Abstract
Objective
To create a comprehensive algorithmic approach to reconstruction after vulvar cancer ablative surgery, which includes both traditional and perforator flaps, evaluating anatomical subunits and shape of the defect.
Methods
We retrospectively reviewed 80 cases of reconstruction after vulvar cancer ablative surgery, performed between June 2006 and January 2016, transferring 101 flaps. We registered the possibility to achieve the complete wound closure, even in presence of very complex defects, and the postoperative complications. On the basis of these experience, analyzing the choices made and considering the complications, we developed an algorithm to help with the selection of the flap in vulvoperineal reconstruction after oncologic ablative surgery for vulvar cancer.
Results
We employed eight types of different flaps, including 54 traditional fasciocutaneous V-Y flaps, 23 rectus abdominis myocutaneous flaps, 11 anterolateral thigh flaps, three V-Y gracilis myocutaneous flaps, three free style perforators V-Y flaps from the inner thigh, two Limberg flaps, two lotus flaps, two deep inferior epigastric artery perforator flap, and one superficial circumflex iliac artery perforator flap. The structures most frequently involved in resection were vulva, perineum, mons pubis, groins, vagina, urethra and, more rarely, rectum, bladder, and lower abdominal wall.
Conclusion
The algorithm we implemented can be a useful tool to help flap selection. The key points in the decision-making process are: anatomical subunits to be covered, overall shape and symmetry of the defect and some patient features such as skin laxity or previous radiotherapy. Perforator flaps, when feasible, must be considered standard in vulvoperineal reconstruction, although in some cases traditional flaps remain the best choice.
Keywords: Algorithm, Perforator Flap, Perineal Reconstruction, Vulvar Neoplasms, Vulvar Reconstruction, Vulvoperineal Reconstruction
INTRODUCTION
Vulvar cancer ablative surgery often causes wide soft tissue defects and, despite tendency to poor wound healing, it requires fast postoperative recovery to allow for adjuvant therapies. Surgical solutions for reconstruction range from secondary healing to free tissue transfer, with pedicled flaps typically being the first choice [1,2]. Primary goals of reconstruction are tension free skin closure, with good quality tissues, maintenance of vaginal and urethral introitus without shrinkage and deviation from their central position, restoration of the anovaginal partition, and simultaneous closure of associated defects, such as mons pubis or inguinal defects if necessary. In presence of pelvic exenteration or abdominoperineal resection, pelvic support can be impaired and a variable amount of dead space may require filling to reduce the risk of complications. Secondary goals include sensitive reconstruction, sexual function, cosmetic restoration of external shape, and minimal flap donor site morbidity. Some algorithms have been proposed to help surgeons to choose among the different flaps available [3,4,5,6], but they present some drawbacks. Historically, they are mainly concerned about the dimension of the defect with minimal consideration for the associated surrounding defects, beyond vulvar and perineal edge. Groin, mons pubis, vaginal or urethral defects are often present in gynecologic surgery for vulvar cancer; this creates singular geometries of defects that must be considered tridimensionally as one single shape, in order to correctly choose the flap for reconstruction. Few papers in literature consider perforator flaps for vulvoperineal reconstruction [7,8,9]; and a true algorithmic approach, including the complete broad armamentarium of traditional and perforator flaps, has not been published. These flaps can be technically demanding but preferable in many cases, because of the longer pedicle, better mobility, and decreased donor site morbidity. Other very important features that must be considered are: possible previous radiotherapy, inner thigh skin laxity, often present if patient is elderly and not obese, asymmetry of the defect, possibly associated abdominoperineal resection or pelvic exenteration, with the possibility of fecal or urinary ostomies. We therefore decided to review our experience, with critical analysis of surgical indications and create an algorithm for flap selection based on the key points above mentioned.
MATERIALS AND METHODS
We performed a retrospective review of the patients operated for reconstruction after vulvar cancer extirpative surgery between June 2006 and January 2016. This study was approved by the Institutional Review Board of Fondazione Policlinico Gemelli. Primary closures and skin grafts were excluded. Demographic and clinical information of the patients were extracted, including age, body mass index, comorbidities (diabetes, hypertension, smoke), histological type, previous local surgery or radiotherapy, size of tumor and defect, anatomical subunits involved in the resection, type and size of flap, flap characteristics (fascia harvest, thinning, splitting), donor site closure, and postoperative complications. In the cases employing perforator flaps, we recorded number and course of perforators.
The incisions planned for extirpative surgery were always discussed preoperatively with the gynecologic oncologist to decide among the reconstructive procedures available. In the case of perforator flap planning, Doppler sonography was performed at this time, to mark the position of the vessels on the skin. The defect was always re-evaluated in the operating room at the end of ablative surgery, in lithotomy position, once the intraoperative pathologic examination had confirmed negative margins. Size, shape, structures involved and distance between the defect and pivot points of flaps or perforators marked preoperatively were considered. Following this, in the case of flaps from the inner surface of the thighs, the position wasn’t changed; while in all other types of reconstruction, lower limbs were moved down during the time of flap harvesting to achieve horizontal position of the thighs, and then moved back to lithotomy position for flap inset. After surgery, all patients were placed on bed rest, with urinary catheter, liquid diet, and loperamide in the case of reconstructions involving perianal area for 1 week. They were then allowed to stand and progressively walk.
RESULTS
A total of 101 flaps were transferred in 80 patients, for vulvoperineal reconstruction, after resection of vulvar cancer. Patients’ age ranged from 44 to 86, with mean age of 68. Thirteen patients were obese and 30 overweight. Thirty-seven were primary cases, while 43 were cancer relapses. Thirty-six patients had undergone previous radiotherapy. In 42 patients ablative surgery consisted of extended vulvectomy, in 19 cases radical vulvectomy was performed, and the remaining 19 patients underwent partial vulvectomy or hemivulvectomy. In 36 cases, there was some resection of mons pubis and in 16 cases some degree of groin defect, with seven cases of butterfly skin incision. In 60 cases, there was some vaginal resection and in 44 cases some urethral resection. Sixteen cases required abdominoperineal resection and seven cases pelvic exenteration. In three cases lower abdominal wall was involved in resection.
Pathologic examination showed in 69 cases squamous cell carcinoma, in seven cases Paget disease with small foci of infiltrating carcinoma, in two cases sarcomatoid carcinoma, in one case endometrioid carcinoma, and in one case dermatofibrosarcoma protuberans.
Eight types of flaps were transferred for vulvoperineal reconstruction, including 54 traditional fasciocutaneous V-Y flaps [10], 23 vertical rectus abdominis myocutaneous flaps (VRAM, extended VRAM, ORAM) [11], 11 anterolateral thigh flap (ALT) [12], three V-Y gracilis myocutaneous flaps [13], three free style perforators V-Y flaps from the inner thigh [7], two Limberg flaps [14], two lotus flaps [15], two deep inferior epigastric artery perforator flap (DIEP) [16], and one superficial circumflex iliac artery perforator flap (SCIP) [17]. Maximum flap size was 330 cm2 and minimum 54 cm2, with mean size of 188 cm2.
There was no case of flap loss. There were 14 cases of wound breakdown in the recipient site, of which only six requiring revisional surgery, and three cases in the donor site. Four cases of infection, one of which had arisen from cutaneous holes of brachytherapy catheters positioned during surgery, requiring reoperation with removal of catheters and washing of surgical bed.
As we could achieve in every case the complete wound closure, even in presence of very complex defects, with very low rate of postoperative complications, we developed an algorithm (Fig. 1), based on this experience, to help with the selection of the flap in vulvoperineal reconstruction, after oncologic ablative surgery for vulvar cancer.
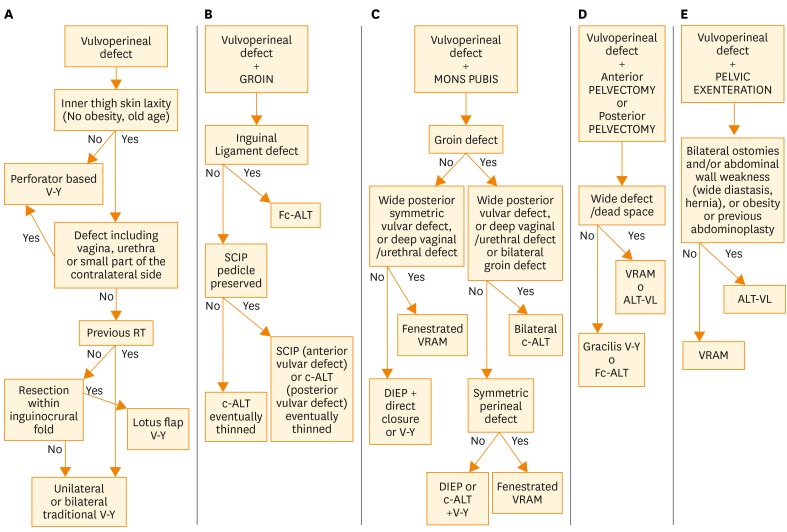
Fig. 1.
Algorithm for flap selection in vulvoperineal reconstruction after vulvar cancer ablative surgery. The five sections are related to the five possible defects associated with the vulvar one. ALT-VL, anterolateral thigh flap with vastus lateralis; c-ALT, cutaneous anterolateral thigh; DIEP, deep inferior epigastric perforator; Fc-ALT, fasciocutaneous anterolateral thigh; RT, radiotherapy; SCIP, superficial circumflex iliac perforator; VRAM, vertical rectus abdominis myocutaneous.
As a first step, we must consider whether the vulvoperineal defect is associated with the resection of other anatomical subunits or not. If the defect is solely vulvar (Fig. 1A), we must then evaluate the inner thigh skin laxity, typically more frequent in normal weight, or thin, old age women. If skin laxity is absent, our first choice is a perforator based V-Y flap from the inner thigh, from one side or both sides, according to the symmetry of the defect. We choose a perforator V-Y flap even when, despite the presence of skin laxity, more mobility than a standard V-Y flap is required. A good example of this is in the case of deep vaginal or urethral defects. Another would be in the case of an asymmetrical defect on the contralateral side which doesn’t necessarily require a contralateral flap, but direct closure would distort the central position of the urethra. In this case, this flap can often cross the midline, encompassing the urethral outlet and avoiding a contralateral flap (Fig. 2). If skin laxity is present, and the greater mobility of a perforator based flap is not required, we must consider the following: in case of previous radiotherapy, we perform a traditional V-Y flap on one side or both, according to the symmetry of defect; in the absence of previous radiotherapy, we perform a lotus flap, provided that the pedicle has not been damaged during resection. In the case of a damaged pedicle, a traditional V-Y flap is required.
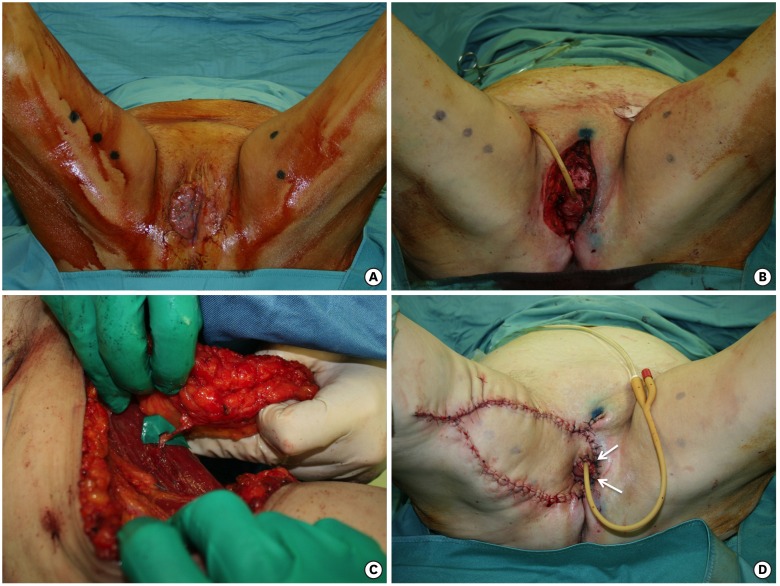
Fig. 2.
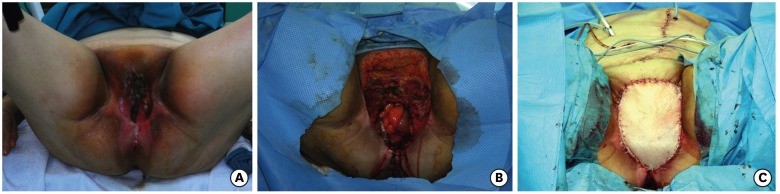
(A) Tumor on the right hemivulva. (B) Radical vulvectomy, wider on the right side. (C) The flap harvested on the medial surface of the right thigh, pedicled on a medial circumflex femoral artery perforator. (D) The flap advanced in a V-Y fashion. The white arrows indicate the part of the flap that goes beyond the midline to surround the urethra and vagina.
When significant involvement of lymph nodes is present, vulvar defect is often associated with groin defect. Resection can affect inguinal skin, the inguinal ligament or simply create wide subcutaneous dead space. In advanced or heavily irradiated cases, even “butterfly incision” is still employed in clinical practice. In the presence of groin defect (Fig. 1B), we must consider whether the inguinal ligament has been damaged; this can occur when positive lymph nodes are in contact with it. If inguinal ligament is undamaged, our first choice is a cutaneous ALT flap, thinned on the plane of fascia superficialis when necessary, or a SCIP flap, if the perforator of the deep branch of the superficial circumflex iliac artery has been preserved. When the inguinal ligament requires reconstruction, we must perform Fasciocutaneous ALT flap, employing the fascia lata to repair the ligament.
When vulvar defect is associated with mons pubis defect (Fig. 1C), we must check the groin region for possible defects. If there are no defects requiring filling in the groins, we prefer an abdominal flap; the choice between DIEP and VRAM is taken according to the need of fenestrating the flap for urethra, vaginal or anal outlet. When the vulvoperineal defect is posterior and symmetric, and flap must be centrally fenestrated, particularly if deep vaginal or urethral resection or a wide defect between anus and vagina are present, we prefer a VRAM flap; reserving DIEP flap to not fenestrated reconstruction, such as anterior vulvar defects or asymmetric defects which allow unilateral DIEP flap inset on the wider side, and contralateral direct closure or V-Y flap.
Conversely, if it is necessary to repair a cutaneous or subcutaneous groin resection, we must evaluate the posterior extension and shape of the perineal defect. If posterior perineal defect is not very wide but is symmetrical, we employ a fenestrated VRAM flap, shield- or banana-shaped, to cover perineum and one or both groins at the same time. If posterior perineal defect is not wide, and is asymmetric, so that it allows the reconstruction of the smaller side with a local flap and one of the groins can be repaired by direct closure, we usually perform a V-Y flap on the smaller side and a banana-shaped cutaneous ALT flap or DIEP flap is employed to repair both perineum and groin on the side of the greater defect. When posterior perineal defect is wide, or simultaneous deep vaginal or urethral defects are present, or groin defect is bilateral, with neither of the two sides allowing direct closure, then we must perform bilateral cutaneous ALT flaps.
In the case of anterior or posterior pelvectomy (Fig. 1D), we evaluate the width of the defect and the presence of dead space; when there is the need to fill wide defect or dead space, we can choose between VRAM flap or ALT flap with vastus lateralis; in absence of wide defect or dead space, our choice is between a fasciocutaneous ALT flap and gracilis V-Y myocutaneous flaps.
When pelvic exenteration is performed (Fig. 1E), there is usually a big dead space to fill and patients have often been previously irradiated. From a reconstructive point of view, a VRAM flap, with possible endopelvic course would likely offer the best reconstructive option. However, very often there is the need for bilateral ostomies on the abdominal wall. In these cases, we prefer not to employ abdominal flaps, and we choose ALT flap with muscular component of vastus lateralis, possibly harvested on different perforators (Fig. 3).
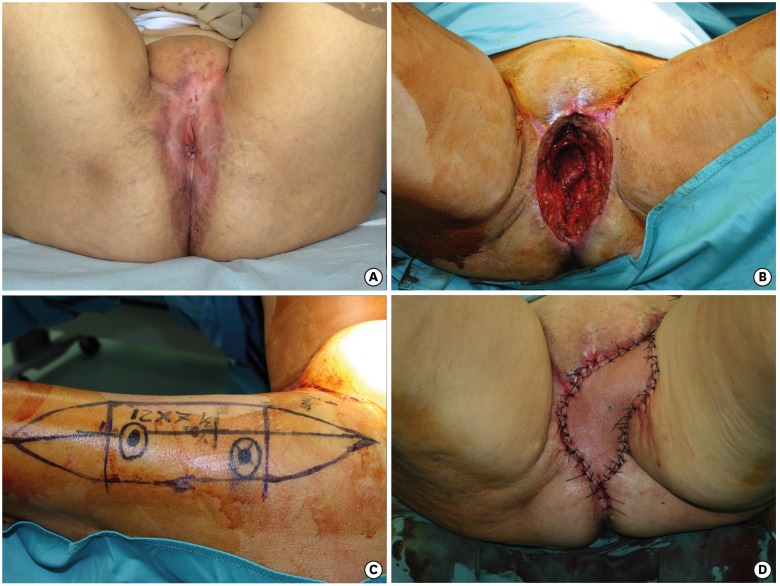
Fig. 3.
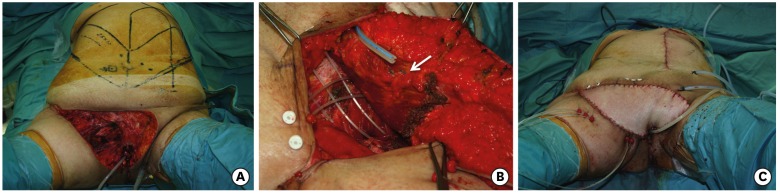
(A) Vulvar cancer relapse inward toward the pelvis, after previous surgery and radiotherapy, showing chronic radiodermatitis of the perineal skin. (B) Pelvic exenteration with pelvic floor defect and dead space. (C) Anterolateral thigh flap, with Vastus Lateralis, planned on the left thigh with two perforators seen with Doppler sonography. (D) Flap inset. The skin, damaged by radiotherapy, has been removed and replaced by the flap.
DISCUSSION
Excluding skin grafts, which we propose only for skin vulvectomy in the case of Paget disease, where there is a thin defect and a high rate of relapse, pedicled flaps are always the best solution for vulvoperineal reconstruction. In our algorithm, the Limberg flap is not included because we believe that its employment is only appropriate in partial vulvectomy to repair the space between vagina and anus.
In other previous algorithms, lotus flap, V-Y flap, and VRAM flap were reported to be the workhorses for vulvoperineal reconstruction [3,4,9]. Although these three flaps together cover about 80% of defects resulting from vulvar cancer resection, this assumption was made before the popularization of perforator flaps in the perineal area, and by papers evaluating only the size of defect as the main guideline for flap selection [3,4,5,6].
The new concept that’s behind our approach is that ablative surgery for vulvar cancer does not involve only vulvoperineal area, but very often it also affects close structures, and the combination of the anatomical subunits involved creates every time a particular configuration of the defect that restricts the indications of the different flaps. Therefore we must consider the defect not only for the size, as in the past other authors reported, but for its shape, moving forward to the next step of the algorithm when a further anatomical subunit is involved in the resection.
The second important point, that is a consequence of the first, is including among reconstructive options both traditional and perforator flaps. In fact, considering in our algorithm a bigger range of defects we need more reconstructive options. Moreover, surgery of perforators increases the possible uses of traditional flaps, such as traditional V-Y flap, and introduced the use of new flaps, with minor morbidity and longer pedicle such as ALT flap or DIEP flap.
The principles of our algorithmic approach derive from the analysis case-by-case of which flap could allow tension free wound closure, for equal combination of anatomical subunits resected, with the lowest possible donor site morbidity and complications incidence. From these principles we have assumed some surgical indications of the different flaps for the anatomical subunits potentially involved in resection. The structures most frequently involved by vulvar cancer ablative surgery are vulva, perineum, mons pubis, groins, vagina, urethra and, more rarely, rectum, bladder, and lower abdominal wall.
Vulva and perineum can be well repaired by lotus flaps and traditional V-Y flaps. When more mobility than a standard V-Y flap is required, as in the case of deep vaginal or urethral resection, we prefer a perforator based V-Y flap. These flaps, advanced from the inner thighs, based on perforators arising from medial circumflex femoral artery, or profunda fermoris artery, can be harvested with a free style method, gaining much more mobility if compared to traditional V-Y, while still allowing an easy donor site closure, if compared to standard perforator based island flaps. This increased mobility is at times such that it is possible to avoid a contralateral flap, when minor defects are present around the urethra or vagina still preventing lateral displacement of their outlets.
When a mons pubis defect is added to vulvoperineal resection, V-Y flaps can become insufficient. This area is well covered by abdominal flaps and ALT flap. According to the geometry of the defect and to the need of fenestrating the flap for vaginal or urethral outlets we can choose between DIEP, VRAM, or ALT flap. DIEP flap is more delicate than VRAM flap, and should not be fenestrated but reserved for unilateral reconstruction.
ALT flap is probably one of the most useful and versatile perforator flaps for vulvoperineal reconstruction; it can reach mons pubis, vulva, perineum, lower abdominal wall, and groin area with strong axial vascularization that allows for thinning and splitting the flap, avoiding sometimes contralateral flaps [18] (Fig. 4). When distal perforators are not available, it is possible to draw the flap not centered on the perforator to increase the length of the pedicle.
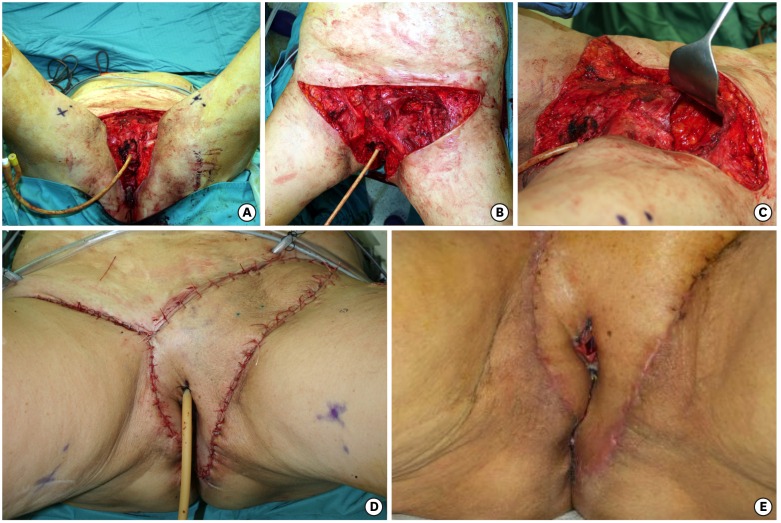
Fig. 4.
(A-C) Vulvoperineal, mons pubis and groins defect, with femoral vessels exposure and partial removal of the left inguinal ligament for cancer relapse after surgery and radiotherapy. (D) Fasciocutaneous anterolateral thigh flap, from left thigh, repairing the defect. Fascia lata reconstructed the inguinal ligament. The flap has been thinned and split. (E) Postoperative view shows uneventful wound healing of the split part of the flap.
Another good option for inguinal, mons pubis and anterior vulvar area is the SCIP flap. In our series, only one SCIP flap was executed because we have only recently introduced this flap in our surgical routine, but when its pedicle is not severed during lymphadenectomy, we believe that it can be very useful for defects including groins, mons pubis and anterior vulva, thanks to its easy and fast dissection and the possibility to rotate it as a propeller flap.
In the presence of abdominoperineal resection, anterior pelvectomy or pelvic exenteration, we need to fill dead space. Therefore, myocutaneous flaps, including gracilis, VRAM, or ALT with vastus lateralis must be preferred. We believe that gracilis flap with proximal skin island, advanced in a V-Y fashion, is very useful for posterior defects following abdominoperineal resection and it is safer than traditional gracilis flap, with a distal skin paddle, for the lower risk of vascular complications [19]. VRAM flap with endopelvic course is the first choice in the case of pelvic exenteration, particularly in presence of previous radiotherapy. However, we prefer to avoid VRAM flap, choosing ALT flap with vastus lateralis, if there is evidence of abdominal weakness that could be further worsened by bilateral ostomies after rectus abdominis sacrifice.
In our opinion, patients must always be evaluated preoperatively together with the oncologic gynecologist to plan incisions precisely. Perforator flaps are an important additional surgical tool. However, we do believe that, in some cases, traditional myocutaneous flaps remain the best choice: when there is wide dead space to fill between pelvis and perineum, preferring endopelvic course of VRAM flap (Fig. 5), when there is the need of central fenestration in the flap and when brachytherapy catheters must be positioned on the surgical bed; in this last case, the catheters must be separated by at least 1 cm. from vessels, and muscular belly can help to protect pedicle (Fig. 6). When possible, we always prefer to keep two different solutions available in the surgical plan, to have a spare wheel in case of complications or unsuitable perforators.
Fig. 5.
(A) Massive cancer relapse after vulvectomy and radiotherapy. (B) Pelvic exenteration with wide dead space and complete loss of pelvic floor. (C) Vertical rectus abdominis myocutaneous flap with endopelvic course.
Fig. 6.
(A) Wide resection of upper vulva, mons pubis and right groin for cancer relapse after surgery and radiotherapy. (B) Brachytherapy catheters positioned in the surgical bed. The muscular belly of vertical rectus abdominis myocutaneous flap (white arrow) separating subcutaneous fat by catheters. (C) Inset of the flap accomplished.
In conclusion, when approaching reconstruction after vulvar cancer ablative surgery we believe that not only the dimension, but mainly the geometry of the defect and the subunits involved in the resection must guide the flap choice. We also think that both traditional and perforator flaps must be included as first line option for reconstruction.Lotus flaps, traditional V-Y flaps and perforator based V-Y flaps are the best option for defects limited to the vulvoperineal area. VRAM flap, DIEP flap, ALT flap, or SCIP flap are the most useful options when groin or mons pubis defects are associated to vulvoperineal resection. In presence of concomitant abdominoperineal resection, anterior pelvectomy or pelvic exenteration, myocutaneous flaps such as gracilis flap, VRAM flap, or ALT flap with vastus lateralis better fill dead space, particularly in the case of previous radiotherapy.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Hollenbeck ST, Toranto JD, Taylor BJ, Ho TQ, Zenn MR, Erdmann D, et al. Perineal and lower extremity reconstruction. Plast Reconstr Surg. 2011;128:551e–563e. doi: 10.1097/PRS.0b013e31822b6b87. [DOI] [PubMed] [Google Scholar]
- 2.Galandiuk S, Jorden J, Mahid S, McCafferty MH, Tobin G. The use of tissue flaps as an adjunct to pelvic surgery. Am J Surg. 2005;190:186–190. doi: 10.1016/j.amjsurg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Salgarello M, Farallo E, Barone-Adesi L, Cervelli D, Scambia G, Salerno G, et al. Flap algorithm in vulvar reconstruction after radical, extensive vulvectomy. Ann Plast Surg. 2005;54:184–190. doi: 10.1097/01.sap.0000141381.77762.07. [DOI] [PubMed] [Google Scholar]
- 4.John HE, Jessop ZM, Di Candia M, Simcock J, Durrani AJ, Malata CM. An algorithmic approach to perineal reconstruction after cancer resection--experience from two international centers. Ann Plast Surg. 2013;71:96–102. doi: 10.1097/SAP.0b013e3182414485. [DOI] [PubMed] [Google Scholar]
- 5.Friedman J, Dinh T, Potochny J. Reconstruction of the perineum. Semin Surg Oncol. 2000;19:282–293. doi: 10.1002/1098-2388(200010/11)19:3<282::aid-ssu10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Saleh DB, Liddington MI, Loughenbury P, Fenn CW, Baker R, Burke D. Reconstruction of the irradiated perineum following extended abdomino-perineal excision for cancer: an algorithmic approach. J Plast Reconstr Aesthet Surg. 2012;65:1537–1543. doi: 10.1016/j.bjps.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Huang JJ, Chang NJ, Chou HH, Wu CW, Abdelrahman M, Chen HY, et al. Pedicle perforator flaps for vulvar reconstruction--new generation of less invasive vulvar reconstruction with favorable results. Gynecol Oncol. 2015;137:66–72. doi: 10.1016/j.ygyno.2015.01.526. [DOI] [PubMed] [Google Scholar]
- 8.Zelken JA, AlDeek NF, Hsu CC, Chang NJ, Lin CH, Lin CH. Algorithmic approach to lower abdominal, perineal, and groin reconstruction using anterolateral thigh flaps. Microsurgery. 2016;36:104–114. doi: 10.1002/micr.22354. [DOI] [PubMed] [Google Scholar]
- 9.Negosanti L, Sgarzani R, Fabbri E, Palo S, Oranges CM, De Iaco P, et al. Vulvar reconstruction by perforator flaps: algorithm for flap choice based on the topography of the defect. Int J Gynecol Cancer. 2015;25:1322–1327. doi: 10.1097/IGC.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 10.Carramaschi F, Ramos ML, Nisida AC, Ferreira MC, Pinotti JA. V-Y flap for perineal reconstruction following modified approach to vulvectomy in vulvar cancer. Int J Gynaecol Obstet. 1999;65:157–163. doi: 10.1016/s0020-7292(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 11.Carlson JW, Carter JR, Saltzman AK, Carson LF, Fowler JM, Twiggs LB. Gynecologic reconstruction with a rectus abdominis myocutaneous flap: an update. Gynecol Oncol. 1996;61:364–368. doi: 10.1006/gyno.1996.0157. [DOI] [PubMed] [Google Scholar]
- 12.Maxhimer JB, Hui-Chou HG, Rodriguez ED. Clinical applications of the pedicled anterolateral thigh flap in complex abdominal-pelvic reconstruction. Ann Plast Surg. 2011;66:285–291. doi: 10.1097/SAP.0b013e3181e78711. [DOI] [PubMed] [Google Scholar]
- 13.Peled IJ. Reconstruction of the vulva with V-Y advanced myocutaneous gracilis flap. Plast Reconstr Surg. 1990;86:1014–1016. doi: 10.1097/00006534-199011000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Burke TW, Morris M, Levenback C, Gershenson DM, Wharton JT. Closure of complex vulvar defects using local rhomboid flaps. Obstet Gynecol. 1994;84:1043–1047. [PubMed] [Google Scholar]
- 15.Ragoowansi R, Yii N, Niranjan N. Immediate vulvar and vaginal reconstruction using the gluteal-fold flap: long-term results. Br J Plast Surg. 2004;57:406–410. doi: 10.1016/j.bjps.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Cheng A, Saint-Cyr M. Split and thinned pedicle deep inferior epigastric perforator (DIEP) flap for vulvar reconstruction. J Reconstr Microsurg. 2013;29:277–282. doi: 10.1055/s-0032-1333322. [DOI] [PubMed] [Google Scholar]
- 17.Koshima I, Nanba Y, Tsutsui T, Takahashi Y, Urushibara K, Inagawa K, et al. Superficial circumflex iliac artery perforator flap for reconstruction of limb defects. Plast Reconstr Surg. 2004;113:233–240. doi: 10.1097/01.PRS.0000095948.03605.20. [DOI] [PubMed] [Google Scholar]
- 18.Gentileschi S, Servillo M, Garganese G, Simona F, Scambia G, Salgarello M. Versatility of pedicled anterolateral thigh flap in gynecologic reconstruction after vulvar cancer extirpative surgery. Microsurgery. 2016 Jun 08; doi: 10.1002/micr.30077. [DOI] [PubMed] [Google Scholar]
- 19.Core GB, Finan MA, Kline RC. Turbo-gracilis myocutaneous flap for perineal reconstruction. Gynecol Oncol. 1997;64:256–261. doi: 10.1006/gyno.1996.4521. [DOI] [PubMed] [Google Scholar]