Abstract
Objective
To investigate the role of radiotherapy (RT) in patients who underwent hysterectomy for uterine carcinosarcoma (UCS).
Methods
Patients with the International Federation of Gynecology and Obstetrics stage I–IVa UCS who were treated between 1990 and 2012 were identified retrospectively in a multi-institutional database. Of 235 identified patients, 97 (41.3%) received adjuvant RT. Twenty-two patients with a history of previous pelvic RT were analyzed separately. Survival outcomes were assessed using the Kaplan-Meier method and Cox proportional hazards model.
Results
Patients with a previous history of pelvic RT had poor survival outcomes, and 72.6% of these patients experienced locoregional recurrence; however, none received RT after a diagnosis of UCS. Univariate analyses revealed that pelvic lymphadenectomy (PLND) and para-aortic lymph node sampling were significant factors for locoregional recurrence-free survival (LRRFS) and disease-free survival (DFS). Among patients without previous pelvic RT, the percentage of locoregional failure was lower for those who received adjuvant RT than for those who did not (28.5% vs. 17.5%, p=0.107). Multivariate analysis revealed significant correlations between PLND and LRRFS, distant metastasis-free survival, and DFS. In subgroup analyses, RT significantly improved the 5-year LRRFS rate of patients who did not undergo PLND (52.7% vs. 18.7% for non-RT, p<0.001).
Conclusion
Adjuvant RT decreased the risk of locoregional recurrence after hysterectomy for UCS, particularly in patients without surgical nodal staging. Given the poorer locoregional outcomes of patients previously subjected to pelvic RT, meticulous re-administration of RT might improve locoregional control while leading to less toxicity in these patients.
Keywords: Locoregional Control; Radiotherapy, Adjuvant; Uterine Carcinosarcoma
INTRODUCTION
Uterine carcinosarcoma (UCS), also known as malignant mixed Müllerian tumor, is a rare gynecologic malignancy with an annual incidence of less than two cases per 100,000 women in the United States. This aggressive type of tumor accounts for less than 5% of all uterine malignancies but more than 15% of all uterine cancer-associated deaths [1]. Patients with UCS have a poor prognosis, with 5-year survival rates of 35% to 39% [2,3].
Surgery is considered the standard treatment for UCS, and the current recommendation is hysterectomy with bilateral salpingo-oophorectomy (BSO), pelvic lymphadenectomy (PLND), and para-aortic lymph node (PALN) sampling with peritoneal washings, although the additive benefit of lymphadenectomy remains undetermined [4,5]. However, high rates of postoperative relapse and metastasis suggest the need for effective adjuvant therapies [6].
Adjuvant radiotherapy (RT), as a component of disease management, is known to improve locoregional control in patients with UCS, although its impact on survival remains controversial [6,7,8,9]. The limited availability of information regarding surgical staging and prognostic factors makes it difficult to draw conclusions from the current literature [7]. The purpose of the present study, therefore, was to investigate retrospectively the role of adjuvant RT in patients with UCS who underwent hysterectomy at multiple institutions.
MATERIALS AND METHODS
Patients with UCS who underwent hysterectomy at 11 institutions in Korea between January 1990 and December 2012 were enrolled. The inclusion criteria were: (1) patients with pathologically confirmed UCS; (2) adult women ≥20 years of age; (3) patients who underwent curative hysterectomy; and (4) stage I–IVa UCS according to the revised the International Federation of Gynecology and Obstetrics (FIGO) staging system [10]. Nodal metastasis was evaluated by surgical staging or via imaging techniques such as pelvic magnetic resonance imaging, abdomen-pelvic computed tomography, or positron emission tomography-computed tomography. Patients with distant metastasis, which was diagnosed via punch biopsy, or those who underwent brachytherapy without pelvic RT were excluded. Finally, 235 patients were included in the analysis. Information regarding patient and tumor characteristics, the extent of surgery, type of adjuvant treatment, and recurrence and survival were collected from a Korean multi-institutional retrospective database. This study was approved by the Korean Radiation Oncology Group (KROG 13-08) and the Institutional Review Boards of all participating hospitals.
All patients underwent hysterectomy with BSO. PLND and para-aortic lymph node sampling or dissection (PALND) were performed in 181 patients (77%) and 131 patients (55.7%), respectively. Adjuvant RT was administered to 97 patients (41.3%). Because of the retrospective nature of this study, there were no specific patient selection criteria for RT or adjuvant treatment modalities. All 97 RT-treated patients received external-beam RT covering the vaginal vault and pelvic nodal area. Fourteen patients underwent RT that included the para-aortic nodal area, and eight underwent brachytherapy. The median external RT dose was 50.4 Gy, and the median brachytherapy dose was 20.5 Gy. Adjuvant chemotherapy was administered to 133 patients (56.6%), and the most commonly used regimen was a combination of ifosfamide and cisplatin. Twenty-two patients (9.4%) underwent pelvic RT for previous malignancies before receiving a diagnosis of UCS; none of these patients had received adjuvant RT as a treatment for UCS. We analyzed these patients separately because of the potential effects of prior malignancies on the study outcome.
Locoregional recurrence was defined as a recurrence in the pelvis, which included the vaginal vault, pelvic lymph node, and PALN areas. Locoregional recurrence-free survival (LRRFS), distant metastasis-free survival (DMFS), and disease-free survival (DFS) were defined respectively as the time from surgery to locoregional recurrence or death, to distant metastasis or death, and to disease recurrence or death. Overall survival (OS) was defined as the time from surgery to death, regardless of cause. In-field failure was defined as any recurrence within the radiation field.
The chi-square test was used to compare the categorical characteristics of patients who did and did not undergo RT. The t-test was used to compare age and tumor size. Survival rates were estimated using the Kaplan-Meier method. A univariate analysis based on the log-rank test was performed to identify prognostic factors affecting survival. A multivariate analysis was conducted using the Cox proportional hazard model. Factors with statistically significant probability values in the univariate analysis were included in the multivariate analysis. All statistical comparisons were two-sided, and p-values <0.05 were considered statistically significant.
RESULTS
1. Patient and tumor characteristics
A total of 213 patients had not previously undergone pelvic RT. The median patient age was 58 years (range, 27 to 87 years). Nine patients (4.2%) had a history of tamoxifen use. PLND and PALND were performed in 173 patients (81.2%) and 126 (59.2%), respectively. Adjuvant RT was performed in 97 patients (45.5%), and adjuvant chemotherapy was administered to 117 patients (54.9%). The median tumor size was 5 cm (range, 0 to 20 cm). Except for 22 patients with missing information, 42 patients (22%) had developed pelvic node metastases. Twenty-four patients (14%) had PALN metastases. Eighteen patients (8.5%) had positive resection margins, and 75 (35.2%) exhibited lymphovascular space invasion (LVI). The clinicopathologic characteristics of patients treated and not treated with adjuvant RT are compared in Table 1. There were no significant differences between patients in the no RT and RT groups with respect to tamoxifen history, extent of lymph node dissection, tumor size, PALN metastasis status, FIGO stage, resection margin status, and LVI status. A higher percentage of patients in the no RT group underwent adjuvant chemotherapy (63.8% vs. 44.3%, p=0.004), and lower percentages of patients in this group exhibited pelvic node metastasis (15.5% vs. 24.7%, p=0.074) and LVI (31% vs. 40.2%, p=0.056).
Table 1. Patient characteristics.
| Characteristic | No RT (n=116) | RT (n=97) | p-value | Previous pelvic RT history (n=22) | |
|---|---|---|---|---|---|
| Age at diagnosis (yr) | 0.108 | ||||
| <60 | 59 (50.9) | 60 (61.9) | 5 (22.7) | ||
| ≥60 | 57 (49.1) | 37 (38.1) | 17 (77.3) | ||
| Tamoxifen history | 0.156 | ||||
| No | 109 (94.0) | 94 (96.9) | 21 (95.5) | ||
| Yes | 7 (6.0) | 2 (2.1) | 0 | ||
| Unknown | 0 | 1 (1.0) | 1 (4.5) | ||
| Pelvic lymphadenectomy | 0.956 | ||||
| No | 21 (18.1) | 18 (18.6) | 14 (63.6) | ||
| Yes | 94 (81.0) | 79 (81.4) | 8 (36.4) | ||
| Unknown | 1 (0.9) | 0 | 0 | ||
| PALND | 0.463 | ||||
| No | 50 (43.1) | 37 (38.1) | 17 (77.3) | ||
| Yes | 66 (56.9) | 60 (61.9) | 5 (22.7) | ||
| Tumor size (cm) | 0.246 | ||||
| ≤6 | 61 (52.6) | 60 (61.9) | 6 (27.3) | ||
| >6 | 47 (40.5) | 33 (34) | 13 (59.1) | ||
| Unknown | 8 (6.9) | 4 (4.1) | 3 (13.6) | ||
| Pelvic node metastasis | 0.074 | ||||
| No | 87 (75.0) | 62 (63.9) | 14 (63.6) | ||
| Yes | 18 (15.5) | 24 (24.7) | 2 (9.1) | ||
| Unknown | 11 (9.5) | 11 (11.4) | 6 (27.3) | ||
| Para-aortic node metastasis | 0.781 | ||||
| No | 78 (67.3) | 69 (71.1) | 15 (68.2) | ||
| Yes | 12 (10.3) | 12 (12.4) | 1 (4.5) | ||
| Unknown | 26 (21.4) | 16 (16.5) | 6 (27.3) | ||
| FIGO stage | 0.267 | ||||
| I | 71 (61.2) | 49 (50.5) | 6 (27.3) | ||
| II | 11 (9.5) | 10 (10.3) | 5 (22.7) | ||
| III | 31 (26.7) | 37 (38.2) | 9 (40.9) | ||
| IVA | 3 (2.6) | 1 (1.0) | 2 (9.1) | ||
| Resection margin | 0.652 | ||||
| Negative | 95 (81.9) | 76 (78.3) | 7 (31.8) | ||
| Positive | 9 (7.8) | 9 (9.3) | 11 (50.0) | ||
| Unknown | 12 (10.3) | 12 (12.4) | 4 (18.2) | ||
| LVI | 0.056 | ||||
| No | 50 (43.1) | 29 (29.9) | 4 (18.2) | ||
| Yes | 36 (31.0) | 39 (40.2) | 12 (54.5) | ||
| Unknown | 30 (25.9) | 29 (29.9) | 6 (27.3) | ||
| Chemotherapy | 0.004 | ||||
| No | 42 (36.2) | 54 (55.7) | 6 (27.3) | ||
| Yes | 74 (63.8) | 43 (44.3) | 16 (72.7) | ||
Values are presented as number (%).
FIGO, the International Federation of Gynecology and Obstetrics; LVI, lymphovascular space invasion; PALND, para-aortic lymph node sampling or dissection; RT, radiotherapy.
The characteristics of patients with a previous history of pelvic RT are also described in Table 1. PLND and PALND were performed in eight patients (36.4%) and five (22.7%), respectively. Adjuvant chemotherapy was administered to 16 patients (72.7%). Two patients (9.1%) and one (4.5%) exhibited pelvic node and PALN metastasis, respectively. Eleven patients (50%) had positive resection margins, and 12 (54.5%) had LVI. Characteristics of these patients’ prior malignancies are described in Table 2.
Table 2. Characteristics related to previous malignancies in patients who underwent pelvic RT history (n=22).
| Characteristic | Value | |
|---|---|---|
| Months between RT and UCS diagnosis (mo) | 123.6 (46.3–293.5) | |
| Type of malignancy | ||
| Uterine cervix | 15 (68.2) | |
| Rectum | 7 (31.8) | |
| Stage of malignancy | ||
| I | 3 (13.6) | |
| II | 8 (36.4) | |
| III | 7 (31.8) | |
| Unknown | 4 (18.2) | |
| Surgical procedure for malignancy | ||
| No | 12 (54.5) | |
| Yes | 10 (45.5) | |
| Treatment | ||
| External RT | 6 (27.3) | |
| External RT followed by brachytherapy | 13 (59.1) | |
| Unknown | 3 (13.6) | |
| RT field | ||
| Pelvis | 16 (72.8) | |
| Semi-extended | 1 (4.5) | |
| Unknown | 5 (22.7) | |
| RT dose (Gy) | 75 (34–93) | |
| CCRT | ||
| No | 13 (59.1) | |
| Yes | 6 (27.3) | |
| Unknown | 3 (13.6) | |
Values are presented as median (range) or number (%).
CCRT, concurrent chemoradiotherapy; RT, radiotherapy; UCS, uterine carcinosarcoma.
2. Patterns of failure
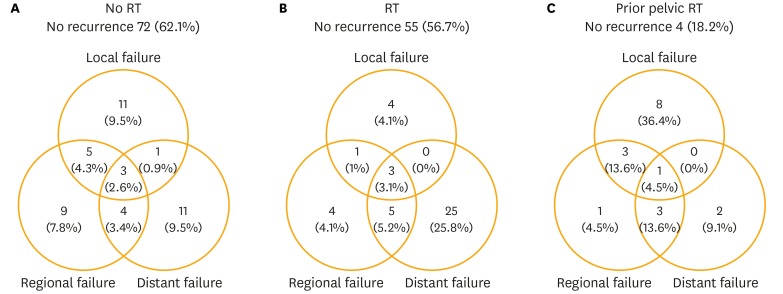
The median follow-up duration of patients without a history of previous pelvic RT, calculated from the date of surgery, was 25 months (range, 0.5 to 273 months). Eighty-six patients (40.4%) had developed recurrences by the time of the analysis. Locoregional recurrence, distant metastasis, and both locoregional and distant failure occurred in 34 patients (16%), 36 (16.9%), and 16 (7.5%), respectively. Forty-four patients (37.9%) in the no RT group and 42 (43.3%) in the RT group developed recurrent disease. In the no RT group, 32 patients (28.5%) and 19 (16.4%) developed locoregional recurrences and distant metastases, respectively (Fig. 1A). In the RT group, 17 patients (17.5%) and 33 (34.1%) developed locoregional recurrences and distant metastases (Fig. 1B), respectively, and nine patients (9.3%) experienced in-field failure.
Fig. 1.
Patterns of failure. (A) The no radiotherapy (RT) group, comprising patients with no previous history of pelvic RT. Thirty-two patients (28.5%) in this group developed locoregional recurrence. (B) The RT group, comprising patients with no previous history of pelvic RT. Seventeen patients (17.5%) in this group developed locoregional recurrences. (C) Patients with a previous history of pelvic RT. Sixteen patients (72.6%) in this group developed locoregional recurrences.
The median follow-up duration of patients with a history of previous pelvic RT, calculated from the date of surgery, was 13.5 months (range, 1 to 92 months). Eighteen patients (71.8%) had developed recurrences by the time of the analysis. Locoregional recurrences and distant metastases were observed in 16 patients (72.6%) and six (27.2%), respectively (Fig. 1C).
3. Survival outcomes and analysis of prognostic factors
During the follow-up period, the 5-year LRRFS, DMFS, DFS, and OS rates of patients without a history of previous pelvic RT were 56.8%, 58.4%, 52.4%, and 67%, respectively; the corresponding 2-year rates of patients with a history of previous pelvic RT were 10.8%, 29.7%, 10.8%, and 44.3%, respectively.
Univariate analyses of the LRRFS, DMFS, DFS, and OS of patients without a history of previous pelvic RT were conducted, and the results of each Kaplan-Meier survival analysis are shown in Supplementary Fig. 1 (A, LRRFS; B, DMFS; C, DFS; D, OS).
A lack of PLND, a lack of PALND, large tumor size, higher FIGO stage, pelvic node metastasis, PALN metastasis, positive resection margin, and LVI correlated significantly with reduced LRRFS. In the multivariate analysis, PLND was the only significant factor associated with LRRFS (hazard ratio [HR], 0.366; 95% CI, 0.163 to 0.820; p=0.015) (Table 3). Adjuvant RT had no significant effect on LRRFS in these patients.
Table 3. Survival outcomes according to the Cox proportional hazards multivariate model in patients without previous pelvic RT history.
| Variable | LRRFS | DMFS | DFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| PLND (no vs. yes) | 0.366 | 0.163–0.820 | 0.015 | 0.401 | 0.187–0.860 | 0.019 | 0.318 | 0.148–0.684 | 0.003 | - | - | - |
| PALND (no vs. yes) | 0.493 | 0.217–1.120 | 0.091 | - | - | - | 0.614 | 0.280–1.348 | 0.224 | - | - | - |
| Tumor size (cm) (≤6 vs. >6) | 1.583 | 0.792–3.161 | 0.193 | 1.546 | 0.837–2.856 | 0.164 | 1.698 | 0.898–3.212 | 0.104 | 1.272 | 0.623–2.597 | 0.508 |
| Pelvic node metastasis (no vs. yes) | 0.926 | 0.328–2.612 | 0.884 | 0.488 | 0.193–1.232 | 0.129 | 0.834 | 0.316–2.198 | 0.713 | 0.421 | 0.145–1.216 | 0.110 |
| Para-aortic node metastasis (no vs. yes) | 1.381 | 0.506–3.769 | 0.529 | 2.297 | 0.995–5.303 | 0.051 | 2.504 | 1.008–6.220 | 0.048 | 1.162 | 0.436–3.100 | 0.764 |
| FIGO stage (I–II vs. III–IVA) | 1.156 | 0.677–1.972 | 0.595 | 1.625 | 1.045–2.527 | 0.031 | 1.189 | 0.728–1.941 | 0.489 | 1.647 | 0.994–2.729 | 0.053 |
| Resection margin (negative vs. positive) | 3.196 | 0.781–13.083 | 0.106 | - | - | - | 2.084 | 0.555–7.831 | 0.277 | - | - | - |
| LVI (negative vs. positive) | 1.976 | 0.891–4.381 | 0.094 | 2.280 | 1.134–4.583 | 0.021 | 1.647 | 0.804–3.377 | 0.173 | 3.497 | 1.489–8.214 | 0.004 |
| RT (no vs. yes) | 0.875 | 0.452–1.693 | 0.691 | 1.323 | 0.722–2.423 | 0.365 | 1.036 | 0.563–1.905 | 0.910 | 1.486 | 0.709–3.114 | 0.294 |
CI, confidence interval; DFS, disease-free survival; DMFS, distant metastasis-free survival; FIGO, the International Federation of Gynecology and Obstetrics; HR, hazard ratio; LRRFS, locoregional recurrence-free survival; LVI, lymphovascular space invasion; OS, overall survival; PALND, para-aortic lymph node sampling or dissection; PLND, pelvic lymphadenectomy; RT, radiotherapy.
The factors significantly associated with reduced DMFS in the univariate analysis were a lack of PLND, large tumor size, higher FIGO stage, pelvic node metastasis, PALN metastasis, and LVI. Adjuvant chemotherapy had no significant effect on DMFS. A lack of PLND (HR, 0.401; 95% CI, 0.187 to 0.860; p=0.019), higher FIGO stage (HR, 1.625; 95% CI, 1.045 to 2.527; p=0.031), and LVI (HR, 2.280; 95% CI, 1.134 to 4.583; p=0.021) remained significant in the multivariate analysis, whereas PALN metastasis exhibited borderline significance (HR, 2.297; 95% CI, 0.995 to 5.303; p=0.051) (Table 3).
The factors significantly associated with DFS in the univariate analysis were the same as those significantly associated with LRRFS. In the multivariate analysis, PLND (HR, 0.318; 95% CI, 0.148 to 0.684; p=0.003) and PALN metastasis (HR, 2.504; 95% CI, 1.008 to 6.220; p=0.048) retained significant associations with DFS (Table 3).
Tumor size, pelvic node metastasis, PALN metastasis, FIGO stage III/IVa, and LVI were significantly associated with OS in the univariate analysis. However, only LVI remained significant in the multivariate analysis (HR, 3.497; 95% CI, 1.489 to 8.214; p=0.004), although an advanced FIGO stage exhibited borderline significance (HR, 1.647; 95% CI, 0.994 to 2.729; p=0.053) (Table 3). Neither adjuvant RT nor chemotherapy was associated with DFS or OS.
A univariate analysis of patients with previous pelvic RT showed that PLND and PALND were significant factors for LRRFS and DFS (Table 4). No other factors correlated significantly with DMFS or OS in this analysis.
Table 4. Univariate analysis of variables in patients with previous pelvic RT history (n=22).
| Variable | LRRFS | DMFS | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2-Year rate (%) | p-value | 2-Year rate (%) | p-value | 2-Year rate (%) | p-value | 2-Year rate (%) | p-value | ||
| Age at diagnosis (yr) | 0.133 | 0.976 | 0.150 | 0.504 | |||||
| <60 | 20.0 | 20.0 | 20.0 | 60.0 | |||||
| ≥60 | 7.5 | 28.0 | 7.5 | 35.6 | |||||
| Pelvic lymphadenectomy | 0.033 | 0.684 | 0.039 | 0.682 | |||||
| No | 0 | 17.0 | 0 | 48.5 | |||||
| Yes | 29.2 | 29.2 | 29.2 | 42.9 | |||||
| PALND | 0.007 | 0.098 | 0.007 | 0.279 | |||||
| No | 0 | 12.9 | 0 | 34.4 | |||||
| Yes | 50.0 | 50.0 | 50.0 | 75.0 | |||||
| Tumor size (cm) | 0.665 | 0.863 | 0.727 | 0.567 | |||||
| ≤6 | 0 | 20.0 | 20.0 | 40.0 | |||||
| >6 | 9.4 | 36.0 | 9.4 | 48.2 | |||||
| Pelvic node metastasis | 0.404 | 0.909 | 0.401 | 0.600 | |||||
| No | 15.4 | 30.8 | 15.4 | 52.7 | |||||
| Yes | 0 | 0 | 0 | 0 | |||||
| Para-aortic node metastasis | 0.508 | 0.987 | 0.507 | 0.814 | |||||
| No | 14.3 | 28.6 | 14.3 | 46.9 | |||||
| Yes | 0 | 0 | 0 | 0 | |||||
| FIGO stage | 0.761 | 0.684 | 0.745 | 0.780 | |||||
| I–II | 20.2 | 33.3 | 20.2 | 53.3 | |||||
| III–IVA | 0 | 0 | 0 | 38.9 | |||||
| Resection margin | 0.882 | 0.885 | 0.950 | 0.230 | |||||
| Negative | 16.7 | 33.3 | 16.7 | 33.3 | |||||
| Positive | 11.7 | 23.4 | 11.7 | 70.0 | |||||
| LVI | 0.473 | 0.543 | 0.473 | 0.557 | |||||
| Negative | 33.3 | 33.3 | 33.3 | 66.7 | |||||
| Positive | 9.3 | 32.1 | 9.3 | 56.1 | |||||
| Chemotherapy | 0.547 | 0.819 | 0.554 | 0.700 | |||||
| No | 20.8 | 25.0 | 20.8 | 50.0 | |||||
| Yes | 7.2 | 21.4 | 7.2 | 43.7 | |||||
DFS, disease-free survival; DMFS, distant metastasis-free survival; FIGO, the International Federation of Gynecology and Obstetrics; LRRFS, locoregional recurrence-free survival; LVI, lymphovascular space invasion; OS, overall survival; PALND, para-aortic lymph node sampling or dissection; RT, radiotherapy.
4. Identification of UCS subgroups that might benefit from adjuvant RT
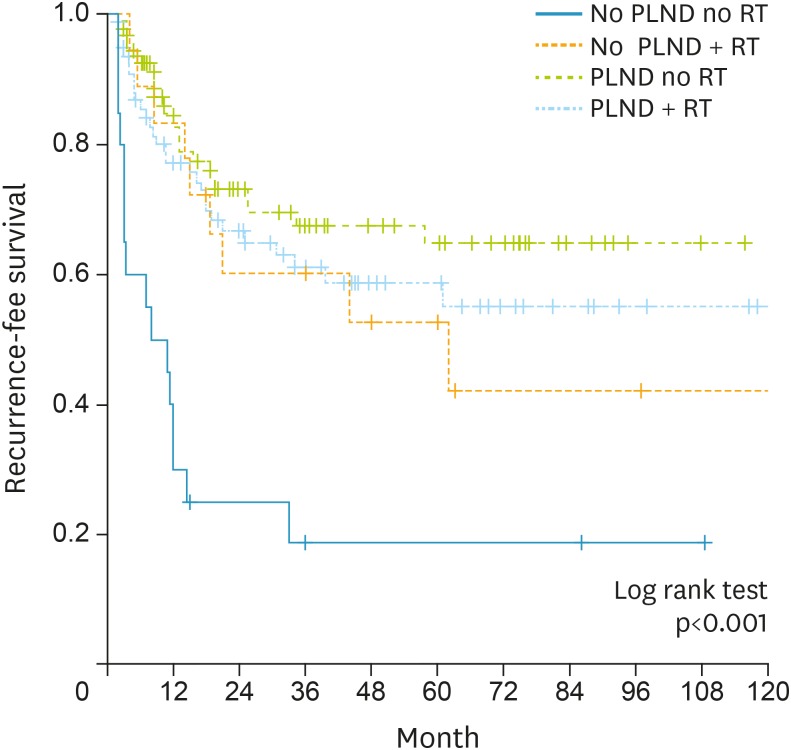
To identify patient subgroups that might benefit from adjuvant RT, we hypothesized that adjuvant RT could benefit patients with poor prognostic factors. Therefore, we performed subgroup analyses based on adjuvant RT and/or PLND statuses, as the latter was the only significant factor associated with LRRFS in the multivariate analysis. The 5-year LRRFS rates were 18.7% for the PLND–/RT– group (n=21), 52.7% for the PLND–/RT+ group (n=18), 64.8% for the PLND+/RT– group (n=94), and 58.8% for the PLND+/RT+ group (n=77; p<0.001, log-rank test) (Fig. 2).
Fig. 2.
Kaplan-Meier survival analyses of subgroups of patients without a previous history of pelvic radiotherapy (RT), classified according to pelvic lymphadenectomy (PLND) and adjuvant RT statuses. The 5-year locoregional recurrence-free survival rates were 18.7% for the PLND–/RT– group, 52.7% for the PLND–/RT+ group, 64.8% for the PLND+/RT– group, and 58.8% for the PLND+/RT+ group.
DISCUSSION
Despite efforts to overcome the dismal outcomes of patients with UCS after surgery alone, the rarity of this disease has made it difficult to establish evidence of the efficacy of adjuvant treatment. We observed beneficial effect of RT on LRRFS in a subgroup analysis, in accordance with a number of previous studies. To date, two randomized trials have been conducted to evaluate the efficacy of adjuvant RT in UCS patients. The Gynecologic Oncology Group 150 phase III randomized trial compared whole abdominal irradiation versus cisplatin, ifosfamide, and mesna as adjuvant therapy in 232 patients with UCS [11]. Although the OS and recurrence rates did not differ significantly between the two groups, patients who underwent chemotherapy had fewer recurrences and better OS rates. The European Organization for the Research and Treatment of Cancer Gynaecological Cancer Group 55874 study [12] compared adjuvant pelvic RT versus observation in patients with uterine sarcoma, including 91 patients with UCS. Although no survival benefit was associated with RT, a marked decrease in the locoregional failure rate was observed in patients with UCS, with local recurrence rates of 47% and 24% in the observation and RT arms, respectively. In addition, several retrospective single institutional studies [6,13,14] and large-scale nationwide database studies [15,16] have evaluated the impact of adjuvant RT on UCS. In most of these studies, adjuvant RT significantly reduced local failure, although OS and distant metastasis were not affected. Sampath and Gaffney [17] analyzed available prospective and retrospective data that addressed the role of adjuvant RT in all histologic types of uterine sarcoma and suggested that adjuvant RT reduced the local failure rate by 50% among cases of UCS, leiomyosarcoma, and endometrial stromal sarcoma.
Imaging-based preoperative nodal staging is insufficient for the detection of micrometastasis, as upstaging frequently occurs after pathologic evaluation in clinically node-negative patients. Therefore, patients with UCS should be subjected to extensive surgical staging, which includes node dissection as well as hysterectomy. The impact of PLND with or without adjuvant RT has been investigated, and 2 retrospective analyses of patient data from the Surveillance, Epidemiology, and End Results (SEER) database have demonstrated the clinical benefits of adjuvant RT in UCS patients, especially those not subjected to PLND [5,18].
In addition to surgical nodal staging, LVI was strongly associated with progression-free survival and weakly associated with OS in a multi-institutional cohort study of 111 patients with early-stage UCS [19]. The Gynecology Oncology Group also found that LVI was predictive of recurrence [20]. In our study, LVI was a significant prognostic factor for LRRFS, DMFS, DFS, and OS in a univariate analysis of patients without previous pelvic RT and was significantly associated with poor OS in a multivariate analysis.
When compared with the no RT group, the RT group in the present study had similar LRRFS and DFS rates and a lower incidence of locoregional failure, despite having higher percentages of patients with nodal metastasis and LVI. Furthermore, adjuvant RT significantly improved LRRFS in subgroups that did not undergo PLND. Therefore, adjuvant RT might be useful for reducing the risk of locoregional recurrence in patients who do not undergo PLND.
The worse DMFS observed in patients who received RT relative to those who did not might be attributable to the higher rates of nodal metastasis and LVI in the former group. Moreover, significantly fewer patients in the RT group received chemotherapy. Several studies have assessed the usefulness of adjuvant chemotherapy for UCS in an attempt to reduce the high incidence of distant failure after treatment. A phase III randomized trial of adjuvant chemotherapy for patients with uterine sarcoma (SARCGYN study) found that a combination of doxorubicin, ifosfamide, and cisplatin increased DFS [21]; however, this study was discontinued early because of the unavailability of participants. A recent study suggested that UCS is a metaplastic form of endometrial carcinoma, the sarcomatous component of which represents a de-differentiation of the carcinomatous component [22]. Therefore, those currently conducting studies are advised to cautiously apply the results of previous studies involving patients selected according to the earlier definition of UCS. Clinical studies that evaluate chemotherapy specifically in patients with UCS are required. A review of the Cochrane database with respect to adjuvant chemotherapy for UCS indicated that combination chemotherapy regimens that include ifosfamide should be considered in cases involving advanced-stage disease or metastasis [23]. In our study, however, chemotherapy had no significant survival benefit.
Patients who did and did not undergo pelvic RT previously exhibited different failure patterns. For example, locoregional recurrence occurred in less than 30% of patients who did not receive pelvic RT previously and in 66.3% of patients who received pelvic RT previously, regardless of the presence of distant metastasis. In addition, positive resection margins were observed in 50% of patients who received pelvic RT previously, but only in 8.9% of those who did not receive pelvic RT previously. Furthermore, 36.4% and 22.7% of patients who received pelvic RT previously underwent PLND and PALND, respectively; these rates were less than half of the corresponding rates among patients who did not receive pelvic RT previously (81.2% and 59.2%, respectively). These findings suggest a tendency toward less aggressive surgery for patients with a previous history of pelvic RT, which could increase the likelihood of surgical complications.
Very limited data were available regarding re-irradiation of the pelvic area for the treatment of gynecologic malignancies. A case report of a patient with recurrent endometrial cancer described favorable outcomes from re-irradiation via conformal RT [24], and another retrospective study that included four re-irradiated patients with recurrent cervical cancer reported one case of complete response, two cases of partial response, and one case of stable disease [25]. Both reports suggested that a sufficient radiation dose is required to achieve tumor control and therefore suggested that conformal RT and, to a greater extent, intensity-modulated radiotherapy (IMRT) could provide safer and more effective treatment [24,25]. Regarding complications, substantial grade 2 to 3 toxicities have been observed in re-irradiated patients [25]. Comparative studies of the dose parameters, outcomes, and complications of postoperative IMRT and conventional or three-dimensional conformal RT for gynecologic cancers demonstrated significant reductions in toxicities without compromising clinical outcomes [26,27,28,29]. Therefore, IMRT should be considered for these patients in order to reduce toxicity while improving locoregional control.
Our study had several limitations in addition to its retrospective nature. First, information about treatment-related toxicities was lacking. Second, complete data were not available for all patients. Third, differences in the percentages of patients who received adjuvant chemotherapy and chemotherapy regimens were observed between the RT and no RT groups; these differences complicated our analyses and precluded an additional analysis of the effects of chemotherapy. Despite these limitations, our study provides valuable information about treatment outcomes in patients treated for UCS during the past 20 years, and has identified prognostic factors in a relatively large number of patients with this rare disease.
In conclusion, adjuvant RT reduced the rate of locoregional recurrence in patients with UCS. The eradication of occult micrometastasis in lymph nodes via PLND is presumably a mandatory step toward improved survival in patients with UCS. In cases involving hysterectomy without surgical nodal staging, adjuvant RT should be considered a viable means of reducing locoregional recurrence. Finally, the re-administration of RT to the pelvic area might improve locoregional control even in patients with a history of pelvic RT prior to the UCS diagnosis and less extensive surgical staging, as these patients had poorer locoregional outcomes when compared to patients without previous pelvic RT; however, further studies are required to confirm this finding.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Supplementary Material
Kaplan-Meier survival analysis of patients without a previous history of pelvic radiotherapy (RT) according to adjuvant RT administration. (A) Locoregional recurrence-free survival (LRRFS). The 5-year LRRFS rates were 55.9% and 57.9% for the no RT and RT groups, respectively. (B) Distant metastasis-free survival (DMFS). The 5-year DMFS rates were 62.3% and 53.8% for the no RT and RT groups, respectively. (C) Disease-free survival (DFS). The 5-year DFS rates were 52.6% and 51.9% for the no RT and RT groups, respectively. (D) Overall survival (OS). The 5-year OS rates were 73% and 61.4% for the no RT and RT groups, respectively.
References
- 1.El-Nashar SA, Mariani A. Uterine carcinosarcoma. Clin Obstet Gynecol. 2011;54:292–304. doi: 10.1097/GRF.0b013e31821ac635. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen SN, Podratz KC, Scheithauer BW, O’Brien PC. Clinicopathologic analysis of uterine malignant mixed müllerian tumors. Gynecol Oncol. 1989;34:372–378. doi: 10.1016/0090-8258(89)90176-5. [DOI] [PubMed] [Google Scholar]
- 3.Dinh TV, Slavin RE, Bhagavan BS, Hannigan EV, Tiamson EM, Yandell RB. Mixed müllerian tumors of the uterus: a clinicopathologic study. Obstet Gynecol. 1989;74:388–392. [PubMed] [Google Scholar]
- 4.Kanthan R, Senger JL. Uterine carcinosarcomas (malignant mixed mullerian tumours): a review with special emphasis on the controversies in management. Obstet Gynecol Int. 2011;2011:470795. doi: 10.1155/2011/470795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemani D, Mitra N, Guo M, Lin L. Assessing the effects of lymphadenectomy and radiation therapy in patients with uterine carcinosarcoma: a SEER analysis. Gynecol Oncol. 2008;111:82–88. doi: 10.1016/j.ygyno.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed Müllerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys. 2004;58:786–796. doi: 10.1016/S0360-3016(03)01561-X. [DOI] [PubMed] [Google Scholar]
- 7.Dusenbery KE, Potish RA, Argenta PA, Judson PL. On the apparent failure of adjuvant pelvic radiotherapy to improve survival for women with uterine sarcomas confined to the uterus. Am J Clin Oncol. 2005;28:295–300. doi: 10.1097/01.coc.0000156919.04133.98. [DOI] [PubMed] [Google Scholar]
- 8.Sartori E, Bazzurini L, Gadducci A, Landoni F, Lissoni A, Maggino T, et al. Carcinosarcoma of the uterus: a clinicopathological multicenter CTF study. Gynecol Oncol. 1997;67:70–75. doi: 10.1006/gyno.1997.4827. [DOI] [PubMed] [Google Scholar]
- 9.Yu T, Kim HJ, Wu HG, Ha SW, Song YS, Park NH, et al. Outcome analysis in patients with uterine sarcoma. Radiat Oncol J. 2015;33:29–35. doi: 10.3857/roj.2015.33.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177–185. doi: 10.1016/j.ygyno.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed NS, Mangioni C, Malmström H, Scarfone G, Poveda A, Pecorelli S, et al. European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874) Eur J Cancer. 2008;44:808–818. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Knocke TH, Weitmann HD, Kucera H, Kölbl H, Pokrajac B, Pötter R. Results of primary and adjuvant radiotherapy in the treatment of mixed Müllerian tumors of the corpus uteri. Gynecol Oncol. 1999;73:389–395. doi: 10.1006/gyno.1999.5400. [DOI] [PubMed] [Google Scholar]
- 14.Park HJ, Kim HJ, Wu HG, Kim H, Ha SW, Kang SB, et al. The influence of adjuvant radiotherapy on patterns of failure and survivals in uterine carcinosarcoma. Radiat Oncol J. 2011;29:228–235. doi: 10.3857/roj.2011.29.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayton Smith D, Kenneth Macdonald O, Gaffney DK. The impact of adjuvant radiation therapy on survival in women with uterine carcinosarcoma. Radiother Oncol. 2008;88:227–232. doi: 10.1016/j.radonc.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Sampath S, Schultheiss TE, Ryu JK, Wong JY. The role of adjuvant radiation in uterine sarcomas. Int J Radiat Oncol Biol Phys. 2010;76:728–734. doi: 10.1016/j.ijrobp.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 17.Sampath S, Gaffney DK. Role of radiotherapy treatment of uterine sarcoma. Best Pract Res Clin Obstet Gynaecol. 2011;25:761–772. doi: 10.1016/j.bpobgyn.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Wright JD, Seshan VE, Shah M, Schiff PB, Burke WM, Cohen CJ, et al. The role of radiation in improving survival for early-stage carcinosarcoma and leiomyosarcoma. Am J Obstet Gynecol. 2008;199:536.e1–536.e8. doi: 10.1016/j.ajog.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Cantrell LA, Havrilesky L, Moore DT, O’Malley D, Liotta M, Secord AA, et al. A multi-institutional cohort study of adjuvant therapy in stage I-II uterine carcinosarcoma. Gynecol Oncol. 2012;127:22–26. doi: 10.1016/j.ygyno.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993;71(Suppl):1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 21.Pautier P, Floquet A, Gladieff L, Bompas E, Ray-Coquard I, Piperno-Neumann S, et al. A randomized clinical trial of adjuvant chemotherapy with doxorubicin, ifosfamide, and cisplatin followed by radiotherapy versus radiotherapy alone in patients with localized uterine sarcomas (SARCGYN study). A study of the French Sarcoma Group. Ann Oncol. 2013;24:1099–1104. doi: 10.1093/annonc/mds545. [DOI] [PubMed] [Google Scholar]
- 22.McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer. 2002;12:687–690. doi: 10.1136/ijgc-00009577-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Galaal K, van der Heijden E, Godfrey K, Naik R, Kucukmetin A, Bryant A, et al. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database Syst Rev. 2013;(2):CD006812. doi: 10.1002/14651858.CD006812.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koukourakis MI, Papadopoulou A, Kyrgias G. Long-term survival of a patient with multiple abdominal metastasis from endometrial carcinoma treated with multi-portal conformal re-irradiation and chemotherapy. Hematol Oncol Stem Cell Ther. 2011;4:45–47. doi: 10.5144/1658-3876.2011.45. [DOI] [PubMed] [Google Scholar]
- 25.Lee YS, Kim YS, Kim JH, Ahn SD, Lee SW, Shin SS, et al. Feasibility and outcome of concurrent chemoradiotherapy for recurrent cervical carcinoma after initial surgery. Tumori. 2010;96:553–559. doi: 10.1177/030089161009600407. [DOI] [PubMed] [Google Scholar]
- 26.Yin YJ, Li HQ, Sheng XG, Du XL, Wang C, Lu CH, et al. The treatment of pelvic locoregional recurrence of cervical cancer after radical surgery with intensity-modulated radiation therapy compared with conventional radiotherapy: a retrospective study. Int J Gynecol Cancer. 2015;25:1058–1065. doi: 10.1097/IGC.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 27.Isohashi F, Mabuchi S, Yoshioka Y, Seo Y, Suzuki O, Tamari K, et al. Intensity-modulated radiation therapy versus three-dimensional conformal radiation therapy with concurrent nedaplatin-based chemotherapy after radical hysterectomy for uterine cervical cancer: comparison of outcomes, complications, and dose-volume histogram parameters. Radiat Oncol. 2015;10:180. doi: 10.1186/s13014-015-0486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu C, Zhu W, Ji Y, Guo J, Pan P, Han J, et al. A comparative study of intensity-modulated radiotherapy and standard radiation field with concurrent chemotherapy for local advanced cervical cancer. Eur J Gynaecol Oncol. 2015;36:278–282. [PubMed] [Google Scholar]
- 29.Lan ML, Yu X, Xiao H, Zhou P, Hu N, Li J, et al. Clinical outcomes and toxicity of postoperative intensity-modulated versus three-dimensional conformal radiation therapy in patients with cervical cancer. Asia Pac J Clin Oncol. 2016 Feb 28; doi: 10.1111/ajco.12476. [Epub] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier survival analysis of patients without a previous history of pelvic radiotherapy (RT) according to adjuvant RT administration. (A) Locoregional recurrence-free survival (LRRFS). The 5-year LRRFS rates were 55.9% and 57.9% for the no RT and RT groups, respectively. (B) Distant metastasis-free survival (DMFS). The 5-year DMFS rates were 62.3% and 53.8% for the no RT and RT groups, respectively. (C) Disease-free survival (DFS). The 5-year DFS rates were 52.6% and 51.9% for the no RT and RT groups, respectively. (D) Overall survival (OS). The 5-year OS rates were 73% and 61.4% for the no RT and RT groups, respectively.