Abstract
Venom composition of parasitoid wasps attracts increasing interest – notably molecules ensuring parasitism success on arthropod pests – but its variation within and among taxa is not yet understood. We have identified here the main venom proteins of two braconid wasps, Psyttalia lounsburyi (two strains from South Africa and Kenya) and P. concolor, olive fruit fly parasitoids that differ in host range. Among the shared abundant proteins, we found a GH1 β-glucosidase and a family of leucine-rich repeat (LRR) proteins. Olive is extremely rich in glycoside compounds that are hydrolyzed by β-glucosidases into defensive toxic products in response to phytophagous insect attacks. Assuming that Psyttalia host larvae sequester ingested glycosides, the injected venom GH1 β-glucosidase could induce the release of toxic compounds, thus participating in parasitism success by weakening the host. Venom LRR proteins are similar to truncated Toll-like receptors and may possibly scavenge the host immunity. The abundance of one of these LRR proteins in the venom of only one of the two P. lounsburyi strains evidences intraspecific variation in venom composition. Altogether, venom intra- and inter-specific variation in Psyttalia spp. were much lower than previously reported in the Leptopilina genus (Figitidae), suggesting it might depend upon the parasitoid taxa.
Hymenopteran parasitoids represent 10 to 20% of all insect species, being as such one of the largest group of venomous organisms. They develop on (ectoparasitoids) or inside (endoparasitoids) other arthropods, consuming their tissues and ultimately killing the host. They are thus important regulators of arthropod populations in nature, and used as pest control auxiliaries in agriculture1. One of the challenges faced by endoparasitoids is overcoming the immune response of the host, i.e. the formation of a multicellular, melanized capsule around the parasitoid egg2. To ensure successful parasitism, endoparasitoids have thus evolved original strategies, the most common being the injection with the egg of various components that manipulate the host physiology (i.e. immunity, metabolism, reproduction, molting) and behaviour (i.e. movements, feeding). These components are often a complex mixture of ovarian and venom proteins3,4, but they also include virus-like particles (VLPs)5 or wasp-specific polydnaviruses (PDVs)6.
Broad studies reporting transcriptomic and/or proteomic analyses have recently improved our knowledge of venom composition in different parasitoid families, evidencing its high complexity and diversity3,4. Indeed, although some venom proteins – e.g. serine proteases, metalloproteases or esterases – are largely shared by parasitoids, others seem specific to a parasitoid group or even species, and some are only found in a few phylogenetically distant species3. This suggests a rapid evolution of parasitoid venom composition, based on unidentified molecular mechanisms.
Strikingly, a high venom diversity was observed between the closely related Drosophila parasitoids Leptopilina boulardi and L. heterotoma (Hymenoptera, Figitidae), with none of the abundant venom proteins in common7. To assess whether this variation between two figitid species that differ in their host range similarly exists in other parasitoid taxa, we compared here the venom composition of two braconid wasps, Psyttalia lounsburyi and P. concolor (Hymenoptera, Braconidae, Opiinae) that belong to the same complex of species8. Both Psyttalia species are used as biological control agents of the olive fruit fly Bactrocera oleae9 and they differ in their host range. P. lounsburyi is specialized on B. oleae9 whereas P. concolor successfully develops in B. oleae and at least 13 other fruit fly species10. Comparison of P. lounsburyi and P. concolor venom was performed using a combined transcriptomic and proteomic approach, and it was extended at the intraspecific level using two geographically distant African strains of P. lounsburyi (South Africa and Kenya). We also compared data with large-scale venomics results from other braconids, either associated with PDVs, as Chelonus inanitus11 and Microplitis demolitor12, or devoid of PDVs, as Aphidius ervi13, since using various parasitism strategies could possibly impact venom evolution and composition.
This study will contribute to a better picture of the diversification of venom components at a short evolutionary scale, opening the way to the characterization of underlying mechanisms.
Results and Discussion
Structure of the Psyttalia venom apparatus
As typically observed for Braconidae, the venom apparatus of both Psyttalia species is composed of venom gland filaments (which secrete venom), a venom reservoir, and a venom duct that extends into the ovipositor (Fig. 1a). As was previously described in Opiinae14,15, P. lounsburyi and P. concolor venom gland is multi-lobed, each lobe displaying an external thick layer of tissue and a central lumen filled by a large volume of venom (Fig. 1b,c). The gland lobes are joined together at the base where the ovoid shaped reservoir is connected. The reservoir is composed of a large muscular layer surrounding a small internal volume of venom, suggesting it may serve as a “pump” for injecting venom at the time of oviposition rather than as a storage organ. The reservoir also shows internal structures that form intricate spirals possibly involved in maintaining the shape of the reservoir, like spiral springs, by passively counteracting the muscular contraction (Fig. 1b,c). At the base of the venom apparatus of P. concolor, we also observed a “round gland” filled with large vesicles (Fig. 1c), already described by Quicke14 and called “basal bulb” by Wharton15. Interestingly, the round gland and the intima spirals of the reservoir showed a strong endogenous green fluorescence (Fig. 1d), suggesting the presence of universal cellular auto-fluorophores such as NAD(P)H and flavins, pigments, or cuticular compounds16.
Figure 1. Microscopy observation of the venom apparatus of Psyttalia species.
Light microscopy: (a) P. lounsburyi female venom apparatus composed of a multi-lobed gland (G), a reservoir (R) and a long ovipositor (O); (b) Dissected P. lounsburyi venom gland showing the thick tissue envelope of the gland and the basal lateral branching of the reservoir; (c) P. concolor venom apparatus evidencing the small round gland at the base of the apparatus (Rg); (d) the same, overlaid with a fluorescence micrograph showing the green auto-fluorescence of the internal spirals of the reservoir and the small round gland. Bars = 100 μm.
No VLP or PDV in P. lounsburyi and P. concolor
Among the few studies on Psyttalia species, two had reported the occurrence of unidentified virus-like particles (VLPs) within the venom secretory cells (in P. concolor, previously Opius concolor17, and the close species O. caricivorae Fisher18). We thus performed electron microscopy on P. concolor venom glands to search for VLPs, as well as on P. lounsburyi and P. concolor ovaries to ensure the absence of PDVs (polydnaviruses). We did not observe VLPs or vesicular material resembling VLPs in venom gland cells or venom (Fig. 2a), nor viral structures or PDV particles in the ovarian cells and fluid close to the eggs (Fig. 2b–d). Accordingly, Pl and Pc transcriptomes contained no transcript having similarities with genes specific of nudiviruses (from which braconid PDVs derive) or the sister group of baculoviruses. As coronaviruses and cypo-like viruses were also described in P. concolor venom glands17, previous observations likely corresponded to small viruses infecting the reproductive tract of the samples, as reported also in other Hymenoptera19. In the absence of VLPs and PDVs, secreted venom proteins are likely the main maternal actors of parasitism success of Psyttalia species.
Figure 2. Microscopy observation of venom glands and ovaries from Psyttalia species.
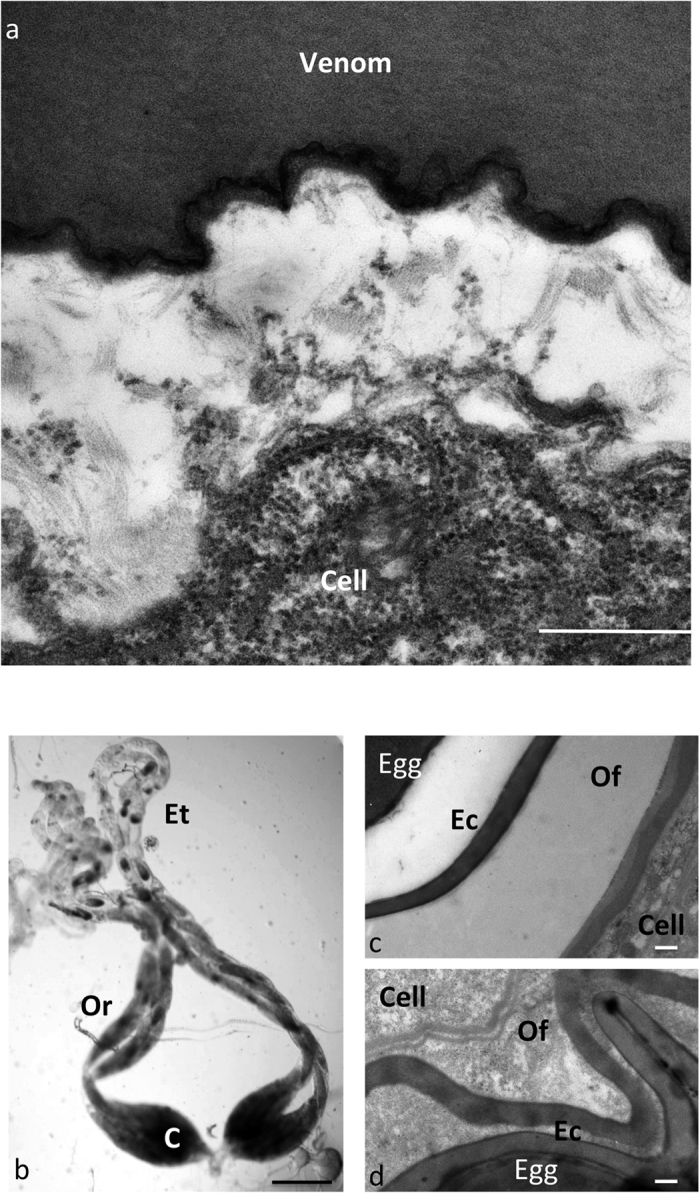
(a) Electron microscopy picture of a transversal section through a venom gland lobe of P. concolor, showing the rough endoplasmic reticulum-rich cytoplasm of the cell and the venom fluid. The empty space is due to retraction during dehydration. No vesicle is observed in the size range of the VLPs described in parasitoids venom (50 to 100 nm). Bar = 500 nm. (b) Picture of whole mounts P. concolor ovaries. Oogenesis occurs in egg tubes (Et), late oocytes being located in reservoirs (Or) and moving down to the calyx (C) where tubes fuse to form the oviduct. Bar = 500 μm. (c,d) TEM micrographs of sections through the calyx region of P. lounsbury (c) and P. concolor (d) ovaries, showing the egg chorion (Ec), the ovarian fluid (Of) and the calyx cells. No PDV particle is observed inside the cells nor in the fluid surrounding the egg chorion. Bar = 500 nm.
Comparison of electrophoretic profiles of Psyttalia venom proteins
Venom samples collected from 50 individuals of P. concolor (Pc) and the P. lounsburyi South African (PlSA) and Kenyan (PlK) strains were analyzed by 1D and 2D gel electrophoresis (Figs 3 and 4). On a 6–16% SDS-PAGE, the protein content of venom glands was resolved into numerous bands from 10 kDa to more than 200 kDa (Fig. 3). On the silver-stained 2D gels, 50 to 100 spots were clearly visible, having a 4 to 8.5–9 isoelectric point, and ranging from 10 to more than 120 kDa (Fig. 4). Some trains of spots were also observed that likely corresponded to post-translational modifications of the same protein.
Figure 3. 1D SDS-PAGE separation of P. lounsbury and P. concolor venom proteins.
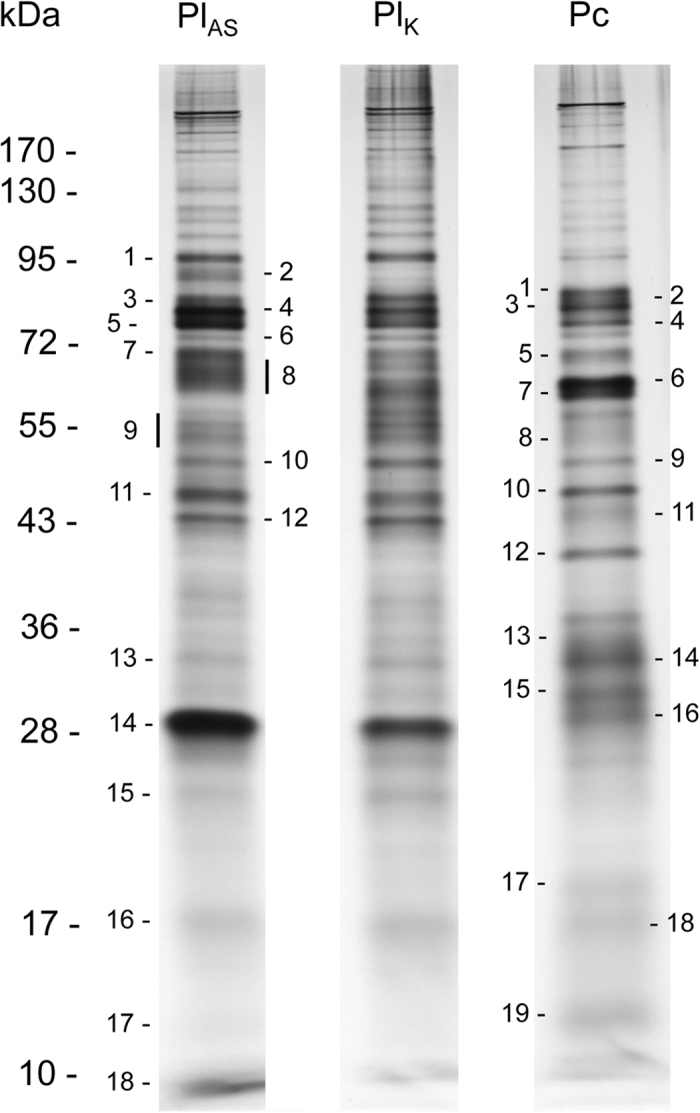
Venom proteins from 50 PlSA, PlK and Pc females were separated on a 6–16% SDS-PAGE under reducing conditions and visualized by silver staining. All stained protein bands numbered on the gel were excised and submitted for protein identification by LC-MS-MS. Molecular weight standard positions are indicated on the left (kDa).
Figure 4. 2D SDS-PAGE separation of P. lounsbury and P. concolor venom proteins.
Venom proteins from 50 PlSA (a), PlK (b) and Pc (c) females were separated by IEF followed by 6–16% SDS-PAGE. Following silver staining, the major spots (numbered) were cut and analyzed by LC-MS-MS. Spots for which a protein with a putative function was identified are indicated on the right. Molecular weight standard positions are indicated on the left (kDa).
The PlSA and PlK 1D electrophoretic profiles were very similar with presence/absence or intensity variation detected for only a few bands (Fig. 3). The distribution of 2D spots evidenced more differences, in particular the absence of the PlSA spot number 10 (55 kDa) in the PlK strain (Fig. 4a,b). We observed a greater variation at the interspecific level with several profile differences in the 25–35 kDa range (Figs 3 and 4).
Comparison of transcriptomic and proteomics data between Psyttalia wasps
All the major bands and spots on 1D and 2D electrophoretic profiles of Pl and Pc venom, and a number of minor spots (70 bands for Pl, 46 bands for Pc, from at least two 1D gels per species; 117 spots for Pl, 57 for Pc, from at least three 2D gels per species) were excised, and tryptic peptides were analyzed by LC-MS-MS. In parallel, a transcriptomic analysis of Pl and Pc venom glands was performed, based on Illumina sequencing. De novo assembly of the Pl transcriptome was improved using additional 454 (full body) and Sanger (venom apparatus) sequence data (Supplementary Figure S1) and it yielded a total of 16,943 and 16,360 unisequences for Pl and Pc, respectively (Supplementary Table S1). Data suggested a similar quality of the transcriptomes, based on general features and similarity searches (Supplementary Table S1), as well as GO terms comparison (Supplementary Figure S2).
The combined transcriptomic and proteomic data resulted in 39 and 40 unisequences for Pl and Pc, respectively, among which 32 and 36 had a putative function (Supplementary Tables S2 and S3). Some of these, such as actin-5C or glyceraldehyde-3-phosphate dehydrogenase 2, were typical cellular proteins with no predicted signal peptide (Supplementary Tables S2 and S3). Whether their identification in venom is due to cellular damage during collection, or these proteins are actually secreted by non-canonical export mechanisms remains unclear. Therefore, we only considered as putative venom proteins the unisequences (i) found in venom proteomics, and (ii) either predicted to be secreted or for which the presence of a signal peptide was not tested due to the incompleteness of the sequence. This resulted in a total of 32 and 30 putative venom proteins for Pl and Pc respectively (Tables 1 and 2), whose relative abundance was compared using (i) the RPKM normalized number of Illumina reads from Pl and Pc venom apparatus, mapped to the assembled transcriptomes and (ii) the number of peptides matches in Mascot searches.
Table 1. Putative P. lounsburyi venom proteins classified based on RPKM values.
| Rank | Sequence | RPKM | Mascot | Putative function | Signal peptide | Homolog in P. concolor |
Homolog in venom of other Braconidae | |
|---|---|---|---|---|---|---|---|---|
| Sequence | Rank | |||||||
| 1 | Pl_004867 | 1963.84 | 71 | Yes | Pc_009390 | 3 | ||
| 2 | Pl_009581 | 1865.04 | 11 | Leucine-rich repeat protein | Yes | Ae | ||
| 3 | Pl_011877 | 1538.27 | 54 | Yes | ||||
| 4 | Pl_014442 | 816.50 | 10 | ? | ||||
| 5 | Pl_010740 | 738.87 | 104 | DUF4803 domain-containing protein | Yes | Pc_014641 | 12 | Ci, Md, Mh |
| 6 | Pl_011340 | 691.22 | 37 | DUF4803 domain-containing protein | Yes | Pc_010911 | 10 | Ci, Md, Mh |
| 7 | Pl_002959 | 659.87 | 5 | Yes | ||||
| 8 | Pl_006410 | 627.83 | 37 | Neprilysin-like metalloprotease | Yes | Pc_006098 | 8 | Mh |
| 9 | Pl_003816 | 574.51 | 33 | DUF4803 domain-containing protein | Yes | Pc_013625 | 1 | Ci, Md, Mh |
| 10 | Pl_006199 | 476.04 | 153 | DUF4803 domain-containing protein | Yes | Pc_014641 | 12 | Ci, Md, Mh |
| 11 | Pl_002819 | 272.37 | 93 | GH1 β-glucosidase | ? | Pc_001157 | 7 | Aeb, Mdc |
| 12 | Pl_010491 | 263.42 | 1 | Calreticulin | ? | Pc_015292 | 17 | Mh |
| 13 | Pl_002333 | 242.50 | 90 | Yes | Pc_006379 | 4 | ||
| 14 | Pl_014829 | 186.32 | 8 | Reprolysin-like metalloprotease | ? | Ci, Md | ||
| 15 | Pl_002212 | 143.39 | 3 | Yes | ||||
| 16 | Pl_006057 | 135.90 | 1 | Esterase/lipase-like | ? | Ci, Md | ||
| 17 | Pl_013024 | 115.21 | 10 | Neprilysin-like metalloprotease | ? | Pc_006098 | 8 | Mh |
| 18 | Pl_014435 | 112.79 | 0d | Protein disulfide isomerase | Yes | Pc_014697 | 15 | Ae |
| 19 | Pl_014734 | 85.45 | 24 | Heat shock protein 70 | ? | Pc_008008 | 18 | Aeb |
| 20 | Pl_002507 | 63.77 | 3 | Protein disulfide isomerase | Yes | Aeb | ||
| 21 | Pl_008373 | 40.85 | 8 | Endoplasmin | Yes | Ae | ||
| 22 | Pl_003563 | 35.95 | 6 | DUF4803 domain-containing protein | Yes | Pc_002889 | Ci, Md, Mh | |
| 23 | Pl_011829 | 22.74 | 16 | Protein disulfide isomerase | Yes | Pc_010489 | 19 | Aeb |
| 24 | Pl_001931 | 21.08 | 6 | Puromycin-sensitive aminopeptidasea | ? | |||
| 25 | Pl_007984 | 17.02 | 2 | Enolase | ? | Pc_009146 | 21 | |
| 26 | Pl_011015 | 6.13 | 5 | Arginine kinase-like protein | ? | |||
| 27 | Pl_010999 | 2.56 | 13 | ? | ||||
| 28 | Pl_009261 | 2.43 | 4 | Esterase/lipase-like | ? | |||
| 29 | Pl_000063 | 2.28 | 14 | Serpin | ? | Pc_007867 | 24 | Ae, Md |
| 30 | Pl_012461 | 2.02 | 6 | Leucine rich repeat protein | ? | Ae | ||
| 31 | Pl_013792 | 1.47 | 2 | Neprilysin-like | Yes | Ae, Mh | ||
| 32 | Pl_004270 | 1.31 | 5 | Glycogen phosphorylasea | ? | |||
Ae, Aphidius ervi13. Ci, Chelonus inanitus11. Md, Microplitis demolitor12. Mh, Microctonus hyperodae20. Abundant proteins (RPKM > 50 and Mascot matches > 10) are in italics.
aUnisequences for which secretion could not be predicted and that are typical cellular proteins.
bProteins identified in the analysis of A. ervi venom apparatus but not considered as venom proteins due to a highly conservative approach.
cSee Burke & Strand12 (Supplementary Table 2, locus comp21422_c0).
dProtein not found in the proteomic analysis but with RPKM > 50 and for which a homolog was found in P. concolor.
Table 2. Putative P. concolor venom proteins classified based on RPKM values.
| Rank | Sequence | RPKM | Mascot | Putative function | Signal peptide | Homolog in P. lounsburyi | Homolog in venom of other Braconidae | |
|---|---|---|---|---|---|---|---|---|
| Sequence | Rank | |||||||
| 1 | Pc_013625 | 3110.46 | 59 | DUF4803 domain-containing protein | ? | Pl_003816 | 9 | Ci, Md, Mh |
| 2 | Pc_015919 | 1626.40 | 12 | ? | ||||
| 3 | Pc_009390 | 1583.31 | 210 | Yes | Pl_004867 | 1 | ||
| 4 | Pc_006379 | 1507.73 | 8 | ? | Pl_002333 | 13 | ||
| 5 | Pc_012023 | 1428.59 | 34 | ? | ||||
| 6 | Pc_012375 | 1326.30 | 8 | Reprolysin-like metalloprotease | ? | Ci, Md | ||
| 7 | Pc_001157 | 1196.52 | 84 | GH1 β-glucosidase | Yes | Pl_002819 | 11 | Aeb, Mdc |
| 8 | Pc_006098 | 1178.46 | 47 | Neprilysin-like metalloprotease | ? | Pl_013024 | 17 | Mh |
| 9 | Pc_014667 | 1152.41 | 9 | DUF4803 domain-containing protein | Yes | Ci, Md, Mh | ||
| 10 | Pc_010911 | 947.92 | 71 | DUF4803 domain-containing protein | Yes | Pl_011340 | 6 | Ci, Md, Mh |
| 11 | Pc_002246 | 580.77 | 3 | Phospholipase A2 | ? | Md | ||
| 12 | Pc_014641 | 441.52 | 57 | DUF4803 domain-containing protein | Yes | Pl_010740 | 10 | Ci, Md, Mh |
| 13 | Pc_007330 | 360.78 | 16 | Annexin | Yes | |||
| 14 | Pc_009900 | 346.13 | 5 | Serine carboxypeptidase | Yes | Md | ||
| 15 | Pc_014697 | 259.63 | 32 | Protein disulfide isomerase | Yes | Aeb | ||
| 16 | Pc_002889 | 243.19 | 2 | DUF4803 domain-containing protein | Yes | Pl_003563 | Ci, Md, Mh | |
| 17 | Pc_015292 | 236.32 | 3 | Calreticulin | Yes | Pl_010491 | 12 | Mh |
| 18 | Pc_008008 | 227.98 | 43 | Heat shock protein 70 | Yes | Pl_014734 | 18 | Aeb |
| 19 | Pc_010489 | 199.42 | 13 | Protein disulfide isomerase | Yes | Pl_011829 | 22 | Aeb |
| 20 | Pc_009911 | 102.94 | 1 | Leucine-rich repeat protein | Yes | Ae | ||
| 21 | Pc_007769 | 60.85 | 0d | Protein disulfide isomerase | ||||
| 22 | Pc_009146 | 53.51 | 9 | Enolase | ? | Pl_007984 | 24 | |
| 23 | Pc_015675 | 39.64 | 3 | Leucine-rich repeat protein | Yes | Ae | ||
| 24 | Pc_002924 | 36.88 | 3 | Ezrin/radixin/moesin familya | ? | |||
| 25 | Pc_007867 | 32.85 | 12 | Serpin | Yes | Pl_000063 | 28 | Ae, Md |
| 26 | Pc_000616 | 29.37 | 5 | Neprilysin-like metalloprotease | ? | Mh | ||
| 27 | Pc_016110 | 12.69 | 4 | Aldehyde dehydrogenasea | ? | |||
| 28 | Pc_005686 | 5.03 | 1 | Leucine-rich repeat protein | ? | Ae | ||
| 29 | Pc_007684 | 4.49 | 1 | Leucine-rich repeat protein | ? | Ae | ||
| 30 | Pc_009846 | 2.31 | 3 | Adenosylhomocysteinasea | ? | |||
Ae, Aphidius ervi13. Ci, Chelonus inanitus11. Md, Microplitis demolito12r. Mh, Microctonus hyperodae20. Abundant proteins (RPKM > 50 and Mascot matches > 10) are in italics.
aUnisequences for which secretion could not be predicted and that are typical cellular proteins.
bProteins identified in the analysis of A. ervi venom apparatus but not considered as venom proteins due to a highly conservative approach.
cSee Burke & Strand12 (Supplementary Table 2, locus comp21422_c0).
Protein not found in the proteomic analysis but with RPKM > 50 and for which a homolog was found in P. lounsburyi.
Interestingly, most of the proteins identified in the proteomics of the reservoir (detection of the most abundant putative venom proteins only, data not shown), such as actin or paramyosin, had a predicted muscular function, as expected from microscopy observations (see above; Fig. 1).
Global analysis of Psyttalia wasps venom proteins and comparison with other wasps
Comparison of venomics data from the two Psyttalia species evidenced that 47% and 43% of the proteins identified in Pl and Pc were shared with the other species, respectively (Tables 1 and 2; Fig. 5a). In comparison, L. boulardi and L. heterotoma shared less than 20% of the identified venom proteins7. When considering only the most abundant venom proteins (RPKM > 50 and Mascot matches > 10), 9 and 8 out of the 11 Pl and 12 Pc proteins, respectively, were shared between P. concolor and P. lounsburyi (Fig. 5b; Tables 1 and 2) whereas the two Leptopilina species had no protein in common7. Finally, 20 Pl and 21 Pc venom proteins (63% and 70%) had already been identified in the venom of another braconid species (Fig. 5c; Tables 1 and 2).
Figure 5. Venn diagrams showing the number of analyzed venom proteins shared between the following species.
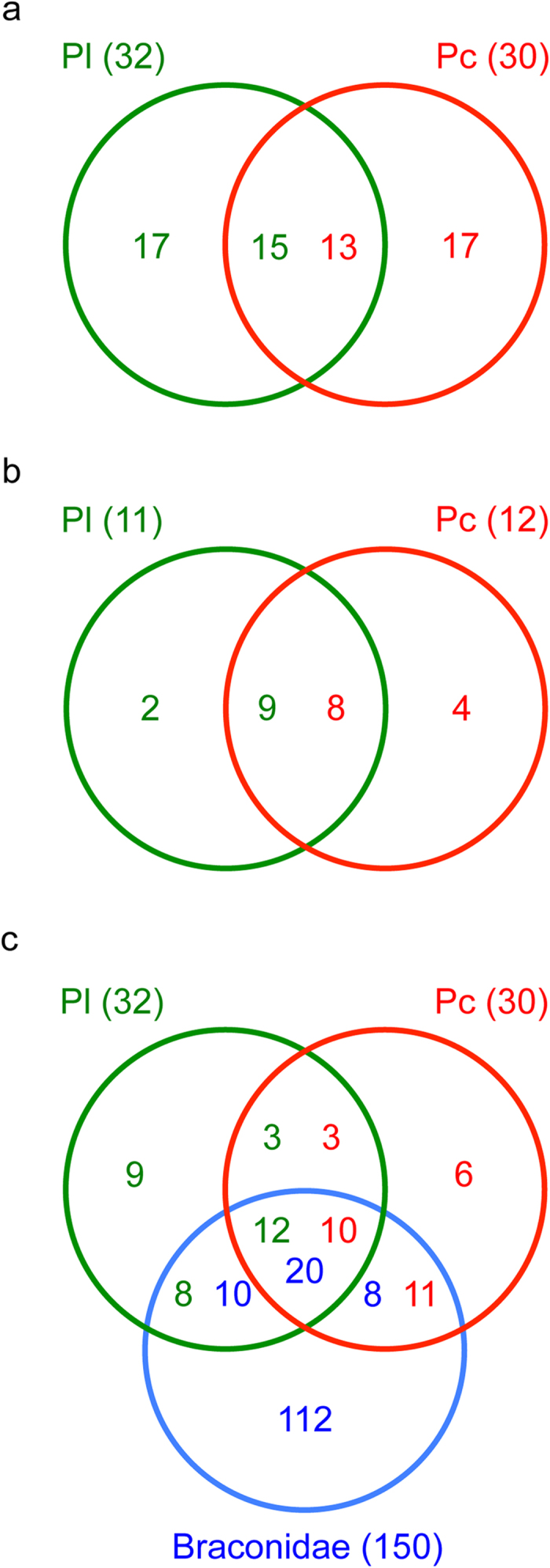
(a) P. lounsburyi and P. concolor; (b) P. lounsburyi and P. concolor, considering only the abundant proteins (RPKM > 50 and Mascot matches > 10); (c) P. lounsburyi, P. concolor and other Braconidae species.
Identified venom proteins
Putative venom proteins described below are classified based on their abundance in venom (RPKM values and Mascot matches; Tables 1 and 2) and their occurrence in the venom of (i) both Psyttalia species, (ii) P. lounsburyi only and (iii) P. concolor only. Venom proteins with a putative function are described in Table 3 (with the proposed biochemical function, previous identification in parasitoid venom, and demonstrated or proposed role in parasitism success). Several proteins with low RPKM values and lacking N-terminal sequence were not considered since they were typical cellular proteins and the number of proteomic matches was low (Tables 1 and 2).
Table 3. Psyttalia venom proteins with a putative function: Biochemical function, occurrence in venom of parasitoids and previously demonstrated or proposed role in parasitism.
| Protein function | General properties and comments |
|---|---|
| Annexin | Annexins are a family of Ca2+-dependent lipid binding proteins believed to be engaged in membrane transport processes, although recent work suggests a more complex set of functions. Annexins normally lack signal sequences for secretion, but some members of the family have been identified extracellularly where they can act as receptors44. Annexins had never been described so far in the venom of parasitoids. However, some data suggest that different mammalian parasite clades possess annexins with unique properties that can be secreted and are likely involved in host-parasite interactions and host immune-modulation45. Moreover, some parasitic nematodes secrete an annexin-like effector into host root cells that may mimic plant annexin function during the parasitic interaction46. At last, it has been shown that annexins are also involved in the binding and internalization of toxins in eukaryotic cells47. |
| Arginine kinase | Arginine kinase plays a crucial role in the energy metabolism of insects and other invertebrates through the use of ATP to catalyze the phosphorylation of arginine in phosphoarginine. This enzyme was detected in the venom of Pteromalus puparum48 and Leptomastix dactylopii49, but its role in the host-parasitoid interaction is unknown. |
| Calreticulin | Calreticulin is a calcium (Ca2+)-binding protein with multifunctional properties including chaperone functions50. Calreticulin was shown to inhibit host cell encapsulation in Cotesia rubecula51 and P. puparum52, although the mechanism is still unclear. Calreticulin was found in the venom of several phylogenetically distant species4 and seems thus largely shared among parasitoids. |
| Endoplasmin | Endoplasmin (alternative names: HSP90B1, GP96, GRP-94), which belongs to the heat shock protein 90 family, is a molecular chaperone located in the ER and involved in the final processing and export of secreted proteins53. Among parasitoids, endoplasmin has only been detected so far in the venom gland of Aphidius ervi13. This venom protein was suggested to play a role in the secretion, stabilization, transport and host cell targeting of the different A. ervi venom proteins. |
| Enolase | Enolase is a key enzyme in cell metabolism which is also associated with virulence of several pathogens54. An extracellular enolase was recently described in the oviposition injecta from A. ervi55 and the venom of Toxoneuron nigriceps56. Enolase is also released by teratocytes surrounding the A. ervi embryo57. This enzyme was proposed to play an important role in the regulation of the host physiology and the host nutritional exploitation57,58. |
| Esterase/lipase-like | Esterases and lipases belong to a superfamily of hydrolytic enzymes that act on carboxylic esters59. Proteins belonging to this functional class were previously found in the venom of several phylogenetically distant species3,4 and appear to be common in parasitoids. The functions of these hydrolase enzymes in host-parasitoid interactions have not been investigated yet. |
| GH1 β-glucosidases | GH1 β-glucosidases are a family of enzymes found from bacteria to mammals that hydrolyze glycosidic bonds from glycosides and oligosaccharides, and remove non reducing terminal glucosyl residues60. Among parasitoids, a β-glucosidase enzymatic activity was detected in the venom of Pimpla hypochondriaca61. A member of this enzyme family was also recently identified, but not abundant, in the venom of Microplitis demolitor12 and, in a low quantity, in a transcriptomic study of the A. ervi venom apparatus13. GH1 β-glucosidases include myrosinases that play a central role in the glucosinolate-myrosinase system, one of the best-studied activated plant defense system33. Some insects have co-opted this system to defend themselves against enemies, by sequestering plant-derived glucosinolates and producing their own myrosinase-like enzyme36,37,38. |
| Heat shock protein 70 | Heat shock proteins 70 (Hsp70; alternative name: GRP-78) are a family of chaperones with distinct sub-cellular localization and function62. An Hsp70 protein was identified in the venom of the parasitoid P. puparum48 whose function remains to be elucidated. |
| Leucine-rich repeat protein | Leucine-rich repeats (LRRs) are motifs involved in protein-protein interactions63. LRRs are generally composed of 20-29 amino acid stretches rich in leucine. To our knowledge, LRR domain-containing proteins were only identified in the venom of A. ervi until now13. They were suggested to act as scavengers for the pea aphid Toll-like receptors, thus impairing the host immune response via the Toll pathway. |
| Neprilysin-like metalloprotease | Neprilysin-like (NEP) proteins are zinc-dependent metalloproteases belonging to the M13 peptidase family. They are involved in the degradation of a number of regulatory peptides in the nervous or immune system of mammals64 and insects65. Although they are typically membrane-bound, ectopeptidases such as NEP may also be shed from the membrane through a proteolytic process and found in the surrounding fluid66. NEP-like proteins were detected in the venom of the Braconidae A. ervi13, Microctonus hyperodae20, M. demolitor12 and T. nigriceps56, as well as of the Figitidae Leptopilina boulardi7. They were also found associated with the VLPs produced in the ovary of Venturia canescens67. NEP-like proteins have been hypothesized to modulate the host immune system by degrading immune-specific peptides67. |
| Phospholipase A2 | Secreted phospholipases A2 (PLA2s) are a family of relatively stable enzymes found in venoms. PLA2 has in vitro and in vivo immunomodulatory effects in bee venom68, and neurotoxic and myotoxic effects in snake venoms69. This enzyme was recently detected in the venom of M. demolitor12 and T. nigriceps56, but its function in the host-parasitoid interaction is unknown. |
| Protein disulfide isomerase | Protein disulfide isomerases (PDIs) are enzymes involved in the folding and stabilizing of nascent polypeptides in the endoplasmic reticulum (ER) through catalysis of disulfide bond formation and isomerization70. Although this protein is normally recycled back to the ER from the Golgi via its C-terminal KDEL motif, secreted PDIs can escape this turnover mechanism71. Among parasitoids, PDIs have only been detected so far in the venom gland of A. ervi13. They have a broad substrate specificity and are involved in the folding of toxin peptides in different venomous organisms72,73. |
| Reprolysin-like metalloprotease | Reprolysin-like (REP) proteins are zinc-dependent metalloproteases belonging to the M12 peptidase family, commonly found as constituents of snake venom. They were previously detected in the venom of the parasitoids Pimpla hypochondriaca22, Eulophus pennicornis23, Chelonus inanitus11, M. demolitor12 and T. nigriceps56. A recombinant E. pennicornis venom REP-like protein was demonstrated to display toxicity toward the host and to manipulate host development23. |
| Serine carboxypeptidase | Classical serine carboxypeptidases are enzymes that hydrolyze a peptide bond at the C-terminal end of peptides and proteins. A related enzyme (Scpep1) that do not show proteolytic activity but is involved in other functions was described in mice74. To our knowledge, serine carboxypeptidase has only been identified so far in the venom of M. demolitor12 and T. nigriceps56. Interestingly, serine carboxypeptidases have also been described as candidate virulence factors in several pathogens75. |
| Serpin | Serpins (serine protease inhibitors) are a large family of functionally diverse protease inhibitors. They share a conserved structural architecture with an exposed reactive center loop (RCL) of about 20 amino acids, which acts as bait for target serine proteases76. The L. boulardi venom serpin LbSPNy was previously shown to be involved in host immune suppression77: it interferes with melanization in the Drosophila host through inhibition of the phenol oxidase activation. More recently, serpins were described in the venom of A. ervi13 and M. demolitor12 but their role in parasitism success is unknown. |
Proteins identified in the venom of both Psyttalia species
Proteins of unknown function
Several abundant unisequences with no predicted function were characterized by a high number of matches (Tables 1 and 2) and rather intense protein spots (Fig. 4). Among these, a family of five related proteins contains a signal peptide and the DUF4803 domain of unknown function (Supplementary Figure S3). They share similarities with venom proteins of the braconids C. inanitus11, M. demolitor12 and M. hyperodae20, and of N. vitripennis21.
Leucine-rich repeat protein
Two and four unisequences encoding leucine-rich repeat (LRR) proteins were identified in Pl and Pc, respectively (Tables 1 and 2; Supplementary Tables S2 and S3). One of these (Pl_009581) was highly abundant (rank 2) in the P. lounsburyi SA strain only, and it corresponded to one of the most intense spot (spot 10, Fig. 4A). The complete unisequences contain a N-terminal signal peptide and 9 to 19 canonical LRR motifs similar to the LRR motif in Toll-like receptors (TLRs). Yet, the majority of TLRs are multidomain proteins while Psyttalia predicted proteins only contain the LRR domain, as already observed for A. ervi venom LRR proteins13. As suggested for A. ervi, Psyttalia truncated LRR proteins might interfere with the host immune response by targeting the Toll pathway.
Neprilysin-like metalloproteases
Three and two unisequences encoded neprilysin-like (NEP) zinc-dependent metalloproteases in Pl and Pc, respectively (Tables 1 and 2; Supplementary Tables S2 and S3), one of which was in high abundance (rank 8; 37 and 47 peptide matches in Pl and Pc venom, respectively). NEP-like proteins occur in the venom of several parasitoid wasps (Table 3).
Another zinc-dependent metalloprotease was identified in each Psyttalia wasp, with low inter-species sequence similarity. Both proteins are weakly related to venom reprolysin-like proteins of P. hypochondriaca22 and Eulophus pennicornis23. However, the sequences were incomplete and the number of matches in the venom was rather low (Tables 1 and 2; Supplementary Tables S2 and S3).
GH1 β-glucosidases
Peptides from major 2D spots at 55–60 kDa (spots 6 and 9 in PlK and PlSA, respectively, spots 8, 9, 10 in Pc) matched with two unisequences (one for each Psyttalia species) that encoded proteins containing a glycosyl hydrolase family 1 (GH1) domain (pfam00232) found in GH1 β-glucosidases (Fig. 4). The high RPKM values and number of peptides matches confirmed that the Pl_002819 and Pc_001157 unisequences, that share 97% identity (Supplementary Figure S4), were among the most abundant in venom (Tables 1 and 2; Supplementary Tables S2 and S3). The Pc sequence seems full-length, with a predicted signal peptide of 18 residues, while the Pl sequence probably lacks the N-terminal part (Supplementary Figure S4). The 56.5 kDa predicted MW of the mature protein is close to that of the observed spot on 2D gels, suggesting none or few glycosylation, although several N-glycosylation sites are predicted. Yet, several spots were observed, having different isoelectric points, suggesting post-translational modification(s) and thus several isoforms (Fig. 4). Two Pl and one Pc additional unisequences, not found in proteomics, shared similarities with GH1 β-glucosidases, suggesting occurrence of a multigene family (Supplementary Figure S5). GH1 β-glucosidases were previously identified in venomics data from M. demolitor and A. ervi, but they did not correspond to abundant proteins (Tables 1, 2 and 3). Finally, the venom of the ichneumonid Pimpla hypochondriaca was reported to display β-glucosidase enzymatic activity (Table 3).
GH1 β-glucosidases are found from bacteria to mammals. They play an essential role in the metabolism of glycolipids and exogenous glycosides by hydrolyzing glycosidic bonds and removing non-reducing terminal glucosyl residues from saccharides and glycosides. This enzyme family includes for instance the myrosinases, well-known for their role in the “glucosinolate-myrosinase” plant defense system (Table 3), and also identified in the cabbage aphid Brevicoryne brassicae that feed on crucifers24.
The alignment of Pl and Pc venom unisequences with that of well-described plant myrosinase (Sinapsis alba) and insect β-glucosidases (B. brassicae, Phyllotreta striolata, Spodoptera frugiperda) shows conservation of all critical enzymatic site residues25,26, suggesting that Psyttalia enzymes are indeed functional (Fig. 6). Insect β-glucosidases differ from plant myrosinases in that they have two glutamates in the catalytic site instead of one glutamate and one glutamine. The possible role of Psyttalia venom GH1 β-glucosidase in host-parasitoid interaction is discussed below.
Figure 6. Multiple alignment of GH1 β-glucosidase sequences.
Identical and similar residues are highlighted in black and grey, respectively. Catalytic residues are printed in white on a red background. Ligands of the Zn2+ ion are printed in white on a green background. Residues involved in glucose-ring recognition are printed in white on a blue background. S_alba, S. alba (1MYR_A); B_brassicae, Brevicoryne brassicae (1WCG_A); P_striolata, Phyllotreta striolata (AHZ59651); S_frugiperda, Spodoptera frugiperda (5CG0_A); P_concolor, P. concolor (Pc_001157); P_lounsburyi, P. lounsburyi (Pl_002819).
Heat shock proteins (HSPs)
Molecular chaperones belonging to the heat shock protein (HSP) class, including HSP70, calreticulin and protein disulfide isomerase (PDI), were found in variable amounts in the two species (Tables 1 and 2; Supplementary Tables S2 and S3). Endoplasmin, also identified in transcriptomic data from both species (RPKM values of 40.85 for Pl and 63.9 for Pc) was detected in Pl venom only, suggesting a lower abundance in Pc venom (Tables 1 and 2; Supplementary Tables S2 and S3). In the endoplasmic reticulum (ER), all these HSPs cooperate to form multi-chaperone complexes involved in the folding and export of secreted proteins. HSP70, PDI and endoplasmin are normally retained in the ER through the binding to specific receptors via a K/R/HDEL motif. Since these proteins are abundant in cells, we cannot totally exclude that their detection in the venom results from tissue contamination during venom collection from the gland. However, one of the venom PDIs, identified in both species, contains a substitution that changes the HDEL motif into a SDEL motif, suggesting a loss of binding and then of retention in the ER. Moreover, the binding of HSPs to ER receptors is labile and the retention is not absolute, allowing HSPs to be secreted and to play a role in intercellular transport and signaling27,28. Endoplasmin, for example, has been associated as a chaperone with the secretion of pancreatic lipases and their further internalization by intestinal cells in rat29.
Venom HSPs may thus play a role in stabilization of other venom proteins and/or their transport and targeting of host cells. Among these, endoplasmin is of particular interest because it is a master chaperone for TLRs, a family of LRR domain-containing proteins30 of which members were found in Psyttalia venom (see above). In accordance with a possible role of endoplasmin as a chaperone of venom TLRs, endoplasmin was only detected in the venom of Pl that contains higher amounts of LRR proteins than the Pc venom (Tables 1 and 2; Supplementary Tables S2 and S3). Interestingly these two proteins are also secreted in the venom of the braconid A. ervi13.
PDIs play a central role in the protection of disulfide bounds of secreted proteins31. Interestingly, the rapid expansion of a large gene family encoding PDIs specifically-expressed in the venom glands of cone snails has been recently suggested to facilitate the folding of the numerous conotoxins produced by these marine predators32. Here, however, phylogenetic analysis shows that Psyttalia venomous PDI sequences group with different PDIs from braconid wasps (Supplementary Figure S6), suggesting that no specific expansion occurred.
Proteins of low abundance
Proteins with similarities with serpin and enolase (Tables 1 and 2; Supplementary Tables S2 and S3) were identified. The Pl and Pc serpin sequences shared 94% identity but only the Pc sequence was complete, the Pl serpin lacking the N- and C-termini (Supplementary Figure S7). The Pc serpin contains a signal peptide as well as the consensus hinge sequence essential for the conformational change involving the RCL and necessary to inhibit the target protease (Supplementary Figure S7). An extracellular enolase was already identified in A. ervi injecta and also produced by specialized extra-embryonic cells (the teratocytes) and suggested to be involved in nutritional host exploitation (Table 3).
Proteins detected in P. lounsburyi venom only
Proteins of unknown function
Among the five different unisequences identified (Table 1; Supplementary Table S2), Pl_011877 and Pl_014442 have the higher RPKM values, and the predicted proteins have a low MW (less than 10 kDa). Abundant transcripts encoding low MW toxin-like peptides were previously identified in A. ervi venom13 but no toxin-like signature was found in Pl venom proteins.
Proteins of low abundance
All the other proteins found in P. lounsburyi venom only were in low abundance (Table 1; Supplementary Table S2). Among these, an arginine kinase-like protein was identified, and similarities were found with members of the esterase/lipase-like superfamily, detected in many parasitic wasp species (Table 3).
Proteins detected in P. concolor venom only
Proteins of unknown function
Two different unisequences (Pc_015919 and Pc_012023) were detected (Table 2; Supplementary Table S3) but the sequences were not complete and the presence of a signal peptide could not be assessed. Pc_012023 (rank 5 based on RPKM values) was one of the most intense 2D spots at less than 15 kDa (Fig. 4). Pc_015919 was better ranked based on RPKM values, but the corresponding spot was less intense (Fig. 4) and the number of peptide matches was lower (Table 2; Supplementary Table S3), suggesting a lower abundance in venom.
Annexin
One unisequence, corresponding to 16 peptide matches, had similarities with annexins (Table 2; Supplementary Table S3). This protein contains a 20 aa N-terminal signal peptide followed by two annexin domains. Based on data from their role in mammalian parasites, P. concolor venom annexin might be involved in binding and cell internalization of other venom proteins (Table 3).
Proteins of low abundance
Among these, a secreted phospholipase A2 (PLA2s) and a retinoid-inducible serine carboxypeptidase (Table 2; Supplementary Table S3) were identified (Table 3).
Conclusions
Large scale combined “omics” studies have recently increased knowledge of the nature and diversity of the venom content of parasitoid wasps3,4. Yet, very few studies were designed to evaluate how far closely-related parasitoid species differ in venom composition. Venom glands of the Braconidae M. hyperodae and M. aethiopoides were shown to express a similar set of genes20 but analyses relied on less than ten genes, and proteomics data were only available for M. hyperodae. More recently, we evidenced striking differences in venom composition of the closely-related Figitidae L. boulardi and L. heterotoma7, Drosophila parasitoids differing in their host range. We observed here a rather similar protein composition of the venom of P. lounsburyi and P. concolor, albeit they also differ in their host specificity level. The host range is determined by both behavioral and physiological traits that include host choice and venom adequacy to the host (i.e. “virulence”). In some taxa, the diversity of venom composition could then be decoupled from the diversity of parasitized hosts in natura if specialization mainly relies on behavioral traits. For instance, although P. lounsburyi is a specialist of the olive fly in the field, it can develop in laboratory conditions on some non-natural hosts such as Ceratitis capitata. Similarly, although intraspecific variation in P. lounsburyi venom was notably evidenced for a LRR protein, the difference between Psyttalia strains from distant geographic origin was much lower than observed between L. boulardi strains7. Whether the level of diversity of venom differs among taxa (being lower in Braconidae than in Figitidae), or the extensive venom variation in Leptopilina wasps is specific of these genus/species remains an open and stimulating question.
Unfortunately, a majority of the most abundant unisequences, either common or specific to each Psyttalia species, had no predicted function. It is thus difficult to make assumptions about how these wasps counteract the host immune defense and regulate the host physiology, and whether they use similar mechanisms. Yet, the identification of a GH1 β-glucosidase as one of the most abundant venom protein in both species suggests a possible role of this protein in parasitoid wasp’s success. In plants, glycoside compounds and hydrolytic enzymes form a classic two-component defense system, with glycosides inducing biological effects after being activated by the enzymes. The vast array of secondary metabolites produced is used as a protection against phytophagous organisms and pathogens33,34. Hydrolysis-products can indeed be repellent or toxic to insects, nematodes, fungi and bacteria, and they also serve as volatile attractants for specialist herbivores and their parasitoids35.
Plant compounds and endogenous glycosidases are usually stored in separated compartments so that activation only occurs upon tissue damage. To reduce toxic effects, some phytophagous insects were shown to downregulate their gut glycosidases while others have even evolved their own glycosylation system to reglycosylate the produced aglycons. The neo-formed glycosides can then possibly be stored, similarly to the ingested plant glycosides that are indeed sequestered by several insect species. By doing so, these insects prevent the production of toxic compounds36,37,38, and some even use them for their own defense39.
The main Psyttalia hosts, B. olea and C. capitata, oviposit in developing olives and fruits that contain large quantities of various glycoside compounds. Olive is particularly rich in phenol-glucosides such as oleuropein, verbascoside and rutin, which accumulate during its development while B. olea is growing inside40. Oleuropein was shown to be converted into a toxic compound with a glutaraldehyde-like structure – a potent protein crosslinker – by a defense-related olive β-glucosidase41. Assuming that fly larvae use a sequestering mechanism to survive within fruits during development, the injection of Psyttalia venom β-glucosidase inside their body might result in a burst of toxic compounds that could weaken the host and increase the parasitoid probability of success. The release of sugar moieties from glycosides could also increase the amount of energy available for the developing parasitoid larvae. Although this is an attractive hypothesis, the fact remains that the alleged role of Psyttalia venom β-glucosidase is based on the sequestration of phenolic glycosides by the olive fly larvae, which has not been tested yet.
The importance of a tri-trophic understanding of plant-herbivore and herbivore-predator/parasitoid interactions is increasingly recognized. For instance, the role of glucosides/glucosinolates has started to be evaluated not only in insect-plant interactions but also for their cascading effects on the performance of herbivore enemies through metabolic impacts or emission of volatile products39. The report of the large production of a β-glucosidase in a parasitoid venom suggests that this enzyme might participate in parasitoid phenotypes such as counter-defense and parasitism success, in addition to its well-described defensive role in plants and herbivore insects.
Altogether, this study illustrates that parasitoid venom is a complex mixture of proteins whose relative abundance in a given species or group is still hardly explained. The study of a new species often reveals new types of abundant venom molecules, as exemplified here with the β-glucosidase, and it highlights the role of gene duplication in the rapid evolution of venom and acquisition of new features. Deciphering the role of major venom proteins in Psyttalia parasitism success, especially those with no predicted function, is a key challenge. This will require further exploration using techniques such as RNAi, demonstrated as an efficient approach for impairing the production of parasitoid venom proteins42.
Material and Methods
Biological material
The South African (SA) and Kenyan (K) strains of P. lounsburyi (Pl) were previously described43. They were reared on a laboratory strain of the fruit fly C. capitata under a 16:8 h light/dark cycle at 22 °C. The P. concolor (Pc) population was collected in 2010 in Sicily (Italy) and reared for one generation on C. capitata under the same conditions, prior to analysis. C. capitata is a natural host for P. concolor but it is used as a substitute host for P. lounsburyi due to the difficulties of rearing the main natural host, Bactrocera oleae (the olive fly).
Light, fluorescence, and transmission electron microscopy
Light and fluorescence microscopies were performed using epifluorescent microscopes fitted with differential interference contrast (DIC) optics (Imager Z1, Zeiss) fitted with a black and white camera (Axiocam MRm, Zeiss). Images were pseudo-colorized digitally (Adobe Photoshop).
For transmission electron microscopy (TEM), blocks were prepared from 10 ovaries or 10 venom glands per sample. Dissected samples were pooled into 100 μl of Ringer’s saline (KCl 182 mM; NaCl 46 mM; CaCl2 3 mM; Tris-HCl 10 mM) in a centrifuge vial on ice. An equivalent volume of fixative (4% glutaraldehyde (Sigma) in 0.2 M sodium cacodylate buffer, pH 7.2) was then added and the sample was kept for 24 h at 4 °C. Fixed samples were centrifuged (500 × g, 10 min) to pellet tissues and remove the fixative prior to post-fixation in 2% osmium tetroxide in cacodylate buffer. Following dehydration in graded series of ethanol solutions, samples were embedded in Epon. Sample sections were cut with a diamond knife using a LKB ultramicrotome, mounted on copper grids, stained with uranyl acetate and lead citrate, and observed with a Zeiss EM10CR electron microscope at 80 kV.
Total RNA isolation and cDNA library construction
The transcriptomic analysis was performed from samples of 100 Pl and 100 Pc venom glands using Illumina RNA-Seq. To improve de novo assembly for Pl, we also generated Sanger sequences from samples of 50 venom glands, and 454 sequences from full insect bodies of 85 males and 85 females obtained from six siblings (Supplementary Fig. S1). Pl and Pc venom glands were dissected in Ringer’s saline and stored at −80 °C. Total RNA was extracted using TRIzol Reagent (Invitrogen) according to manufacturer’s instructions, and quality was checked using an Agilent BioAnalyzer. cDNA library construction for Illumina RNA-Seq and 454 sequencing was performed by Beckman Coulter Genomics (USA). cDNA library used for Sanger sequencing was constructed from 1 μg of total RNA using the Creator SMART cDNA Library Construction Kit (Clontech). Ligation products were transformed into ElectroMax DH10 B Escherichia coli competent cells (Invitrogen).
Sequencing and assembly
Illumina RNA-Seq sequencing (HiSeq 2000, 2 × 75 pb), 454 sequencing (454 GS-FLX Titanium platform) and trimming were performed by Beckman Coulter Genomics. Quality of Illumina raw reads was controlled using FastQC software and reads were cleaned by removing low quality sequences and reads containing N or adaptor sequences. For Sanger sequencing, a total of 2,000 clones were analyzed by the Genoscope (CEA, Evry, France) on an ABI sequencer using the standard M13 forward primer and BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA, USA). Sanger ESTs were then trimmed using TIGR SeqClean software.
For each species, we performed de novo transcriptome assembly using Velvet/Oases assembler (https://www.ebi.ac.uk/~zerbino/oases/) after the filtering process of Illumina raw reads. The first assembly step used a multiple kmer approach with kmer size ranging from 45 to 65 (k = 45, 55, 65 and coverage = 2). A meta-assembly (kmeta = 51, coverage = 1) was then performed using all previously obtained transcripts (>100 bases long) (Supplementary Fig. S1). At both assembly steps, we used CD-HIT-EST to remove the shorter redundant transcripts entirely covered by other transcripts with more than 99% identity. Finally, a clustering of transcripts was performed using TIGR-TGICL. To improve the quality of the assembly for Pl, we included the cleaned 454 and Sanger sequences as long sequences (minimum size of 200 bases) or otherwise as short sequences, in addition to the short Illumina reads.
Sequence annotation and analysis
To identify similarities with known proteins, the unisequences were compared to NCBI non-redundant protein sequence database, UniProtKB/Swiss-Prot database, insect predicted proteome databases (Drosophila melanogaster v5.46 and Nasonia vitripennis v1.2) and all braconid venom proteins found in UniProtKB (96 venom proteins from 9 different braconid species), using blastx with a cut-off e-value of 1e-7. Comparisons with previously published venom gland transcriptomes of A. ervi13 and Leptopilina spp7 were performed using tblastx with a cut-off e-value of 1e-7. Search for nudivirus/baculovirus-related specific genes was performed using tblastn with a cut-off e-value of 1e-1.
ORF prediction and translation were performed with FrameDP (https://iant.toulouse.inra.fr/FrameDP/), signal peptide prediction with SignalP (http://www.cbs.dtu.dk/services/SignalP/), and prediction of N-Glycosylation sites with the NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/). Search for protein domains was achieved using PfamScan (ftp://ftp.sanger.ac.uk/pub/databases/Pfam/Tools/) and CD-Search against Conserved Domain Database (CDD) at NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). Identification of leucine-rich repeats (LRR) in protein sequences was done using LRRfinder (http://www.lrrfinder.com/).
Search for DUF4803 domain-containing, GH1 β-glucosidase and protein disulfide isomerase sequences was performed using blastp at NCBI (http://www.ncbi.nlm.nih.gov/blast/) and HMMsearch from the HMMER package (http://hmmer.org/). Pairwise and multiple amino acid sequence alignments were respectively obtained using the Needleman-Wunsch algorithm and MAFFT implemented in Geneious software (Biomatters). Phylogenetic analyses were performed using maximum likelihood (ML) with PhyML (http://phylogeny.lirmm.fr/). ProtTest was used to select the best fit model of amino acid substitution for ML phylogeny (https://github.com/ddarriba/prottest3).
Gene functions and GO terms were automatically assigned to the predicted proteins based on the identification of domains with PfamScan. Only the root domain of the hierarchical domain organization available from EBI was conserved. Comparison of GO terms between Pc and Pl unisequences and homogenization of the annotation level were performed using GO slim terms.
Differential expression analysis
For each species, we used bowtie (http://bowtie-bio.sourceforge.net/) to map back all input trimmed Illumina raw reads (minimum size of 30 bases) to the assembled transcriptome with up to 3 nucleotides mismatches allowed. To compare the unisequence expression levels, the number of mapped raw reads for each transcript was normalized with the RPKM (reads per kilobase per million reads), using the R package edgeR (https://bioconductor.org/packages/release/bioc/html/edgeR.html).
SDS-polyacrylamide gel electrophoresis of venom and protein identification
The proteomic analysis was performed independently on Pc and Pl wasp venom (Supplementary Fig. S1). Venom apparatus were dissected from 50 individuals per sample and glands were collected in 25–50 μl of Ringer’s saline supplemented with a protease inhibitor cocktail (Sigma). Glands were opened to release the venom and centrifuged for 5 min at 500 × g to remove residual tissues.
For 1D SDS-PAGE, samples were mixed with 4× Laemmli buffer containing β-mercaptoethanol (v/v) and boiled for 5 min. Proteins were then separated on a 6–16% linear gradient SDS-PAGE and the gel was silver stained as previously described7,13.
For isoelectric focusing (IEF), samples were prepared by boiling the protein solution for 5 min with 4% (v:v) of a denaturing solution (0.15 M dithioerythritol, 10% SDS). After cooling, the samples were mixed with an equal volume of a solution containing 9.2 M urea, 0.1 M dithioerythritol, and 2% CHAPS. IEF was performed using slab gel. Slab gels were made on glass tubes 14 cm in length (1.5 mm internal diameter) that were filled with 4% acrylamide, 9.2 M urea, 2% ampholytes [1% pH 3–10 (Pharmacia) and 1% pH 2–11 (Servalytes)], and 2% CHAPS. Isoelectric focusing was run in two steps: a first run at 20 mA, 0.1 W/tube, 700 V for a total of 10,000 V/h, followed by a second run at 20 mA, 0.1 W/tube, 3,000 V for a total of 2,000 V/h. For the second dimension, 6–16% linear gradient SDS-PAGE was used. IEF gels were incubated with 4x Laemmli buffer containing ß-mercaptoethanol and loaded on top of the 1D SDS-PAGE. After separation, proteins were silver stained as previously described7,13.
Identification of proteins by mass spectrometry was performed on 1D bands and 2D spots excised from the gels as previously described7,13. MS/MS data analysis was performed with the Mascot software (http://www.matrixscience.com) licensed in house using the combined Pc and Pl unisequences and non-redundant NR (NCBI). Data validation criteria were (i) one peptide with individual ion score above 50 (the Mascot significant identity threshold corresponding to p <0.05 is 45 in our case) or (ii) at least two peptides of individual ion score above 20 (corresponding to 1% probability that a peptide spectrum match is a random event). The Mascot score was calculated as -10*LOG10(P). The calculated FDR (based on an automatic decoy database search) ranged from 0 to 1.4% depending of the individual gel analysis. Mascot analysis was performed with a fragment ion mass tolerance of 0.30 Da and a parent ion tolerance of 0.30 Da. Carbamidomethyl of cysteine was specified in Mascot as a fixed modification, and oxidation of methionine as a variable modification. The maximum missed cleavage allowed was set to 2.
Additional Information
Accession codes: P. concolor and P. lounsburyi raw Illumina sequencing data are available in the GenBank Sequence Read Archive (SRA) database under the accessions SRR1593901, SRR1593902, SRR1593906 and SRR1593907, and raw 454 sequencing data for P. lounsburyi under the accession SRR1593908. All trimmed ESTs for P. lounsburyi can be found in the GenBank dbEST repository under the accessions: JZ818733 - JZ820447. The Transcriptome Shotgun Assembly (TSA) projects were deposited in GenBank under the accessions GCDX00000000 (P. concolor) and GCEQ00000000 (P. lounsburyi). Versions in this paper are the first versions GCDX01000000 and GCEQ01000000
How to cite this article: Mathé-Hubert, H. et al. Comparative venomics of Psyttalia lounsburyi and P. concolor, two olive fruit fly parasitoids: a hypothetical role for a GH1 β-glucosidase. Sci. Rep. 6, 35873; doi: 10.1038/srep35873 (2016).
Supplementary Material
Acknowledgments
We are grateful to M. Thaon for help in insect rearing, N. Ris and T. Malausa for fruitful discussions, M.C. Bon for providing P. lounsburyi samples, and K. Varikou and V. Caleca for providing P. concolor samples. We also thank A. Schmitz, J. Villalba, and the Microscopy Platform of the Sophia Agrobiotech Institute (ISA) for technical advices. We are grateful to the anonymous reviewers for their careful reading of our manuscript. This work received support from the Genoscope (Parasitoid venoms project), the Association Française Interprofessionnelle de l’Olive (AFIDOL), the Department of Plant Health and Environment (SPE) from the French National Institute for Agricultural Research (INRA), the French National Research Agency through the PARATOXOSE project (ANR-09-BLAN-0243-01), and the “Investments for the Future” LABEX SIGNALIFE: program reference ANR-11-LABX-0028. Hugo Mathé-Hubert PhD was funded by the “Provence Alpes Côte d’Azur” (PACA) region and the INRA SPE Division.
Footnotes
Author Contributions H.M.-H. performed light and fluorescence microscopy, 1D and 2D SDS-PAGE of venom, and RNA isolation for Illumina and 454 sequencing. M.R. performed transmission electron microscopy. D.C. performed RNA isolation and constructed cDNA library for Sanger sequencing. J.P. and C.D. coordinated ESTs sequencing and quality controls. E.D. performed de novo transcriptome assembly and GO analysis. D.C. and E.D. annotated and analyzed the sequences. C.D., E.D. and H.M.-H. performed the RPKM analysis. M.B. carried out the LC-MSMS analysis and interpretation. D.C. and J.-L.G. performed protein sequence analysis. D.C., E.D., H.M.-H., J._L.G. and M.P. wrote the manuscript. J.-L.G. and M.P. designed and coordinated the study. All authors read and approved the final manuscript.
References
- Quicke D. L. J. Parasitic Wasps (Chapman & Hall, 1997). [Google Scholar]
- Carton Y., Poirié M. & Nappi A. J. Insect immune resistance to parasitoids. Insect Sci. 15, 67–87 (2008). [Google Scholar]
- Poirié M., Colinet D. & Gatti J.-L. Insights into function and evolution of parasitoid wasp venoms. Curr. Opin. Insect Sci. 6, 52–60 (2014). [DOI] [PubMed] [Google Scholar]
- Moreau S. J. M. & Asgari S. Venom proteins from parasitoid wasps and their biological functions. Toxins (Basel) 7, 2385–2412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti J.-L., Schmitz A., Colinet D. & Poirié M. Diversity of virus-like particles in parasitoids’ venom: viral or cellular origin? In Parasitoid Viruses (eds Beckage N. E. & Drezen J. M.) 181–192 (Academic Press, 2012). [Google Scholar]
- Strand M. R. Polydnavirus gene products that interact with the host immune system. In Parasitoid Viruses (eds Beckage N. E. & Drezen J. M.) 149–161 (Academic Press, 2012). [Google Scholar]
- Colinet D. et al. Extensive inter- and intraspecific venom variation in closely related parasites targeting the same host: The case of Leptopilina parasitoids of Drosophila. Insect Biochem. Mol. Biol. 43, 601–611 (2013). [DOI] [PubMed] [Google Scholar]
- Rugman-Jones P. F., Wharton R., Noort T. V. & Stouthamer R. Molecular differentiation of the Psyttalia concolor (Szépligeti) species complex (Hymenoptera: Braconidae) associated with olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), in Africa. Biol. Control 49, 17–26 (2009). [Google Scholar]
- Daane K. M. et al. Biological controls investigated to aid management of olive fruit fly in California. Calif. Agric. 65, 21–28 (2011). [Google Scholar]
- Benelli G., Gennari G. & Canale A. Host discrimination ability in the tephritid parasitoid Psyttalia concolor (Hymenoptera: Braconidae). J. Pest Sci. 86, 245–251 (2012). [Google Scholar]
- Vincent B. et al. The venom composition of the parasitic wasp Chelonus inanitus resolved by combined expressed sequence tags analysis and proteomic approach. BMC Genomics 11, 693 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke G. R. & Strand M. R. Systematic analysis of a wasp parasitism arsenal. Mol. Ecol. 23, 890–901 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet D. et al. Identification of the main venom protein components of Aphidius ervi, a parasitoid wasp of the aphid model Acyrthosiphon pisum. BMC Genomics 15, 342 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke D. L., Achterberg K. & Godfray H. C. J. Comparative morphology of the venom gland and reservoir in opiine and alysiine braconid wasps (Insecta, Hymenoptera, Braconidae). Zoologica Scripta 26, 23–50 (1997). [Google Scholar]
- Wharton R. A. Generic relationships of opiine Braconidae (Hymenoptera) parasitic on fruit-infesting Tephritidae (Diptera). Contributions of the American Entomological Institute 30, 1–53 (1997). [Google Scholar]
- Lee S., Brown R. L. & Monroe W. Use of confocal laser scanning microscopy in systematics of insects with a comparison of fluorescence from different stains. System. Entomol. 34, 10–14 (2009). [Google Scholar]
- Jacas J. A., Budia F., Rodriguez-Cerezo E. & Vinuela E. Virus-like particles in the poison gland of the parasitic wasp Opius concolor. Ann. Appl. Biol. 130, 587–592 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z. W., Wang H. Y. & Chen X. X. Venom apparatus of the endoparasitoid wasp Opius caricivorae Fischer (Hymenoptera: Braconidae): morphology and ultrastructure. Microsc. Res. Tech. 69, 820–825 (2006). [DOI] [PubMed] [Google Scholar]
- Reineke A. & Asgari S. Presence of a novel small RNA-containing virus in a laboratory culture of the endoparasitic wasp Venturia canescens (Hymenoptera: Ichneumonidae). J. Insect Physiol. 51, 127–135 (2005). [DOI] [PubMed] [Google Scholar]
- Crawford A. M. et al. The constituents of Microctonus sp. parasitoid venoms. Insect Mol. Biol. 17, 313–324 (2008). [DOI] [PubMed] [Google Scholar]
- de Graaf D. C. et al. Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatic and proteomic studies. Insect Mol. Biol. 19, 11–26 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson N., Conyers C. & Smith I. A venom protein from the endoparasitoid wasp Pimpla hypochondriaca is similar to snake venom reprolysin-type metalloproteases. J. Invertebr. Pathol. 79, 129–131 (2002). [DOI] [PubMed] [Google Scholar]
- Price D. et al. A venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae). Insect Mol. Biol. 18, 195–202 (2009). [DOI] [PubMed] [Google Scholar]
- Pontoppidan B., Ekbom B., Eriksson S. & Meijer J. Purification and characterization of myrosinase from the cabbage aphid (Brevicoryne brassicae), a brassica herbivore. Eur. J. Biochem. 268, 1041–1048 (2001). [DOI] [PubMed] [Google Scholar]
- Burmeister W. P. et al. The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure 5, 663–675 (1997). [DOI] [PubMed] [Google Scholar]
- Husebye H. et al. Crystal structure at 1.1 Å resolution of an insect myrosinase from Brevicoryne brassicae shows its close relationship to β-glucosidases. Insect Biochem. Mol. Biol. 35, 1311–1320 (2005). [DOI] [PubMed] [Google Scholar]
- Takemoto H. et al. Heavy chain binding protein (BiP/GRP78) and endoplasmin are exported from the endoplasmic reticulum in rat exocrine pancreatic cells, similar to protein disulfide-isomerase. Arch. Biochem. Biophys. 296, 129–136 (1992). [DOI] [PubMed] [Google Scholar]
- Nganga A., Bruneau N., Sbarra V., Lombardo D. & Le Petit-Thevenin J. Control of pancreatic bile-salt-dependent-lipase secretion by the glucose-regulated protein of 94 kDa (Grp94). Biochem. J. 352, 865–874 (2000). [PMC free article] [PubMed] [Google Scholar]
- Bruneau N. et al. Roles of molecular chaperones in pancreatic secretion and their involvement in intestinal absorption. Microsc. Res. Tech. 49, 329–345 (2000). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Science 26, 215–226 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang X. & Wang C. C. Protein disulfide-isomerase, a folding catalyst and a redox-regulated chaperone. Free Radic. Biol. Med. 83, 305–313 (2015). [DOI] [PubMed] [Google Scholar]
- Safavi-Hemami H. et al. Rapid expansion of the protein disulfide isomerase gene family facilitates the folding of venom peptides. Proc. Natl. Acad. Sci. USA 113, 3227–3232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R. J., van Dam N. M. & van Loon J. J. A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 54, 57–83 (2009). [DOI] [PubMed] [Google Scholar]
- Matile P. “The Mustard Oil Bom”: Compartmentation of the myrosinase system. Biochemie Und Physiologie Der Pflanzen 175, 722–731 (1980). [Google Scholar]
- Winde I. & Wittstock U. Insect herbivore counteradaptations to the plant glucosinolate-myrosinase system. Phytochemistry 72, 1566–1575 (2011). [DOI] [PubMed] [Google Scholar]
- Opitz S. E. W. & Müller C. Plant chemistry and insect sequestration. Chemoecology 19, 117–154 (2009). [Google Scholar]
- Beran F. et al. Phyllotreta striolata flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system. Proc. Natl. Acad. Sci. USA 111, 7349–7354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentzold S., Zagrobelny M., Roelsgaard P. S., Møller B. L. & Bak S. The multiple strategies of an insect herbivore to overcome plant cyanogenic glucoside defence. PLoS ONE 9, e91337 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos M. et al. Herbivore-mediated effects of glucosinolates on different natural enemies of a specialist aphid. J. Chem. Ecol. 38, 100–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsopoulos G., Papageorgiou V., Komaitis M. & Hagidimitriou M. Phenolic profile of leaves and drupes in major Greek olive varieties. Not. Bot. Horti. Agrobo. 44, 162–166 (2016). [Google Scholar]
- Koudounas P. et al. A defence-related Olea europaea β-glucosidase hydrolyses and activates oleuropein into a potent protein cross-linking agent. J. Exp. Bot. 66, 2093–2106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet D. et al. Development of RNAi in a Drosophila endoparasitoid wasp and demonstration of its efficiency in impairing venom protein production. J. Insect Physiol. 63, 56–61 (2014). [DOI] [PubMed] [Google Scholar]
- Mathé-Hubert H., Gatti J. L., Poirié M. & Malausa T. A PCR-based method for estimating parasitism rates in the olive fly parasitoids Psyttalia concolor and P. lounsburyi (Hymenoptera: Braconidae). Biol. Control 67, 44–50 (2013). [Google Scholar]
- Moss S. E. & Morgan R. O. The annexins. Genome Biol 5, 219 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. et al. Parasite annexins - New molecules with potential for drug and vaccine development. Bioessays 32, 967–976 (2010). [DOI] [PubMed] [Google Scholar]
- Patel N. et al. A nematode effector protein similar to annexins in host plants. J. Exp. Bot. 61, 235–248 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somarajan S. R. et al. Annexin A2 mediates Mycoplasma pneumoniae community-acquired respiratory distress syndrome toxin binding to eukaryotic cells. MBio 5, e01497–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.-Y., Fang Q., Wang L., Hu C. & Ye G. Y. Proteomic analysis of the venom from the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae). Arch. Insect Biochem. Physiol. 75, 28–44 (2010). [DOI] [PubMed] [Google Scholar]
- Labella C. et al. Identification of two arginine kinase forms of endoparasitoid Leptomastix dactylopii venom by bottom up-sequence tag approach. J. Mass Spectrom. 50, 756–765 (2015). [DOI] [PubMed] [Google Scholar]
- Michalak M., Corbett E. F., Mesaeli N., Nakamura K. & Opas M. Calreticulin: one protein, one gene, many functions. Biochem. J. 344, 281e292 (1999). [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Schmidt O. & Asgari S. A calreticulin-like protein from endoparasitoid venom fluid is involved in host hemocyte inactivation. Dev. Comp. Immunol. 30, 756–764 (2006). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. Inhibition of host cell encapsulation through inhibiting immune gene expression by the parasitic wasp venom calreticulin. Insect Biochem. Mol. Biol. 43, 936–946 (2013). [DOI] [PubMed] [Google Scholar]
- Marzec M., Eletto D. & Argon Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta 1823, 774–787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K. & Jacobs-Lorena M. Surface-expressed enolases of Plasmodium and other pathogens. Mem. Inst. Oswaldo Cruz 106, 85–90 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T. A. et al. Early presence of an enolase in the oviposition injecta of the aphid parasitoid Aphidius ervi analyzed with chitosan beads as artificial hosts. J. Insect Physiol. 59, 11–18 (2013). [DOI] [PubMed] [Google Scholar]
- Laurino S. et al. Identification of major Toxoneuron nigriceps venom proteins using an integrated transcriptomic/proteomic approach. Insect Biochem. Mol. Biol. 76, 49–61 (2016). [DOI] [PubMed] [Google Scholar]
- Falabella P. et al. Aphidius ervi teratocytes release an extracellular enolase. Insect Biochem. Mol. Biol. 39, 801–813 (2009). [DOI] [PubMed] [Google Scholar]
- Grossi G. et al. Extracellular matrix degradation via enolase/plasminogen interaction: Evidence for a mechanism conserved in Metazoa. Biol. Cell. 108, 161–178 (2016). [DOI] [PubMed] [Google Scholar]
- Wong H. & Schotz M. C. The lipase gene family. J. Lipid Res. 43, 993–999 (2002). [DOI] [PubMed] [Google Scholar]
- Ketudat Cairns J. R. & Esen A. β-Glucosidases. Cell. Mol. Life Sci. 67, 3389–3405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani M. P., Edwards J. P. & Richards E. H. Hydrolase activity in the venom of the pupal endoparasitic wasp, Pimpla hypochondriaca. Comp. Biochem. Physiol. B 141, 373–381 (2005). [DOI] [PubMed] [Google Scholar]
- Daugaard M., Rohde M. & Jäättelä M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 581, 3702–3710 (2007). [DOI] [PubMed] [Google Scholar]
- Kobe B. & Kajava A. V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11, 725–732 (2001). [DOI] [PubMed] [Google Scholar]
- Turner A., Isaac R. & Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. BioEssays 23, 261–269 (2001). [DOI] [PubMed] [Google Scholar]
- Isaac R. E., Bland N. D. & Shirras A. D. Neuropeptidases and the metabolic inactivation of insect neuropeptides. Gen. Comp. Endocrinol. 162, 8–17 (2009). [DOI] [PubMed] [Google Scholar]
- Antczak C., De Meester I. & Bauvois B. Ectopeptidases in pathophysiology. Bioessays 23, 251–260 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S., Reineke A., Beck M. & Schmidt O. Isolation and characterization of a neprilysin-like protein from Venturia canescens virus-like particles. Insect Mol. Biol. 11, 477–485 (2002). [DOI] [PubMed] [Google Scholar]
- Lee G. & Bae H. Bee venom phospholipase A2: yesterday’s enemy becomes today’s friend. Toxins 8, 48 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco C., Gutiérrez J. M. & Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cell. Mol. Life Sci. 65, 2897–2912 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. & Gilbert H. F. Protein disulfide isomerase. Biochim. Biophys. Acta 1699, 35–44 (2004). [DOI] [PubMed] [Google Scholar]
- Terada K. et al. Secretion, surface localization, turnover, and steady state expression of protein disulfide isomerase in rat hepatocytes. J. Biol. Chem. 270, 20410–20416 (1995). [DOI] [PubMed] [Google Scholar]
- di Luccio E. et al. Parameters affecting in vitro oxidation/folding of maurotoxin, a four-disulphide-bridged scorpion toxin. Biochem. J. 358, 681–692 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi-Hemami H., et al. Modulation of conotoxin structure and function is achieved through a multienzyme complex in the venom glands of cone snails. J. Biol. Chem. 287, 34288–34303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X. et al. Serine carboxypeptidase SCPEP1 and cathepsin A play complementary roles in regulation of vasoconstriction via inactivation of endothelin-1. PLoS Genet. 10, e1004146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez V. E., Niemirowicz G. T. & Cazzulo J. J. The peptidases of Trypanosoma cruzi: Digestive enzymes, virulence factors, and mediators of autophagy and programmed cell death. Biochim. Biophys. Acta 1824, 195–206 (2012). [DOI] [PubMed] [Google Scholar]
- Huntington J. A. Serpin structure, function and dysfunction. J. Thromb. Haemost. 9, 26–34 (2011). [DOI] [PubMed] [Google Scholar]
- Colinet D. et al. A serpin from the parasitoid wasp Leptopilina boulardi targets the Drosophila phenoloxidase cascade. Dev. Comp. Immunol. 33, 681–689 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





