Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is common in individuals with tetraplegia and associated with adverse health outcomes. The causes of the high prevalence of OSA in this population are unknown, but it is important to understand as standard treatments are poorly tolerated in tetraplegia. Nasal congestion is common in tetraplegia, possibly because of unopposed parasympathetic activity. Further, nasal obstruction can induce OSA in healthy individuals. We therefore aimed to compare nasal resistance before and after topical administration of a sympathomimetic between 10 individuals with tetraplegia (T) and 9 able-bodied (AB) controls matched for OSA severity, gender, and age.
Methods:
Nasal, pharyngeal, and total upper airway resistance were calculated before and every 2 minutes following delivery of ≈0.05 mL of 0.5% atomized phenylephrine to the nostrils and pharyngeal airway. The surface tension of the upper airway lining liquid was also assessed.
Results:
At baseline, individuals with tetraplegia had elevated nasal resistance (T = 7.0 ± 1.9, AB = 3.0 ± 0.6 cm H2O/L/s), that rapidly fell after phenylephrine (T = 2.3 ± 0.4, p = 0.03 at 2 min) whereas the able-bodied did not change (AB = 2.5 ± 0.5 cm H2O/L/s, p = 0.06 at 2 min). Pharyngeal resistance was non-significantly higher in individuals with tetraplegia than controls at baseline (T = 2.6 ± 0.9, AB = 1.2 ± 0.4 cm H2O/L/s) and was not altered by phenylephrine in either group. The surface tension of the upper airway lining liquid did not differ between groups (T = 64.3 ± 1.0, AB = 62.7 ± 0.6 mN/m).
Conclusions:
These data suggest that the unopposed parasympathetic activity in tetraplegia increases nasal resistance, potentially contributing to the high occurrence of OSA in this population.
Citation:
Gainche L, Berlowitz DJ, LeGuen M, Ruehland WR, O'Donoghue FJ, Trinder J, Graco M, Schembri R, Eckert DJ, Rochford PD, Jordan AS. Nasal resistance is elevated in people with tetraplegia and is reduced by topical sympathomimetic administration. J Clin Sleep Med 2016;12(11):1487–1492.
Keywords: quadriplegia, nasal congestion, upper airway physiology, sleep apnea
INTRODUCTION
Sleep is an integral component of a healthy and productive life, yet a good night's sleep is a rare event for people with tetraplegia.1,2 Obstructive sleep apnea (OSA), as defined by an apneahypopnea index (AHI) of > 10 events per hour, is two to seven times more prevalent in chronic tetraplegia than in the general population.2–7 Untreated OSA is a significant issue in tetraplegia as it is associated with significant neurocognitive deficits, sleepiness, and reduced quality of life.2–6,8,9
Tetraplegia is likely to compromise the patency of the nose because sympathetic outflow to the upper airway is typically disrupted after cervical spinal cord injury. In an animal model, unopposed parasympathetic activation of the upper airway was observed to increase nasal airflow resistance.10 Consistent with this, subjective nasal obstruction and stuffiness is frequently reported as a clinically significant symptom in tetraplegia.11 In the able-bodied, nasal obstruction increases pharyngeal resistance12 and the frequency of upper airway collapse during sleep.13 Furthermore, in tetraplegia, this nasal obstruction has been specifically identified by patients with OSA as a predominant reason for non-adherence with the mainstay treatment for OSA, continuous positive airway pressure (CPAP).9 Despite these observations, measurement of nasal resistance in tetraplegia has not previously been reported.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Patients with tetraplegia often report nasal congestion and suffer from obstructive sleep apnea (OSA). High nasal resistance contributes to OSA in the able-bodied and may therefore predispose people with tetraplegia to the condition. Until now, nasal resistance has not previously been documented in tetraplegia.
Study Impact: This paper found that nasal resistance was higher in patients with tetraplegia and OSA compared to OSA alone and was reduced with topical phenylephrine, possibly providing a reason and a line of therapy for OSA in tetraplegia.
Upper airway patency and collapsibility are also influenced by the surface tension of the liquid lining the upper airway.14 Patients with OSA have a stickier airway lining liquid (higher surface tension) than healthy controls,15 and reducing this improves OSA.14 Breathing route also influences the surface tension, with oral breathing drying out the airways and making the liquid lining stickier.16 This partially explains the increased likelihood17,18 and severity16 of OSA in mouth breathers. Individuals with tetraplegia may have altered surface tension of their airway lining liquid due to either altered route of breathing, their autonomic disturbance altering salivary output and/or consistency, or due to medications further predisposing their airways to collapse. However, this has not been tested.
Thus, the aims of this experiment were to measure the surface tension of the upper airway lining fluid and the nasal, pharyngeal, and total upper airway resistance in people with tetraplegia compared to able-bodied controls. We also examined whether the application of a topical sympathomimetic vasoconstrictor would alter the resistance of the upper airway.
METHODS
Thirteen spontaneously breathing patients with chronic (> 1 year since injury), motor and sensory complete (as classified by the American Spinal Injuries Association Impairment Scale (AIS A) at the time of initial post-injury discharge) traumatic tetraplegia (T1 or higher) were recruited from the Victorian Spinal Cord Service in Melbourne, Australia. Seventeen able-bodied (AB) controls were recruited through advertisements. Participants were aged between 18–70 years. Able-bodied participants were initially matched to participants with tetraplegia (T) by gender and age (within 10 years) and subsequently by supine AHI. Exclusion criteria for both groups included allergy to lignocaine or phenylephrine and preexisting medical conditions beyond OSA and tetraplegia. No participants reported any anatomical reasons for nasal obstruction (e.g., nasal polyps, deviated septum). All participants gave informed consent, and the study procedures were approved by the Austin Health human research ethics committee.
Screening Polysomnography
Full overnight polysomnography (PSG) was conducted in the home (SomtePSG, Compumedics, Abbottsford, Australia) for all participants, except when full PSG had been performed within 6 months prior, when the screening study was omitted. For the participants with tetraplegia, 2 sleep scientists attended the home approximately 3 h prior to usual bedtime. All equipment was attached, data recording commenced and the scientists left. The study ended in the morning at approximately 06:30 when the participants' carers would arrive and remove the equipment. Able-bodied participants attended the institutional sleep laboratory approximately 2 h before their usual bedtime, were instrumented, data recording commenced, and they returned home by taxi. The study ended in the morning when the participant removed the equipment. A staff member collected the equipment on the same day.
Able-bodied controls whose supine AHI did not match to a tetraplegia participant within 15 events/h were excluded at this point because people with tetraplegia typically sleep supine all night. Eight able-bodied participants could not be AHI matched and were excluded from further study. Three participants with tetraplegia became unwell or declined to continue, leaving 10 tetraplegic and 9 able-bodied participants to complete the physiology study.
Physiology Study Procedure
Any participant who used nasal decongestion chronically, abstained from use for the week prior to the study (Table 1; T4, T6 and AB7). Participants arrived at the physiology laboratory around 16:00 and were given an early dinner before being transferred to a bed with the back raised to approximately 45 degrees. At least 60 min after dinner was finished, two 5–10 μL upper airway lining liquid samples were collected from under the tongue using polyethylene tubing attached to a 1 mL syringe. Sampling from under the tongue was chosen because it is easier to obtain than posterior pharyngeal wall samples and the surface tension (γ) of fluid under the tongue and posterior wall do not differ during nasal breathing.15 To ensure a sample was obtained even if the participant did not usually breathe through the nose, liquid was sampled first without control of breathing route and then after 15 minutes of enforced nasal breathing.
Table 1.
Demographic characteristics of the participants.
Samples were stored in the polyethylene sample tubing inside a 1.5 mL tube (Safe-Lock micro test tubes, Eppendorf), frozen (−80°C) until packed in dry ice and shipped for assessment (Ludwig Engel Centre for Respiratory Research, Westmead Hospital, Sydney, Australia). The γ of the upper airway lining liquid was measured via the “pull-off” force technique.15 This approach evaluates the force required to separate 2 silica surfaces bridged by a droplet (∼0.2 μL) of the test liquid to estimate γ in mN/m.
A leak-proof nasal mask (modified profile-lite gel, Respironics, Murrysville, PA) was fitted to the subject, secured with a head strap and suspended to minimize facial discomfort and muscle activity. A heated pneumotachograph (model 3700; Hans Rudolph, Shawnee, KS) was attached to the mask and together with a differential pressure transducer (Validyne Model CD223 city) monitored inspiratory airflow. The inspiratory side of the pneumotachograph was open to atmosphere and the total deadspace of the nasal mask and pneumotachograph was approximately 125 mL. The mask and pneumotachograph configuration was kept constant within participants and pre- and post-phenylephrine administration.
Two pressure transducer tipped catheters (Mikro-tip catheter transducers Model MPC-500 Millar Instruments, Inc., Houston, TX) were inserted into one nostril at least 3 h after the participant finished dinner to reduce the risk of regurgitation and aspiration. The tip of one catheter was passed until it reached the level of the epiglottis (about 1.5 cm below the tongue base as viewed through the mouth), the other to the level of the choanae. Catheters were secured at the nose using tape. Mask pressure was measured from a port in the mask. An electroencephalogram and an electrooculogram were recorded to confirm the participant did not fall asleep. All data were acquired on Spike2 (Cambridge Electronic Design, Cambridge, UK) at 1,000Hz.
Ten minutes of quiet, resting breathing was recorded to determine baseline airflow and resistance measurements before ∼0.05 mL 0.5% phenylephrine (Minims Phenylephrine Eye Drops, Bausch & Lomb (Australia) Pty Ltd) atomised decongestant was administered to each nostril and directly onto the posterior pharyngeal wall via the mouth. Another 10 minutes of resting breathing was recorded immediately after decongestant administration.
Analysis
All sleep studies were sleep staged and respiratory events scored by a single, experienced sleep scientist using the “Chicago criteria.”19,20 Sleep indices of primary interest included: sleep quality (sleep efficiency, number of awakenings, arousal index, REM and sleep latency, % of sleep in each stage of sleep), sleep apnea severity (AHI, oxygenation (% of sleep with SpO2 < 90%, oxygen desaturation index) and periodic limb movement index. Disease severity was classified as; no OSA AHI < 10, mild OSA 10 ≥ AHI < 30, moderate OSA 30 ≥ AHI < 60, and severe OSA AHI ≥ 60 events/h sleep.
Nasal and pharyngeal resistances were calculated at an inspiratory flow rate of 0.2 L/s (ΔPressure/Flow) on a breath by breath basis using custom Spike2 software. Breathing frequency, minute ventilation, and peak inspiratory flow rate were calculated for each breath. Data were averaged within a participant for the baseline 10 minutes and over each 2 minutes after phenylephrine administration. Breaths during swallows, talking, and body movements were excluded. Separate 2 × 6 repeated measures ANOVA (2 subject groups, 6 time points) were used to assess resistance changes over time for nasal, pharyngeal, and total upper airway resistance. A 2 × 2 repeated measures ANOVA was also used to compare surface tension between patient groups and measurement routes. If significant asphericity was observed, the Huynh-Feldt correction was employed. Student's t-tests were used for post hoc testing. Statistics were calculated using SPSS (version 22). Means ± SEM are presented and p < 0.05 was considered statistically significant.
RESULTS
Patients with tetraplegia did not differ from able-bodied controls in terms of age, BMI, AHI, or gender (Table 1). Patients with tetraplegia were taking more medications. Overall, the majority of participants were of normal weight and had mild obstructive sleep apnea.
Surface Tension
Two participants with tetraplegia had insufficient volume of lining liquid sampled in the natural breathing condition. Similarly, two able-bodied controls had insufficient samples for analysis following the enforced nasal breathing period. Analysis is therefore limited to 8 tetraplegic and 7 able-bodied participants. The surface tension of the airway lining liquid did not differ between groups during natural (62.7 ± 1.0 and 62.0 ± 0.7 mN/m in T and AB, respectively) or enforced nasal breathing (64.3 ± 1.2 and 62.7 ± 0.5 mN/m, no significant ANOVA main effect for group or interaction). However, surface tension was higher following enforced nasal breathing in both groups (ANOVA main effect of breathing route p = 0.015, group p = 0.37, interaction p = 0.36).
Resistance
The pharyngeal pressure trace in one tetraplegic participant was consistently unacceptable due to nasal secretions obscuring the catheter tip prior to and after phenylephrine administration. Another tetraplegic participant and one able-bodied control subject fell asleep during the post phenylephrine period. These subjects were awoken but any data obtained during sleep was omitted. As a result, nasal resistance was measured in 10 tetraplegic participants and 9 able-bodied controls while pharyngeal and total resistance in 9 tetraplegic participants and 9 able-bodied controls.
Nasal, pharyngeal, and total upper airway resistance are illustrated in Figure 1. Nasal resistance fell significantly over time (p < 0.01) and tended to differ between groups over time (interaction p = 0.05). There was no significant main effect for group (p = 0.28). Post hoc t-tests indicated that resistance fell significantly in the group with tetraplegia between baseline and 2 minutes (p = 0.03), whereas the change in able-bodied was nonsignificant. In contrast to nasal resistance, pharyngeal resistance showed no time or group by time interaction effects, but showed a trend towards pharyngeal resistance being higher in tetraplegia (p = 0.08). Total upper airway resistance also showed a significant main effect for time (p < 0.01). There were no significant group (p = 0.10) or group by time interaction (p = 0.10) effects.
Figure 1. Airway resistance in people with tetraplegia (T) and the able-bodied (AB) before and after phenylephrine.
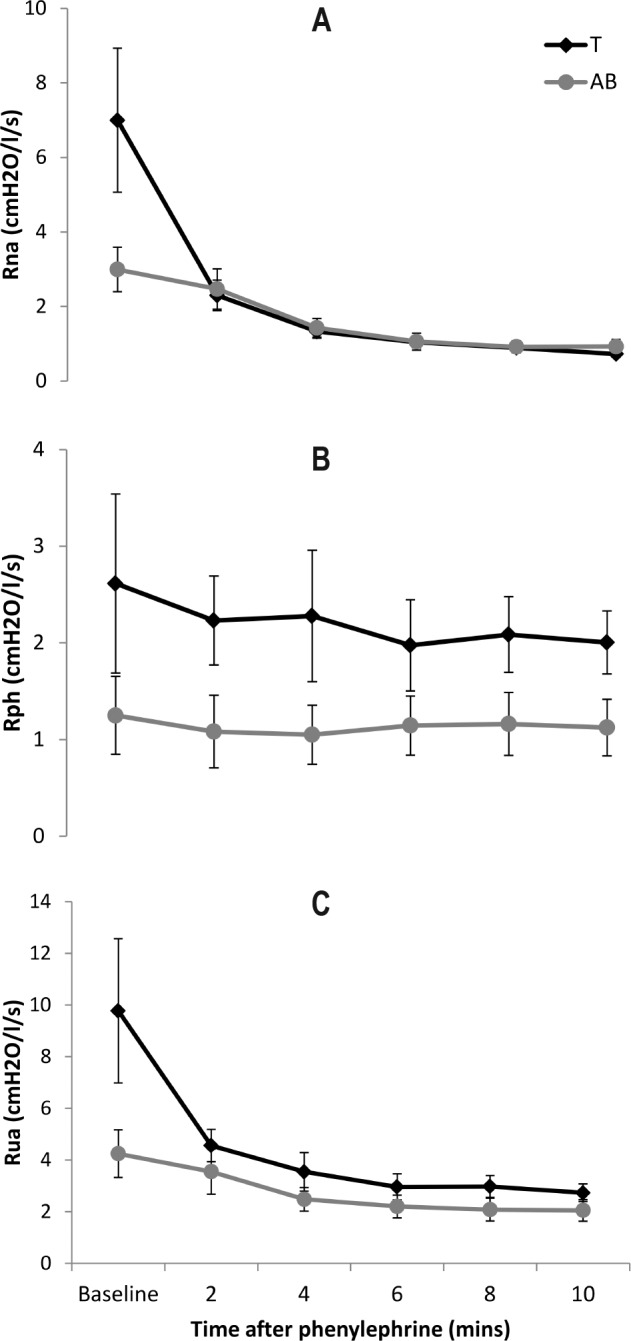
Mean nasal (Rna), pharyngeal (Rph), and total upper airway (Rua) resistance before and after ∼0.05 mL of 0.5% phenylephrine was atomized into the nostrils and pharyngeal airway in 10 individuals with tetraplegia (T) and 9 able-bodied (AB) volunteers.
The ventilatory changes that occurred following administration of phenylephrine were small. Tidal volume and duty cycle did not differ between groups nor change with time. Both groups increased peak inspiratory flow following phenylephrine (Figure 2). The increase was larger in the able-bodied than the tetraplegic participants; time (p < 0.01) and group (p = 0.04) effects but no interaction (p = 0.91).
Figure 2. Peak inspiratory flow in people with tetraplegia (T) and the able-bodied (AB) before and after phenylephrine.
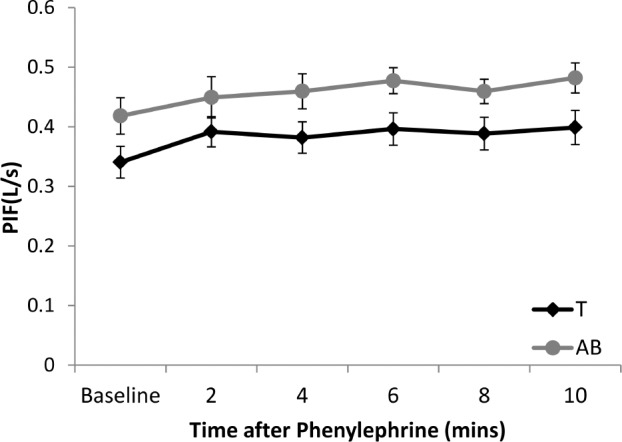
Peak inspiratory flow (PIF) before and after ∼0.05 mL of 0.5% phenylephrine was atomized into the nostrils and pharyngeal airway in 10 individuals with tetraplegia (T) and 9 able-bodied (AB) volunteers.
DISCUSSION
The main finding of this study was that nasal resistance was high in tetraplegia and that topical phenylephrine administration reduced this to the same level as that observed in matched able-bodied controls. Pharyngeal resistance was not different between the participant groups and was not significantly modified by topical vasoconstriction. Notwithstanding this, the nasal resistance reduction over time was large enough to also reduce total upper airway resistance. The upper airway lining liquid in people with tetraplegia was not found to be different to their able-bodied counterparts.
As highlighted above, OSA is common in individuals with tetraplegia and adherence to CPAP is often poor.9 It is likely that the increased nasal resistance observed in this study contributes to both of these observations. In otherwise healthy individuals, nasal packing13 can give rise to OSA and nasal decongestion with steroids and an α-adrenergic agent (dexamethasone and tramazoline) can reduce OSA severity in the able-bodied.21 Taken together these data suggest that pharmacological nasal decongestion may be an effective management approach for OSA in tetraplegia.
In an isolated feline upper airway model, unopposed, para-sympathetic activation of the upper airway was observed to increase nasal airflow resistance.10 It is believed that a similar autonomic derangement is present in people with high, complete tetraplegia. The marked elevation in nasal resistance and the immediate reduction with phenylephrine in the current study of people with tetraplegia and OSA is consistent with this. In a recent study that examined upper airway mechanics in people with tetraplegia, paraplegia and controls,22 upper airway resistance was three times higher in those with tetraplegia compared with paraplegia and controls; however, the difference was not significant (p = 0.24). The lack of statistical significance was likely attributable to the small sample and the wide variance in measured response.
We were unable to demonstrate a significant reduction in pharyngeal resistance with phenylephrine in either of our participant groups. This contrasts with the data of Wasicko,23 who found a small (approximately 0.7 cm H2O/L/sec) but statistically significant reduction in pharyngeal resistance. Our participants' overall pharyngeal resistance reduced by only 0.3 cm H2O/L/sec. However, the effective “dose” of phenylephrine delivered in the Wasicko paper was approximately 10 times larger than that administered in the current study. The participants with tetraplegia had a tendency towards higher pharyngeal resistance and a larger relative reduction with phenylephrine (Figure 1B). Further research could explore whether a larger dose or an alternative preparation may result in a clinically important effect.
There was no difference between the groups in upper airway lining liquid surface tension measured either before or after enforced nasal breathing. There was an overall increase in surface tension observed when we enforced nasal breathing. This finding contrasts with previous research, where 120 minutes of oral rather than nasal breathing increased lining liquid surface tension by 13 mN/m.16 Further, Kirkness et al.24 demonstrated that a reduction in surface tension by approximately 15 mN/m with surfactant gave rise to an average reduction in the respiratory disturbance index of 15 events per hour. Our observed increase in surface tension of approximately 1 mN/m is thus unlikely to be clinically relevant, may represent a type I error and requires replication.
Methodological Considerations
Studies involving people with tetraplegia are both clinically and technically challenging. A larger sample size would have been desirable but was simply not feasible. Despite the limited sample size, the results we obtained in our able-bodied controls are similar to previous studies and the tetraplegia data are consistent with the clinical impression, patient report and the theoretical effect of isolated parasympathetically mediated upper airway vascular engorgement. As anticipated, there were clear differences in the medication profiles of the groups. The group with tetraplegia included more participants with a history of using allergy and decongestion medications (Table 1). If these medications blunted the response in the people with tetraplegia, the group differences observed may have been under estimated. There was no matching for body mass index undertaken in this trial. People with tetraplegia have substantially altered body composition after injury,25 such that typical indices such as BMI are not comparable with the able-bodied. Specifically, people with tetraplegia have more fat for any given BMI and as such the greater BMI in the control participants may not signify more adiposity.
Summary and Clinical Implications
People with tetraplegia and OSA have deficits in the areas of attention, concentration, memory, and learning skills.8 In acute tetraplegia, these deficits are likely to prolong rehabilitation, and in chronic tetraplegia these same deficits will reduce independence and limit vocational outcomes.1,26 CPAP is poorly adhered to in people with tetraplegia, and as such alternative effective therapies are urgently needed. The identified, modifiable contributor to OSA of increased nasal resistance may present a therapeutic target for future research.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the National Health and Medical Research Council (NHMRC) of Australia (APP1065913) and proudly supported by the Transport Accident Commission. Dr Jordan was supported by the Australian Research Council (FT100100203). Dr Eckert is supported by a NHMRC RD Wright Fellowship (1049814). This work was performed at The Institute for Breathing and Sleep, Austin Health, Heidelberg, VIC Australia. Dr. Berlowitz has received research support from ResMed. Dr. Jordan has received the use of investigational devices or drugs from Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the staff of the Ludwig Engel Centre for Respiratory Research Westmead Hospital, and specifically Manisha Verma for the airway lining liquid surface tension analysis. We also thank the patients and controls for their contribution to the study.
Author contributions: Drs. Berlowitz, O'Donoghue, Trinder, Rochford, and Jordan designed the study. Ms. Gainche, Drs. Berlowitz, LeGuen,Graco, Schembri, Rochford and Jordan assisted with data collection. Ms. Gainche, Dr. Berlowitz, Mr. Ruehland, and Jordan performed data analysis. Dr. Berlowitz, Ms. Gainche, Dr. O'Donoghue, Dr. Eckert, Trinder and Jordan interpreted the study and all authors contributed to the preparation of the manuscript.
ABBREVIATIONS
- AB
able bodied
- AHI
apnea-hypopnea index
- AIS
American Spinal Injuries Association Impairment Scale
- BMI
body mass index
- CPAP
continuous positive airway pressure
- OSA
obstructive sleep apnea
- PIF
peak inspiratory flow
- PSG
polysomnography
- Rna
nasal resistance
- Rph
pharyngeal resistance
- Rua
upper airway resistance
- T
tetraplegia
- SEM
standard error of the mean
- γ
surface tension
REFERENCES
- 1.Biering-Sorensen F, Biering-Sorensen M. Sleep disturbances in the spinal cord injured: an epidemiological questionnaire investigation, including a normal population. Spinal Cord. 2001;39:505–13. doi: 10.1038/sj.sc.3101197. [DOI] [PubMed] [Google Scholar]
- 2.Berlowitz DJ, Spong J, Gordon I, Howard ME, Brown DJ. Relationships between objective sleep indices and symptoms in a community sample of people with tetraplegia. Archives of Phys Med Rehabil. 2012;93:1246–52. doi: 10.1016/j.apmr.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Leduc BE, Dagher JH, Mayer P, Bellemare F, Lepage Y. Estimated prevalence of obstructive sleep apnea-hypopnea syndrome after cervical cord injury. Arch Phys Med Rehabil. 2007;88:333–37. doi: 10.1016/j.apmr.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy RD, Mykytyn I, Sajkov D, et al. Sleep apnoea in patients with quadriplegia. Thorax. 1995;50:613–19. doi: 10.1136/thx.50.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns SP, Little JW, Hussey JD, Lyman P, Lakshminarayanan S. Sleep apnea syndrome in chronic spinal cord injury: associated factors and treatment. Arch Phys Med Rehabil. 2000;81:1334–39. doi: 10.1053/apmr.2000.9398. [DOI] [PubMed] [Google Scholar]
- 6.Stockhammer E, Tobon A, Michel F, et al. Characteristics of sleep apnea syndrome in tetraplegic patients. Spinal Cord. 2002;40:286–94. doi: 10.1038/sj.sc.3101301. [DOI] [PubMed] [Google Scholar]
- 7.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajkov D, Marshall R, Walker P, et al. Sleep apnoea related hypoxia is associated with cognitive disturbances in patients with tetraplegia. Spinal Cord. 1998;36:231–39. doi: 10.1038/sj.sc.3100563. [DOI] [PubMed] [Google Scholar]
- 9.Berlowitz DJ, Spong J, Pierce RJ, Ross J, Barnes M, Brown DJ. The feasibility of using auto-titrating continuous positive airway pressure to treat obstructive sleep apnoea after acute tetraplegia. Spinal Cord. 2009;47:868–73. doi: 10.1038/sc.2009.56. [DOI] [PubMed] [Google Scholar]
- 10.Lung MA, Wang JC. Autonomic nervous control of nasal vasculature and airflow resistance in the anaesthetized dog. J Physiol. 1989;419:121–39. doi: 10.1113/jphysiol.1989.sp017864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansel JK, Norman JR. Respiratory complications and management of spinal cord injuries. Chest. 1990;97:1446–52. doi: 10.1378/chest.97.6.1446. [DOI] [PubMed] [Google Scholar]
- 12.Gleeson K, Zwillich CW, Bendrick TW, White DP. Effect of inspiratory nasal loading on pharyngeal resistance. J Appl Physiol. 1986;60:1882–6. doi: 10.1152/jappl.1986.60.6.1882. [DOI] [PubMed] [Google Scholar]
- 13.Suratt PM, Turner BL, Wilhoit SC. Effect of intranasal obstruction on breathing during sleep. Chest. 1986;90:324–29. doi: 10.1378/chest.90.3.324. [DOI] [PubMed] [Google Scholar]
- 14.Kirkness JP, Christenson HK, Garlick SR, et al. Decreased surface tension of upper airway mucosal lining liquid increases upper airway patency in anaesthetised rabbits. J Physiol. 2003;547:603–11. doi: 10.1113/jphysiol.2002.031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Surface tension of upper airway mucosal lining liquid in obstructive sleep apnea/ hypopnea syndrome. Sleep. 2005;28:457–63. doi: 10.1093/sleep/28.4.457. [DOI] [PubMed] [Google Scholar]
- 16.Verma M, Seto-Poon M, Wheatley JR, Amis TC, Kirkness JP. Influence of breathing route on upper airway lining liquid surface tension in humans. J Physiol. 2006;574:859–66. doi: 10.1113/jphysiol.2005.102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwillich CW, Pickett C, Hanson FN, Weil JV. Disturbed sleep and prolonged apnea during nasal obstruction in normal men. Am Rev Respir Dis. 1981;124:158–60. doi: 10.1164/arrd.1981.124.2.158. [DOI] [PubMed] [Google Scholar]
- 18.Taasan V, Wynne JW, Cassisi N, Block AJ. The effect of nasal packing on sleep-disordered breathing and nocturnal oxygen desaturation. Laryngoscope. 1981;91:1163–72. doi: 10.1288/00005537-198107000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. Washington DC: National Institutes of Health; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. [Google Scholar]
- 21.Koutsourelakis I, Minaritzoglou A, Zakynthinos G, Vagiakis E, Zakynthinos S. The effect of nasal tramazoline with dexamethazone in obstructive sleep apnoea patients. Eur Respir J. 2013;42:1055–63. doi: 10.1183/09031936.00142312. [DOI] [PubMed] [Google Scholar]
- 22.Sankari A, Bascom AT, Badr MS. Upper airway mechanics in chronic spinal cord injury during sleep. J Appl Physiol. 2014;116:1390–95. doi: 10.1152/japplphysiol.00139.2014. [DOI] [PubMed] [Google Scholar]
- 23.Wasicko MJ, Leiter JC, Erlichman JS, Strobel RJ, Bartlett D., Jr Nasal and pharyngeal resistance after topical mucosal vasoconstriction in normal humans. Am Rev Respir Dis. 1991;144:1048–52. doi: 10.1164/ajrccm/144.5.1048. [DOI] [PubMed] [Google Scholar]
- 24.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Relationship between surface tension of upper airway lining liquid and upper airway collapsibility during sleep in obstructive sleep apnea hypopnea syndrome. J Appl Physiol. 2003;95:1761–66. doi: 10.1152/japplphysiol.00488.2003. [DOI] [PubMed] [Google Scholar]
- 25.Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 1993;95:2398–407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy P, Sherlock O, McClelland M, Short D, Royle J, Wilson C. A multi-centre study of the community needs of people with spinal cord injuries: the first 18 months. Spinal Cord. 2009;48:15–20. doi: 10.1038/sc.2009.65. [DOI] [PubMed] [Google Scholar]


