Abstract
Study Objectives:
To investigate whether sleep perception (SP), defined by the ratio of subjective and objective total sleep time, and habitual sleep time in various sleep disorders may be based on comorbid insomnia status.
Methods:
We enrolled 420 patients (age 20–79 y) who underwent polysomnography (PSG). They were divided into three groups based on chief complaints: chronic insomnia (CI, n = 69), patients with both obstructive sleep apnea and insomnia (OSA-I, n = 49) or OSA only (OSA, n = 149). Healthy volunteers were also recruited (normal controls [NC], n = 80). We compared differences in PSG parameters and habitual sleep duration and investigated the discrepancy between objective and subjective total sleep time (TST) and sleep latency among four groups. Subjective TST was defined as sleep time perceived by participants the next morning of PSG.
Results:
SP for TST was highest in the OSA group (median 92.9%), and lowest in the CI group (80.3%). SP of the NC group (91.4%) was higher than the CI, but there was no difference between OSA-I and OSA groups. OSA-I had higher depressive mood compared to the OSA group (p < 0.001). SP was positively associated with the presence of OSA and habitual sleep duration and negatively related to the presence of insomnia and arousal index of PSG. Insomnia patients with (OSA-I) or without OSA (CI) reported the smallest discrepancy between habitual sleep duration and objective TST.
Conclusions:
Patients with OSA with or without insomnia have different PSG profiles, which suggests that objective measures of sleep are an important consideration for differentiating subtypes of insomnia and tailoring proper treatment.
Commentary:
A commentary on this articles appears in this issue on page 1437.
Citation:
Choi SJ, Suh S, Ong J, Joo EY. Sleep misperception in chronic insomnia patients with obstructive sleep apnea syndrome: implications for clinical assessment. J Clin Sleep Med 2016;12 (11):1517–1525.
Keywords: chronic insomnia, obstructive sleep apnea, sleep perception
INTRODUCTION
Insomnia is a chronic condition characterized by difficulty initiating sleep and/or maintaining sleep that causes significant distress or impairment in daily functioning. It is an established finding that insomnia patients have difficulty recognizing sleep state, with the tendency to overestimate their wake time and underestimate their sleep time.1,2 Research using polysomnography (PSG) in insomnia patients have revealed that there is a remarkable discrepancy between the subjective experience of insomnia and objective sleep measured with PSG.3,4 Sleep misperception has been associated with several negative psychological dimensions, and longer periods of wakefulness following sleep onset, greater self-perceived sleep impairment.5 Sleep misperception in insomnia patients is problematic because it makes the patient believe that he or she is getting insufficient sleep, which subsequently leads to heightened anxiety and worry about sleep. Increased anxiety toward sleep further hinders propensity to sleep, and a vicious cycle occurs where sleep misperception ends up exacerbating insomnia symptoms.1,6,7
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although sleep perception may vary in patients with insomnia and obstructive sleep apnea (OSA), it is unknown how sleep perception changes when patients have both comorbid conditions. The objective of this study was to investigate differences in sleep perception and self-reported habitual sleep duration in four groups—patients with insomnia, obstructive sleep apnea (OSA), patients with both insomnia and OSA, and those without diagnosed OSA or insomnia.
Study Impact: Patients with insomnia, regardless of OSA status, have lower sleep perception and showed smaller discrepancy between objective sleep time and habitual sleep duration compared to patients with OSA or good sleepers. This suggests that sleep studies may be necessary in patients with insomnia for tailoring proper treatment based on insomnia subtype.
However, although previous studies have noted that using PSG in insomnia patients can help differentiate physiologically based sleep disturbances from sleep state misperception, the American Academy of Sleep Medicine standard of practice does not recommend PSG for the routine evaluation of insomnia.8,9 Insomnia is a clinical diagnosis that does not require a laboratory PSG unless there is a suspicion of another disorder. This may have negative implications for treatment, as recent studies recognize that insomnia is highly comorbid with other disorders.10,11 This has been reflected in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, which changed its diagnostic guidelines to “insomnia disorder” instead of using the previous term, “secondary insomnia.” One of the important implications of this change is investigating insomnia in the presence of other sleep disorders, especially obstructive sleep apnea (OSA). Previous studies examining the comorbidity of OSA and insomnia have reported 22% to 67% co-occurrence of the two sleep disorders.10,12 Although insomnia is comorbid with OSA, the mechanisms of sleep perception by the patient with multiple sleep disorders are largely unknown. In contrast to insomnia patients, studies suggest that patients with OSA have intact sleep perception compared to insomnia patients, even if they sleep less.2 One study reported that decreased oxyhemoglobin saturation and microarousals in the occurrence of respiratory events do not seem to interfere with sleep perception.2 Only a few studies have investigated sleep perception in the presence of both insomnia and OSA,2,13,14 and the underpinning mechanisms of sleep perception are still largely unknown. Another important reason to consider using PSG with insomnia patients is to elucidate various subtypes of insomnia. Studies by Fernandez-Mendoza et al.15 and Vgontzas and colleagues16 have found that using PSG can identify insomnia patients with objective short-sleep phenotypes, which has been shown to be associated with greater cardiovascular consequences and also more strongly associated with sleep misperception compared to other insomnia phenotypes.
Additionally, when administering PSG to sleep disorder patients, there are alterations in sleep architecture that depart from typical night's sleep, mainly due to discomfort in the procedure and potential psychological scrutiny of being watched while sleeping.17 This phenomenon is of significant importance to sleep-related breathing disorders such as OSA, which is frequently described as a limitation of PSG.17 OSA patients display less total sleep time (TST) and altered sleep architecture the first night of receiving PSG when compared to subsequent nights, and is often different from typical sleep.17–19 In contrast, patients with insomnia tend to display a ‘paradoxical’ effect, where they tend to sleep better the first night when receiving PSG compared to their typical sleep.20 However, little is known about how habitual sleep duration differs from PSG total sleep duration when insomnia is comorbid with OSA.
The aim of this study was to investigate differences in sleep perception in four groups: Individuals with chronic insomnia (CI), combined insomnia with OSA (OSA-I), OSA only (OSA), and normal controls (NC). The second aim was to compare the discrepancy of TST from PSG and TST from self-reported habitual sleep duration in these four groups. Additionally, we also investigated clinical correlates of sleep perception.
METHODS
Subjects
Patients who had OSA and insomnia were included retrospectively. Data were collected at a sleep center of a university hospital from the patients who visited between December 2008 and September 2009. Healthy volunteers were recruited prospectively in the local community by the advertisements for NC from September 2010 to April 2011. All patients and healthy volunteers underwent overnight PSG and were instructed to complete self-report questionnaires before and after the sleep study.
Exclusion criteria for both patients and healthy volunteers were as follows: (1) younger than 20 y, (2) non-Korean speaking foreigners, (3) serious medical, mental or neurological conditions, (4) any sleep disorders such as restless legs syndrome (RLS), periodic limb movement disorder (PLMD) confirmed by PSG (movement arousal index > 5 events/h or total PLM index ≥ 15 events/h), narcolepsy, or rapid eye movement (REM) sleep behavior disorder, (5) shift workers, or (6) subjects with a split-night PSG or insufficient data of the self-reported questionnaires.
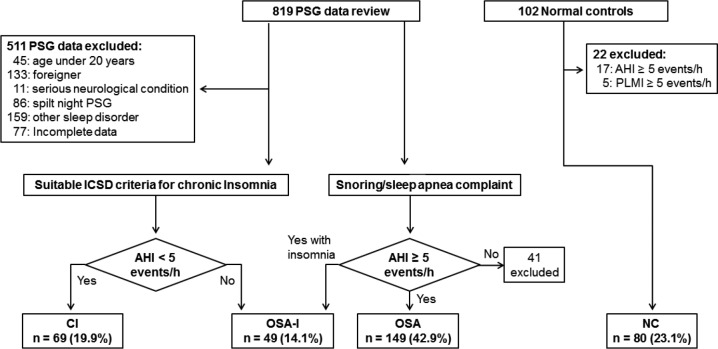
Participants were divided into four groups. The first group consisted of patients with chronic insomnia without OSA (apnea-hypopnea index, AHI < 5 events/h) (CI). Insomnia diagnosis was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition21 and International Classification of Sleep Disorders, Second Edition criteria.22 Insomnia symptoms which were associated with other medical or mental disorders were also excluded. The second group consisted of patients who had OSA (AHI ≥ 5 events/h) and presented with complaints of insomnia-related symptoms (OSA-I). Participants with an AHI < 5 events/h or who had an insomnia complaint less than 6 mo were excluded. The third group consisted of OSA patients without insomnia (OSA). The fourth group was healthy volunteers for NC who did not present with complaints of sleep-related symptoms with AHI < 5 events/h, did not take medications or any other substances that interfered with their sleep, and had no reported neurological diseases or mental disorders. Among 819 participants, 511 were excluded due to the following factors: age younger than 20 y (n = 45), being a non-Korean foreigner (n = 133), having a diagnosis of a serious neurological condition (n = 11), a split night with continuous positive airway pressure (n = 86), other sleep disorders [PLMD/RLS (n = 131), REM behavior disorder (n = 11), or narcolepsy (n = 17)], or incomplete or missing data (n = 77). Patients who had AHI < 5 events/h (n = 41) were excluded from the OSA or OSA-I groups. Among 102 NC, 17 people who had OSA (AHI ≥ 5 events/h) or PLMD were excluded. A total of 360 subjects (CI, n = 69; OSA-I, n = 49; OSA, n = 149; NC, n = 80) were included in the study for final analyses (Figure 1).
Figure 1. Study enrollment flow.
AHI, apnea-hypopnea index; CI, chronic insomnia; ICSD, International Classification of Sleep Disorders; NC, normal controls; OSA, obstructive sleep apnea; PLMI, periodic limb movement index; PSG, polysomnography.
Self-Report Questionnaires
Subjects were asked to respond to the following self-report questionnaires prior to undergoing PSG.
Daytime Sleepiness
The effect of subjective daytime sleepiness was measured using the Epworth Sleepiness Scale (ESS).23 The ESS consists of eight items, and each item was measured on a 0 (no napping) to 3 (high chance for napping) scale, with total scores ranging from 0 to 24. Subjects with an ESS score ≥ 10 were considered as having clinically significant daytime sleepiness.
Depression
Depressive symptoms were measured using the Beck Depression Inventory (BDI), which is a 21-item self-report questionnaire measuring severity of depressive symptoms over the past 2 weeks.24 Each item is rated on a Likert scale of 0 (absence of symptom) to 3 (most severe level). Total scores range from 0 to 63 with a higher score reflecting more depressive mood. The cutoff value reflecting clinical levels of depression in the Korean version of BDI is 16.25
Habitual Sleep Duration
Participants were asked about how much time they slept on average on weekdays and weekends during their PSG visit.
Polysomnography
PSG sleep studies were recorded during 1 night of observation with standard electrodes and sensors by using Embla N7000 (Medcare Flaga, Iceland). Electroencephalography electrodes were applied at C3-A2, C4-A1, F3-A2, F4-A1, O3-A2, and O2-A1, and four electrooculography electrodes were applied at both lateral sides, superior and inferior of the eyes, to record horizontal and vertical eye movements. Chin and both anterior tibialis electromyogram, and electrocardiography sensors were applied. Two plethysmography belts were used to monitor thoracic and abdominal movements. Nasal and oral airflow was measured with a nasal pressure transducer and a thermistor. Oxygen saturation was measured by pulse oximetry via index finger. Synchronized video monitoring was used to monitor abnormal sleep breathing or movements. We collected data from PSG as sleep parameters (TST, sleep latency, waking after sleep onset, sleep efficiency), sleep stage (N1, N2, N3, REM sleep, %), and AHI. Apnea was defined as the complete cession of airflow for at least 10 seconds, whereas hypopnea was defined as a moderate reduction in airflow (> 30%) for at least 10 sec with oxygen desaturation (≥ 4%) or arousal.26
While performing the PSG, we attempted to modify “lights out” to match the patient's habitual sleep period as closely as possible, when feasible. All participants were discouraged from having a “lights out” time that largely deviated from their habitual sleep time.
Sleep Perception
After completion of overnight PSG, subjects were asked to fill out a questionnaire about their perceived sleep (subjective TST and sleep latency) the next morning. Sleep perception was defined as the percentage of the ratio between the perceived TST by subject and the TST measured by PSG: (Subjective TST / Objective TST) * 100.2
Statistical Analysis
The current study used statistical package R (Institute for Statistics and Mathematics, Vienna, Austria, version 2.15.0, www.R-porject.org) and the Statistical Package for Social Science, SPSS for Windows (version 18.0, SPSS, Chicago, IL) for all analyses, and statistical significance level was set at p < 0.05. To test normality assumptions, the Shapiro-Wilks test was used. All continuous variables were analyzed using non-parametric Kruskal-Wallis tests, and categorical variables were analyzed using chi-square tests or Fisher exact test. Post hoc analyses were conducted using the Tukey test.
Multiple linear regression analysis was performed to determine the relationships between sleep perception and various factors such as the presence of insomnia or OSA, habitual sleep duration, TST and arousal index based on PSG, and depressive mood in patients controlling for age and sex. Because the distribution of PSG-arousal index was highly skewed, logarithmically transformed values of PSG-arousal index (log AI) was used in the analysis. After log transformation, the distribution of the residuals from the fitted models became normally distributed.
RESULTS
Subject Characteristics
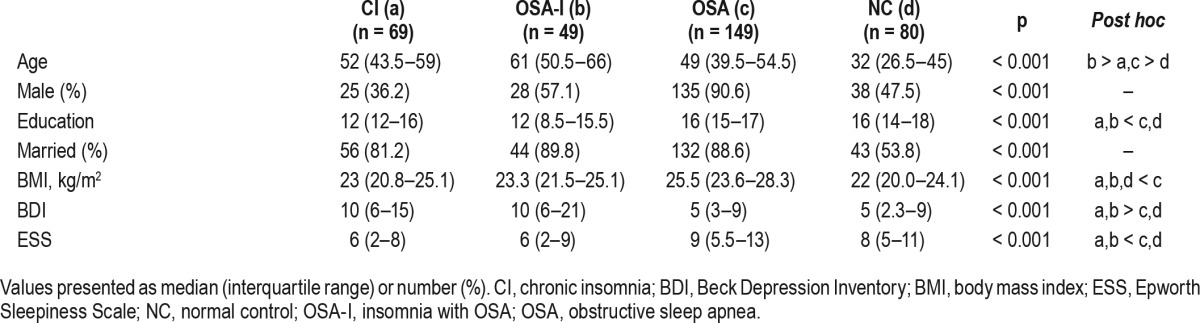
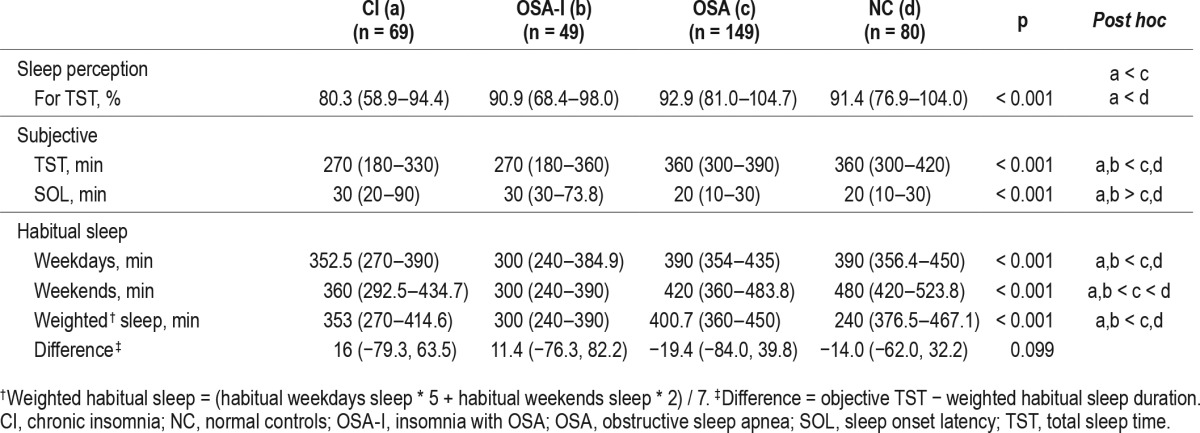
Demographic characteristics of the subjects are summarized in Table 1. Patients in the CI, OSA-I, and OSA groups were older than the NC group. Among patients, mean age was higher in the CI and the OSA-I groups compared to the OSA group (p < 0.001). There was a higher percentage of males in the OSA group, and education level was significantly lower in the CI and the OSA-I groups compared to others (both p ≤ 0.005). Body mass index was also significantly higher in the OSA group (p < 0.001). There were also significant differences in depression and excessive daytime sleepiness among groups. Patients with insomnia were significantly more depressed regardless of OSA status (median 10 in CI and OSA-I groups, respectively) than the OSA and NC groups (median 5, respectively; p < 0.001). The OSA and NC groups reported the higher level of daytime sleepiness than CI and OSA-I (p < 0.05). There was no association between sleep stages and sleep perception in the sample (ps > 0.54).
Table 1.
Demographic characteristics of the four groups (n = 347).
Differences in Sleep and Sleep Perception among Groups
All sleep parameters obtained from PSG were different among groups and were consistent with the characteristics of each sleep disorder (Table 2). The range of PSG “lights out” was 20:50–01:00, and “lights on” ranged between 03:39–09:27.
Table 2.
Polysomnography sleep parameters of the four groups (n = 347).
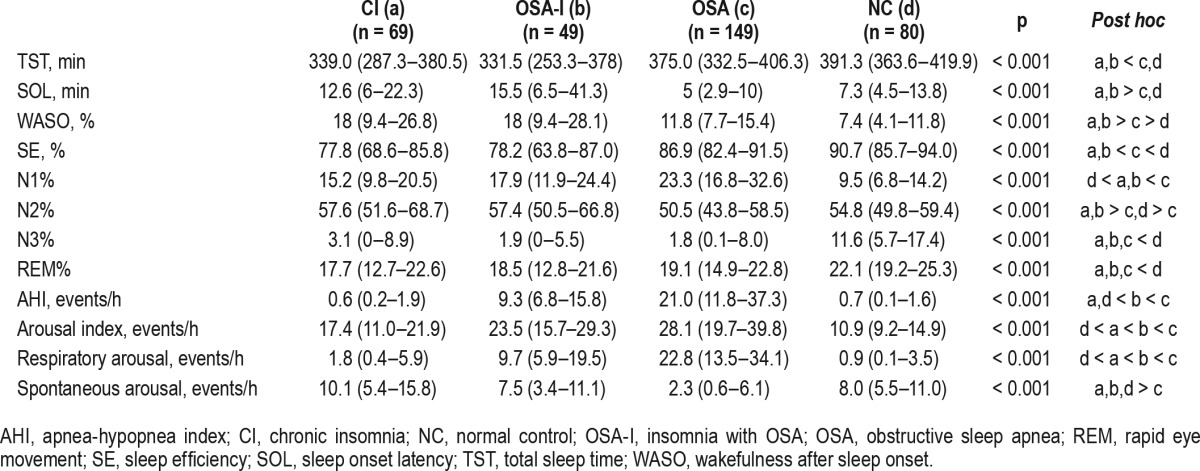
As expected, patients with insomnia (the CI and the OSA-I groups) had decreased TST, prolonged sleep latency, increased wake after sleep onset, and decreased sleep efficiency compared to the OSA and the NC groups. Sleep architecture was fragmented (increased N1 and N2 sleep and decreased N3 sleep), whereas REM sleep was preserved in patient groups. The OSA group had the highest AHI, arousal index, and sleep efficiency (85.3%) compared to the other patient groups. The OSA-I group had significantly higher AHI compared to the CI group (median 9.3 events/h vs. 0.6 events/h, respectively) and arousal index (median 23.5 events/h vs. 17.4 events/h, respectively). Interestingly, sleep efficiency in the OSA-I group compared to the CI group (median 78.2% vs. 77.8%, respectively) and wake after sleep onset (median 18.0, respectively) were not different between patient groups with insomnia.
Median sleep perception for TST was lowest in the CI (80.3%) and highest in the OSA (92.9%) group (Figure 2). Post hoc analyses of sleep perception for TST revealed that there was a significant difference between the CI and the OSA groups (p < 0.001), and CI and NC groups (p = 0.004), but not between the CI and the OSA-I groups (p = 0.304).
Figure 2. Difference of sleep perception of total sleep time between the four groups.
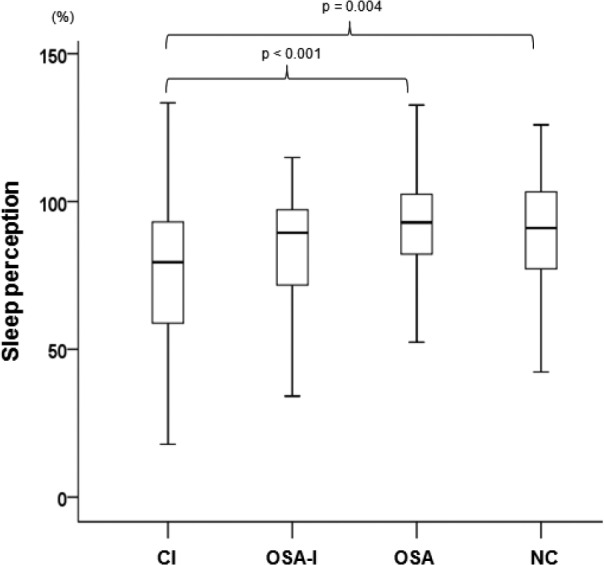
CI, chronic insomnia; NC, normal control; OSA, obstructive sleep apnea.
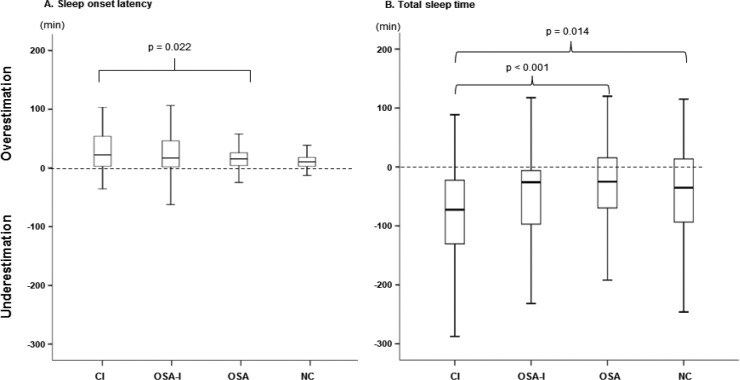
Discrepancy of subjective and objective sleep latency and TST is demonstrated in Figure 3. In all groups, sleep latency was overestimated and TST was underestimated. Post hoc analyses using Tukey methods indicated that sleep latency of patients with insomnia (the CI and the OSA-I groups) were significantly different from the NC group (p < 0.001, respectively), and TST of the OSA group (p < 0.001).
Figure 3. Discrepancy between subjective and objective sleep between the four groups.
CI, chronic insomnia; NC, normal control; OSA, obstructive sleep apnea.
Discrepancy of Habitual Sleep Duration and Objective TST Based on PSG
Among all four groups, the CI and OSA-I groups reported a smaller amount of total habitual sleep duration than the OSA and NC groups (p < 0.001) (Table 3). A difference score was calculated between habitual sleep duration during weekdays and TST measured by PSG (Table 3) because sleep studies were usually performed on weekdays. However, there was no significant difference between groups.
Table 3.
Sleep perception for total sleep time and habitual sleep duration in the four groups (n = 347).
Association Between Sleep Perception and Clinical Variables
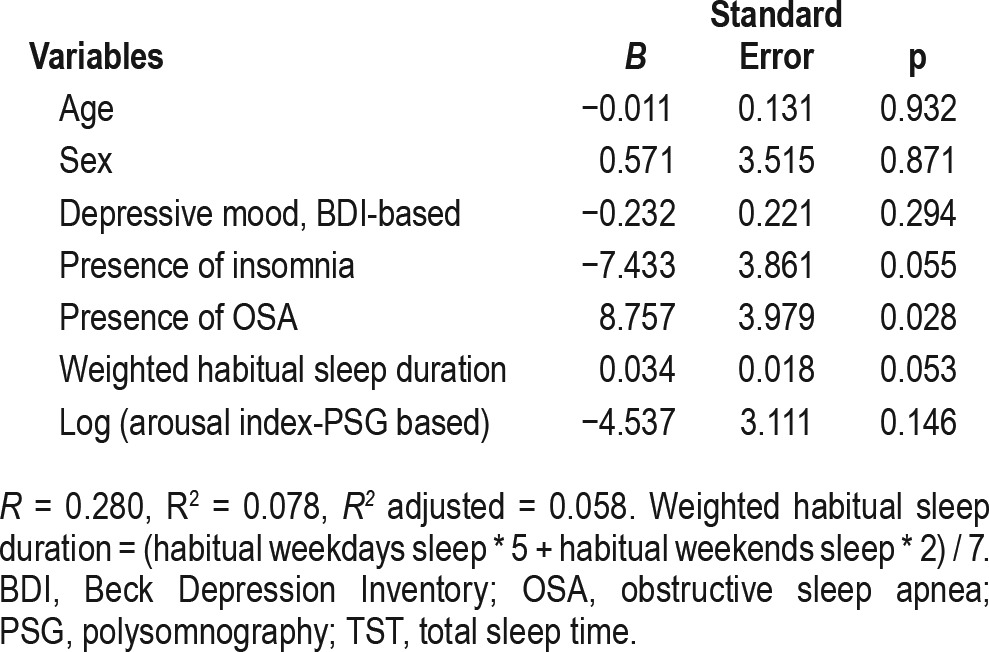
Multivariate linear regression analysis after adjusting for age and sex revealed that sleep perception was positively related to presence of OSA (B = 8.757, p = 0.028) (Table 4). Other clinical variables (depressive mood, presence of insomnia, weighted habitual sleep duration, PSG-based arousal index) were not associated with sleep perception.
Table 4.
Multiple linear regression of the association of sleep perception with clinical variables.
DISCUSSION
The current study investigated sleep perception in four different groups—those with insomnia (chronic insomnia only, the CI and comorbid insomnia with OSA, the OSA-I) and OSA only (the OSA), and good sleepers (the NC). The main focus of our study was to investigate sleep perception in patients who had insomnia comorbid with OSA, and how they differed from patient groups who had either only OSA or insomnia, without comorbidity.
Differences in Sleep Perception in Various Sleep Disorders
A main finding of this study was that patients in the OSA-I group had significantly lower sleep perception than those in the OSA group, although there was no difference with the CI group. This suggests that sleep perception in individuals with insomnia have low sleep perception, regardless of comorbidity with OSA. The OSA-I group and the OSA group had clearly distinct characteristics. Both were male dominant, although the OSA group showed higher body mass index and ESS scores and lower BDI scores than the OSA-I group. Additionally, the OSA group had significantly higher AHI scores compared to the OSA-I group (median AHI [interquartile range] = 21.0 (11.8– 37.3) vs. 9.3 (6.8–15.8), respectively). Nevertheless, subjective sleep perception was higher in the OSA group (median 92.9%), with a median discrepancy of subjective and objective TST being 25 min. In contrast, those in the CI group had the lowest sleep perception (median 80.3%), with the median discrepancy of subjective and objective TST being 72.5 min, respectively.
The OSA-I group consisted of patients who had complaints of insomnia-related symptoms (difficulty initiating and maintaining sleep). We differentiated the OSA-I group from the CI group by AHI scores (> 5 events/h) and PSG evidence that sleep-disordered breathing and respiratory arousals triggered difficulty falling asleep or maintaining sleep. As a result, PSG parameters of the two groups who complain of insomnia commonly were not statistically different except for AHI and arousal index. Although previous studies have suggested that increased arousal index within total sleep time may be associated with strong increase in REM sleep, this was not consistent with our findings.27 Additionally, there was no association with sleep perception and stages of sleep in our study. For demographic information, there were no differences in body mass index, BDI, and ESS, except that females were predominant in the CI (63.8%) and males were predominant in the OSA-I group (57.1%). Sleep perception for TST in the insomnia groups (CI and OSA-I groups) were lower than the OSA group, but not significantly different between the CI and OSA-I groups. Additionally, patients in these two groups significantly underestimate their sleep latencies compared to the OSA and the NC groups.
This interesting finding suggests to clinicians that incorporating objective sleep measurement such as PSG or actigraphy should be considered in practice when identifying subgroups of insomnia or tailoring treatment for insomnia patients.28 Edinger and Krystal28 suggested that sleep misperception is not ubiquitous in all insomnia patients, but may be a valuable discriminator in differentiating between certain types of insomnia subgroups, such as those with subjective and objective insomnia. Additionally, PSG indicators such as alpha-delta sleep may be used as a physiological index for sleep state misperception.29,30 For treatment, one study by Bonnet and Arand31 showed that waking patients 27 times per night and providing them with PSG feedback about their sleep or wake state was effective in correcting sleep misperception.31 It has also been shown that demonstrating the discrepancy between subjective and objective sleep in behavioral experiments during therapy for insomnia may be helpful in correcting this misperception. Additionally, as cognitive behavioral therapy for insomnia (CBTI) is recommended as the first line of treatment for insomnia, having knowledge of accurate sleep parameters is important in implementing treatment modules, especially sleep restriction, which has been shown to be one of the most active components of CBTI, may help reduce sleep misperception. Considering that sleep misperception is associated with physiological arousal and cognitive arousal28,32,33 knowing the degree of sleep misperception may be an important aspect of insomnia that is often important but difficult to measure. Additionally, based on our results, middle-aged males who have both OSA and insomnia may benefit from combined continuous positive airway pressure therapy and cognitive behavioral therapy for insomnia.34 However, sleep perception did not differentiate between the CI and the OSA-I groups.
We also found that the OSA group had the highest sleep efficiency (median 86.9%) despite having the highest AHI and arousal index among patient groups, and the presence of OSA was positively associated with higher sleep perception, which consistently support this paradox. Low sleep perception in the CI group is consistent with the study by Pinto et al.2 in that sleep perception was lowest in individuals with insomnia, although in their study, the normal control group had the highest sleep perception. One reason insomnia patients have sleep misperception may be because they tend to exaggerate their subjective report of sleep problems.7 The association between the presence of insomnia and higher arousal index and low sleep perception may support sleep misperception in patients with insomnia in our study. This may also have been the case in our study, which found that insomnia patients, regardless of OSA status, reported higher levels of depressive mood. Additionally, cognitive arousal, which is typically seen in insomnia patients, has been found to be closely associated with distorted perception of sleep in insomnia patients.32,35,36 Median sleep perception in the NC group was 91.4%, higher than patients with insomnia, but not statistically different from the OSA group. In one study, 60% of normal sleepers did not recognize awakenings 4 to 8 min after arousal from their first sleep spindles.37 In the same study, 10% of the normal sleepers did not recognize sleep onset 16 min after entering stage 2 sleep.37 Therefore, it is possible that the NC group showed a bigger discrepancy of sleep latency compared to other previous studies. We also found that those with a larger discrepancy between subjective and objective sleep had higher levels of depressive mood. This may also be a reflection of recent studies by Vgontzas and colleagues16 that proposes two subtypes of insomnia – insomnia with objective short sleep duration, and insomnia with normal sleep duration. Among two subgroups, Fernandez-Mendoza et al.15 from the same group found that sleep misperception is prevalent only among insomniacs with objective normal sleep duration, along with other psychological characteristics such as anxiety and rumination.
Group Differences of Habitual Sleep Time Compared to Objective TST
Another purpose of our study was to investigate the discrepancy of habitual sleep duration and objective TST from PSG between the four groups. Results from our study indicated that patients with insomnia with or without OSA reported the smallest discrepancy between habitual sleep duration and objective TST in the four groups. This is consistent with past studies that have reported that insomnia patients show a reverse first-night effect.20 Among these two groups, the OSA-I group especially showed the least discrepancy, and actually slept longer than their reported habitual sleep duration.
We investigated habitual sleep duration during both week-days and weekends. However, comparison of objective sleep time based on PSG with sleep duration were only performed on weekdays, because all sleep studies were only performed during weekdays. In addition, there were considerable differences of sleep duration between weekdays and weekends, especially, in the OSA (38.1 ± 70.4 min less sleep during week-days) and the NC groups (79.3 ± 99.5 min less) compared to the CI (22.6 ± 60.4 min less) and the OSA-I groups (6.7 ± 27.8 min less). This suggests that patients with insomnia may be more rigid about keeping regular sleep schedules or suffer from short sleep regardless of the days of the week. However, the OSA and NC groups who seldom have insomnia-related symptoms tend to compensate for their sleep loss during weekdays by sleeping more during weekends.
To the best of our knowledge, this is the first study that has reported on the effects of the discrepancy of habitual sleep duration and objective TST from PSG in an OSA comorbid with insomnia patient group. One previous study by Newell et al.38 compared intra-patient night-to-night variability to compare first night effect in sleep-related breathing disorders, insomnia, movement and behavioral disorders, and health controls, although that study did not include a group with OSA comorbid with insomnia. They found a reduced first- night effect, although it was not the typical inverse or paradoxical first-night effect for the individuals with psychophysiological insomnia only.38 We observed that patients with OSA and good sleepers had larger discrepancy of habitual sleep time compared to PSG TST compared to patients with insomnia. This may be associated with previous research suggesting that reduced first-night effect is commonly found in insomnia patients, which did not differ regardless of the presence of OSA.
Limitations
The current study has several limitations. First, we relied entirely on measure of sleep duration of only 1 night of PSG. Thus, it is possible that PSG measures did not reflect the usual sleep state due to first-night effect. Second, from a methodological standpoint, collecting information using sleep diaries or actigraphy may have been more accurate in determining habitual sleep duration. Third, it is difficult to explain the causality between sleep misperception and relating factors using a cross-sectional study design. Therefore, the current study results show that further well-designed longitudinal studies are required to confirm the causality. Fourth, although we tried to match “lights off” with habitual bedtime in each patient (about 22:30 to 12:00), it is possible that there was a time discrepancy between habitual sleep time and PSG TST unless the patients woke up at their self-reported habitual wake time. Finally, the methodology about assessing depressive mood involved subjects completing self-report questionnaires without structured or semistructured interviews, which limits generalizability to diagnoses of depression or mood disorders.
CONCLUSIONS
We observed that sleep perception may be different based on which sleep complaint a patient has, and may also affect objective TST when administering PSG. Patients with insomnia, regardless of OSA status, have lower sleep perception and showed smaller discrepancy between objective sleep time and habitual sleep duration compared to patients with OSA or good sleepers. Although the CI and the OSA-I groups both have insomnia-related symptoms in common, they have different etiologies for insomnia, which was revealed by PSG profiles. This suggests that objective measures of sleep are an important consideration in patients with insomnia for tailoring proper treatment based on insomnia subtype.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (No. 2014R1A1A3049510) and by Samsung Biomedical Research Institute grant (#SMO1162071). Dr. Ong has consulted for Big Health Inc., and receives royalties from the American Psychological Association. The other authors have indicated no financial conflicts of interest. This study was conducted at Samsung Medical Center located in Seoul, Korea.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
arousal index
- BDI
Beck Depression Inventory
- BMI
body mass index
- CBTI
cognitive behavioral therapy for insomnia
- CI
chronic insomnia
- ESS
Epworth Sleepiness Scale
- NC
normal controls
- OSA
obstructive sleep apnea
- OSA-I
both obstructive sleep apnea and insomnia
- PLM
periodic limb movement
- PLMD
periodic limb movement disorder
- PSG
polysomnography
- REM
rapid eye movement
- RLS
restless legs syndrome
- SE
sleep efficiency
- SOL
sleep onset latency
- SP
sleep perception
- TST
total sleep time
- WASO
wakefulness after sleep onset
REFERENCES
- 1.Mercer JD, Bootzin RR, Lack LC. Insomniacs' perception of wake instead of sleep. Sleep. 2002;25:564–71. [PubMed] [Google Scholar]
- 2.Pinto LR, Jr., Pinto MC, Goulart LI, et al. Sleep perception in insomniacs, sleep-disordered breathing patients, and healthy volunteers--an important biologic parameter of sleep. Sleep Med. 2009;10:865–8. doi: 10.1016/j.sleep.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Voderholzer U, Al-Shajlawi A, Weske G, Feige B, Riemann D. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depress Anxiety. 2003;17:162–72. doi: 10.1002/da.10101. [DOI] [PubMed] [Google Scholar]
- 4.Feige B, Al-Shajlawi A, Nissen C, et al. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17:180–90. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 5.Vanable PA, Aikens JE, Tadimeti L, Caruana-Montaldo B, Mendelson WB. Sleep latency and duration estimates among sleep disorder patients: variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep. 2000;23:71–9. [PubMed] [Google Scholar]
- 6.Tang NK, Harvey AG. Time estimation ability and distorted perception of sleep in insomnia. Behav Sleep Med. 2005;3:134–50. doi: 10.1207/s15402010bsm0303_2. [DOI] [PubMed] [Google Scholar]
- 7.Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Littner M, Hirshkowitz M, Kramer M, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]
- 9.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–9. [PubMed] [Google Scholar]
- 10.Beneto A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13:287–93. doi: 10.1016/j.smrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34:859–67. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith S, Sullivan K, Hopkins W, Douglas J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS) Sleep Med. 2004;5:449–56. doi: 10.1016/j.sleep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Castillo J, Goparaju B, Bianchi MT. Sleep-wake misperception in sleep apnea patients undergoing diagnostic versus titration polysomnography. J Psychosom Res. 2014;76:361–7. doi: 10.1016/j.jpsychores.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi MT, Williams KL, McKinney S, Ellenbogen JM. The subjective-objective mismatch in sleep perception among those with insomnia and sleep apnea. J Sleep Res. 2013;22:557–68. doi: 10.1111/jsr.12046. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73:88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Basta M, Fernandez-Mendoza J. Subjective short sleep duration: what does it mean? Sleep Med Rev. 2014;18:291–2. doi: 10.1016/j.smrv.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Le Bon O, Hoffmann G, Tecco J, et al. Mild to moderate sleep respiratory events: one negative night may not be enough. Chest. 2000;118:353–9. doi: 10.1378/chest.118.2.353. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadi N, Shapiro GK, Chung SA, Shapiro CM. Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography. Sleep Breath. 2009;13:221–6. doi: 10.1007/s11325-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 19.Gouveris H, Selivanova O, Bausmer U, Goepel B, Mann W. First-night-effect on polysomnographic respiratory sleep parameters in patients with sleep-disordered breathing and upper airway pathology. Eur Arch Otorhinolaryngol. 2010;267:1449–53. doi: 10.1007/s00405-010-1205-3. [DOI] [PubMed] [Google Scholar]
- 20.Riedel BW, Winfield CF, Lichstein KL. First night effect and reverse first night effect in older adults with primary insomnia: does anxiety play a role? Sleep Med. 2001;2:125–33. doi: 10.1016/s1389-9457(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 21.American Psychology Association. Washington, DC: American Psychiatric Association; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 22.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: diagnostic & coding manual. [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 25.Shin MS KZ, Park KB. The cut-off score for the Korean version of Beck depression inventory. Korean J Clin Psychology. 1993;12:71–81. [Google Scholar]
- 26.Iber C, Anchli-Israel S, Chesson A, Ouan S. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 27.Feige B, Al-Shajlawi A, Nissen C, et al. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17:180–90. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 28.Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep Med Rev. 2003;7:203–14. doi: 10.1053/smrv.2002.0253. [DOI] [PubMed] [Google Scholar]
- 29.Martinez D, Breitenbach TC, Lenz Mdo C. Light sleep and sleep time misperception - relationship to alpha-delta sleep. Clin Neurophysiol. 2010;121:704–11. doi: 10.1016/j.clinph.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Parrino L, Milioli G, De Paolis F, Grassi A, Terzano MG. Paradoxical insomnia: the role of CAP and arousals in sleep misperception. Sleep Med. 2009;10:1139–45. doi: 10.1016/j.sleep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Bonnet MH, Arand DL. Physiological activation in patients with Sleep State Misperception. Psychosom Med. 1997;59:533–40. doi: 10.1097/00006842-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Tang NK, Harvey AG. Effects of cognitive arousal and physiological arousal on sleep perception. Sleep. 2004;27:69–78. doi: 10.1093/sleep/27.1.69. [DOI] [PubMed] [Google Scholar]
- 33.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133:1382–8. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 34.Ong JC, Crisostomo MI. The more the merrier? Working towards multidisciplinary management of obstructive sleep apnea and comorbid insomnia. J Clin Psychology. 2013;69:1066–77. doi: 10.1002/jclp.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedman RR, Sattler HL. Physiological and psychological factors in sleep-onset insomnia. J Abnorm Psychol. 1982;91:380–9. doi: 10.1037//0021-843x.91.5.380. [DOI] [PubMed] [Google Scholar]
- 36.Lichstein KL, Rosenthal TL. Insomniacs' perceptions of cognitive versus somatic determinants of sleep disturbance. J Abnorm Psychol. 1980;89:105–7. doi: 10.1037//0021-843x.89.1.105. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet MH, Moore SE. The threshold of sleep: perception of sleep as a function of time asleep and auditory threshold. Sleep. 1982;5:267–76. doi: 10.1093/sleep/5.3.267. [DOI] [PubMed] [Google Scholar]
- 38.Newell J, Mairesse O, Verbanck P, Neu D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res. 2012;200:795–801. doi: 10.1016/j.psychres.2012.07.045. [DOI] [PubMed] [Google Scholar]