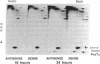
Abstract
The function of prothymosin alpha has been investigated by using four different antisense oligodeoxyribonucleotides directed at selected regions of its mRNA. In every case, when synchronized human myeloma cells were released from stationary phase by incubation in fresh medium containing antisense oligomers, cell division was prevented or inhibited; sense oligomers and random antisense oligomers had no effect. A detailed analysis of synchronized cell populations indicated that sense-treated and untreated cells divided approximately 17 hr after growth initiation, whereas cells incubated with antisense oligomer 183, a 16-mer targeted 5 bases downstream of the initiation codon, entered mitosis approximately one cell division late. The failure to divide correlated directly with a deficit in prothymosin alpha and with the continued presence of intact intracellular antisense oligomers over a period of at least 24 hr. Because antisense oligomers had no effect either on the timing of the induction of prothymosin alpha mRNA upon growth stimulation or on mRNA levels seen throughout the cell cycle, we concluded that antisense DNA caused specific hybrid arrest of translation. Our data suggest that prothymosin alpha is required for cell division. However, there is no evidence that prothymosin alpha directly regulates mitosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D., Meier C. B., Herlyn M. Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J. 1989 Dec 1;8(12):3685–3691. doi: 10.1002/j.1460-2075.1989.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L. Isolation of cytoplasmic RNA: ribonucleoside-vanadyl complexes. Methods Enzymol. 1987;152:227–234. doi: 10.1016/0076-6879(87)52024-9. [DOI] [PubMed] [Google Scholar]
- Berger S. L. Quantifying 32P-labeled and unlabeled nucleic acids. Methods Enzymol. 1987;152:49–54. doi: 10.1016/0076-6879(87)52009-2. [DOI] [PubMed] [Google Scholar]
- Ch'ng J. L., Mulligan R. C., Schimmel P., Holmes E. W. Antisense RNA complementary to 3' coding and noncoding sequences of creatine kinase is a potent inhibitor of translation in vivo. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10006–10010. doi: 10.1073/pnas.86.24.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenfeldt W. H., Berger S. L. The human prothymosin alpha gene is polymorphic and induced upon growth stimulation: evidence using a cloned cDNA. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9403–9407. doi: 10.1073/pnas.83.24.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenfeldt W. H., Manrow R. E., Krug M. S., Berger S. L. Isolation and partial sequencing of the human prothymosin alpha gene family. Evidence against export of the gene products. J Biol Chem. 1989 May 5;264(13):7546–7555. [PubMed] [Google Scholar]
- Eschenfeldt W. H., Puskas R. S., Berger S. L. Homopolymeric tailing. Methods Enzymol. 1987;152:337–342. doi: 10.1016/0076-6879(87)52040-7. [DOI] [PubMed] [Google Scholar]
- Franco F. J., Diaz C., Barcia M., Arias P., Gomez-Marquez J., Soriano F., Mendez E., Freire M. Synthesis and apparent secretion of prothymosin alpha by different subpopulations of calf and rat thymocytes. Immunology. 1989 Jun;67(2):263–268. [PMC free article] [PubMed] [Google Scholar]
- Gómez-Márquez J., Segade F., Dosil M., Pichel J. G., Bustelo X. R., Freire M. The expression of prothymosin alpha gene in T lymphocytes and leukemic lymphoid cells is tied to lymphocyte proliferation. J Biol Chem. 1989 May 25;264(15):8451–8454. [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hannappel E., Davoust S., Horecker B. L. Isolation of peptides from calf thymus. Biochem Biophys Res Commun. 1982 Jan 15;104(1):266–271. doi: 10.1016/0006-291x(82)91969-6. [DOI] [PubMed] [Google Scholar]
- Haritos A. A., Horecker B. L. A radioimmunoassay for thymosin alpha 1 that detects the native polypeptide, prothymosin alpha. J Immunol Methods. 1985 Aug 2;81(2):199–205. doi: 10.1016/0022-1759(85)90204-2. [DOI] [PubMed] [Google Scholar]
- Haritos A. A., Tsolas O., Horecker B. L. Distribution of prothymosin alpha in rat tissues. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1391–1393. doi: 10.1073/pnas.81.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruys V., Wathelet M., Poupart P., Contreras R., Fiers W., Content J., Huez G. The 3' untranslated region of the human interferon-beta mRNA has an inhibitory effect on translation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6030–6034. doi: 10.1073/pnas.84.17.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre M., Bayard B., Lebleu B. Specific antiviral activity of a poly(L-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc Natl Acad Sci U S A. 1987 Feb;84(3):648–652. doi: 10.1073/pnas.84.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova T., Grebenshikov N., Egorov C., Vartapetian A., Bogdanov A. Prothymosin alpha is an evolutionary conserved protein covalently linked to a small RNA. FEBS Lett. 1989 Nov 6;257(2):247–250. doi: 10.1016/0014-5793(89)81544-3. [DOI] [PubMed] [Google Scholar]
- Marcus-Sekura C. J. Techniques for using antisense oligodeoxyribonucleotides to study gene expression. Anal Biochem. 1988 Aug 1;172(2):289–295. doi: 10.1016/0003-2697(88)90447-2. [DOI] [PubMed] [Google Scholar]
- Panneerselvam C., Haritos A. A., Caldarella J., Horecker B. L. Prothymosin alpha in human blood. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4465–4469. doi: 10.1073/pnas.84.13.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer K. F., Ingles C. J., Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990 Jun 28;345(6278):783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Antimessenger oligodeoxyribonucleotides: an alternative to antisense RNA for artificial regulation of gene expression--a review. Gene. 1988 Dec 10;72(1-2):51–58. doi: 10.1016/0378-1119(88)90127-8. [DOI] [PubMed] [Google Scholar]
- Zhao L. J., Padmanabhan R. Nuclear transport of adenovirus DNA polymerase is facilitated by interaction with preterminal protein. Cell. 1988 Dec 23;55(6):1005–1015. doi: 10.1016/0092-8674(88)90245-0. [DOI] [PubMed] [Google Scholar]