Abstract
Study Objectives:
Patients with circadian rhythm sleep disorders (CRSDs) often have coincidence of orthostatic dysregulation (OD). Both disorders have many common clinical features. However, the prevalence of OD in patients with CRSD has not been examined.
Methods:
Thirty-eight patients with CRSD with either delayed sleep phase disorder or free-running disorder were tested for OD using the new orthostatic test, which was originally established by Tanaka et al. (< 20 years) and the Schellong test, i.e., the active standing test (≥ 20 years).
Results:
The overall prevalence of OD in patients with CRSD was 57.9% (22/38), and prevalence of OD was 70% in patients under 20 years of age (14/20). These rates exceed the previously reported values in adolescents aged 14–15 years (15%), regarded as the age with highest OD prevalence. Prevalence was not significantly associated with CRSD severity and medications used.
Conclusions:
We observed a high prevalence of OD in patients with CRSD, suggesting some relationship between CRSD and OD. Large-scale case-control studies are warranted to investigate the underlying mechanisms for this comorbidity.
Citation:
Tsuchiya A, Kitajima T, Tomita S, Esaki Y, Hirose M, Iwata N. High prevalence of orthostatic dysregulation among circadian rhythm disorder patients. J Clin Sleep Med 2016;12(11):1471–1476.
Keywords: circadian rhythm sleep disorders, orthostatic dysregulation, prevalence
INTRODUCTION
Circadian rhythm sleep disorders (CRSDs) are characterized by persistent or recurrent patterns of sleep disturbance primarily caused by alterations in the circadian timing system or misalignment between the endogenous circadian rhythm and external schedules. CRSD is associated with impairments in social, occupational, and other areas of functioning.1 Delayed sleep phase disorder (DSPD) is the most common CRSD diagnosis (excluding shift work disorder and jet lag disorder), with a prevalence of 0.1% to 0.4%.2,3 DSPD is more common among adolescents and young adults. Many of these school-age patients often have school refusal; sometimes it is difficult to distinguish clinically whether school refusal is a cause or a result of the circadian rhythm disturbance. Previous studies reported that the nadir of core body temperature (CBT) was markedly delayed, and that the amplitude of circadian CBT rhythm was significantly smaller in subjects with school refusal behavior than in healthy controls.4,5 These observations suggest that the clinical psychosomatic symptoms often observed in patients with school refusal behavior are related to the desynchronization of the circadian rhythm of body temperature and sleep-wake rhythm.4,5
It is our clinical experience that patients with CRSD often have a diagnosis of orthostatic dysregulation (OD), a functional physical disorder with an impairment of circulatory adjustment against gravitational stress because of dysregulation of the autonomic nervous system.6,7 OD is common in adolescents and often affected by psychosocial factors; therefore, it is regarded as a psychosomatic disorder. The characteristics of OD among adults, alternatively named as orthostatic intolerance (OI) in America and Europe, has not been as clearly defined as those of OD among children.8,9 It was reported that more than half of the Japanese children with symptoms of OD had school refusal behavior, and conversely 30% to 40% of children with school refusal behavior had OD.7,10 In addition, approximately 50% of children with OD had disturbed sleep-wake patterns and were unable to get up until noon, but they usually recovered their vigor in the evening; these symptoms could seriously impair multiple areas of functioning and quality of life.7
BRIEF SUMMARY
Current Knowledge/Study Rationale: Circadian rhythm sleep disorders (CRSDs) and orthostatic dysregulation (OD) have many common clinical characteristics, including association with school refusal of adolescence. However, the prevalence of OD in patients with CRSD has not been examined.
Study Impact: The overall prevalence of OD in patients with CRSD was 57.9%, and prevalence of OD was 70% in those aged under 20 years. This result suggests some relationship between CRSD and OD.
Interestingly, CRSD and OD have many common clinical features. The sleep-wake pattern is usually disrupted to varying degrees in children with OD, while patients with CRSD often complain of somatic symptoms such as headache, fatigue, nausea, and abdominal pain. Melatonin, which has a property of circadian rhythm modulation, has been reported to be effective for both CRSD11,12 and OD.6 We hypothesized that the pathophysiology of OD is closely related to that of CRSD, specifically internal desynchronization of circadian rhythms. Hence, one would expect high rates of comorbidity, but the prevalence of OD in patients with CRSD has not been examined. In this study, we performed orthostatic testing in both adolescent and adult patients with CRSD to determine the prevalence of OD.
METHODS
Patients
Consecutive patients with CRSD treated at the sleep clinic in the Department of Psychiatry, Fujita Health University Hospital (Aichi, Japan), from April 2011 to September 2011 were included in this study. Inclusion criteria were as follows: (1) patients who met the International Classification of Sleep Disorders, Second Edition (ICSD-2) diagnostic criteria for CRSD (delayed sleep phase disorder: DSPD or free-running disorder: FRD) according to at least two physicians, including patients whose symptoms had improved after treatment, (2) patients who were able to provide informed consent (in the case of a minor, the consent was provided by both the patient and one of his/her parents), and (3) patients who were able to undergo an orthostatic test (described below). Patients with any ongoing treatments or comorbid psychiatric conditions were also included. Patients who took psychotropic drugs with alpha-adrenergic receptor action (antipsychotics, except drugs such as sulpiride, tricyclic or tetracyclic antidepressants, and trazo-done) or those who had other disorders that could cause similar clinical symptoms of orthostatic intolerance (such as hypo/hyperthyroidism, brain tumors, iron deficiency anemia, Addison disease, cardiomyopathy, and primary pulmonary hypertension) were excluded from the study.
This study was approved by the Ethics Committee of the Fujita Health University, and all participants gave their verbal and written informed consent before participation.
Measures
Patients were given different orthostatic tests depending on their age. We applied the Schellong test, i.e., the active standing test for the adult patients (≥ 20 years), and the new ortho-static test (described below), for the adolescent patients (< 20 years).7 We evaluated the state of sleep phase during the investigation using the severity level criteria for delayed sleep phase syndrome (DSPS) devised by Ohta et al.13 We also confirmed the use of medications (vitamin B12, GABAergic agents [such as benzodiazepines and non-benzodiazepine hypnotics], and ramelteon). We performed the OD test when the patient came for a routine medical examination.
Test Protocol for OD
The protocol for the new orthostatic test established by Tanaka et al.6,7 for use in adolescents was as follows: (1) Rest for 10 min in the supine position. Put a manometer cuff on the arm. A stethoscope should be fixed on the arm around the brachial artery. (2) Measure blood pressure and heart rate 3 times after a 10-min rest. Determine and record the middle value. (3) Inflate the air to the cuff at the level of the middle value of systolic blood pressure and pinch a rubber tube of the manometer by a clamp in order not to deflate. Use a stethoscope and hear the Korotkoff sounds of the brachial artery, which you can hear very slightly if the instructions have been correctly followed. (4) Tell the child to stand up actively and start measurement using a stopwatch while listening using a stethoscope. (5) At the beginning of standing, the Korotkoff sounds disappear once, and then appear again (after 17 s in average). Stop the stopwatch. The time (s) displayed on the stopwatch corresponds to the recovery time of blood pressure. (6) Take off the clamp and deflate the cuff. (7) Measure blood pressure and heart rate by conventional method at 1, 3, 5, 7, and 10 min.
The convention Schellong test14 employed for adults followed the same procedure as the new orthostatic test, except that steps 3 and 5 were omitted and we measured blood pressure immediately after standing.
Evaluation of OD
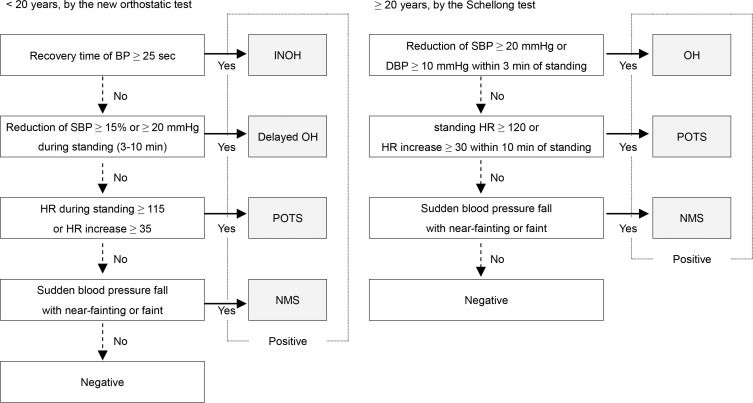
In the adolescent patients, we diagnosed OD when test results matched the diagnostic criteria for one of the following four OD subcategories established by the Japanese Society of Psychosomatic Pediatrics7: (1) instantaneous orthostatic hypotension (INOH), (2) postural tachycardia syndrome (POTS), (3) neurally mediated syncope (NMS), and (4) delayed orthostatic hypotension (delayed OH). Adult patients were diagnosed with one of the following 3 OD subcategories based on the diagnostic criteria of the American Autonomic Society15–17: (1) ortho-static hypotension (OH), (2) POTS, and (3) NMS. A flowchart of the subtype categorization is shown in Figure 1.
Figure 1. Flowchart of categorization.
The diagram on the left adapted from Tanaka et al.7 shows the classification of orthostatic dysregulation (OD) subcategory in adolescent patients (< 20 years) according to the results of the new orthostatic test (modified Schellong test). The right panel shows the OD subcategory classification of the adult patients (≥ 20 years) by the conventional Schellong test. BP, blood pressure; SBP, systolic blood pressure; HR, heart rate; DBP, diastolic blood pressure. INOH, instantaneous orthostatic hypotension; POTS, postural tachycardia syndrome; delayed OH, delayed orthostatic hypotension; NMS, neurally mediated syncope; OH, orthostatic hypotension.
If a patient was previously diagnosed with OD by either the new orthostatic test or the conventional Schellong test, they were considered as positive.
Severity Level Criteria for DSPS
The severity of DSPD was determined using the severity level criteria for delayed sleep phase syndrome (DSPS; an alternative name of DSPD) devised by Ohta et al.,13 which categorizes the severity by the extent of the sleep phase delay according to sleep onset and sleep offset times. Both sleep onset and sleep offset times were scored using standardized procedures. For sleep onset time, the scale was as follows: (1) 0 point if the patient could fall asleep before 00:00, (2) 1 point if the patient could fall asleep between 00:00 and 2:00, (3) 2 points if the patient could fall asleep between 02:00 and 04:00, and (4) 3 points if the patient could fall asleep after 04:00. For sleep offset time, the scale was as follows: (1) 0 points if the patient was able to wake up before 08:00, (2) 1 point if the patient was able to wake up between 08:00 and 09:00, (3) 2 points if the patient was able to wake up between 09:00 and 10:00, and (4) 3 points if the patient was able to wake up after 10:00. The severity of DSPS was then determined by adding the scores for the sleep onset and sleep offset times; a total score of 0–1 points represented remission; 2–3 points, a mild disease state; 4–5 points, a moderate disease state; and 6 points, a severe disease state. In patients with FRD, the state was considered to be severe if the sleep-wake rhythm was free-running, regardless of sleep onset and sleep offset times.
Statistical Analysis
The χ2 test or Fisher exact test was used to assess the relationships between the presence of OD and each potentially associated factor (CRSD disease severity and medications used). We divided patients into the following group pairs and compared OD prevalence: CRSD severity total score of 0–3 points (“remission” to “mild disease state”) vs. total score of 4–6 points (“moderate disease state” to “severe disease state”) and use vs. non-use of vitamin B12, GABAergic agents (benzodiazepines and non-benzodiazepine hypnotics), or ramelteon. We used the JMP 8.0.1J software package (SAS Institute Japan, Tokyo, Japan) for all statistical analyses. A p value < 0.05 was regarded as statistically significant. We did not correct for multiple testing because of the explorative nature of this study.
RESULTS
Patient Demographics and CRSD Status
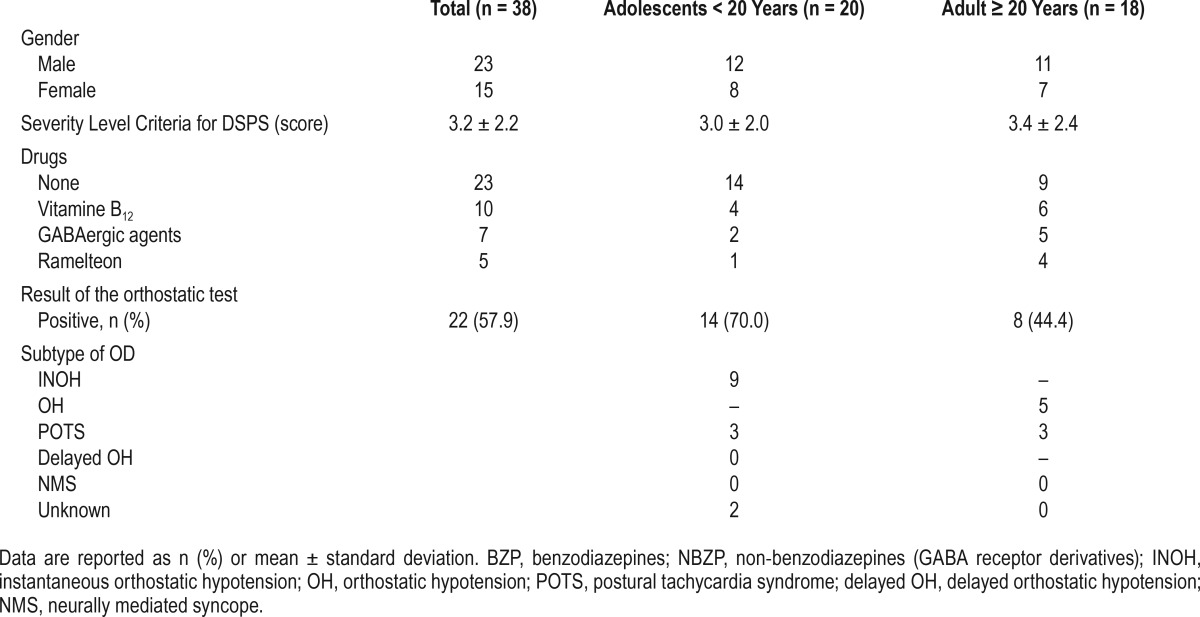
Subject demographics are summarized in Table 1 and statistical tests in Table 2. Thirty-eight patients (23 male and 15 female, aged between 12 and 50 years, 20 adolescents and 18 adults) with CRSD (34 DSPD and 4 FRD) were included in this study. Males outnumbered females in the total cohort as well as in both adolescent (< 20 years) and adult (≥ 20 years) groups. The mean DSPS severity level criteria score did not differ significantly between age groups. A total of 19 patients took no medications. Fifteen patients were currently taking medications to aid sleep, including vitamin B12, benzodiazepines or non-benzodiazepines (zolpidem, zopiclone), and ramelteon (with some overlap).
Table 1.
Study population demographics by age.
Table 2.
Relations of the standing test result and patient characteristic.
Prevalence of OD
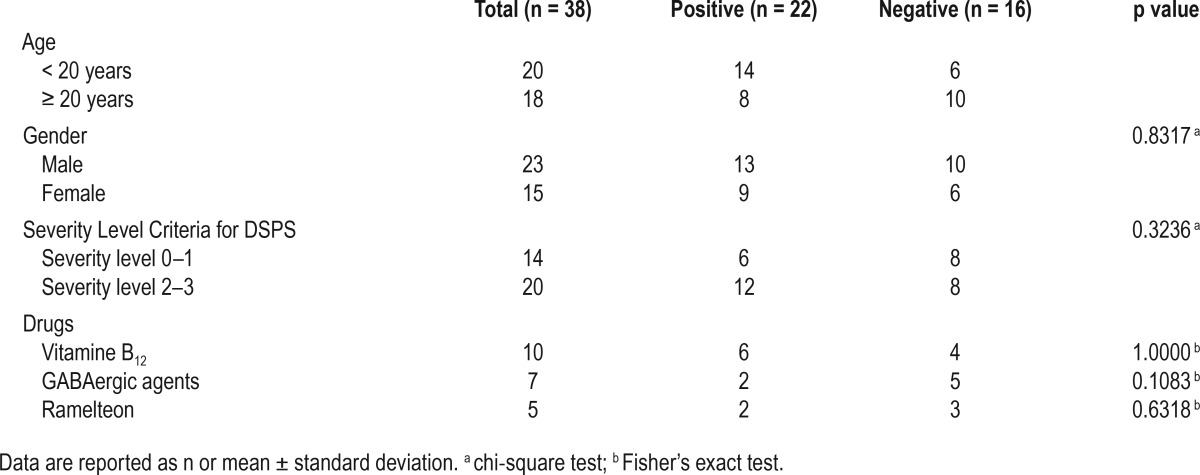
A total of 57.9% (22/38), of patients had OD according to the orthostatic test; 70% (14/20) of patients in the adolescent group were found to be positive for OD by the new orthostatic test, and 44.4% (8/18) adults were positive by the Schellong test. The results of the OD subtypes were showed on Table 1. There was no significant difference in OD prevalence between males and females (p = 0.8317: χ2 test).
Associations of OD Prevalence with CRSD Severity and Sleep Aid Use
There was no relationship between CRSD disease severity and OD risk because 42.9% of patients with CRSD with mild severity (severity level of 0 or 1) had OD compared with 60.0% (12/20) of patients with CRSD with a high severity (severity level of 2 or 3; p = 0.3236: χ2 test). There was no relationship between medications and OD prevalence because 42.1% (8/19) of patients with CRSD, with some kind of drug use, had OD compared with 68.4% (13/19) of patients with CRSD who took no medications (p = 0.3245; χ2 test). There was no significant difference in OD prevalence between users and non-users of the 3 types of sleep aid medications (Table 2: vitamin B12, p = 1.0000; GABAergic agents, p = 0.1083; ramelteon, p = 0.6318: Fisher exact test).
DISCUSSION
A high prevalence of OD was observed among patients with CRSD in this study. The prevalence of OD in the general population is currently unknown, but Japanese studies have reported that OD prevalence peaks at 15% in adolescents aged 14–15 years.7,18 According to a large-scale investigation performed by the Japanese Ministry of Welfare and Labor in 1999, OD is one of the most common disorders treated in pediatric clinics, with a prevalence of 8.5% of outpatients aged 10–15 years.7 In this study, the prevalence of OD in patients with CRSD under 20 years of age was even higher than that documented in adolescents aged 14–15 years, thereby strongly suggesting enhanced OD prevalence among patients with CRSD. The prevalence of OD in the general adult population using criteria of the American Autonomic Society has not been reported; however, it is thought that OD prevalence in adults is lower than that in adolescence. Nonetheless, the prevalence of OD in adults with CRSD was also higher than that in adolescents aged 14–15 years, supporting substantially higher OD prevalence in adult patients with CRSD than in general adult population. In this study, the prevalence of OD was nominally higher in patients with CRSD under 20 years of age than in adult patients with CRSD; however, a direct comparison cannot be made because of the difference in test protocols and diagnostic criteria.
Despite this high rate of comorbidity, there was no association between OD prevalence and CRSD severity. The most parsimonious explanation is that patients with CRSD have an intrinsic autonomic vulnerability to OD unrelated to the temporal sleep phase or to internal desynchronization. Another possible explanation is that the severity criteria that we used in this study did not reflect the degree of internal desynchronization. However, it is difficult to discuss these factors only from the results of this study.
Patients who consumed agents acting on alpha-adrenergic receptors were excluded from this study because these agents may affect the orthostatic test, whereas patients who took other medications to aid sleep (vitamin B12, GABAergic agents, and ramelteon) were included. Previous studies reported that diazepam and melatonin reduced muscle sympathetic nerve activity and affected the regulation of blood pressure19,20; therefore, it is conceivable that these drugs may interfere with the results of the orthostatic test. However, as there was no significant difference in OD prevalence between users and non-users, the interference of the drugs with the results of the orthostatic test appeared unlikely.
Miura et al. compared the prevalence of OD among healthy young women classified as “morning,” “evening,” or “intermediate” type on the morningness−eveningness questionnaire (MEQ) and found that OD was significantly more common in the evening type (50%) than in the intermediate and morning types (13% and 11%, respectively).21 It was suggested that a circadian rhythm disturbance may be related to OD, and our finding that approximately half of the patients with CRSD met the OD criteria supports this notion.
A possible pathophysiological relationship between these two conditions should be discussed. A large arterial pressure drop after active standing occurs in OH, whereas POTS involves marked tachycardia during upright posture without obvious hypotension.7,15–17 One possible explanation may be that these abnormal cardiovascular responses and distress might simply make it difficult for subjects to arise in the morning, resulting in a delay in the sleep phase and internal circadian rhythm. However, this cannot explain the entire pathophysiology of CRSD because a substantial population of patients with CRSD did not have OD. Another explanation may be that CRSD and OD share some common pathophysiology. OD occurs when mechanisms for the regulation of orthostatic BP control fails. Such regulation depends on baroreflexes, normal blood volume, and defenses against excessive venous pooling.6,15 On the other hand, there have been several evidence suggesting a relationship between circadian rhythm and cardiac autonomic control.22,23 Therefore, disturbance in circadian regulation may result in both sleep-wake disturbance and OD. One more possibility may be that the occurrence of OD in a patient with CRSD is a presentation of a mere shift in the timing of normal orthostatic responses in CRSD, not a qualitative difference or pathological failure in the mechanisms of ortho-static regulation. It is unknown whether normal people display orthostatic intolerance very early in the morning. In addition, we did not examine the impact of the time of the day on testing in any identical patient in this study; we are, therefore, unable to comment on this further. However, we consider that, at least, the fact that there was orthostatic intolerance during normal activity time in many patients with CRSD highlights a link of definite clinical significance. Alternatively, we could consider that the shift in the timing of orthostatic responses itself might have a pathophysiological meaning. Either way, the underlying mechanisms for this comorbidity remain unclear, and the clinical value of finding OD in patients with CRSD as a new target for therapeutic intervention can also be pursued.
This study has several limitations. The small sample size might have affected the results. We did not enroll a control group for the direct comparison of prevalence using the same diagnostic method; therefore, we did not conduct a statistical comparison nor did we calculate the required sample size. Additionally, this study was performed at our specialized sleep clinic with many refractory cases; therefore, there might be sampling bias. A properly designed case-control study, adjusting for confounding factors (i.e., age, sex, physical constitution, and related clinical features), will be needed in the future. However, in simple comparison with the OD prevalence in the generation of the peak, the prevalence in patients with CRSD was clearly high. Thus, we believe that our study has definite value reporting firstly that OD prevalence in patients with CRSD was markedly higher than that in the general population. As for measuring of OD, the reliability between the assessors of OD has not been fully established, and this may need to be examined in the future. Japanese guidelines indicate that orthostatic testing should be performed during the morning because the test often results in false negatives if performed during the afternoon.6 However, in this study, we performed the test when the patient came for a routine medical examination, which included tests performed in the afternoon as well. Thus, the OD prevalence in this study might actually be underestimated, further suggesting a strong link between CRSD and OD.
CONCLUSIONS
The prevalence of OD as assessed by the new orthostatic test or the Schellong test was higher in patients with CRSD than in the general population (57.9% vs. < 15%) and was even higher in patients with CRSD under 20 years (70%). The probability of OD was statistically unrelated to the present severity of CRSD or sleep aid use. These findings suggest some relationship between CRSD and OD and warrant large-scale case-control studies to confirm this finding and investigate its possible pathophysiological link.
DISCLOSURE STATEMENT
This was not an industry-sponsored study. Dr. Iwata has received research support from Eli Lilly, Janssen, and Daiichi-Sankyo and has consulted for Eli Lilli. Dr. Kitajima has received research support from Takeda and MSD and is on the speakers bureau for Takeda, MSD, Yoshitomiyakuhin Corp., Fukuda Life Tech Chubu KK, Dainippon Suimitomo Pharma, and Shionogi & Co. The other authors have indicated no financial conflicts of interest. This work was performed at Department of Psychiatry, Fujita Health University School of Medicine, Aichi, Japan.
ABBREVIATIONS
- CBT
core body temperature
- CRSDs
circadian rhythm sleep disorders
- DSPD
delayed sleep phase disorder
- DSPS
delayed sleep phase syndrome
- ICSD
International Classification of Sleep Disorders
- INOH
instantaneous orthostatic hypotension
- MEQ
morningness−eveningness questionnaire
- NMS
neurally mediated syncope
- OD
orthostatic dysregulation
- OH
orthostatic hypotension
- OI
orthostatic intolerance
- POTS
postural tachycardia syndrome
REFERENCES
- 1.American Academy of Sleep Medicine. Diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 2.Yazaki M, Shirakawa S, Okawa M, Takahashi K. Demography of sleep disturbances associated with circadian rhythm disorders in Japan. Psychiatry Clin Neurosci. 1999;53:267–8. doi: 10.1046/j.1440-1819.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Hazama GI, Inoue Y, Kojima K, Ueta T, Nakagome K. The prevalence of probable delayed-sleep-phase syndrome in students from junior high school to university in Tottori, Japan. Tohoku J Exp Med. 2008;216:95–8. doi: 10.1620/tjem.216.95. [DOI] [PubMed] [Google Scholar]
- 4.Tomoda A, Miike T, Uezono K, Kawasaki T. A school refusal case with biological rhythm disturbance and melatonin therapy. Brain Dev. 1994;16:71–6. doi: 10.1016/0387-7604(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 5.Tomoda A, Miike T, Yonamine K, Adachi K, Shiraishi S. Disturbed circadian core body temperature rhythm and sleep disturbance in school refusal children and adolescents. Biol Psychiatry. 1997;41:810–3. doi: 10.1016/S0006-3223(96)00179-5. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Fujita Y, Ishitani N, editors. Japanese Society of Psychosomatic Pediatrics; 2005. Guidelines for the diagnosis, treatment of child orthostatic dysregulation. [Google Scholar]
- 7.Tanaka H, Fujita Y, Takenaka Y, et al. Japanese clinical guidelines for juvenile orthostatic dysregulation version 1. Pediatr Int. 2009;51:169–79. doi: 10.1111/j.1442-200X.2008.02783.x. [DOI] [PubMed] [Google Scholar]
- 8.Lkhagvasuren B, Oka T. Orthostatic syncope and autonomic imbalance in psychosomatic internal medicine. Jpn J Psychosom Intern Med. 2010;14:98–101. [Google Scholar]
- 9.Tanaka H. Understanding orthostatic dysregulation in chirdren from the view point of psychosomatic medicine. Jpn J Psychosom Intern Med. 2010;14:81–7. [Google Scholar]
- 10.Tanaka H, Yamaguchi H, Takenaka Y. School refusal or orthostatic dysregulation? Psychosomatic diagnosis by orthostatic test with Finapres and treatment outcome in Japanese school absentees. J Jpn Soc Psychosom Pediatr. 1999;7:125–30. [Google Scholar]
- 11.van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010;33:1605–14. doi: 10.1093/sleep/33.12.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlitz M, Alvarez B, Vignau J, English J, Arendt J, Parkes JD. Delayed sleep phase syndrome response to melatonin. Lancet. 1991;337:1121–4. doi: 10.1016/0140-6736(91)92787-3. [DOI] [PubMed] [Google Scholar]
- 13.Ando K, Hayakawa T, Ohta T, et al. Long-term follow-up study of 10 adolescent patients with sleep-wake schedule disorders. Jpn J Psychiatr Neurol. 1994;48:37–41. doi: 10.1111/j.1440-1819.1994.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 14.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 15.Bradley JG, Davis KA. Orthostatic hypotension. Am Fam Physician. 2003;68:2393–8. [PubMed] [Google Scholar]
- 16.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Autonom Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 17.Diehl RR, Linden D. [Differential orthostatic dysregulation disorders diagnosis] Der Nervenarzt. 1999;70:1044–51. doi: 10.1007/s001150050538. [DOI] [PubMed] [Google Scholar]
- 18.Tanimura M, Honda K, Nose T, Tanaka K, Yoshida N. Reproducibility of the orthostatic responses and orthostatic dysregulation complaints in Japanese junior and senior high school students. Jpn Circ J. 1977;41:287–98. doi: 10.1253/jcj.41.287. [DOI] [PubMed] [Google Scholar]
- 19.Ray CA. Melatonin attenuates the sympathetic nerve responses to orthostatic stress in humans. J Physiol. 2003;551:1043–8. doi: 10.1113/jphysiol.2003.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitajima T, Kanbayashi T, Saito Y, et al. Diazepam reduces both arterial blood pressure and muscle sympathetic nerve activity in human. Neurosci Lett. 2004;23;355:77–80. doi: 10.1016/j.neulet.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Miura Y, Yamauchi T, Sugishita Y. The association between the morningnesseveningness and orthostatic dysregulation, Cornell medical Index, and 24 hours blood pressure in the young woman. Autonom Nerv Syst. 1992;29:480–90. [Google Scholar]
- 22.Boudreau P, Yeh WH, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36:1919–28. doi: 10.5665/sleep.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheer FA, Ter Horst GJ, van Der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol. 2001;280:H1391–9. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]