Abstract
The primary aim of this review is to summarize the current literature on the effects of acute exercise and regular exercise on nuclear factor erythroid 2-related factor 2 (Nrf2) activity and downstream targets of Nrf2 signaling. Nrf2 (encoded in humans by the NFE2L2 gene) is the master regulator of antioxidant defenses, a transcription factor that regulates expression of more than 200 cytoprotective genes. Increasing evidence indicates that Nrf2 signaling plays a key role in how oxidative stress mediates the beneficial effects of exercise. Episodic increases in oxidative stress induced through bouts of acute exercise stimulate Nrf2 activation and when applied repeatedly, as with regular exercise, leads to upregulation of endogenous antioxidant defenses and overall greater ability to counteract the damaging effects of oxidative stress. The evidence of Nrf2 activation in response to exercise across variety of tissues may be an important mechanism of how exercise exerts its well-known systemic effects that are not limited to skeletal muscle and myocardium. Additionally there are emerging data that results from animal studies translate to humans.
Keywords: Acute exercise, Regular exercise, Phase II enzymes, Reactive oxygen species, Cell signaling, NFE2L2, HO-1, SOD
Graphical abstract
Highlights
-
•
This review summarizes the current literature on the effects of exercise on Nrf2 signaling.
-
•
Acute exercise stimulates Nrf2 signaling through ROS production.
-
•
Nrf2 signaling plays a key role in how ROS mediate the beneficial effects of exercise.
-
•
Emerging data support translation of data from animal studies to humans.
1. Introduction
The understanding of cell signaling induced by acute exercise resulting in physiological adaptations that accumulate after multiple bouts, has been greatly enhanced with the advancement of molecular biology and its application to exercise. Acute exercise activates primary messengers such as adenosine monophosphate (AMP), calcium, and mechanical stress and subsequent secondary messengers including AMP-activated protein kinase (AMPK), calcium/calmodulin-dependent protein kinase (CAMK), and p38 mitogen-activated protein kinases, leading to acute changes in mRNA transcription [1]. Another important primary messenger that is stimulated by acute exercise is the temporary change in redox balance (or redox potential) towards a more oxidized state through production of reactive oxygen species (ROS) [2]. While ROS were initially designated as damaging, there is now a greater understanding of the important role of ROS in cell signaling, regulation of immune function, gene transcription, and apoptosis [3], [4], [5], [6]. A prime example is the role of ROS in activation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), the master regulator of antioxidant enzymes and cellular stress resistance [7], [8], [9].
Data from rodent studies illustrate the therapeutic potential of targeting Nrf2 activity for lowering disease risk. Targeted Nrf2 knockout (Nrf2–/–) models exhibit substantially impaired resistance to toxic and oxidative stressors in all tissues and as a result, Nrf2–/– mice are more susceptible to chronic diseases [10]. Pharmacologic and genetic (negative regulator knockout) approaches targeting Nrf2 activation effectively ameliorate the damage from oxidative stressors and restore resistance to chronic disease [11]. Increased pathology in the presence of disrupted Nrf2 signaling, and subsequent reversal in the presence of Nrf2 activators have been reviewed elsewhere [12].
Increasing evidence indicates that the Nrf2 pathway plays a key role in how oxidative stress mediates the beneficial effects of exercise. Episodic increases in oxidative stress induced through bouts of acute exercise stimulate Nrf2 activation and when applied repeatedly, as with regular exercise, this may lead to upregulation of endogenous antioxidant defenses and overall greater ability to counteract the damaging effects of oxidation to nucleic acids, proteins, and lipids.
The primary aim of this review is to summarize the current literature on the effects of acute exercise and regular exercise or exercise interventions on Nrf2 activity and downstream targets of Nrf2 signaling. We also examine the evidence for or against using antioxidant supplementation and phytonutrient Nrf2-activators in conjunction with exercise. Because the effect of an intervention can be more readily seen when the target is impaired, we will highlight studies on aging throughout as a non-disease model of impaired cell signaling, which often manifest coordinately with age related pathologies [13], [14], [15]. We begin with a brief overview of Nrf2 regulation.
2. Nrf2: the master regulator of cellular defense
The transcription factor Nrf2, (encoded in humans by the NFE2L2 gene) is the master regulator of antioxidant defenses, regulating more than 200 cytoprotective genes in response to oxidative stress [16]. Nrf2 is a member of the basic leucine zipper (bZIP) family of transcription factors that is repressed through binding to the homodimeric protein Kelch-like erythroid cell-derived protein with CNC homology associated protein 1 (Keap1) in the cytosol under unstressed conditions [17]. The interaction between Nrf2 and Keap1 is highly conserved across species, indicating its important regulatory role [18]. In this state, Keap1 functions as an adapter for Cul3/Rbx1-mediated degradation of Nrf2 by promoting ubiquitination and subsequent degradation of Nrf2 by the 26 s proteasome. The Keap1/Cul3/Rbx1 also exists in the nucleus as an additional negative regulator [19].
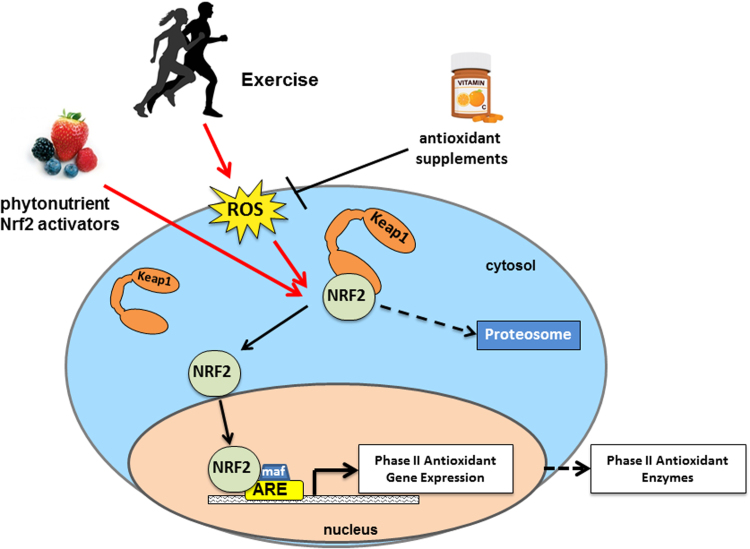
In response to an oxidative or electrophilic stimulus, cysteine residues are modified in a unique format, whereby structurally dissimilar inducers react with differing combinations of cysteine residues on Keap1 resulting in the same biological response – specifically the unhinging from Nrf2 and activation of the Nrf2-antioxidant response element (ARE) response, or by stabilization of the Keap1-Nrf2 complex slowing degradation at the proteasome [20], [21], [22], [23]. Once unhinged from Keap1, Nrf2 is translocated into the nucleus where it has the capacity to heterodimerize with small MAF proteins and bind to Cis-acting AREs, effectively activating transcription of phase II detoxifying enzymes (see Fig. 1).
Fig. 1.
Nrf2 signaling. Nrf2 is activated by exercise-induced ROS or phytonutrient Nrf2 activators. Antioxidant supplementation inhibits the signaling of exercise-induced ROS and thereby downstream Nrf2 signaling.
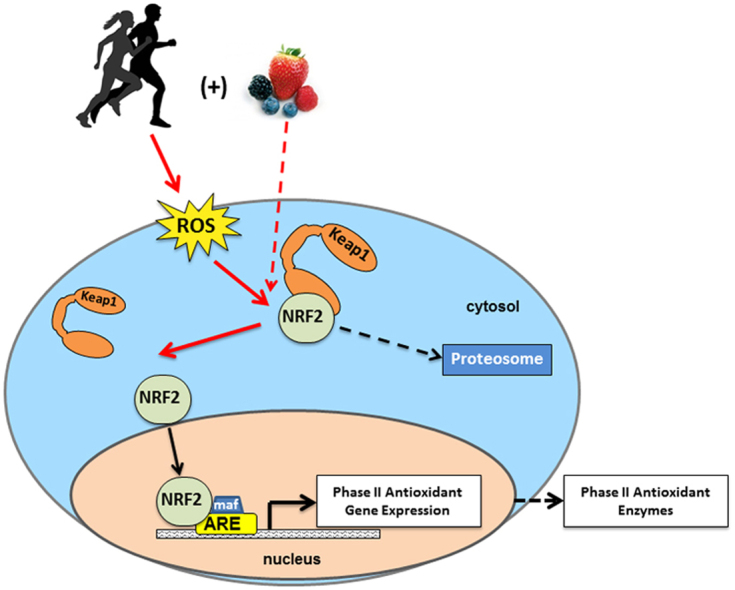
In terms of therapeutic potential, this cysteine code offers great interest as multiple compounds and stimuli act as potent Nrf2 inducers independent of each other [24], [25], [26]. For an example, exercise-induced ROS activation of Nrf2 likely occurs through oxidation of these cysteine residues. Similarly certain phytonutrients have been shown to activate Nrf2 and this process may occur through modifications of cysteine residues different from those targeted through exercise. If that is in fact the case, there may be potential for synergistic effects between exercise training and Nrf2 activating compounds. There are some recent data in support of this and those are presented in section 6 of this review [27], [28]. For those interested in a more detailed background on mechanisms of Nrf2 activation we refer readers to an excellent recent review in this area [16].
It is worth noting that in certain conditions, such as cancer, Nrf2 activation and signaling may have detrimental effects. Under normal conditions Nrf2 signaling protects from cancer as seen with the effects of phytonutrients in cruciferous vegetables that stimulate Nrf2 activation [29], [30], [31]. However, in cancer cells Nrf2 activation may confer cellular stress resistance which can make the cells less susceptible to chemotherapeutic treatment [32], [33]. Nrf2 has been shown to be upregulated in cancer tumors and using Nrf2 inhibitors during chemotherapy may be necessary to increase the efficacy of treatment [16], [34].
3. Nrf2 signaling in response to acute exercise
Cell culture studies using C2C12 skeletal muscle cells provide evidence that Nrf2 is activated by ROS and this activation is suppressed when antioxidants, such as N-acetylcysteine, are applied to the culture medium [35], [36]. Horie et al. [35] used an electrical pulse stimulation (EPS) to mimic acute exercise and demonstrated that Nrf2 expression was associated with both intensity and duration of the stimulus. In addition, EPS stimulated Nrf2-related antioxidant gene expression and the response was blunted when siRNA transfection was used to knock down Nrf2 in the cells [35]. Other studies have shown an increase in Nrf2 protein expression after treating myotubes or rat cardiomyocytes with H2O2 [36], [37].
Translating these in vitro results to in vivo has primarily been done in animal models with one recent study in humans. These studies are listed in Table 1. It is worth noting that some of the earlier studies targeted a non-specific band for Nrf2 (57 kDa) in their immunoblots. Several groups have since identified the 90–110 kDa band as the biologically relevant Nrf2 target, despite the predicted molecular weight of Nrf2 being 57 kDa [38], [39].
Table 1.
Acute exercise induced Nrf2 signaling.
| Ref | Description of study | Ex stimulus | Model,n, and tissue | Gender, age, and fitness | Nrf2 | Nrf2 target expression | |
|---|---|---|---|---|---|---|---|
| Malaguti et al. [51] | Four groups: control (C), exercise (EX), sulforaphane, (SFN), and EX+SFN (ES). SFN supplementation at 72, 48, and 24-h prior to exhaustive EX. | Exercise to exhaustion on treadmill; 24 m/min, 7% grade. | Rat (Wistar) n=8/grp SMT | Male young (4-months) untrained | mRNA: NR Protein: WC: ↔ EX; ↑ ES | mRNA: NR Protein: ↑ GST, GR, NQO1 in SFN and ES groups; ↔ EX and C groups. | |
| Gounder et al. [43] | Y and O mice were exercised for two consecutive days followed by sacrifice immediately following the second bout. | Endurance exercise on treadmill; 90 min/day, 20 m/min, 12% grade, for 2 consecutive days. | Mice (C57/Bl6/SJ; WT and KO) n=4–6/grp myocardium | Male young (8–10 weeks) vs old (>23 months) untrained | mRNA: NR Protein: NUC: ↑ young; ↔ old | mRNA: Young - ↑ NQO1, HO1, GCLM, GCLC, CAT, SOD2, GSR, G6PDX, GPX1; Old - ↔ Protein: Young - ↑ NQO1, HO1, GCLM, CAT, SOD2, GSR, G6PD, GPX1; Old - ↔ | |
| Muthusamy et al. [42] | Wild-type and Nrf2 KO mice were exercised for two consecutive days followed by sacrifice immediately following the second bout. | Endurance exercise on treadmill; 60 min/day, 14 m/min, 10% grade, for 2 days. | Mice (WT and KO) n=4–6/grp myocardium | Male young (2-months) untrained | mRNA: NR Protein: NUC: ↑ WT; ↔ KO NRF2/ARE binding: ↑ WT; ↔ KO | mRNA: WT - ↑ CAT, GCLM, NQO1, GCLC, GPX1, G6PDX, GSR; KO - ↔ Protein: WT- ↑ G6PD, GCLM, HO1; KO - ↔ | |
| Narasimhan et al. [41] | Old WT and KO mice were exercised for two-weeks. *Authors report as acute endurance exercise stress, although not truly acute. | Exhaustive endurance exercise; 45 min/day, 10–15 m/min, 0% grade, daily for two weeks. | Mice (C57/Bl6/SJ; WT and KO) n=3–4/grp SMT | Male old (>23 months) untrained* |
mRNA: NR Protein: NR |
mRNA: WT - ↑ NQO1, G6PD, CAT, GSR; KO - ↑ GPX1, GSR Protein: WT - ↑CAT, ↑G6PD; KO - ↔ | |
| Wang et al. [40] | Five groups for comparing role of exercise duration: Control, 45-, 90-, 120-, and 150-min exercise conditions. | Incremental treadmill, duration corresponding to assigned group. Stage 1: 0°, 8.2 m/min, 53% VO2max stage 2: 5°, 15 m/min, 64% VO2max stage 3: 10°, 19.3 m/min, 76% VO2max | Mice (ICR/CD-1) n=6/grp SMT | Male young (8-weeks) untrained | mRNA: ↔ 45; ↑90, 120, 150 Protein: WC: ↔ 45; ↑90, 120, 150 | mRNA: NR Protein: NR | |
| Li et al. [50] | Three groups for comparing role of exercise duration: Control, 1-h, and 6-h exercise conditions. | 1-h and 6-h treadmill running groups; 20 m/min, 5% grade. | Mice (C57/Bl6) n=10/grp SMT | Male young (2-months) untrained | mRNA: NR Protein: NUC: ↔ 1-h, ↑ 6-h NRF2/ARE binding: ↔ 1-h, ↑ 6-h | mRNA: 1-hr - ↑ GCLC, CAT; 6-h - ↑ GCLC, SOD2, CAT, HO1, GCLM, SOD1, CAT Protein: NR | |
| Done et al. [45] | Y and O males completed one exercise bout with blood draws pre-, and five time points post exercise spanning a 24-h period. | Cycle ergometer; 30 min at 70% VO2max | Human n=10/grp PBMC | Male young (23±1 y) and older (63±1 y) recreationally active | mRNA: NR Protein: WC: ↑ young; ↑ old NUC: ↑ young; ↔ old | mRNA: Young - ↑ NQO1, HO1; Old - ↔ Protein: Young - ↔; Old - ↔ | |
| Merry and Ristow [36] | Wild-type and Nrf2 KO mice randomized into groups sacrificed 30-min, 2-h, or 16-h post exercise. | Endurance exercise on treadmill; 60 min, 12 m/min, 10% grade. | Mice (C57/Bl6; WT and KO) n=5–10/grp SMT | Male young (15–30 weeks) untrained | mRNA: ↑ 30-min, ↔ 2-h, 6-h Protein: NR | mRNA: 30-min - ↑ GSR, 16-h - ↑ GST Protein: NR | |
NR not reported or not measured, SMT skeletal muscle tissue, PBMC peripheral blood mononuclear cells, NUC nuclear, WC, whole-cell.
A single bout of acute exercise in wild-type mice has been shown to increase Nrf2 gene expression [36], [40] as well as Nrf2 protein abundance in skeletal muscle [40] and Nrf2-dependent phase II enzymes [36], [41]. Two additional studies that measured the effects of exercise on Nrf2 signaling in mouse myocardium are essentially in agreement with the data from skeletal muscle [42], [43]. However, these studies used an “acute exercise stress” that was composed of 60-min treadmill exercise on two consecutive days. While clearly not long enough to be classified as exercise training, it is known that consecutive exercise bouts lead to cumulative responses of mRNA expression and may be necessary to observe measurable changes in protein abundance [44]. Nevertheless, Muthusamy et al. [42] provided one of the most comprehensive analyses of Nrf2-signaling in response to exercise and compared young wild-type (WT) and Nrf2–/– mice. Despite both groups experiencing similar increases in ROS, the exercise bouts elicited significant increases in nuclear accumulation and Nrf2-ARE binding in the myocardium of WT mice, while no change in Nrf2 activity was observed in Nrf2–/– mice. Gene expression of nearly all measured phase II enzymes was significantly increased in the WT; the only exception being heme oxygenase-1 (HO-1). Changes in protein expression of phase II antioxidants were less consistent, with changes only observed in HO-1, glutamate-cysteine ligand modifier (GCLM), and glucose-6-phosphate dehydrogenase (G6PD).
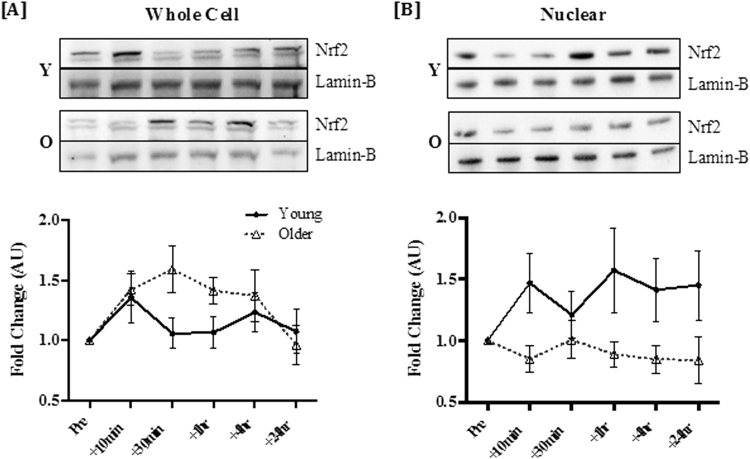
Data on exercise-induced Nrf2 signaling in humans are still very limited but a recent study showed for the first time that acute exercise increased Nrf2 protein abundance at the whole-cell level measured in peripheral blood mononuclear cells (PBMCs) in young and older men [45]. Furthermore, nuclear accumulation of Nrf2 was only observed in the young group, demonstrating that aging is associated with impairment in the nuclear import of Nrf2 (see Fig. 2). Not surprisingly, the exercise bout stimulated increases in gene expression of HO-1 and NAD(P)H quinone dehydrogenase 1 (NQO1) in the young but not the older men [45]. Age-related decreases in cytosolic and nuclear Nrf2 as well as Nrf2/ARE binding capacity were previously shown in mouse myocardium [43] and rat liver [46] with concomitant attenuation of Nrf2-dependent antioxidant gene expression in response to acute exercise [43].
Fig. 2.
Exercise induces increased Nrf2 at the whole-cell level, but nuclear accumulation is attenuated in older adults. Nrf2 response to exercise in the whole cell [A] and nuclear fraction [B] of PBMCs. Values are means±SEM. Representative blots of changes in Nrf2 and loading control in young (Y) and older (O) are provided above the corresponding graphs. There was a significant main effect of time in fold change of whole-cell Nrf2 (P=0.003). Nuclear accumulation only increased significantly in the young, but not the older group (main effect of age, P=0.031). Reprinted from [45] with permission from Elsevier.
Two additional human studies, although not directly investigating Nrf2 signaling, are relevant because they included measures of Nrf2 mRNA in response to acute exercise. Gene expression of Nrf2 and target SOD2 were shown to increase significantly in skeletal muscle of young fit males following an acute bout of cycling exercise lasting 90-min in normoxic recovery conditions [47]. Interestingly, these responses were not seen when recovery was performed under hypoxic conditions [47]. Similarly Nrf2 mRNA was significantly increased at 2-h post 30-min of moderate treadmill exercise in middle-aged women who were regular exercisers; however, sedentary women showed no change in gene expression for Nrf2 or targets in response to the exercise bout [48], suggesting that fitness may play a role in maintaining the acute Nrf2 response with aging.
Similar to the cell culture studies, some of the animal studies have demonstrated that the increases in Nrf2 signaling are dependent on the duration of exercise. Treadmill exercise lasting less than one hour elicited no change in Nrf2 mRNA, or protein expression [40], [49], [50]. As duration was increased to 90-min or more, Nrf2 activation became apparent in both skeletal muscle tissue and in brain homogenate with significant increases in Nrf2 protein expression in skeletal muscle and gene expression increasing in both tissues [40], [49]. In an extreme example of endurance exercise, Li and colleagues [50] demonstrated no difference in Nrf2 activity in mice running for 1-h on the treadmill, but robust increases in Nrf2 nuclear accumulation and Nrf2-ARE binding following a 6-h session on the treadmill. In addition, gene expression of several phase II enzymes was significantly increased over non-exercising controls and the 1-h group [50]. In contrast to these results, other studies have reported increases in Nrf2 protein abundance after 1-h of treadmill running [36] and 30-min of moderate intensity cycling [45]. The differences observed between these studies are likely a result of differences in protocol intensities (summarized in Table 1).
None of the existing studies have investigated the effects of exercise intensity on the Nrf2 signaling response. However, Wang et al. [40] found a positive correlation between exercise-induced mitochondrial H2O2 content and Nrf2 gene expression and Nrf2 protein expression (measured in response to exercise of different duration). However, under the assumption that higher intensity induces greater oxidative stress; these data along with the results from the electrical pulse stimulation in myotubes suggest that intensity may affect Nrf2 signaling. There may be an upper limit though to the stimulatory effects of exercise (whether by duration or intensity) as rats who ran until exhaustion did not exhibit increases in Nrf2 protein or phase II enzyme protein levels or enzyme activity including glutathione reductase (GR), NQO1, and glutathione-S-transferase (GST), unless they received a previous treatment of sulforaphane, a potent Nrf2 activator [51].
A further mechanism to consider comes from recent work from Xue et al. [52] who demonstrated that Nrf2 accumulation may not be as important in stimulating Phase II antioxidant expression as the frequency of import/export of Nrf2 into and out of the nucleus. In their model, the rate and amplitude of Nrf2 cycling determined the intensity of downstream signaling – and while it is difficult to translate these in vitro data, the concept is intriguing. Mode, intensity, and duration of exercise could each impact the rate and amplitude of Nrf2 cycling in vivo. For example, maximal bouts of exercise even as short as 30-seconds in duration induce systemic changes in redox balance; and as intensity and duration increase, the magnitude of the shift in redox balance also increases [40], [50], [53]. Thus, delivering repeated shifts in redox balance through high-intensity interval training may differentially affect the frequency of import/export of Nrf2 as compared to traditional aerobic exercise. Determining the optimal “dose” or delivery of exercise for Nrf2 activation offers great opportunity for future research.
4. Nrf2 signaling in response to regular exercise
The effect of regular exercise training on the Nrf2 response has been studied more extensively than acute exercise. A comparison of these studies is outlined in Table 2. Regardless of duration (4–24 weeks) or training regimen (traditional moderate intensity or high-intensity interval training), regular aerobic exercise in rodent models has consistently been shown to activate Nrf2 signaling across multiple tissues including skeletal muscle, kidney, brain, liver, testes, prostate, and myocardium [27], [42], [43], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63].
Table 2.
Regular exercise induced Nrf2 signaling.
| Description | Description of study | Ex program | Model,n, and tissue | Gender, age, and fitness | Nrf2 | Nrf2 target expression |
|---|---|---|---|---|---|---|
| Asghar et al. [55] | Two groups, sedentary control and exercise intervention in older animals. | Length: 6-weeks Treadmill; 5 days/wk, 60 min/day, 10 m/min, 15% grade. | Rat (Fischer) n=5–6/grp renal | Male Old (>23 months) untrained | mRNA: NR Protein: NUC: ↑ | mRNA: NR Protein: ↑ SOD1 |
| George et al. [63] | Four groups: Y and O exercise and sedentary control groups. | Length: 12-weeks Treadmill; 5 days/wk, 60 min/day, 12 m/min, 15% grade. | Rat (Fischer) n=4–5/grp renal | Male young (3 months) and old (>23 months) untrained | mRNA: NR Protein: NUC: ↑ old, ↔ young | mRNA: NR Protein: NR |
| Kumar et al. [27] | Four groups: control (C), exercise (EX), cordyceps sinensis (CS), and EX+CS (ECS). Time to exhaustion test on final day. Sacrifice 6-h post exercise. | Length: 15-days Swimming; 6 days/wk, 30–60 min/day, swim to exhaustion on final exercise day | Rat (Sprague-Dawley) n=6/grp SMT | Male age NR untrained | mRNA: NR Protein: NUC: ↑ | mRNA: NR Protein: ↑ SOD1, TRX in all groups except Control |
| Gounder et al. [43] | Y and O mice completed moderate intensity aerobic training program; compared to sedentary control group (n=15). | Length: 6-weeks Treadmill; 7 days/wk, 50 min/day, 10 m/min, 7% grade | Mice (C57/Bl6/SJ; WT and KO) n=4–6/grp myocardium | Male young (8–10 weeks) vs old (>23 months) untrained | mRNA: NR Protein: NUC: ↑ young; ↑ old | mRNA: NR Protein: Young - ↑ NQO1, HO1, GSR, G6PD, CAT; Old - ↑ GSR, G6PD, CAT |
| Sun et al. [61] | Three groups: sedentary control (C), exercise (EX), and Ginsenoside (Rg3). | Length: 8-weeks Treadmill; 6 days/wk, 60 min/day, 20 m/min, 5% grade. | Rat (Sprague-Dawley) n=12/grp myocardium | Male young (8 weeks) untrained | mRNA: NR Protein: NUC: ↑ EX, ↑ Rg3 | mRNA: NR Protein: EX - ↑ NQO1, CAT, SOD2; Rg3 ↑ CAT, SOD2 |
| Zhao et al. [62] | Four groups comparing regular exercise over the lifespan or interventions at mid- and late-life: sedentary (SED), lifelong (LIFE), early (EAR), and late-life (LATE). | Length: LIFE - 6-months, EAR and LATE - 3-months Swimming; 5 days/wk, 15 min/day | Mice (SAMP8) n=20/grp testes | Male LL (2–7months), EAR (3 months), LATE (5 months) untrained | mRNA: NR Protein: WC: ↑ LIFE, EAR, LATE; sig greater in LIFE and EAR compared to LATE Nrf2-ARE binding: ↑ LIFE, EAR | mRNA: ↑ SOD1, SOD2, GPX in LL and EAR Protein: NR |
| Camiletti-Moiron et al. [57] | Four groups comparing regular protein and high-protein intake combined with high-intensity resistance exercise. Regular protein+exercise (EX), high protein+exercise (PRX) | Length: 12-weeks Treadmill, HIE consisting of weighted treadmill running, 10–12 intervals at 60–85% body weight | Rat (Albino Wistar) n=8/grp brain | Male age NR untrained | mRNA: NR Protein: WC: ↑ in both EX and PRX relative to SED | mRNA: NR Protein: NR |
| Jiang et al. [49] | Three groups comparing interval training on myocardial infarction outcomes: control (CON), sedentary MI (MI), aerobic interval training+MI (AIT) | Length: 8-weeks Treadmill adjusted to maintain relative intensities as follows: warmup ~55% VO2max, high-intensity 80–90% VO2max, recovery 65–75% VO2max. 10 min warmup, 7 intervals 4×3 min | Rat (model NR) n=28/grp myocardium | Male age NR untrained | mRNA: NR Protein: WC: ↑ AIT relative to MI | mRNA: NR Protein: ↑ NQO1, y-GCL AIT relative to MI |
| Tsou et al. [67] | Four groups testing the effects of exercise on coutneracting damage from MPP toxicity in neurons: Sedentary+PBS or MPP toxicity, exercise+PBS or MPP toxicity. | Length: 4-weeks Treadmill; 5 days/wk, 60 min/day, 70% VO2max | Rat (Wistar) n=5/grp brain | Male young (4 weeks) untrained | mRNA: NR Protein: WC: ↑ in MPP+ toxic with exercise relative to sedentary MPP+ | mRNA: NR Protein: ↔ in EX group proteins |
| Zampieri et al. [66] | Cross-sectional study comparing young fit (YF) older sedentary (OS) and older fit (OF) | Cross-sectional study | Human YF (n=5), OS (n=9), OF (n=15) SMT | Male YF (27±4 y), OS (71±3 y), OF (70±4 y) Group specific | mRNA: OS ↑ than YF and OF; OF ↑ YF Protein: NR | mRNA: NR Protein: NR |
| Gomes et al. [68] | Multiple groups comparing effects of Nandrolone supplementation. Only non-treated groups reported here. Four groups: Sedentary older (SO) and young (SY), exercise older (EXO) and young (EXY) | Length: 8-weeks Resistance; jump exercise with weighted vest, 50–70% body weight, 3 days/wk, 4-sets of jumps 60 s each | Rat (Sprague-Dawley) n=7/grp ventral prostate | Male young (13 weeks at start) untrained | mRNA: NR Protein: Basal Expression ↑ in SO compared to SY; ↓ in response to exercise regimen in EXO, ↔ in EXY | mRNA: NR Protein: NR |
| Pala et al. [28] | Four groups: control (CON), Q10 sedentary (Q10), exercise (EX) exercise+Q10 (SYN) | Length: 6-weeks Treadmill; 5 days/wk, 45 min/day, 25 m/min, grade (NR) | Rat (Wistar) n=9/grp cardiac, liver, SMT | Male young (8 weeks) untrained | mRNA: NR Protein: ↑ EX, ↑ EX+Q10 for all tissues | mRNA: NR Protein: Heart: HO1: ↑ EX; ↑↑ EX+Q10 Liver & SMT: HO1: ↑ EX; ↑↑ EX+Q10 |
| Aguiar et al. [54] | Study compared role of exercise in hemiparkinsonism model; only control sedentary (SED) and control exercise (EX) reported here | Length: 6-weeks Treadmill; 5 days/wk, to exahustion, incremental 16 m/min with 2 m/min increase every 3 min | Mice (C57BL/6) n=8–10/grp brain | Male young (8–10 weeks) untrained | mRNA: ↑ EX Protein: NR | mRNA: NR Protein:EX ↑ HO1 |
| Merry and Ristow [36] | Wild-type and Nrf2 KO mice randomized into groups for exercise intervention: trained (TR) untrained (UN) | Length: 4–6 weeks Endurance exercise on treadmill; 4–5 days/wk, 30–60 min/day, 10–15 m/min, 10% grade. | Mice (C57/Bl6; WT and KO) n=5–10/grp SMT | Male young (15–30 weeks) untrained | mRNA: ↑ TR Protein: NR | mRNA: TR ↑ GSR, GST, SOD1, SOD2, CAT Protein: NR |
NR not reported or not measured, SMT skeletal muscle tissue, PBMC peripheral blood mononuclear cells, NUC nuclear, WC whole cell.
Taken together, the data from these animal studies demonstrate that regular exercise upregulates Nrf2 protein abundance and phase II enzyme amounts and/or enzyme activity. To date there have been no exercise intervention studies conducted on Nrf2 signaling in humans. However, a cross-sectional study comparing Nrf2 and Keap1 protein content from a single muscle biopsy in sedentary and active older adults showed that the physically active individuals had significantly greater Nrf2 protein content and higher Nrf2-to-Keap1 ratio [64], suggesting that regular exercise may attenuate age-related changes in Nrf2 signaling. The question still remains whether an exercise intervention can restore redox balance in individuals who already have impairment in Nrf2 signaling. Exercise intervention data from older animals support that there is a potential of restoring Nrf2 signaling in older age [43]. Another interesting question is whether it matters when an individual starts exercising during their life time. Two recent studies, although not measuring Nrf2 signaling, demonstrated that lifelong training in older individuals was associated with compensatory adaptations to the age-related changes of a more oxidized redox balance in skeletal muscle [65], [66]. This question was more directly investigated by Zhao et al. [62] in a mouse model of accelerated senescence (SAMP8). They had 3 groups of animals that were subjected to swimming exercise, either lifelong or starting early versus late in the life span. These groups were also compared to a non-exercising control group. Overall, the late group still had some benefits over the sedentary group supporting the old adage that it is never too late to start exercising. However, the lifelong and the early group showed significantly greater benefits over the late group suggesting that starting earlier provides greater protection against age-related deficits in exercise-induced signaling. Importantly, regular exercise has been shown to increase the resilience to subsequent insults. For an example, rats that had either undergone treadmill training or remained sedentary, received an intracerebral infusion of a drug to destroy dopamine receptors to mimic Parkinson's disease [67]. The exercise training protected the rats from neurodegeneration, oxidative stress, and lowering of Nrf2 in response to the drug. Furthermore, the protective effects of exercise were not seen when Nrf2 was blocked, supporting the role of Nrf2 in the exercise-mediated neuroprotection [67].
To our knowledge there have not been any studies conducted to date investigating Nrf2 signaling in response to traditional resistance training. Gomes et al. [68] used a jump protocol with weighted vests as a form of resistance training in Sprague-Dawley rats. No changes in Nrf2 expression were observed in young animals, while older animals exposed to the same training regimen showed a decrease in Nrf2 [68]. Baseline levels of Nrf2 expression were significantly higher in the aged animals than in the young, which may explain in part the observed decrease in Nrf2 in response to exercise training. It is possible that older animals upregulated Nrf2 to counteract the increasingly pro-oxidant environment. Exercise training could then improve the Nrf2-inducible response, resulting in lower baseline values. Measuring the response to acute exercise before and after the exercise intervention may be needed to elucidate these observed differences in Nrf2 expression. A similar scenario has been observed in humans who were subjected to bed rest, with or without concomitant resistance training [69]. Bed rest controls (no exercise) displayed elevated levels of Nrf2 mRNA in skeletal muscle; however, bed rest coupled with resistance training elicited no change in Nrf2 expression when compared to pre-bed rest levels, suggesting maintenance of the redox balance with resistance exercise. Both of these studies did not focus on Nrf2 signaling per se, and only included measures of changes in Nrf2 gene expression. Further studies are needed to elucidate whether resistance training can induce Nrf2 signaling.
5. Effects of antioxidant supplementation on Nrf2 signaling
While the evidence clearly support exercise as a viable method of inducing endogenous antioxidant defenses through activation of Nrf2, there is always interest in whether the same can be achieved through exogenous supplementation or pharmaceuticals. Given the premise of the role of oxidative stress in aging, exogenous antioxidant supplementation should slow aging, or at minimum slow the rate of ROS derived cellular damage and age-related pathology by reducing free radicals prior to contact with cellular components and promoting a more balanced cellular redox environment. This assumption has led to a tremendous number of studies looking at the relationship between exogenous supplementation and successful aging and/or lifespan extension. In fact, a Pubmed search of “antioxidants and aging” yields over 13,000 returns. Despite the overwhelming body of literature, no consensus has been reached regarding any benefit of antioxidant supplementation. Many studies have shown no impact, and some studies have even shown negative impacts of antioxidant supplements [70]. Currently over 40% of the adult population in the United States consumes vitamin supplements equivalent at minimum to a daily multi-vitamin, generating a $32 billion per year industry in the US alone [71]. Two of the most common vitamins studied are Vitamins C and E.
Vitamin C (ascorbic acid/ascorbate) is a potent hydrophilic antioxidant capable of directly neutralizing ROS although at a very slow rate constant [72]. Vitamin E (α-tocopherol) is the primary hydrophobic antioxidant in cell membranes and a powerful inhibitor of lipid peroxidation in vivo. Because of its hydrophobic nature it is stored in cell membranes where it can scavenge peroxyl radicals much faster than they can react with the membrane itself. In addition, α-tocopherol can directly scavenge ROS in the mitochondrial membrane. Given its antioxidant properties, Vitamin E has been touted for its atherosclerosis preventing potential by inhibition of oxidative modification to circulating lipoproteins [73]. Based on these reactions Vitamins C and E should yield increased cell survival, however, a recent meta-analysis demonstrated that supplementation with antioxidant vitamins had no effect, and may even increase risk for morbidity [70]. The interaction between exercise and antioxidant vitamin supplementation further complicates the picture [74], [75]. Exogenous antioxidants may attenuate signal transduction by reducing ROS prior to the initiation of cell-signaling (see Fig. 1).
As a result, many of the beneficial roles of ROS are disrupted, which is in direct contrast to the controlled reduction of ROS by endogenous antioxidant defenses mediated through Nrf2. In a double-blind, placebo controlled Vitamin C and E supplementation study, Morrison et al. [76] clearly demonstrated no benefit of supplementation for reducing oxidative stress as measured by F2-isoprostanes in response to an acute exercise challenge. Additionally, they demonstrated attenuated SOD1 and SOD2 protein expression following a 4-week exercise intervention in supplemented adults, while the placebo group showed significant increases in these proteins. The maladaptive response to exercise with concurrent supplementation has also been shown at the functional level, with supplementation attenuating improvements in time to exhaustion tests, blood pressure control, and glucose tolerance [74], [75]. At the muscle level, antioxidant supplementation represses several markers of mitochondrial adaptations to endurance training; although it is unclear whether supplementation dampens endurance exercise performance in humans [77].
6. Are there synergistic effects of exercise and phytonutrient Nrf2 activators?
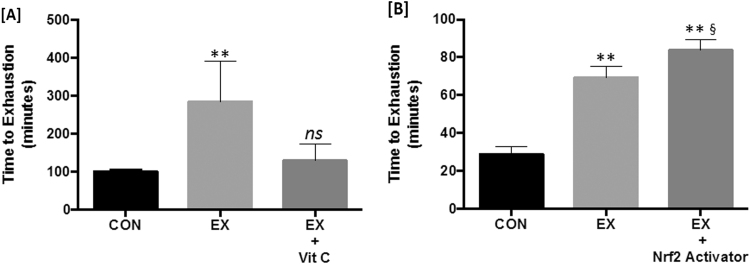
A thorough discussion of the effects of phytonutrients or phytochemicals on Nrf2 signaling is beyond the scope of this review (see the following references for review: [78], [79]). Rather we will focus on recent work that has demonstrated the potential synergistic effect of combining exercise with known Nrf2 activators to further improve redox balance. Pala et al. [28] demonstrated a synergistic effect between Coenzyme Q10 (CoQ10) supplementation and exercise training in male Wistar rats. Independent of each other, exercise and CoQ10 supplementation significantly increased Nrf2 expression in heart, liver, and skeletal muscle, over sedentary controls. The combined effect of supplementation and exercise was significantly greater in all tissues for Nrf2 expression with similar patterns observed for inducible HO-1. Sprague-Dawley rats supplemented with a known Nrf2 activator, Cordyceps sinensis [80], during a 15-day swimming program increased Nrf2 protein expression and phase II enzyme expression to levels significantly higher than those measured with exercise alone [27]. This area warrants further research. Fig. 3 shows data adapted from earlier studies demonstrating how the effects of exercise are inhibited by concomitant supplementation of antioxidants and enhanced by concomitant use of phytonutrient Nrf2 activator.
Fig. 3.
Exercise training with antioxidant vitamin supplementation inhibits training adaptations while Nrf2 activators enhance training effects. Rodents trained with concomitant vitamin C supplementation failed to improve their time to exhaustion relative to sedentary control mice [A]. In direct contrast, rats supplemented with an Nrf2 activating compound during their training program performed significantly better in a time to exhaustion test relative to both sedentary controls and the non-supplemented exercise group suggesting a potential synergistic effect [B]. Data adapted from Gomez-Cabrera et al. [77] and Kumar et al. [27].
Another possible mechanism of activating Nrf2 and the phase II response is through caloric restriction [81], [82], [83]. To our knowledge there are no published randomized controlled studies on the effects of combining caloric restriction and exercise on Nrf2 and downstream signaling.
7. Conclusion and future directions
In conclusion, the data are convincing that exercise exerts many of its benefits through redox activation of Nrf2 signaling. The evidence of Nrf2 activation across variety of tissues including brain, kidney, and testes may be an important mechanism of how exercise results its well-known systemic effects that are not just limited to skeletal muscle and myocardium. Additionally there are emerging data that these results do in fact translate to humans. Clearly more studies are needed, in particular randomized controlled trials of exercise intervention studies in humans. Both the animal studies and the human studies have been almost exclusively limited to male subjects. Results need to be extended to females and possible gender differences need to be elucidated.
The use of Nrf2 knockout mice has been extremely valuable in enhancing our understanding of the important role of this transcription factor in health and disease. Nrf2−/− exhibit phenotypes that are remarkably similar to aged mice suggesting disruption of Nrf2-Keap1 signaling as a contributor to biological aging [84] making Nrf2 a promising target especially for treating biological aging in the pursuit of prevention of age-related diseases.
One caveat of the animal studies is that there are often only two time points available (pre/post acute exercise or pre/post exercise intervention). Many of the Nrf2-regulated genes as well as proteins may have different time course of response so that multiple time points may be needed to capture the overall change. These need to be further clarified in future studies.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Coffey V.G., Hawley J.A. The molecular bases of training adaptation. Sports. Med. 2007;37(9):737–763. doi: 10.2165/00007256-200737090-00001. [DOI] [PubMed] [Google Scholar]
- 2.Camera D.M., Smiles W.J., Hawley J.A. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic. Biol. Med. 2016 doi: 10.1016/j.freeradbiomed.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Allen R.G., Tresini M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000;28(3):463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 4.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Cabrera M.C., Domenech E., Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Shih P.H., Yeh C.T., Yen G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric Food Chem. 2007;55(23):9427–9435. doi: 10.1021/jf071933i. [DOI] [PubMed] [Google Scholar]
- 8.Owuor E.D., Kong A.N. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharmacol. 2002;64(5–6):765–770. doi: 10.1016/s0006-2952(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen T., Sherratt P.J., Pickett C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 10.Copple I.M. The Keap1-Nrf2 cell defense pathway – a promising therapeutic target? Adv. Pharmacol. 2012;63:43–79. doi: 10.1016/B978-0-12-398339-8.00002-1. [DOI] [PubMed] [Google Scholar]
- 11.Ohkoshi A., Suzuki T., Ono M., Kobayashi T., Yamamoto M. Roles of Keap1-Nrf2 system in upper aerodigestive tract carcinogenesis. Cancer Prev. Res. 2013;6(2):149–159. doi: 10.1158/1940-6207.CAPR-12-0401-T. [DOI] [PubMed] [Google Scholar]
- 12.Gao B., Doan A., Hybertson B.M. The clinical potential of influencing Nrf2 signaling in degenerative and immunological disorders. Clin. Pharmacol. 2014;6:19–34. doi: 10.2147/CPAA.S35078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson M.E., Silva H.S., Conboy I.M. Aging of signal transduction pathways, and pathology. Exp. Cell Res. 2008;314(9):1951–1961. doi: 10.1016/j.yexcr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seals D.R., Kaplon R.E., Gioscia-Ryan R.A., LaRocca T.J. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology. 2014;29(4):250–264. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seals D.R., Melov S. Translational geroscience: emphasizing function to achieve optimal longevity. Aging. 2014;6(9):718–730. doi: 10.18632/aging.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tebay L.E., Robertson H., Durant S.T., Vitale S.R., Penning T.M., Dinkova-Kostova A.T., Hayes J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015;88(Pt B):108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaspar J.W., Niture S.K., Jaiswal A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi M., Itoh K., Suzuki T., Osanai H., Nishikawa K., Katoh Y., Takagi Y., Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7(8):807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Paonessa J.D., Zhang Y. Mechanism of chemical activation of Nrf2. PLoS One. 2012;7(4):e35122. doi: 10.1371/journal.pone.0035122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi A., Kang M.I., Watai Y., Tong K.I., Shibata T., Uchida K., Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell Biol. 2006;26(1):221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell Biol. 2009;29(2):493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi M., Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme RegulAdv. Enzym. Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi S., Izawa Y., Suzuki N. Astrogliopathy as a loss of astroglial protective function against glycoxidative stress under hyperglycemia. Rinsho Shinkeigaku. 2012;52(1):41–51. doi: 10.5692/clinicalneurol.52.41. [DOI] [PubMed] [Google Scholar]
- 25.Niture S.K., Jain A.K., Jaiswal A.K. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J. Cell Sci. 2009;122(Pt 24):4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Takaya K., Suzuki T., Motohashi H., Onodera K., Satomi S., Kensler T.W., Yamamoto M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2012;53(4):817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar R., Negi P.S., Singh B., Ilavazhagan G., Bhargava K., Sethy N.K. Cordyceps sinensis promotes exercise endurance capacity of rats by activating skeletal muscle metabolic regulators. J. Ethnopharmacol. 2011;136(1):260–266. doi: 10.1016/j.jep.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 28.Pala R., Orhan C., Tuzcu M., Sahin N., Ali S., Cinar V., Atalay M., Sahin K. Coenzyme Q10 supplementation modulates NFkappaB and Nrf2 pathways in exercise training. J. Sports Sci. Med. 2016;15(1):196–203. [PMC free article] [PubMed] [Google Scholar]
- 29.Saw C.L., Kong A.N. Nuclear factor-erythroid 2-related factor 2 as a chemopreventive target in colorectal cancer. Expert Opin. Ther. Targets. 2011;15(3):281–295. doi: 10.1517/14728222.2011.553602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuentes F., Paredes-Gonzalez X., Kong A.T. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3′-diindolylmethane: anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr. Pharmacol. Rep. 2015;1(3):179–196. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L., Palliyaguru D.L., Kensler T.W. Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin. Oncol. 2016;43(1):146–153. doi: 10.1053/j.seminoncol.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X.J., Sun Z., Villeneuve N.F., Zhang S., Zhao F., Li Y., Chen W., Yi X., Zheng W., Wondrak G.T., Wong P.K., Zhang D.D. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29(6):1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu L., Miao W., Loignon M., Kandouz M., Batist G. Putative chemopreventive molecules can increase Nrf2-regulated cell defense in some human cancer cell lines, resulting in resistance to common cytotoxic therapies. Cancer Chemother. Pharmacol. 2010;66(3):467–474. doi: 10.1007/s00280-009-1182-7. [DOI] [PubMed] [Google Scholar]
- 34.Kensler T.W., Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31(1):90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horie M., Warabi E., Komine S., Oh S., Shoda J. Cytoprotective role of Nrf2 in electrical pulse stimulated C2C12 myotube. PLoS One. 2015;10(12):e0144835. doi: 10.1371/journal.pone.0144835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merry T.L., Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and antioxidant response in mice. J. Physiol. 2016 doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purdom-Dickinson S.E., Sheveleva E.V., Sun H., Chen Q.M. Translational control of nrf2 protein in activation of antioxidant response by oxidants. Mol. Pharmacol. 2007;72(4):1074–1081. doi: 10.1124/mol.107.035360. [DOI] [PubMed] [Google Scholar]
- 38.Kemmerer Z.A., Ader N.R., Mulroy S.S., Eggler A.L. Comparison of human Nrf2 antibodies: a tale of two proteins. Toxicol. Lett. 2015;238(2):83–89. doi: 10.1016/j.toxlet.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau A., Tian W., Whitman S.A., Zhang D.D. The predicted molecular weight of Nrf2: it is what it is not. Antioxid. Redox Signal. 2013;18(1):91–93. doi: 10.1089/ars.2012.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P., Li C.G., Qi Z., Cui D., Ding S. Acute exercise stress promotes Ref/Nrf signaling and increases mitochondrial antioxidant activity in skeletal muscle. Exp. Physiol. 2015 doi: 10.1113/EP085493. [DOI] [PubMed] [Google Scholar]
- 41.Narasimhan M., Hong J., Atieno N., Muthusamy V.R., Davidson C.J., Abu-Rmaileh N., Richardson R.S., Gomes A.V., Hoidal J.R., Rajasekaran N.S. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radic. Biol. Med. 2014;71:402–414. doi: 10.1016/j.freeradbiomed.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muthusamy V.R., Kannan S., Sadhaasivam K., Gounder S.S., Davidson C.J., Boeheme C., Hoidal J.R., Wang L., Rajasekaran N.S. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012;52(2):366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gounder S.S., Kannan S., Devadoss D., Miller C.J., Whitehead K.J., Odelberg S.J., Firpo M.A., Paine R., 3rd, Hoidal J.R., Abel E.D., Rajasekaran N.S. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One. 2012;7(9):e45697. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry C.G., Lally J., Holloway G.P., Heigenhauser G.J., Bonen A., Spriet L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010;588(Pt 23):4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Done A.J., Gage M.J., Nieto N.C., Traustadottir T. Exercise-induced Nrf2-signaling is impaired in aging. Free Radic. Biol. Med. 2016;96:130–138. doi: 10.1016/j.freeradbiomed.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Shenvi S.V., Smith E., Hagen T.M. Identification of age-specific Nrf2 binding to a novel antioxidant response element locus in the Gclc promoter: a compensatory means for the loss of glutathione synthetic capacity in the aging rat liver? Aging Cell. 2012;11(2):297–304. doi: 10.1111/j.1474-9726.2011.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ballmann C., McGinnis G., Peters B., Slivka D., Cuddy J., Hailes W., Dumke C., Ruby B., Quindry J. Exercise-induced oxidative stress and hypoxic exercise recovery. Eur. J. Appl Physiol. 2014;114(4):725–733. doi: 10.1007/s00421-013-2806-5. [DOI] [PubMed] [Google Scholar]
- 48.Scott H.A., Latham J.R., Callister R., Pretto J.J., Baines K., Saltos N., Upham J.W., Wood L.G. Acute exercise is associated with reduced exhaled nitric oxide in physically inactive adults with asthma. Ann. Allergy Asthma Immunol. 2015;114(6):470–479. doi: 10.1016/j.anai.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Bos I., De Boever P., Int Panis L., Sarre S., Meeusen R. Negative effects of ultrafine particle exposure during forced exercise on the expression of Brain-Derived Neurotrophic Factor in the hippocampus of rats. Neuroscience. 2012;223:131–139. doi: 10.1016/j.neuroscience.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 50.Li T., He S., Liu S., Kong Z., Wang J., Zhang Y. Effects of different exercise durations on Keap1-Nrf2-ARE pathway activation in mouse skeletal muscle. Free Radic. Res. 2015;49(10):1269–1274. doi: 10.3109/10715762.2015.1066784. [DOI] [PubMed] [Google Scholar]
- 51.Malaguti M., Angeloni C., Garatachea N., Baldini M., Leoncini E., Collado P.S., Teti G., Falconi M., Gonzalez-Gallego J., Hrelia S. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J. Appl Physiol. 2009;107(4):1028–1036. doi: 10.1152/japplphysiol.00293.2009. [DOI] [PubMed] [Google Scholar]
- 52.Xue M., Momiji H., Rabbani N., Barker G., Bretschneider T., Shmygol A., Rand D.A., Thornalley P.J. Frequency modulated translocational oscillations of Nrf2 mediate the antioxidant response element cytoprotective transcriptional response. Antioxid. Redox Signal. 2015;23(7):613–629. doi: 10.1089/ars.2014.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamprecht M., Greilberger J.F., Schwaberger G., Hofmann P., Oettl K. Single bouts of exercise affect albumin redox state and carbonyl groups on plasma protein of trained men in a workload-dependent manner. J. Appl Physiol. 1985;104(6):1611–1617. doi: 10.1152/japplphysiol.01325.2007. [DOI] [PubMed] [Google Scholar]
- 54.Aguiar A.S., Jr., Duzzioni M., Remor A.P., Tristao F.S., Matheus F.C., Raisman-Vozari R., Latini A., Prediger R.D. Moderate-intensity physical exercise protects against experimental 6-hydroxydopamine-induced hemiparkinsonism through Nrf2-antioxidant response element pathway. Neurochem. Res. 2016;41(1–2):64–72. doi: 10.1007/s11064-015-1709-8. [DOI] [PubMed] [Google Scholar]
- 55.Asghar M., George L., Lokhandwala M.F. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am. J. Physiol. Ren. Physiol. 2007;293(3):F914–F919. doi: 10.1152/ajprenal.00272.2007. [DOI] [PubMed] [Google Scholar]
- 56.Camiletti-Moiron D., Aparicio V.A., Nebot E., Medina G., Martinez R., Kapravelou G., Andrade A., Porres J.M., Lopez-Jurado M., Aranda P. High-intensity exercise modifies the effects of stanozolol on brain oxidative stress in rats. Int. J. Sports Med. 2015;36(12):984–991. doi: 10.1055/s-0035-1548941. [DOI] [PubMed] [Google Scholar]
- 57.Camiletti-Moiron D., Aparicio V.A., Nebot E., Medina G., Martinez R., Kapravelou G., Andrade A., Porres J.M., Lopez-Jurado M., Aranda P. High-protein diet induces oxidative stress in rat brain: protective action of high-intensity exercise against lipid peroxidation. Nutr. Hosp. 2014;31(2):866–874. doi: 10.3305/nh.2015.31.2.8182. [DOI] [PubMed] [Google Scholar]
- 58.Fiorin F., Ferreira A.P., Ribeiro L.R., Silva L.F., Castro M.R., Silva L.R., Silveira M.E., Junior, Zemolin A.P., Dobrachinski F., Marchesan S., Franco J.L., Soares F.A., Furian A.F., Oliveira M.S., Fighera M.R., Royes L.F. The impact of previous physical training on redox signaling after traumatic brain injury in rats: behavioral and neurochemical approach. J. Neurotrauma. 2015 doi: 10.1089/neu.2015.4068. [DOI] [PubMed] [Google Scholar]
- 59.Jiang H.K., Miao Y., Wang Y.H., Zhao M., Feng Z.H., Yu X.J., Liu J.K., Zang W.J. Aerobic interval training protects against myocardial infarction-induced oxidative injury by enhancing antioxidase system and mitochondrial biosynthesis. Clin. Exp. Pharmacol. Physiol. 2014;41(3):192–201. doi: 10.1111/1440-1681.12211. [DOI] [PubMed] [Google Scholar]
- 60.Strobel N.A., Peake J.M., Matsumoto A., Marsh S.A., Coombes J.S., Wadley G.D. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2011;43(6):1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 61.Sun M., Huang C., Wang C., Zheng J., Zhang P., Xu Y., Chen H., Shen W. Ginsenoside Rg3 improves cardiac mitochondrial population quality: mimetic exercise training. Biochem. Biophys. Res. Commun. 2013;441(1):169–174. doi: 10.1016/j.bbrc.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 62.Zhao X., Bian Y., Sun Y., Li L., Wang L., Zhao C., Shen Y., Song Q., Qu Y., Niu S., Wu W., Gao F. Effects of moderate exercise over different phases on age-related physiological dysfunction in testes of SAMP8 mice. Exp. Gerontol. 2013;48(9):869–880. doi: 10.1016/j.exger.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 63.George L., Lokhandwala M.F., Asghar M. Exercise activates redox-sensitive transcription factors and restores renal D1 receptor function in old rats. Am. J. Physiol. Ren. Physiol. 2009;297(5):F1174–F1180. doi: 10.1152/ajprenal.00397.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Safdar A., deBeer J., Tarnopolsky M.A. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 2010;49(10):1487–1493. doi: 10.1016/j.freeradbiomed.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Cobley J.N., Sakellariou G.K., Owens D.J., Murray S., Waldron S., Gregson W., Fraser W.D., Burniston J.G., Iwanejko L.A., McArdle A., Morton J.P., Jackson M.J., Close G.L. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic. Biol. Med. 2014;70:23–32. doi: 10.1016/j.freeradbiomed.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Zampieri S., Pietrangelo L., Loefler S., Fruhmann H., Vogelauer M., Burggraf S., Pond A., Grim-Stieger M., Cvecka J., Sedliak M., Tirpakova V., Mayr W., Sarabon N., Rossini K., Barberi L., De Rossi M., Romanello V., Boncompagni S., Musaro A., Sandri M., Protasi F., Carraro U., Kern H. Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70(2):163–173. doi: 10.1093/gerona/glu006. [DOI] [PubMed] [Google Scholar]
- 67.Tsou Y.H., Shih C.T., Ching C.H., Huang J.Y., Jen C.J., Yu L., Kuo Y.M., Wu F.S., Chuang J.I. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp. Neurol. 2015;263:50–62. doi: 10.1016/j.expneurol.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 68.Gomes F.C., Chuffa L.G., Scarano W.R., Pinheiro P.F., Favaro W.J., Domeniconi R.F. Nandrolone decanoate and resistance exercise training favor the occurrence of lesions and activate the inflammatory response in the ventral prostate. Andrology. 2016;4(3):473–480. doi: 10.1111/andr.12162. [DOI] [PubMed] [Google Scholar]
- 69.Salanova M., Schiffl G., Gutsmann M., Felsenberg D., Furlan S., Volpe P., Clarke A., Blottner D. Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse. Redox Biol. 2013;1:514–526. doi: 10.1016/j.redox.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bjelakovic G., Nikolova D., Gluud C. Antioxidant supplements and mortality. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17(1):40–44. doi: 10.1097/MCO.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 71.Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994), U.S. Department of Heatlh and Human Services: Centers for Disease Control and Prevention: Washington, DC, 2011.
- 72.Sadowska-Bartosz I., Bartosz G. Effect of antioxidants supplementation on aging and longevity. Biomed. Res. Int. 2014;2014:404680. doi: 10.1155/2014/404680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang F., Lu M., Zhang S., Mei M., Wang T., Liu P., Wang H. Vitamin E conditionally inhibits atherosclerosis in ApoE knockout mice by anti-oxidation and regulation of vasculature gene expressions. Lipids. 2014;49(12):1215–1223. doi: 10.1007/s11745-014-3962-z. [DOI] [PubMed] [Google Scholar]
- 74.Ristow M., Zarse K., Oberbach A., Kloting N., Birringer M., Kiehntopf M., Stumvoll M., Kahn C.R., Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wray D.W., Uberoi A., Lawrenson L., Bailey D.M., Richardson R.S. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin. Sci. 2009;116(5):433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 76.Morrison D., Hughes J., Della Gatta P.A., Mason S., Lamon S., Russell A.P., Wadley G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015;89:852–862. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 77.Gomez-Cabrera M.C., Domenech E., Romagnoli M., Arduini A., Borras C., Pallardo F.V., Sastre J., Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 78.Son T.G., Camandola S., Mattson M.P. Hormetic dietary phytochemicals. Neuromol. Med. 2008;10(4):236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Upadhyay S., Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell. Longev. 2015;2015:504253. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh M., Tulsawani R., Koganti P., Chauhan A., Manickam M., Misra K. Cordyceps sinensis increases hypoxia tolerance by inducing heme oxygenase-1 and metallothionein via Nrf2 activation in human lung epithelial cells. Biomed. Res. Int. 2013;2013:569206. doi: 10.1155/2013/569206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hine C.M., Mitchell J.R. NRF2 and the phase II response in acute stress resistance induced by dietary restriction. J. Clin. Exp. Pathol. 2012;S4(4) doi: 10.4172/2161-0681.S4-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Csiszar A., Gautam T., Sosnowska D., Tarantini S., Banki E., Tucsek Z., Toth P., Losonczy G., Koller A., Reglodi D., Giles C.B., Wren J.D., Sonntag W.E., Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2014;307(3):H292–H306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin-Montalvo A., Villalba J.M., Navas P., de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30(5):505–520. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]