Abstract
Obstructive sleep apnea (OSA) is characterized by repeated involuntary closure of the pharyngeal airspace during sleep. Normal activity of the genioglossus (GG) muscle is important in maintaining airway patency, and inhibition of GG activity can contribute to airway closure. Neurons in the hypoglossal motor nucleus (HMN) regulate GG activity. Adrenergic tone is an important regulator of HMN neuronal excitability. In laboratory models α1-adrenergic antagonists inhibit HMN neurons and GG activity, suggesting that α1-adrenergic antagonism might adversely affect patients with OSA. To date there has been no report of such a case. Case Summary: The patient was a 67-year old man with a 27-month history of obstructive sleep apnea. Diagnostic polysomnography demonstrated a baseline apnea-hypopnea index (AHI) of 21.3 and a trough oxygen saturation of 84%. Treatment with continuous positive airway pressure (CPAP) was initiated. The AHI in year 1 averaged 1.0 ± 0.1 (mean ± SD) and 0.8 ± 0.1 in year 2. Other medical conditions included hypertension controlled with losartan and benign prostatic hypertrophy not well controlled by finasteride monotherapy. The α1-adrenergic receptor antagonist tamsulosin 0.4 mg daily was added. Shortly after initiation of tamsulosin, subjective sleep quality deteriorated. Significant surges in obstructive events, apneic episodes, and AHI were also recorded, and nocturnal airway pressure was frequently sustained at the CPAP device maximum of 20 cm H2O. Tamsulosin was discontinued. CPAP parameters and sleep quality returned to the pre-tamsulosin baselines within 10 days. These findings suggest that α1-adrenergic blockade with tamsulosin may exacerbate sleep-disordered breathing in susceptible patients.
Keywords: AHI, CPAP, Obstructive sleep apnea, Prostatic hypertrophy, Tamsulosin, Alpha-adrenergic antagonist
1. Introduction
Obstructive sleep apnea (OSA) is a common and sometimes serious disorder, affecting an estimated 20–30% of men and 10–15% of women [1], [2], [3]. It is characterized by recurrent collapse of the oropharyngeal musculature during sleep, leading to partial or complete obstruction of the upper airway [4]. These changes are most pronounced during rapid eye movement (REM) sleep, when skeletal muscle tone reaches its lowest point, resulting in reduced genioglossus (GG) and intercostal muscle tone and increased susceptibility to upper airway obstruction. Remmers and colleagues provided some of the earliest clinical evidence that GG muscle relaxation is involved in the pathophysiology of obstructive sleep apnea [5]. Several groups have investigated the neuroanatomy and control of GG activity during both sleep and wakefulness, demonstrating that neurons in the hypoglossal motor nucleus (HMN) regulate GG activity [4], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15].
The work of Horner's group in Toronto is of particular relevance to the case presented in this paper. He and his colleagues have developed a model of freely-moving, instrumented rodents well-suited for examining the pharmacological manipulation of HMN function. Adding to other work [16], [17], they have recently demonstrated the effects on respiratory muscle activity of stimulation and blockade of HMN α1-adrenergic receptors [12]. Microdialysis perfusion with the α1-adrenergic agonist phenylephrine increased HMN neuronal and GG activity while perfusion with the α1-antagonist terazosin markedly decreased both. These agonistic and antagonistic effects were demonstrated during both wakefulness and sleep.
Alpha1-adrenergic antagonists are in common clinical use. Adverse respiratory effects in patients with OSA, including that of reversible airway obstruction, have not been reported to date. In the current case, a patient with stable OSA treated with CPAP received the α1-adrenergic antagonist tamsulosin for treatment of benign prostatic hypertrophy (BPH). Tamsulosin dosing was associated with a rapid increase in both the number and severity of symptomatic episodes of airway obstruction. These changes reversed upon discontinuation of the drug.
2. Case
A 67-year old male with a 27-month history of obstructive sleep apnea treated with CPAP presented to the clinic with complaints of fatigue and a marked deterioration of subjective sleep quality, changing as the patient said from “fairly good” to “now very bad”. The adverse changes had begun approximately four weeks prior to the clinic visit, with a marked deterioration reported for the most recent seven days. His only other complaint was mild constipation, also beginning during the previous month. The patient had continued his usual professional activities as a physician, although he felt “much less efficient than usual.” The patient typically used CPAP between the hours of 11:00 p.m. and 6:00 a.m. He was not a shift worker. He traveled frequently for work, usually involving 3–9 h changes in time zones. He used CPAP during overnight flights. There had, however, been no air travel during the four weeks prior to the clinic visit. He continued to exercise daily. There were no known infectious exposures. He continued to report that he did not smoke, consume alcohol, ingest herbal or natural-product remedies such as yohimbine [18] or use illicit drugs.
Physical examination revealed an alert, mildly fatigued man in no acute distress. Vital signs and the remainder of his examination were normal for his age. In particular, the cardiopulmonary examination was unremarkable. There were no pulmonary or tracheal adventitial breath sounds. The ear/nose/throat examination was also unchanged from his initial presentation (described further below). His weight was 65 kg and BMI 21.8 kg/m2. His weight had decreased gradually over 18 months from a peak of 77 kg. Most but not all of the weight loss was described by the patient as voluntary. Epworth Sleepiness Scale (ESS) was 4.
Other medical conditions and medications included:
-
•
hypertension for 6 years treated with losartan 100 mg daily
-
•
gastroesophageal reflux for 1 year treated with ranitidine 300 mg daily
-
•
prostatic hypertrophy for 13 years treated for the most recent nine months with finasteride 5 mg daily to which tamsulosin 0.4 mg daily had been added four weeks before the clinic visit (and which resulted in a 40% increase in peak urinary flow rate after three weeks of dosing)
2.1. Past sleep history
The patient originally presented with symptoms of insomnia and severe nocturnal diaphoresis. At initial presentation, the patient's physical examination was noteworthy for a slight left inferior nasal septal deviation, a high/narrow hard palate, Mallampati score = 4, class 2 malocclusion with 4 mm overjet, and retrognathia. Diagnostic polysomnography demonstrated an AHI of 21.3, trough oxygen saturation of 84%, oxygen desaturation index of 7.0, and Periodic Limb Movement Index of 45.6. The patient did not report symptoms of restless leg syndrome. There was no other known sleep disorder.
He began empiric CPAP (Resmed® Auto S-9, Rescan™ software Version 5.6.0.9419). The CPAP prescription was then adjusted monthly over the ensuing four months based on detailed outpatient data downloads. The patient reported symptomatic improvement beginning approximately six months after initiation of CPAP. Repeat polysomnography was declined. Minor alterations in CPAP settings were made periodically in an effort to improve patient comfort. Regular follow-up visits demonstrated excellent compliance (98% in year 1 and 97% in year 2 and beyond).
His CPAP prescription on presentation for the current clinic visit had been unchanged for more than six months: auto-PAP 12.6–20 cm H2O (1.24–1.96 kPa) with an EPR (expiratory pressure relief) of 1 cm H2O (0.098 kPa). There was a 5-min linear pressure ramp to the target minimum from a starting airway pressure of 7 cm H2O (0.69 kPa).
2.2. CPAP data
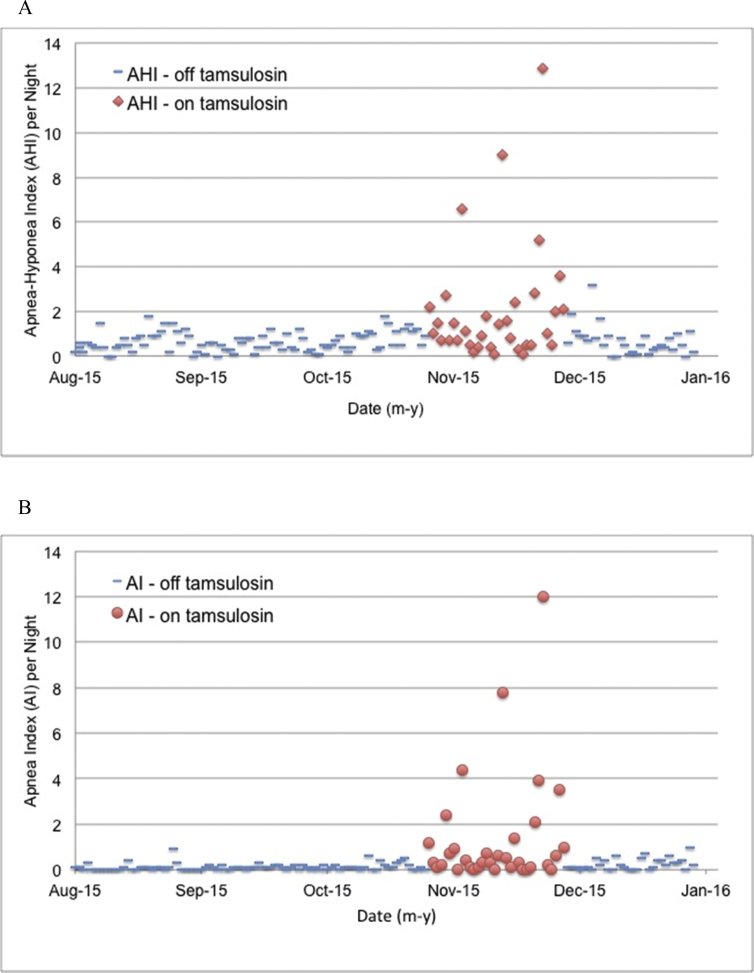
CPAP unit data were available for approximately two years with approximately 85% of nights also having detailed data available. Both detailed and summary data were recorded for all nights during the nine months immediately preceding the clinic visit. During the one-year period preceding the clinic visit (months 16–27 after the formal diagnosis of OSA), AHI averaged 0.8 ± 0.1 (mean ± SD), AI = 0.1, HI = 0.7. The maximal single-night values recorded during this same one-year period were AHI = 2.7, AI = 0.9, and HI = 2.2 (not all occurring on the same night). The patient's data for AHI and AI for the three months immediately prior to the tamsulosin dosing period, the period of tamsulosin dosing, and one month after discontinuation appear in Fig. 1.
Fig. 1.
Values for AHI (panel A) and AI (panel B) are shown by night in monthly intervals before and after tamsulosin exposure (“off tamsulosin”, blue dashes) and during tamsulosin exposure (“on tamsulosin”, AHI solid red diamonds, AI solid red circles). The on-tamsulosin data points begin with the first dose of tamsulosin and, to accommodate the time needed to clear tamsulosin from the body, continue until four days after the final tamsulosin dose. As shown, both AHI and AI had been low and stable for months prior to the initiation of dosing with tamsulosin. Within two days after the first dose of tamsulosin, both AHI and AI exceeded values that had not been exceeded for months. Both AHI and AI reached apex values just before tamsulosin dosing was terminated specifically because of these changes and attendant symptoms. After termination of tamsulosin, AHI and AI declined over several days, returning to and then remaining at pre-tamsulosin levels (During the five calendar months shown in Fig. 1, the patient used CPAP for a total of 1064:06 h:min, for an average of 6 h 57 min per night, >4 h per night on 153 nights, <4 h per night on 0 nights.). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
On a night-to-night basis, airway obstruction varies in patients with OSA [19]. Although the variability in the current patient's data appears to be greater during the on-tamsulosin period, the coefficient of variation is actually similar in both the on- and off-periods (on = 1.6 vs off = 1.3). Notwithstanding this similarity, during the 119 off-tamsulosin days of Fig. 1 AI did not exceed 0.9. Phrased in another way, AI exceeded 0.9 on 0 of 119 days. (A review of historical data showed that AI had not exceeded 0.9 in more than 450 days.) During the on-tamsulosin days in Fig. 1B, AI exceeded the previous 18 months' maximum on 9 of 34 days. The probability that this finding regarding days in excess of the prior AI maximum (0 of 119 days vs. 9 of 34 days) is due to chance alone is less than 1 × 10−8 (chi-square, Microsoft® Excel® v14.6.8).
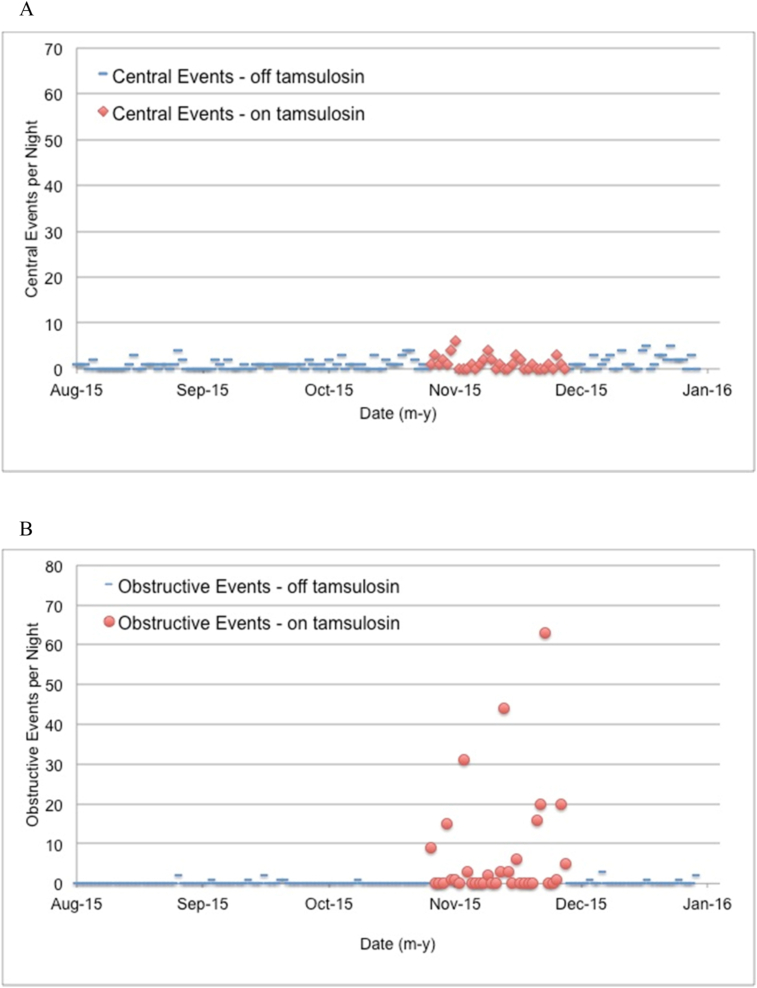
The events during tamsulosin exposure were obstructive rather than central in origin (Fig. 2).
Fig. 2.
The number of central events (panel A) and obstructive events (panel B) by night in monthly intervals before and after tamsulosin exposure (“off tamsulosin”, blue dashes) and during tamsulosin exposure (“on tamsulosin”, solid red diamonds [central events] or solid red circles [obstructive events]). The on-tamsulosin data points begin with the first dose of tamsulosin and, to accommodate the time needed to clear tamsulosin from the body, continue until four days after the final tamsulosin dose. The incidence of central events did not change with α1-adrenergic blockade while both the incidence and duration (not shown) of obstructive events increased markedly during tamsulosin exposure. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
During the three months preceding initiation of tamsulosin, the patient experienced a total of 93 recorded events (9 obstructive, 84 central). During the approximately one month (34 days) of tamsulosin exposure, the patient experienced 281 events (240 obstructive, 41 central). In the month following discontinuation of tamsulosin there were 62 events recorded (9 obstructive, 53 central). The probability that this increase in both the number and proportion of obstructive events during tamsulosin exposure occurred at random is less than 1 × 10−10 (chi-square).
While the absolute values of AHI and AI may appear to be numerically modest, the increased frequency and duration of obstructive events resulted for this patient in both disturbed sleep and compromised cognition. Moreover, the incidence and severity of symptomatic obstruction appeared to be increasing with continued tamsulosin dosing.
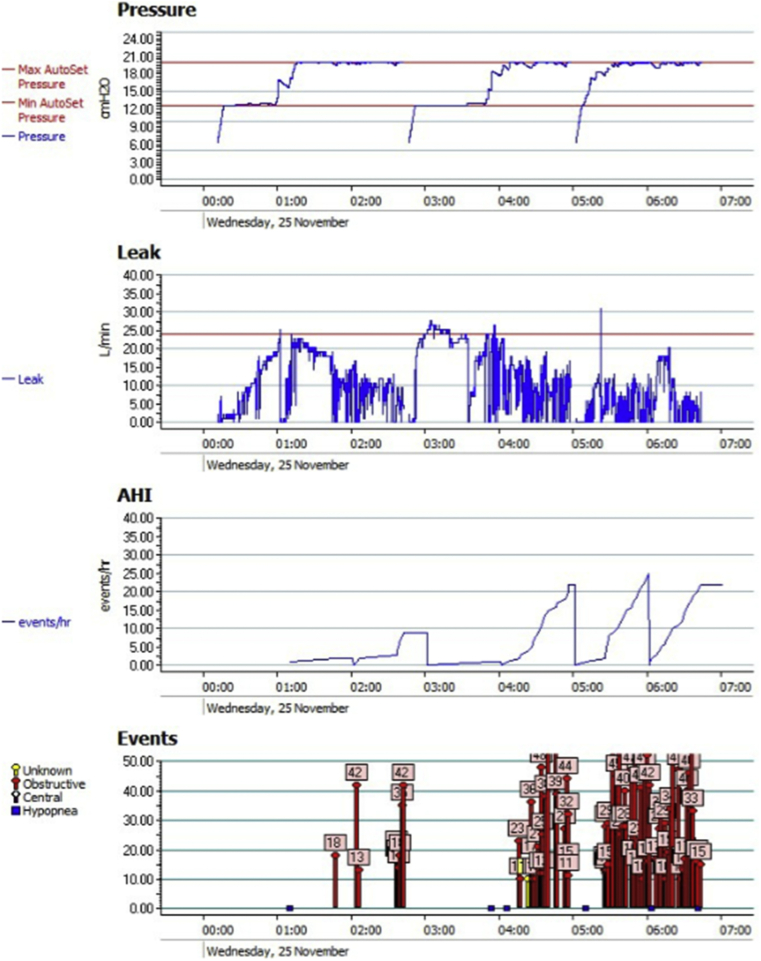
More detailed evidence of airway obstruction for the night of the peak AI, and which then resulted in the immediate, next-morning termination of tamsulosin, is shown in Fig. 3.
Fig. 3.
Detailed CPAP device data output (ResScan™) from 25 November 2015 after what proved to be the final dose of tamsulosin on the evening of 24 November 2015. Data collection began at 00:15 a.m. and ended at 06:39 a.m., a total of 6 h 24 min. Airway pressure was maintained at the machine maximum of 20 cm H2O (1.96 kPa*) for approximately 3h 20 min (52% of the total data collection time). Median leak rate for the night was 9.6 L/min. Leak exceeded 24 L/min for fewer than 20 min. AHI peaked at 23 events/h, almost entirely due to episodes of obstruction (red arrows), each obstructive event lasting 10–46 s, with the majority lasting 25 s or longer. Note that when the obstructive event rate was at its highest, between 04:15 and 06:39 a.m., the leak rate was near its lowest for the night. [*Multiply cm H2O by 0.098 to convert to kPa.].
Before the initiation of tamsulosin therapy, and from the more than 700 nights of detailed historical CPAP data available for this patient, the combination of persistent high-frequency obstructive events and sustained maximal elevation in airway pressure shown had never been observed.
3. Discussion
The current case report appears to be the first to document a reversible deterioration in the signs and symptoms of OSA following systemic α1-adrenergic blockade. The incidence of OSA and its associated morbidity and mortality are significant [1], [2], [3], [20]. An estimated four million men in the United States who have BPH (6% of men over 40) receive one of the α1-adrenergic blockers [21], [22]. If these two disorders segregate independently, then there could readily be some 200,000 men in the U.S. with OSA and BPH who are also receiving an α1-adrenergic antagonist. Some of these OSA patients will not be treated with CPAP. As a result, the OSA-CPAP/BPH-α1-antagonist subset is probably smaller in number and may explain how previous cases - if any - could have been missed. On the other hand, any worsening of sleep quality in patients may not be reported or could be attributed to other more common causes.
From an examination of the current patient's data, increased airway obstruction began to appear shortly after tamsulosin dosing was initiated. The half-life of tamsulosin is approximately 15 h [23]. Standard pharmacokinetic-pharmacodynamic modeling [24], [25] would suggest that three or more days of dosing could be required to achieve peak steady-state plasma levels of tamsulosin and a similar period of time to eliminate the drug from circulation. Even if peak levels required several days to establish in the current patient, with continued tamsulosin dosing there was a trend toward progressively higher values in the patient's AHI and AI. This suggests the possibility of an increased pharmacodynamic airway response developing over several weeks. The same type of observation – a progressively greater response to tamsulosin during weeks of exposure – has been made previously in the population of patients with prostatic hypertrophy [23]. Up to eight weeks of dosing were required to achieve maximal clinical increases in urinary flow rates. Such pharmacodynamic response data also suggest that adverse airway effects could still be observed during the first few days after the final tamsulosin dose, after which – if tamsulosin were cause of increased airway obstruction - CPAP data would then return to pre-tamsulosin values. This was the pattern observed in the current patient.
There are multiple marketed α1-adrenergic antagonists, differing in specificity, receptor affinity and probably in blood-brain-barrier permeability [26]. When adjusted for clinical dose, however, pharmacological effects appear more uniform across the class, at least for indicated uses. Tamsulosin, though, is a drug that partitions into the brain (at least in some species) [27], has high α1-receptor specificity and affinity [26], [28], a slow offset from its receptor [29], a prolonged half-life in older patients [23], and reduced clearance in some patients who concomitantly receive, as the current patient did, an H2-antagonist like ranitidine [23]. These pharmacological characteristics of tamsulosin, and perhaps the concomitant use of ranitidine, may substantially account for the observations in the current case.
While the preceding drug-specific considerations support a role for tamsulosin in precipitating the respiratory compromise observed in this patient, it is appropriate to ask if there were also patient-specific contributory factors. There is adrenergic genetic variation of relevance to OSA. However, it is a polymorphism in the β1-, and not the α1-, adrenergic receptor that has been identified as important, at least in those patients who also have - like the patient of this report - hypertension [30]. In addition, the current patient's BMI had decreased meaningfully from approximately 25 to 21 while on CPAP during the 18 months preceding the initiation of tamsulosin therapy. A significant weight loss in an OSA patient beginning with a normal-range BMI could perhaps unfavorably alter genioglossus mass or strength [31], [32]. If so, then in this patient any sensitivity to adverse muscular effects from α1-adrenergic blockade may have been increased.
The strengths of this report include the availability of extensive and detailed data: longitudinal clinical recordings of airway pressures, flows, and obstructive events from hundreds of nights preceding initiation of α1-adrenergic blockade with tamsulosin and similarly detailed data during and after tamsulosin exposure. The observed pattern of airway obstruction in this patient (onset, worsening, and offset) fit well with known tamsulosin pharmacokinetics. Finally, preclinical data from multiple laboratories have identified α1-adrenergic blockade as a plausible mechanism for interference with genioglossus activity and consequential airway obstruction.
Potential weaknesses of the report include the absence of clinical reports for other drugs in the same class and the unavoidable fact that data from laboratory models cannot substitute for their clinical counterparts. Whether a second polysomnography could have yielded probative data for this patient that would have altered the apparent response to tamsulosin is, of course, unknown.
It is very unlikely that the reversible obstruction observed in this patient occurred at random. The only known pharmacological changes were tamsulosin administration and withdrawal. The clinical changes were consistent with preclinical observations. The onset and offset of adverse respiratory effects were compatibly timed with tamsulosin exposure. The statistics are familiar, simple, and robust. In brief, one month's exposure to the α1-adrenergic antagonist tamsulosin was associated with a reversible deterioration in the signs and symptoms of obstructive sleep apnea.
Acknowledgement
The author thanks Mitchell Miglis, M.D., Stanford University School of Medicine, for his comments.
References
- 1.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013;177(9):1006. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T., Palta M., Dempsey J., Skatrud J., Weber S., Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Horner R.L. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 5.Remmers J.E., deGroot W.J., Sauerland E.K., Anch A.M. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 6.Aston-Jones G., Bloom F.E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep- waking cycle. J. Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldes L.D., Chapman M.E., Chronister R.B., Haycock J.W. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res. Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- 8.Goh A.S., Issa F.G., Sullivan C.E. Upper airway dilating forces during wakefulness and sleep in dogs. J. Appl. Physiol. 1986;61:2148–2155. doi: 10.1152/jappl.1986.61.6.2148. [DOI] [PubMed] [Google Scholar]
- 9.Kuna S, Remmers JE. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine, third ed.. Philadelphia, PA; WB Saunders. pp. 840–858.
- 10.Tangel D.J., Mezzanotte W.S., White D.P. Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J. Appl. Physiol. 1991;70:2574–2581. doi: 10.1152/jappl.1991.70.6.2574. [DOI] [PubMed] [Google Scholar]
- 11.Tangel D.J., Mezzanotte W.S., Sandberg E.J., White D.P. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J. Appl. Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- 12.Chan E., Steenland H.W., Liu H., Horner R.L. Endogenous excitatory drive modulating respiratory muscle activity across sleep–wake states. Am. J. Respir. Crit. Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 13.Chase M.H., Morales F.R. Control of motoneurons during sleep. In: Kryger M.H., Roth T., Dement W.C., editors. Principles and Practice of Sleep Medicine. 3 ed. WB Saunders; Philadelphia, PA: 2000. pp. 155–168. [Google Scholar]
- 14.Fuller D.D., Williams J.S., Janssen P.L., Fregosi R.F. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J. Physiol. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin X.-T., Cui N., Zhong W., Jin X., Wu Z., Jiang C. Pre- and postsynaptic modulations of hypoglossal motoneurons by α-adrenoceptor activation in wild type and Mecp2(-/Y) mice. Am. J. Physiol. Cell Physiol. 2013;305:C1080–C1090. doi: 10.1152/ajpcell.00109.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sood S., Morrison J.L., Liu H., Horner R.L. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am. J. Respir. Crit. Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 17.Sood S., Raddatz E., Liu X., Liu H., Horner R.L. Inhibition of serotonergic medullary raphe obscurus neurons suppresses genioglossus and diaphragm activities in anesthetized but not conscious rats. J. Appl. Physiol. 2006;100:1807–1821. doi: 10.1152/japplphysiol.01508.2005. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsen S., Pedersen K., Smith D.F., Jensen S.B., Munk O.L., Cumming P. Detection of α2-adrenergic receptors in the brain of living pig with 11C-Yohimbine. J. Nucl. Med. 2006;47:2008–2015. [PubMed] [Google Scholar]
- 19.Bittencourt L.R.A., Suchecki D., Tufik S., Peres C., Togeiro S.M., Bagnato M.D.C., Nery L.E. The variability of the apnoea-hypopnoea index. J. Sleep. Res. 2001;10:245–251. doi: 10.1046/j.1365-2869.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 20.Phillipson E.A. Sleep apnea: a major public health problem. N. Engl. J. Med. 1993;328:1271–1273. doi: 10.1056/NEJM199304293281712. [DOI] [PubMed] [Google Scholar]
- 21.Vuichoud C., Loughlin K.R. Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can. J. Urol. 2015;22(Suppl. 1):1–6. [PubMed] [Google Scholar]
- 22.http://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age/.
- 23.FDA label: www.accessdata.fda.gov; document 020579s016lbl-2.pdf.
- 24.Meibohm B., Derendorf H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modeling. Int. J. Clin. Pharmacol. Ther. 1997;35:401–413. [PubMed] [Google Scholar]
- 25.Zhang L., Pfister M., Meibohm B. Concepts and challenges in quantitative pharmacology and model-based drug development. AAPS J. 2008;10:552–559. doi: 10.1208/s12248-008-9062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito K., Ohtani H., Sawada Y. Assessment of a1-adrenoceptor antagonists in benign prostatic hyperplasia based on the receptor occupancy theory. Br. J. Clin. Pharmacol. 2007;63:394–403. doi: 10.1111/j.1365-2125.2006.02783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada S., Ohkura T., Deguchi Y., Kimura R. Vivo measurement by [3H]Tamsulosin of α1-adrenoceptors in rat tissues in relation to the pharmacokinetics. JPET. 1999;289:1575–1583. [PubMed] [Google Scholar]
- 28.Muramatsu I., Taniguchi T., Okada K. Tamsulosin: alpha1-adrenoceptor subtype-slectivity and comparison with terazosin. Jpn. J. Pharmacol. 1998;78:331–335. doi: 10.1254/jjp.78.331. [DOI] [PubMed] [Google Scholar]
- 29.Sato S., Hatanaka T., Yuyama H., Ukai M., Noguchi Y., Ohtake A., Taguchi K., Sasamata M., Miyata K. Tamsulosin potently and selectively antagonizes human recombinant α(1A/1D)-adrenoceptors: slow dissociation from the α(1A)-adrenoceptor may account for selectivity for α(1A)-adrenoceptor over α(1B)- adrenoceptor subtype. Biol. Pharm. Bull. 2012;35(1):72–77. doi: 10.1248/bpb.35.72. [DOI] [PubMed] [Google Scholar]
- 30.Boström K.B., Hedner J., Grote L., Melander O., von Wowern F., Råstam L., Groop L., Lindblad U. Polymorphisms in α- and β-adrenergic receptor genes, hypertension, and obstructive sleep apnea: the skaraborg sleep study. Int. J. Hypertens. 2010:2010–2023. doi: 10.4061/2010/458410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanezaki M., Ogawa T., Izumi T. Tongue protrusion strength in arousal state is predictive of the airway patency in obstructive sleep apnea. J. Exp. Med. 2015;236(4):241–245. doi: 10.1620/tjem.236.241. [DOI] [PubMed] [Google Scholar]
- 32.Almeida L.D., Furlan R.M., Las Casas E.B., Motta A.R. Influence of height, weight and body mass index in the axial tongue force. J. Soc. Bras. Fonoaudiol. 2012;24(4):381–385. doi: 10.1590/s2179-64912012000400015. [DOI] [PubMed] [Google Scholar]