19. doi: 10.1016/j.phrs.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Fukai T., Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants & Redox Signaling. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J., Shuvaev V. V., Muzykantov V. R. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. The Journal of Pharmacology and Experimental Therapeutics. 2011;338(1):82–91. doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X., Morris-Natschke S. L., Lee K.-H. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Medicinal Research Reviews. 2007;27(1):133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 34.Hung Y.-C., Tseng Y.-J., Hu W.-L., et al. Demographic and prescribing patterns of Chinese herbal products for individualized therapy for ischemic heart disease in Taiwan: population-based study. PLoS ONE. 2015;10(8) doi: 10.1371/journal.pone.0137058.e0137058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung I. L., Hung Y. C., Wang L. Y., et al. Chinese herbal products for ischemic stroke. The American Journal of Chinese Medicine. 2015;43(7):1365–1379. doi: 10.1142/S0192415X15500779. [DOI] [PubMed] [Google Scholar]
- 36.Fu J., Huang H., Liu J., Pi R., Chen J., Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. European Journal of Pharmacology. 2007;568(1–3):213–221. doi: 10.1016/j.ejphar.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Liu J.-Q., Lee T.-F., Miedzyblocki M., Chan G. C. F., Bigam D. L., Cheung P.-Y. Effects of tanshinone IIA, a major component of Salvia miltiorrhiza, on platelet aggregation in healthy newborn piglets. Journal of Ethnopharmacology. 2011;137(1):44–49. doi: 10.1016/j.jep.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 38.Chan K., Chui S. H., Wong D. Y. L., Ha W. Y., Chan C. L., Wong R. N. S. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sciences. 2004;75(26):3157–3171. doi: 10.1016/j.lfs.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Hung Y.-C., Wang P.-W., Pan T.-L., Bazylak G., Leu Y.-L. Proteomic screening of antioxidant effects exhibited by Radix Salvia miltiorrhiza aqueous extract in cultured rat aortic smooth muscle cells under homocysteine treatment. Journal of Ethnopharmacology. 2009;124(3):463–474. doi: 10.1016/j.jep.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Pang H., Wu L., Tang Y., Zhou G., Qu C., Duan J. Chemical analysis of the herbal medicine salviae miltiorrhizae radix et rhizoma (Danshen) Molecules. 2016;21(1, article 51) doi: 10.3390/molecules21010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J.-Z., Li S.-L., Zhou Y., Qiao C.-F., Chen S.-L., Xu H.-X. A novel approach to rapidly explore analytical markers for quality control of Radix Salviae Miltiorrhizae extract granules by robust principal component analysis with ultra-high performance liquid chromatography-ultraviolet-quadrupole time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2010;53(3):279–286. doi: 10.1016/j.jpba.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Jiang P., Ye M., Kim S.-H., Jiang C., Lü J. Tanshinones: sources, pharmacokinetics and anti-cancer activities. International Journal of Molecular Sciences. 2012;13(10):13621–13666. doi: 10.3390/ijms131013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su C.-Y., Ming Q.-L., Rahman K., Han T., Qin L.-P. Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chinese Journal of Natural Medicines. 2015;13(3):163–182. doi: 10.1016/s1875-5364(15)30002-9. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y.-B., Ni Z.-Y., Shi Q.-W., et al. Constituents from Salvia species and their biological activities. Chemical Reviews. 2012;112(11):5967–6026. doi: 10.1021/cr200058f. [DOI] [PubMed] [Google Scholar]
- 45.Niu X.-L., Ichimori K., Yang X., et al. Tanshinone II-A inhibits low density lipoprotein oxidation in vitro. Free Radical Research. 2000;33(3):305–312. doi: 10.1080/10715760000301471. [DOI] [PubMed] [Google Scholar]
- 46.Lee D.-S., Lee S.-H., Noh J.-G., Hong S.-D. Antibacterial activities of cryptotanshinone and dihydrotanshinone I from a medicinal herb, Salvia miltiorrhiza bunge. Bioscience, Biotechnology and Biochemistry. 1999;63(12):2236–2239. doi: 10.1271/bbb.63.2236. [DOI] [PubMed] [Google Scholar]
- 47.Kim S. Y., Moon T. C., Chang H. W., Son K. H., Kang S. S., Kim H. P. Effects of tanshinone I isolated from Salvia miltiorrhiza Bunge on arachidonic acid metabolism and in vivo inflammatory responses. Phytotherapy Research. 2002;16(7):616–620. doi: 10.1002/ptr.941. [DOI] [PubMed] [Google Scholar]
- 48.Liu T., Jin H., Sun Q.-R., Xu J.-H., Hu H.-T. The neuroprotective effects of tanshinone IIA on β-amyloid-induced toxicity in rat cortical neurons. Neuropharmacology. 2010;59(7-8):595–604. doi: 10.1016/j.neuropharm.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Mosaddik M. A. In vitro cytotoxicity of Tanshinones isolated from Salvia miltiorrhiza Bunge against P388 lymphocytic leukemia cells. Phytomedicine. 2003;10(8):682–685. doi: 10.1078/0944-7113-00321. [DOI] [PubMed] [Google Scholar]
- 50.Qiu F., Wang G., Zhang R., Sun J., Jiang J., Ma Y. Effect of danshen extract on the activity of CYP3A4 in healthy volunteers. British Journal of Clinical Pharmacology. 2010;69(6):656–662. doi: 10.1111/j.1365-2125.2010.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang R.-W., Lau K.-M., Hon P.-M., Mak T. C. W., Woo K.-S., Fung K.-P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza . Current Medicinal Chemistry. 2005;12(2):237–246. doi: 10.2174/0929867053363397. [DOI] [PubMed] [Google Scholar]
- 52.Ge G., Zhang Q., Ma J., et al. Protective effect of Salvia miltiorrhiza aqueous extract on myocardium oxidative injury in ischemic-reperfusion rats. Gene. 2014;546(1):97–103. doi: 10.1016/j.gene.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Yu J., Wang L., Akinyi M., et al. Danshensu protects isolated heart against ischemia reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling. International Journal of Clinical and Experimental Medicine. 2015;8(9):14793–14804. [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W., Zheng L., Zhang Z., Hai C.-X. Protective effect of a water-soluble polysaccharide from Salvia miltiorrhiza Bunge on insulin resistance in rats. Carbohydrate Polymers. 2012;89(3):890–898. doi: 10.1016/j.carbpol.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 55.Jiang Y.-Y., Wang L., Zhang L., et al. Characterization, antioxidant and antitumor activities of polysaccharides from Salvia miltiorrhiza Bunge. International Journal of Biological Macromolecules. 2014;70:92–99. doi: 10.1016/j.ijbiomac.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 56.Rhoades D. A., Welty T. K., Wang W., et al. Aging and the prevalence of cardiovascular disease risk factors in older American Indians: the strong heart study. Journal of the American Geriatrics Society. 2007;55(1):87–94. doi: 10.1111/j.1532-5415.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 57.Peiris D., Thompson S. R., Beratarrechea A., et al. Behaviour change strategies for reducing blood pressure-related disease burden: findings from a global implementation research programme. Implementation Science. 2015;10(1) doi: 10.1186/s13012-015-0331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang P.-R., Shih W.-T., Chu Y.-H., Chen P.-C., Wu C.-Y. Frequency and co-prescription pattern of Chinese herbal products for hypertension in Taiwan: A Cohort Study. BMC Complementary and Alternative Medicine. 2015;15(1, article 163) doi: 10.1186/s12906-015-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z.-W., Xie X.-L., Zhou S.-F., Li C. G. Mechanism of reversal of high glucose-induced endothelial nitric oxide synthase uncoupling by tanshinone IIA in human endothelial cell line EA.hy926. European Journal of Pharmacology. 2012;697(1–3):97–105. doi: 10.1016/j.ejphar.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 60.Wang P., Wu X., Bao Y., et al. Tanshinone IIA prevents cardiac remodeling through attenuating NAD (P)H oxidase-derived reactive oxygen species production in hypertensive rats. Die Pharmazie. 2011;66(7):517–524. doi: 10.1691/ph.2011.0806. [DOI] [PubMed] [Google Scholar]
- 61.Jia L.-Q., Yang G.-L., Ren L., et al. Tanshinone IIA reduces apoptosis induced by hydrogen peroxide in the human endothelium-derived EA.hy926 cells. Journal of Ethnopharmacology. 2012;143(1):100–108. doi: 10.1016/j.jep.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Kang D. G., Oh H., Chung H. T., Lee H. S. Inhibition of angiotensin converting enzyme by lithospermic acid B isolated from radix Salviae miltiorrhiza Bunge. Phytotherapy Research. 2003;17(8):917–920. doi: 10.1002/ptr.1250. [DOI] [PubMed] [Google Scholar]
- 63.Kang D. G., Yun Y. G., Ryoo J. H., Lee H. S. Anti-hypertensive effect of water extract of Danshen on renovascular hypertension through inhibition of the renin angiotensin system. American Journal of Chinese Medicine. 2002;30(1):87–93. doi: 10.1142/S0192415X02000107. [DOI] [PubMed] [Google Scholar]
- 64.Ouyang X., Takahashi K., Komatsu K., et al. Protective effect of Salvia miltiorrhiza on angiotensin II-induced hypertrophic responses in neonatal rat cardiac cells. Japanese Journal of Pharmacology. 2001;87(4):289–296. doi: 10.1254/jjp.87.289. [DOI] [PubMed] [Google Scholar]
- 65.Leung S. W. S., Zhu D.-Y., Man R. Y. K. Effects of the aqueous extract of Salvia Miltiorrhiza (danshen) and its magnesium tanshinoate B-enriched form on blood pressure. Phytotherapy Research. 2010;24(5):769–774. doi: 10.1002/ptr.3047. [DOI] [PubMed] [Google Scholar]
- 66.Lam F. F. Y., Seto S. W., Kwan Y. W., Yeung J. H. K., Chan P. Activation of the iberiotoxin-sensitive BKCa channels by salvianolic acid B of the porcine coronary artery smooth muscle cells. European Journal of Pharmacology. 2006;546(1–3):28–35. doi: 10.1016/j.ejphar.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 67.Tang Y., Wang M., Chen C., Le X., Sun S., Yin Y. Cardiovascular protection with danshensu in spontaneously hypertensive rats. Biological & Pharmaceutical Bulletin. 2011;34:1596–1601. doi: 10.1248/bpb.34.1596. [DOI] [PubMed] [Google Scholar]
- 68.Xia Z., Gu J., Ansley D. M., Xia F., Yu J. Antioxidant therapy with Salvia miltiorrhiza decreases plasma endothelin-1 and thromboxane B2 after cardiopulmonary bypass in patients with congenital heart disease. The Journal of Thoracic and Cardiovascular Surgery. 2003;126(5):1404–1410. doi: 10.1016/s0022-5223(03)00970-x. [DOI] [PubMed] [Google Scholar]
- 69.Pirie K., Peto R., Reeves G. K., Green J., Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. The Lancet. 2013;381(9861):133–141. doi: 10.1016/s0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Isik B., Ceylan A., Isik R. Oxidative stress in smokers and non-smokers. Inhalation Toxicology. 2007;19(9):767–769. doi: 10.1080/08958370701401418. [DOI] [PubMed] [Google Scholar]
- 71.Pujades-Rodriguez M., George J., Shah A. D., et al. Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1937 360 people in England: lifetime risks and implications for risk prediction. International Journal of Epidemiology. 2015;44(1):129–141. doi: 10.1093/ije/dyu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang T., Xu J., Li D., et al. Salvianolic acid A, a matrix metalloproteinase-9 inhibitor of Salvia miltiorrhiza, attenuates aortic aneurysm formation in apolipoprotein E-deficient mice. Phytomedicine. 2014;21(10):1137–1145. doi: 10.1016/j.phymed.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Lam F. F. Y., Yeung J. H. K., Cheung J. H. Y. Mechanisms of the dilator action of Danshen (Salvia miltiorrhiza) on rat isolated femoral artery. Journal of Cardiovascular Pharmacology. 2005;46(3):361–368. doi: 10.1097/01.fjc.0000175439.94906.e9. [DOI] [PubMed] [Google Scholar]
- 74.Lam F. Y., Ng S. C. W., Cheung J. H. Y., Yeung J. H. K. Mechanisms of the vasorelaxant effect of Danshen (Salvia miltiorrhiza) in rat knee joints. Journal of Ethnopharmacology. 2006;104(3):336–344. doi: 10.1016/j.jep.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 75.Yi-ju Cheng F. W., Hu Y., Wu M.-X., Du J., Cheng M.-L. Compound injection of Danshen root suppresses cigarettes smoking-induced lung inflammation: a SD rat model. Inflammation and Cell Signaling. 2015;2(4):1–6. [Google Scholar]
- 76.Singer D. E., Nathan D. M., Anderson K. M., Wilson P. W. F., Evans J. C. Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham heart study. Diabetes. 1992;41(2):202–208. doi: 10.2337/diab.41.2.202. [DOI] [PubMed] [Google Scholar]
- 77.Kannel W. B., McGee D. L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham Study. Diabetes Care. 1979;2(2):120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 78.Aydin A., Orhan H., Sayal A., Özata M., Şahin G., Isimer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clinical Biochemistry. 2001;34(1):65–70. doi: 10.1016/s0009-9120(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 79.Dave G. S., Kalia K. Hyperglycemia induced oxidative stress in type-1 and type-2 diabetic patients with and without nephropathy. Cellular and Molecular Biology. 2007;53(5):68–78. doi: 10.1170/T820. [DOI] [PubMed] [Google Scholar]
- 80.Sasaki N., Yamashita T., Takaya T., et al. Augmentation of vascular remodeling by uncoupled endothelial nitric oxide synthase in a mouse model of diabetes mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(6):1068–1076. doi: 10.1161/ATVBAHA.107.160754. [DOI] [PubMed] [Google Scholar]
- 81.Gupta S., Chough E., Daley J., et al. Hyperglycemia increases endothelial superoxide that impairs smooth muscle cell Na+-K+-ATpase activity. American Journal of Physiology—Cell Physiology. 2002;282(3):C560–C566. doi: 10.1152/ajpcell.00343.2001. [DOI] [PubMed] [Google Scholar]
- 82.Monaghan K., McNaughten J., McGahon M. K., et al. Hyperglycemia and diabetes downregulate the functional expression of TRPV4 channels in retinal microvascular endothelium. PLoS ONE. 2015;10(6, article e0128359) doi: 10.1371/journal.pone.0128359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qian Q., Qian S., Fan P., Huo D., Wang S. Effect of Salvia miltiorrhiza hydrophilic extract on antioxidant enzymes in diabetic patients with chronic heart disease: a randomized controlled trial. Phytotherapy Research. 2012;26(1):60–66. doi: 10.1002/ptr.3513. [DOI] [PubMed] [Google Scholar]
- 84.Qian S., Huo D., Wang S., Qian Q. Inhibition of glucose-induced vascular endothelial growth factor expression by Salvia miltiorrhiza hydrophilic extract in human microvascular endothelial cells: evidence for mitochondrial oxidative stress. Journal of Ethnopharmacology. 2011;137(2):985–991. doi: 10.1016/j.jep.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 85.Huang M., Wang P., Xu S., et al. Biological activities of salvianolic acid B from Salvia miltiorrhiza on type 2 diabetes induced by high-fat diet and streptozotocin. Pharmaceutical Biology. 2015;53(7):1058–1065. doi: 10.3109/13880209.2014.959611. [DOI] [PubMed] [Google Scholar]
- 86.Jung S. H., Seol H. J., Jeon S. J., Son K. H., Lee J. R. Insulin-sensitizing activities of tanshinones, diterpene compounds of the root of Salvia miltiorrhiza Bunge. Phytomedicine. 2009;16(4):327–335. doi: 10.1016/j.phymed.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 87.National Cholesterol Education Program (NCEP) Expert Panel on Detection-Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 88.Wouters K., Shiri-Sverdlov R., van Gorp P. J., van Bilsen M., Hofker M. H. Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and LDLR mice. Clinical Chemistry and Laboratory Medicine. 2005;43(5):470–479. doi: 10.1515/cclm.2005.085. [DOI] [PubMed] [Google Scholar]
- 89.Hulsmans M., Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. Journal of Cellular and Molecular Medicine. 2010;14(1-2):70–78. doi: 10.1111/j.1582-4934.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang C.-C., Chu C.-F., Wang C.-N., et al. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine. 2014;21(3):207–216. doi: 10.1016/j.phymed.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 91.Yang T.-L., Lin F.-Y., Chen Y.-H., et al. Salvianolic acid B inhibits low-density lipoprotein oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolaemic rabbits. Journal of the Science of Food and Agriculture. 2011;91(1):134–141. doi: 10.1002/jsfa.4163. [DOI] [PubMed] [Google Scholar]
- 92.Karmin O., Lynn E. G., Vazhappilly R., Au-Yeung K. K., Zhu D. Y., Siow Y. L. Magnesium tanshinoate B (MTB) inhibits low density lipoprotein oxidation. Life Sciences. 2001;68(8):903–912. doi: 10.1016/s0024-3205(00)00989-9. [DOI] [PubMed] [Google Scholar]
- 93.Au-Yeung K. K. W., Karmin O., Choy P. C., Zhu D.-Y., Siow Y. L. Magnesium tanshinoate B protects endothelial cells against oxidized lipoprotein-induced apoptosis. Canadian Journal of Physiology and Pharmacology. 2007;85(11):1053–1062. doi: 10.1139/Y07-096. [DOI] [PubMed] [Google Scholar]
- 94.Stevens J., Oakkar E. E., Cui Z., Cai J., Truesdale K. P. US adults recommended for weight reduction by 1998 and 2013 obesity guidelines, NHANES 2007–2012. Obesity. 2015;23(3):527–531. doi: 10.1002/oby.20985. [DOI] [PubMed] [Google Scholar]
- 95.Poirier P., Eckel R. H. Obesity and cardiovascular disease. Current Atherosclerosis Reports. 2002;4(6):448–453. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- 96.Wajchenberg B. L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocrine Reviews. 2000;21(6):697–738. doi: 10.1210/er.21.6.697. [DOI] [PubMed] [Google Scholar]
- 97.Nishimura S., Manabe I., Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discovery Medicine. 2009;8(41):55–60. [PubMed] [Google Scholar]
- 98.Poirier P., Giles T. D., Bray G. A., et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(5):968–976. doi: 10.1161/01.atv.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 99.Rahman N., Jeon M., Song H.-Y., Kim Y.-S. Cryptotanshinone, a compound of Salvia miltiorrhiza inhibits pre-adipocytes differentiation by regulation of adipogenesis-related genes expression via STAT3 signaling. Phytomedicine. 2016;23(1):58–67. doi: 10.1016/j.phymed.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 100.Wang P., Xu S., Li W., et al. Salvianolic acid B inhibited pparγ expression and attenuated weight gain in mice with high-fat diet-induced obesity. Cellular Physiology and Biochemistry. 2014;34(2):288–298. doi: 10.1159/000362999. [DOI] [PubMed] [Google Scholar]
- 101.Tan Y., Kamal M. A., Wang Z.-Z., Xiao W., Seale J. P., Qu X. Chinese herbal extracts (SK0506) as a potential candidate for the therapy of the metabolic syndrome. Clinical Science. 2011;120(7):297–305. doi: 10.1042/CS20100441. [DOI] [PubMed] [Google Scholar]
- 102.Wu G. B., Zhou E. X., Qing D. X. TanshinoneII(A) elicited vasodilation in rat coronary arteriole: roles of nitric oxide and potassium channels. European Journal of Pharmacology. 2009;617(1–3):102–107. doi: 10.1016/j.ejphar.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 103.Yang Y., Cai F., Li P.-Y., et al. Activation of high conductance Ca2+-activated K+ channels by sodium tanshinoneII-A sulfonate (DS-201) in porcine coronary artery smooth muscle cells. European Journal of Pharmacology. 2008;598(1–3):9–15. doi: 10.1016/j.ejphar.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 104.Lam F. F. Y., Yeung J. H. K., Kwan Y. W., Chan K. M., Or P. M. Y. Salvianolic acid B, an aqueous component of danshen (Salvia miltiorrhiza), relaxes rat coronary artery by inhibition of calcium channels. European Journal of Pharmacology. 2006;553(1–3):240–245. doi: 10.1016/j.ejphar.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 105.Jia Y., Huang F., Zhang S., Leung S.-W. Is danshen (Salvia miltiorrhiza) dripping pill more effective than isosorbide dinitrate in treating angina pectoris? A systematic review of randomized controlled trials. International Journal of Cardiology. 2012;157(3):330–340. doi: 10.1016/j.ijcard.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 106.Maione F., De Feo V., Caiazzo E., De Martino L., Cicala C., Mascolo N. Tanshinone IIA, a major component of Salvia milthorriza Bunge, inhibits platelet activation via Erk-2 signaling pathway. Journal of Ethnopharmacology. 2014;155(2):1236–1242. doi: 10.1016/j.jep.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Carden D. L., Granger D. N. Pathophysiology of ischaemia-reperfusion injury. Journal of Pathology. 2000;190(3):255–266. doi: 10.1002/(sici)1096-9896(200002)190:3<255::aid-path526>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 108.Han J.-Y., Fan J.-Y., Horie Y., et al. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacology & Therapeutics. 2008;117(2):280–295. doi: 10.1016/j.pharmthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Hu H., Zhai C., Qian G., et al. Protective effects of tanshinone IIA on myocardial ischemia reperfusion injury by reducing oxidative stress, HMGB1 expression, and inflammatory reaction. Pharmaceutical Biology. 2015;53(12):1752–1758. doi: 10.3109/13880209.2015.1005753. [DOI] [PubMed] [Google Scholar]
- 110.Ren Z. H., Tong Y. H., Xu W., Ma J., Chen Y. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine. 2010;17(3-4):212–218. doi: 10.1016/j.phymed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 111.Zhou Z., Liu Y., Miao A.-D., Wang S.-Q. Protocatechuic aldehyde suppresses TNF-α-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells. European Journal of Pharmacology. 2005;513(1-2):1–8. doi: 10.1016/j.ejphar.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 112.Xu T., Wu X., Chen Q., et al. The anti-apoptotic and cardioprotective effects of salvianolic acid a on rat cardiomyocytes following ischemia/reperfusion by DUSP-mediated regulation of the ERK1/2/JNK pathway. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0102292.e102292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fan H., Yang L., Fu F., et al. Cardioprotective effects of salvianolic acid a on myocardial ischemia-reperfusion injury in vivo and in vitro. Evidence-Based Complementary and Alternative Medicine. 2012;2012:9. doi: 10.1155/2012/508938.508938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deng Y., Yang M., Xu F., et al. Combined salvianolic acid B and ginsenoside Rg1 exerts cardioprotection against ischemia/reperfusion injury in rats. PLoS ONE. 2015;10(8) doi: 10.1371/journal.pone.0135435.e0135435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao Y., Zhang K., Zhu F., et al. Salvia miltiorrhiza (Danshen) inhibits L-type calcium current and attenuates calcium transient and contractility in rat ventricular myocytes. Journal of Ethnopharmacology. 2014;158:397–403. doi: 10.1016/j.jep.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 116.Song M., Huang L., Zhao G., Song Y. Beneficial effects of a polysaccharide from Salvia miltiorrhiza on myocardial ischemia-reperfusion injury in rats. Carbohydrate Polymers. 2013;98(2):1631–1636. doi: 10.1016/j.carbpol.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 117.Konstam M. A., Kramer D. G., Patel A. R., Maron M. S., Udelson J. E. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC: Cardiovascular Imaging. 2011;4(1):98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 118.Reis Filho J. R. D. A. R., Cardoso J. N., Cardoso C. M. D. R., Pereira-Barretto A. C. Reverse cardiac remodeling: a marker of better prognosis in heart failure. Arquivos Brasileiros de Cardiologia. 2015;104(6):502–506. doi: 10.5935/abc.20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yagi A., Takeo S. Anti-inflammatory constituents, aloesin and aloemannan in Aloe species and effects of tanshinon VI in Salvia miltiorrhiza on heart. Yakugakuzasshi: Journal of the Pharmaceutical Society of Japan. 2003;123(7):517–532. doi: 10.1248/yakushi.123.517. [DOI] [PubMed] [Google Scholar]
- 120.Chan P., Liu J.-C., Lin L.-J., et al. Tanshinone IIA inhibits angiotensin II-induced cell proliferation in rat cardiac fibroblasts. The American Journal of Chinese Medicine. 2011;39(2):381–394. doi: 10.1142/s0192415x11008890. [DOI] [PubMed] [Google Scholar]
- 121.Yang L., Zou X.-J., Gao X., et al. Sodium tanshinone IIA sulfonate attenuates angiotensin II-induced collagen type I expression in cardiac fibroblasts in vitro. Experimental and Molecular Medicine. 2009;41(7):508–516. doi: 10.3858/emm.2009.41.7.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mao S., Li W., Qa'aty N., Vincent M., Zhang M., Hinek A. Tanshinone IIA inhibits angiotensin II induced extracellular matrix remodeling in human cardiac fibroblasts—implications for treatment of pathologic cardiac remodeling. International Journal of Cardiology. 2016;202:110–117. doi: 10.1016/j.ijcard.2015.08.191. [DOI] [PubMed] [Google Scholar]
- 123.Jiang B., Wu W., Li M., et al. Cardioprotection and matrix metalloproteinase-9 regulation of salvianolic acids on myocardial infarction in rats. Planta Medica. 2009;75(12):1286–1292. doi: 10.1055/s-0029-1185669. [DOI] [PubMed] [Google Scholar]
- 124.Jiang B., Li D., Deng Y., et al. Salvianolic acid A, a novel matrix metalloproteinase-9 inhibitor, prevents cardiac remodeling in spontaneously hypertensive rats. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059621.e59621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang B., Chen J., Xu L., et al. Salvianolic acid B functioned as a competitive inhibitor of matrix metalloproteinase-9 and efficiently prevented cardiac remodeling. BMC Pharmacology. 2010;10, article 10 doi: 10.1186/1471-2210-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y., Xu F., Chen J., et al. Matrix metalloproteinase-9 induces cardiac fibroblast migration, collagen and cytokine secretion: inhibition by salvianolic acid B from Salvia miltiorrhiza . Phytomedicine. 2011;19(1):13–19. doi: 10.1016/j.phymed.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 127.Chang C. C., Lee Y. C., Lin C. C., et al. Characteristics of traditional Chinese medicine usage in patients with stroke in Taiwan: a nationwide population-based study. Journal of Ethnopharmacology. 2016;186:311–321. doi: 10.1016/j.jep.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 128.Tang C., Xue H., Bai C., Fu R., Wu A. The effects of Tanshinone IIA on blood-brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine. 2010;17(14):1145–1149. doi: 10.1016/j.phymed.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 129.Zhou L., Bondy S. C., Jian L., et al. Tanshinone IIA attenuates the cerebral ischemic injury-induced increase in levels of GFAP and of caspases-3 and -8. Neuroscience. 2015;288:105–111. doi: 10.1016/j.neuroscience.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 130.Yang X., Yan J., Feng J. Treatment with tanshinone IIA suppresses disruption of the blood-brain barrier and reduces expression of adhesion molecules and chemokines in experimental autoimmune encephalomyelitis. European Journal of Pharmacology. 2016;771:18–28. doi: 10.1016/j.ejphar.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 131.Liu X., Ye M., An C., Pan L., Ji L. The effect of cationic albumin-conjugated PEGylated tanshinone IIA nanoparticles on neuronal signal pathways and neuroprotection in cerebral ischemia. Biomaterials. 2013;34(28):6893–6905. doi: 10.1016/j.biomaterials.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 132.Liu X., An C., Jin P., Liu X., Wang L. Protective effects of cationic bovine serum albumin-conjugated PEGylated tanshinone IIA nanoparticles on cerebral ischemia. Biomaterials. 2013;34(3):817–830. doi: 10.1016/j.biomaterials.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 133.Jiang Y.-F., Liu Z.-Q., Cui W., et al. Antioxidant effect of salvianolic acid B on hippocampal CA1 neurons in mice with cerebral ischemia and reperfusion injury. Chinese Journal of Integrative Medicine. 2015;21(7):516–522. doi: 10.1007/s11655-014-1791-1. [DOI] [PubMed] [Google Scholar]
- 134.Lv H., Wang L., Shen J., et al. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Research Bulletin. 2015;115:30–36. doi: 10.1016/j.brainresbull.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 135.Zhu H., Zou L., Tian J., Du G., Gao Y. SMND-309, a novel derivative of salvianolic acid B, protects rat brains ischemia and reperfusion injury by targeting the JAK2/STAT3 pathway. European Journal of Pharmacology. 2013;714(1–3):23–31. doi: 10.1016/j.ejphar.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 136.Guo C., Yin Y., Duan J., et al. Neuroprotective effect and underlying mechanism of sodium danshensu [3-(3,4-dihydroxyphenyl) lactic acid from Radix and Rhizoma Salviae miltiorrhizae = Danshen] against cerebral ischemia and reperfusion injury in rats. Phytomedicine. 2015;22(2):283–289. doi: 10.1016/j.phymed.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 137.Ming G.-L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhuang P., Zhang Y., Cui G., et al. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035636.e35636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Elbayoumi T. A., Torchilin V. P. Current trends in liposome research. Methods in Molecular Biology. 2010;605:1–27. doi: 10.1007/978-1-60327-360-2_1. [DOI] [PubMed] [Google Scholar]
- 140.Cai Y., Zhang W., Chen Z., Shi Z., Chengwei H., Chen M. Recent insights into the biological activities and drug delivery systems of tanshinones. International Journal of Nanomedicine. 2016;11:121–130. doi: 10.2147/IJN.S84035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao X., Liu X., Gan L., Zhou C., Mo J. Preparation and physicochemical characterizations of tanshinone IIA solid dispersion. Archives of Pharmacal Research. 2011;34(6):949–959. doi: 10.1007/s12272-011-0612-3. [DOI] [PubMed] [Google Scholar]