Abstract
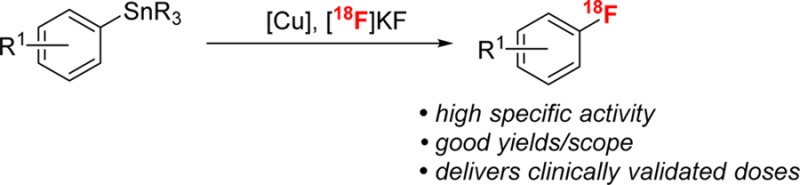
A copper-mediated nucleophilic radiofluorination of aryl- and vinylstannanes with [18F]KF is described. This method is fast, uses commercially available reagents, and is compatible with both electron-rich and electron-deficient arene substrates. This method has been applied to the manual synthesis of a variety of clinically relevant radiotracers including protected [18F]F-phenylalanine and [18F]F-DOPA. In addition, an automated synthesis of [18F]MPPF is demonstrated that delivers a clinically validated dose of 200 ± 20 mCi with a high specific activity of 2400 ± 900 Ci/mmol.
Positron emission tomography (PET) imaging is a noninvasive diagnostic imaging technique that provides in vivo physiochemical information.1−4 Fluorine-18 (18F) is the most commonly used radionuclide for PET, largely due to its attractive half-life of 110 min. This half-life enables the sophisticated synthesis of target molecules, allows distribution of 18F-labeled tracers to facilities that lack cyclotrons, and facilitates in vivo pharmacokinetic studies.2 With the increasing number of PET scans, there is a need for new, practical methods for the late-stage introduction of 18F into bioactive molecules. Methods that enable the nucleophilic radiofluorination of readily accessible and stable precursors are in particularly high demand due to the availability of [18F]fluoride from small medical cyclotrons.5,6
Arylstannanes are appealing precursors for PET radiolabeling for a number of reasons. First, they can be easily prepared from inexpensive starting materials,7,8 and important examples are already known intermediates and/or commercially available (i.e., TriBoc-l-DOPA methyl ester).9 Second, the Sn–C bond is stable to most functional group manipulations,10,11 which enables flexible and modular syntheses of complex precursors that can then undergo selective late-stage radiofluorination. Third, arylstannanes have already been validated as precursors to radiopharmaceuticals for human clinical trials,12−15 thereby mitigating concerns about toxicity and the feasibility of Sn removal. However, to date, the major limitation in this field is that existing methods for the fluorination16,17 and radiofluorination18,19 of arylstannanes require electrophilic fluorine sources (e.g., F2, N-fluoropyridinium salts, N-fluorobenzenesulfonimide, Selectfluor) (Scheme 1a).20 These reagents are much more expensive and less available than fluoride (particularly in the 18F form). Furthermore, electrophilic radiofluorination methods result in products with dramatically lower specific activity (ratio of 18F/19F), which greatly reduces imaging sensitivity.3
Scheme 1. Radiofluorination of Arylstannanes.
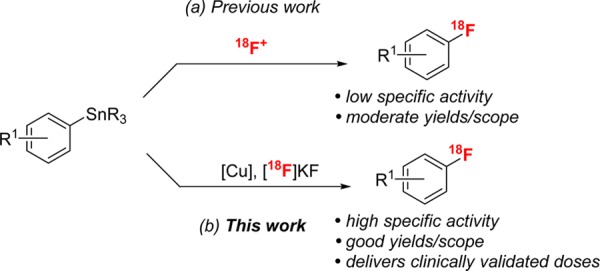
We sought to address this limitation through the development of a method for the Cu-mediated nucleophilic radiofluorination of arylstannanes (Scheme 1b). This work builds on recent reports from our group6e,21 and others6c,6f demonstrating a related Cu-mediated (radio)fluorination of arylboron precursors. In addition to the generally attractive features of arylstannane precursors discussed above, we anticipated that they would be particularly well suited for Cu-mediated radiofluorination. Specifically, a key fundamental step of the Cu-mediated process, the transmetalation of the aryl group to Cu, is expected to proceed significantly faster from tin versus boron, which should result in faster reaction rates (crucial for radiolabeling applications) as well as fewer side reactions and higher yields.22,23 We report herein the development, optimization, and scope of the Cu-mediated radiofluorination of arylstannanes with [18F]KF. This transformation exhibits high functional group tolerance and has been applied to the late-stage radiofluorination of a number of complex molecules. Furthermore, it has been scaled to an automated synthesis module and used to prepare a clinically validated high specific activity dose of the radiotracer 2′-methoxyphenyl-(N-2′-pyridinyl)-p-18F-fluorobenzamidoethylpiperazine ([18F]MPPF).
Our initial investigations focused on the Cu(OTf)2-mediated fluorination of 1-SnBu3 with KF under conditions analogous to those demonstrated for aryltrifluoroborate substrates.21 After 18 h at 60 °C with 4 equiv of KF in CH3CN, the fluorinated product 1 was obtained in 42% yield as determined by 19F NMR spectroscopy (Table 1, entry 1). While this yield is lower than that reported with 1-BF3K under closely analogous conditions (70%, 20 h), the addition of 18-crown-6 as a phase-transfer catalyst could be used to boost the yield with 1-SnBu3 to 55% (entry 2). Furthermore, as predicted, the fluorination of 1-SnBu3 is significantly faster than that of 1-BF3K. For example, 1-SnBu3 affords 51% yield after just 15 min under these conditions. In contrast, the analogous reaction of 1-BF3K requires more than 2 h to afford 50% yield. The faster rate with 1-SnBu3 is highly desirable for PET applications. We also examined the sensitivity of this reaction to the stoichiometry of fluoride. This is another key consideration for translation because [18F]fluoride is typically the limiting reagent during radiofluorination. We were encouraged to see that this reaction still proceeds (albeit in reduced yield) with KF as the limiting reagent (Table 1, entry 6).
Table 1. Cu-Mediated Nucleophilic Fluorination of Arylstannanes in Acetonitrile with Excess Fluoridea.
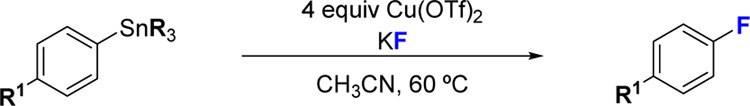
| entry | R | R1 | time (h) | additive | KF (equiv) | yield (%) |
|---|---|---|---|---|---|---|
| 1 | Bu | F | 18 | none | 4 | 42 |
| 2 | Bu | F | 18 | 18-crown-6 | 4 | 55 |
| 3 | Bu | F | 2 | none | 4 | 42 |
| 4 | Bu | F | 2 | 18-crown-6 | 4 | 53 |
| 5 | Bu | F | 0.25 | 18-crown-6 | 4 | 51 |
| 6 | Bu | F | 0.25 | 18-crown-6 | 0.5 | 15b |
| 7 | Bu | MeO | 0.25 | 18-crown-6 | 4 | 23 |
| 8 | Me | MeO | 0.25 | 18-crown-6 | 4 | 24 |
| 9 | Bu | Ph | 0.25 | 18-crown-6 | 4 | 34 |
| 10 | Me | Ph | 0.25 | 18-crown-6 | 4 | 64 |
| 11 | Bu | Ac | 0.25 | 18-crown-6 | 4 | 57 |
| 12 | Me | Ac | 0.25 | 18-crown-6 | 4 | 65 |
General conditions: Arylstannane (0.025 mmol, 1 equiv), Cu(OTf)2 (4 equiv), KF, 18-crown-6 (4 equiv), CH3CN (0.083 M), 60 °C. Yield determined by 19F NMR spectroscopic analysis of the crude reaction mixture using 1,2-difluorobenzene as an internal standard.
Yield based on KF.
We next applied this fluorination to a small set of arylstannanes (Table 1, entries 7–12) to establish the effectiveness of our 15 min nucleophilic fluorination protocol for electronically diverse arylstannanes and examine the impact of the alkyl substituents on tin (R) on the reaction. As shown in Table 1 (entries 7–12), stannanes bearing electron-donating (p-MeO), electron-neutral (p-Ph), and electron-withdrawing (p-Ac) substituents react to form fluorinated products in moderate to good yields within just 15 min at 60 °C. The p-MeO derivative is particularly noteworthy, as the corresponding aryltrifluoroborate is poorly reactive, affording <5% yield under any of the fluorination conditions examined.21 Substitution of the alkyl group on the stannane had a significant impact on yield over this short reaction time, with the Me-substituted stannanes affording comparable or higher yield than the Bu derivatives in all cases. This likely reflects a faster rate of transmetalation from the less hindered tin center.24
We next focused on translating this nucleophilic fluorination to achieve the 18F-fluorination of 3-SnBu3. As is common in the radiofluorination field,3,25 significant reoptimization was required, as the best cold fluorination conditions afforded no detectable 18F-labeled product (Table 2, entry 1). This is likely a consequence of the dramatic change in fluoride stoichiometry (from 0.3 M with KF to approximately 1 nM with [18F]KF).26 Three modifications were found to be critical for achieving radiofluorination in this system: (1) changing from CH3CN to an amide-based solvent (DMF or DMA); (2) the addition of pyridine (which was also an important additive in related radiofluorinations of arylborons);6e and (3) increasing the reaction temperature to between 110 and 140 °C. The optimal radiofluorination conditions for 3-SnBu3 were as follows: 2 equiv of Cu(OTf)2, 15 equiv of pyridine in 0.1 M DMA at 140 °C. This afforded [18F]3 in 65 ± 2% radiochemical conversion (RCC) within just 5 min (entry 8).
Table 2. Cu-Mediated Nucleophilic Fluorination of 3-SnBu3 with [18F]KFa.
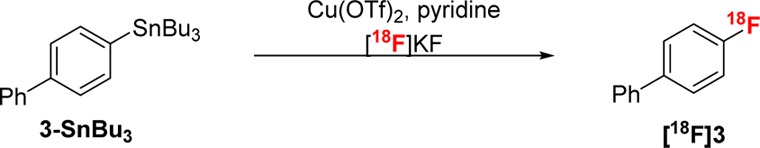
| entry | solvent | temp (°C) | pyridine (equiv) | RCCb (%) |
|---|---|---|---|---|
| 1 | CH3CN | 60 | 0 | nd |
| 2 | CH3CN | 60 | 50 | nd |
| 3 | DMF | 60 | 50 | nd |
| 4 | DMF | 110 | 50 | 22 ± 2 |
| 5 | DMA | 110 | 50 | 51 ± 1 |
| 6 | DMA | 110 | 15 | 53 ± 1 |
| 7 | DMA | 140 | 15 | 55 ± 10 |
| 8c | DMA | 140 | 15 | 65 ± 2 |
| 9d | DMA | 140 | 15 | 44 ± 1 |
General conditions: 3-SnBu3 (0.01 mmol, 1 equiv), Cu(OTf)2 (2 equiv), pyridine (15 equiv), [18F]KF, DMA (0.01 M), 140 °C, 30 min. RCC determined by radio-TLC (n ≥ 2).
nd = no product detected by radio-TLC or HPLC.
Reaction time = 5 min.
3-SnMe3 used as substrate.
The optimal conditions were applied to a series of aryl-, heteroaryl-, and vinylstannane precursors. As summarized in Figure 1, this method is compatible with aromatic substrates bearing electron-neutral (3), electron-withdrawing (4, 13), and electron-donating substituents (2, 5–10) as well as heteroaromatic (11) and vinylstannane (12) derivatives. Substrates such as 13-SnBu3 include a functional handle that can be used for further elaboration. Ortho-substitution was well-tolerated, with the o-MeO substrate 6-SnBu3 affording a yield comparable to that of the p-MeO substrate 2-SnBu3 (RCC = 57% versus 48%, respectively). This is in contrast to other metal-mediated nucleophilic radiofluorinations, which typically afford much lower yields with ortho-substituted aromatic substrates (see Table S20). The electron-rich substrates (2, 5–10) are noteworthy, as they are challenging to radiofluorinate using traditional SNAr.1 Overall, the RCCs are comparable, and in many cases significantly higher, than those for the state-of-the-art metal-mediated radiofluorinations of analogous substrates (see Table S20 for a comparison of metal-mediated nucleophilic radiofluorination methods).
Figure 1.
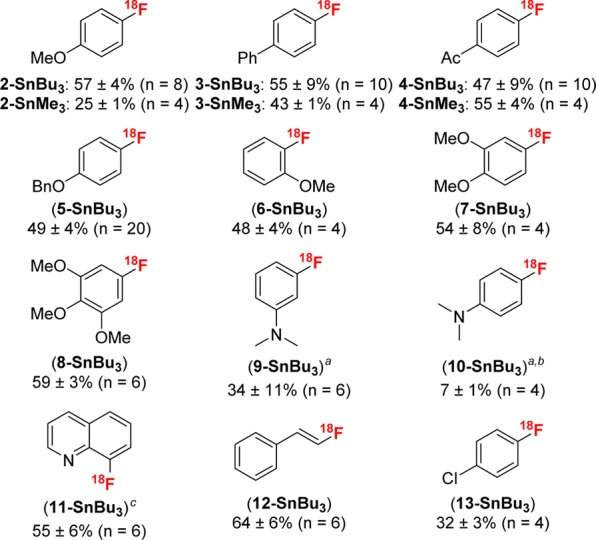
Scope of arylstannane substrates. Conditions: aryltributyltin substrate (0.01 mmol, 1 equiv), Cu(OTf)2 (2 equiv), pyridine (15 equiv), [18F]KF, DMA (0.01 M), 140 °C, 5–30 min. RCC determined by radio-TLC; (a) 100 °C; (b) 18-crown-6 (0.5 equiv); (c) 30 equiv of pyridine.
We next applied our method to the preparation of products currently being evaluated as radiotracers in clinical trials or already FDA approved.27,37 Initial studies were conducted via manual synthesis, which provided useful RCCs (Figure 2). For instance, this method is effective for the synthesis of protected phenylalanine derivatives ([18F]14 and [18F]15) for studying amino acid transport.28 We also targeted 3-[18F]fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile ([18F]F-PEB, [18F]18), whose previous radiosyntheses suffer from low yields (1–5% RCY, nondecay corrected).6e,29−31 Our new method, starting from the readily available and stable arylstannane precursor 18-SnBu3, delivers the product in 11 ± 2% RCC.
Figure 2.
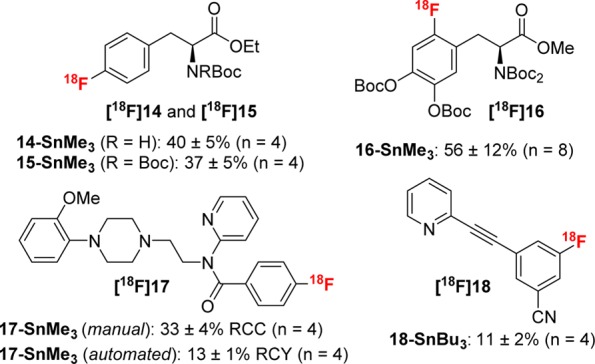
Substrate scope of relevant radiotracers.
This method is also effective for the radiofluorination of the protected l-DOPA stannane 16-SnMe3, affording [18F]16 in 56 ± 12% yield. This result is noteworthy for several reasons. First, nucleophilic methods for radiolabeling l-DOPA remain highly sought after,1,28,32,33 and the obtained RCC is among the best reported for this type of transformation (see Figure S10 for a comparison of nucleophilic methods for [18F]F-DOPA derivatives). Second, the precursor 16-SnMe3 is a single step from a commercial stannane whose derivatives have been used in the clinical production of [18F]F-DOPA via electrophilic radiofluorination.12,13 As such, the successful radiofluorination of 16-SnMe3 offers the potential for a direct nucleophilic replacement for this method.
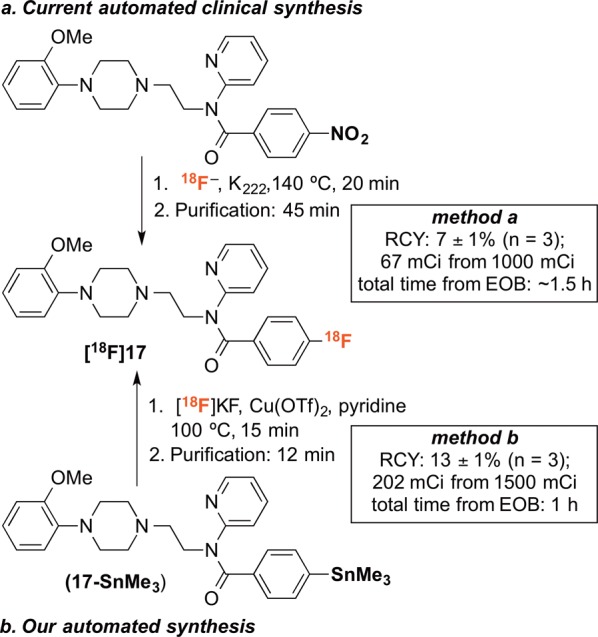
Finally, we targeted [18F]MPPF ([18F]17), a serotonin receptor ligand currently synthesized by SNAr radiofluorination of the NO2 precursor (Scheme 2a). Our manual Cu-mediated procedure from stannane 17-SnMe3 delivered [18F]17 in 33 ± 4% RCC. The [18F]MPPF synthesis was scaled and automated using a TRACERLab FXFN module (Scheme 2b). The reaction using 1500 mCi of initial activity afforded a formulated and validated 200 ± 20 mCi dose (13% RCY) after radiolabeling and HPLC purification.34 The dose prepared by this method passed all cGMP quality control testing necessary for clinical use, as outlined in the US Pharmacopeia and 21CFR212, including residual Cu and Sn levels below the allowed limits specific in the ICH Guidelines (see the Supporting Information for complete information).6g,35 As shown in Scheme 2, our new method affords nearly double the RCY and reduces the overall time from end of bombardment (EOB) to end of purification by one-third relative to the current commercial synthesis of this tracer.36
Scheme 2. Comparison of Current Methods To Synthesize [18F]MPPF ([18F]17)36,37.
In conclusion, this paper discloses a mild, copper-mediated method for the nucleophilic fluorination and radiofluorination of arylstannanes. This method represents the first practical nucleophilic fluorination of stannanes using 18F, is compatible with aryl, heteroaryl, and vinylstannanes, and fills an important gap in available late-stage fluorination methods. Furthermore, this process can be readily automated and scaled on a commercial radiochemistry synthesis module and applied to clinically relevant radiotracers. Finally, we have shown that the method is tolerant of a reasonable number of functional groups common in drug targets. To expand the utility further, exploration of the compatibility of this method with medicinal chemistry space is ongoing and will be reported in due course.
Acknowledgments
We acknowledge the NIH (R01EB021155 to M.S.S. and P.J.H.S.), US DOE/NIBIB (DE-SC0012484 to P.J.H.S.), and Merck (M.S.S. and P.J.H.S.) for financial support. We thank Sydonie D. Schimler (U. of Michigan) for conducting comparative studies of the fluorination of p-methoxyphenyl trifluoroborate.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.6b02911.
Experimental procedures, optimization details, radio-HPLC/TLC traces, and spectral data for all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Brooks A. F.; Topczewski J. J.; Ichiishi N.; Sanford M. S.; Scott P. J. H. Chem. Sci. 2014, 5, 4545–4553. 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. W.; Long N. J.; Vilar R.; Gee A. D. Angew. Chem., Int. Ed. 2008, 47, 8998–9033. 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- Campbell M. G.; Ritter T. Chem. Rev. 2015, 115, 612–633. 10.1021/cr500366b. [DOI] [PubMed] [Google Scholar]
- Preshlock S.; Tredwell M.; Gouverneur V. Chem. Rev. 2016, 116, 719–766. 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- For recent examples of uncatalyzed nucleophilic radiofluorinations of arenes, see:; a Mu L.; Fischer C. R.; Holland J. P.; Becaud J.; Schubiger P. A.; Schibli R.; Ametamey S. M.; Graham K.; Stellfeld T.; Dinkelborg L. M.; Lehmann L. Eur. J. Org. Chem. 2012, 2012, 889–892. 10.1002/ejoc.201101730. [DOI] [Google Scholar]; b Rotstein B. H.; Stephenson N. A.; Vasdev N.; Liang S. H. Nat. Commun. 2014, 5, 4365–4371. 10.1038/ncomms5365. [DOI] [PubMed] [Google Scholar]; c Stephenson N. A.; Holland J. P.; Kassenbrock A.; Yokell D. L.; Livni E.; Liang S. H.; Vasdev N. J. Nucl. Med. 2015, 56, 489–492. 10.2967/jnumed.114.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Rotstein B. H.; Wang L.; Liu R. Y.; Patteson J.; Kwan E. E.; Vasdev N.; Liang S. H. Chem. Sci. 2016, 7, 4407–4417. 10.1039/C6SC00197A. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Neumann C. N.; Hooker J. M.; Ritter T. Nature 2016, 534, 369–373. 10.1038/nature17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent examples of metal-mediated nucleophilic radiofluorination of arenes, see:; a Lee E.; Kamlet A. S.; Powers D. C.; Neumann C. N.; Boursalian G. B.; Furuya T.; Choi D. C.; Hooker J. M.; Ritter T. Science 2011, 334, 639–642. 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee E.; Hooker J. M.; Ritter T. J. Am. Chem. Soc. 2012, 134, 17456–17458. 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tredwell M.; Preshlock S. M.; Taylor N. J.; Gruber S.; Huiban M.; Passchier J.; Mercier J.; Genicot C.; Gouverneur V. Angew. Chem., Int. Ed. 2014, 53, 7751–7755. 10.1002/anie.201404436. [DOI] [PubMed] [Google Scholar]; d Ichiishi N.; Brooks A. F.; Topczewski J. J.; Rodnick M. E.; Sanford M. S.; Scott P. J. H. Org. Lett. 2014, 16, 3224–3227. 10.1021/ol501243g. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Mossine A. V.; Brooks A. F.; Makaravage K. J.; Miller J. M.; Ichiishi N.; Sanford M. S.; Scott P. J. H. Org. Lett. 2015, 17, 5780–5783. 10.1021/acs.orglett.5b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Zlatopolskiy B. D.; Zischler J.; Krapf P.; Zarrad F.; Urusova E. A.; Kordys E.; Endepols H.; Neumaier B. Chem. - Eur. J. 2015, 21, 5972–5979. 10.1002/chem.201405586. [DOI] [PubMed] [Google Scholar]; g Hoover A. J.; Lazari M.; Ren H.; Narayanam M. K.; Murphy J. M.; van Dam R. M.; Hooker J. M.; Ritter T. Organometallics 2016, 35, 1008–1014. 10.1021/acs.organomet.6b00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina V.; Krishnamurthy V.; Scott W. J. Org. React. 1997, 50, 1–384. 10.1002/0471264180.or050.01. [DOI] [Google Scholar]
- Qiu D.; Meng H.; Jin L.; Wang S.; Tang S.; Wang X.; Mo F.; Zhang Y.; Wang J. Angew. Chem., Int. Ed. 2013, 52, 11581–11584. 10.1002/anie.201304579. [DOI] [PubMed] [Google Scholar]
- TriBoc-l-DOPA methyl ester is commercially available from ABX (CAS no. 857502-21-7).
- Flemming I., Allylsilanes, Allylstannanes, and Related Systems. In Comprehensive Organic Synthesis, 2nd ed.; Knochel P., Molander G., Ed.; Elsevier, 2014; Vol. 2, pp 72–147. [Google Scholar]
- Molloy K. C., Organometallic Compounds of Tetravalent Tin. In Chemistry of Tin, 2nd ed.; Smith P. J., Ed.; Chapman & Hall: London, 1998; pp 138–175. [Google Scholar]
- Cools R.; Frank M. J.; Gibbs S. E.; Miyakawa A.; Jagust W.; D’Esposito M. J. Neurosci. 2009, 29, 1538–1543. 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. T.; Palotti M.; Holden J. E.; Oh J.; Okonkwo O.; Christian B. T.; Bendlin B. B.; Buyan-Dent L.; Harding S. J.; Stone C. K.; Dejesus O. T.; Nickles R. J.; Gallagher C. L. Synapse 2014, 68, 325–331. 10.1002/syn.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher C. L.; Christian B. T.; Holden J. E.; Dejesus O. T.; Nickles R. J.; Buyan-Dent L.; Bendlin B. B.; Harding S. J.; Stone C. K.; Mueller B.; Johnson S. C. Mov. Disord. 2011, 26, 2032–8. 10.1002/mds.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridler K.; Cunningham V.; Huiban M.; Martarello L.; Pampols-Maso S.; Passchier J.; Gunn R. N.; Searle G.; Abi-Dargham A.; Slifstein M.; Watson J.; Laruelle M.; Rabiner E. A. EJNMMI Res. 2014, 4, 1–12. 10.1186/s13550-014-0066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of metal-catalyzed/mediated electrophilic fluorination of arylstannanes, see:; a Furuya T.; Strom A. E.; Ritter T. J. Am. Chem. Soc. 2009, 131, 1662–1663. 10.1021/ja8086664. [DOI] [PubMed] [Google Scholar]; b Tang P.; Furuya T.; Ritter T. J. Am. Chem. Soc. 2010, 132, 12150–12154. 10.1021/ja105834t. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ye Y.; Sanford M. S. J. Am. Chem. Soc. 2013, 135, 4648–4651. 10.1021/ja400300g. [DOI] [PubMed] [Google Scholar]
- For an example of metal-free electrophilic fluorination of arylstannanes, see:Bryce M. R.; Chambers R. D.; Mullins S. T.; Parkin A. J. Chem. Soc., Chem. Commun. 1986, 1623–1624. 10.1039/c39860001623. [DOI] [Google Scholar]
- For examples of metal-mediated electrophilic radiofluorination of arylstannanes, see:Teare H.; Robins E. G.; Kirjavainen A.; Forsback S.; Sandford G.; Solin O.; Luthra S. K.; Gouverneur V. Angew. Chem., Int. Ed. 2010, 49, 6821–6824. 10.1002/anie.201002310. [DOI] [PubMed] [Google Scholar]
- For examples of metal-free electrophilic radiofluorination of arylstannanes, see:; a Adam M. J.; Pate B. D.; Ruth T. J.; Berry J. M.; Hall L. D. J. Chem. Soc., Chem. Commun. 1981, 733–733. 10.1039/C39810000733. [DOI] [Google Scholar]; b Adam M. J.; Ruth T. J.; Jivan S.; Pate B. D. J. Fluorine Chem. 1984, 25, 329–337. 10.1016/S0022-1139(00)81207-5. [DOI] [Google Scholar]; c Coenen H. H.; Moerlein S. M. J. Fluorine Chem. 1987, 36, 63–75. 10.1016/S0022-1139(00)82054-0. [DOI] [Google Scholar]; d Eskola O.; Gronroos T.; Bergman J.; Haaparanta M.; Marjamaki P.; Lehikoinen P.; Forsback S.; Langer O.; Hinnen F.; Dolle F.; Halldin C.; Solin O. Nucl. Med. Biol. 2004, 31, 103–110. 10.1016/S0969-8051(03)00098-2. [DOI] [PubMed] [Google Scholar]
- We communicated this Cu-mediated fluorination of arylstannanes in May 2016; see:Mossine A.; Makaravage K.; Ichiishi N.; Brooks A.; Miller J.; Sanford M.; Scott P.. J. Nucl. Med. 2016, Vol. 57, (Suppl. 2), pp. 2. [Google Scholar]; Following submission of this manuscript in August 2016, a related copper-mediated fluorination of arylstannanes using K19F was also disclosed:Gamache R. F.; Waldmann C.; Murphy J. M. Org. Lett. 2016, 18, 4522–4525. 10.1021/acs.orglett.6b02125. [DOI] [PubMed] [Google Scholar]
- Ye Y.; Schimler S. D.; Hanley P. S.; Sanford M. S. J. Am. Chem. Soc. 2013, 135, 16292–16295. 10.1021/ja408607r. [DOI] [PubMed] [Google Scholar]
- For enhancement in Cu-mediated electrophilic fluorination of arylstannanes versus arylboron compounds, see ref (16c) and compare with ref (6e).
- For enhancement in Ag-mediated electrophilic fluorination of arylstannanes versus arylborons, see:Huang C.; Liang T.; Harada S.; Lee E.; Ritter T. J. Am. Chem. Soc. 2011, 133, 13308–13310. 10.1021/ja204861a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoufik M.; Cordonnier M.-A.; Santini C. C.; Basset J.-M.; Candy J.-P. New J. Chem. 2004, 28, 1531–1537. 10.1039/b407850k. [DOI] [Google Scholar]
- Tredwell M.; Gouverneur V. Angew. Chem., Int. Ed. 2012, 51, 11426–11437. 10.1002/anie.201204687. [DOI] [PubMed] [Google Scholar]
- 1.5 Ci of activity at a specific activity of 10000 Ci/mmol corresponds to 0.170 μmol of fluoride, of which only about 1 nmol is 18F.
- Bell C.; Dowson N.; Puttick S.; Gal Y.; Thomas P.; Fay M.; Smith J.; Rose S. Nucl. Med. Biol. 2015, 42, 788–795. 10.1016/j.nucmedbio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Edwards R.; Wirth T. J. Labelled Compd. Radiopharm. 2015, 58, 183–187. 10.1002/jlcr.3285. [DOI] [PubMed] [Google Scholar]
- Hamill T. G.; Krause S.; Ryan C.; Bonnefous C.; Govek S.; Seiders T. J.; Cosford N. D. P.; Roppe J.; Kamenecka T.; Patel S.; Gibson R. E.; Sanabria S.; Riffel K.; Eng W.; King C.; Yang X.; Green M. D.; O’Malley S. S.; Hargreaves R.; Burns H. D. Synapse 2005, 56, 205–216. 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- Wang J.-Q.; Tueckmantel W.; Zhu A.; Pellegrino D.; Brownell A.-L. Synapse 2007, 61, 951–961. 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]
- Metal-free fluorination of an iodonium ylide afforded 20% RCY; however, these precursors can be challenging to prepare (ref (5d)).
- Preshlock S.; Calderwood S.; Verhoog S.; Tredwell M.; Huiban M.; Hienzsch A.; Gruber S.; Wilson T. C.; Taylor N. J.; Cailly T.; Schedler M.; Collier T. L.; Passchier J.; Smits R.; Mollitor J.; Hoepping A.; Mueller M.; Genicot C.; Mercier J.; Gouverneur V. Chem. Commun. 2016, 52, 8361–8364. 10.1039/C6CC03295H. [DOI] [PubMed] [Google Scholar]
- Kuik W.-J.; Kema I. P.; Brouwers A. H.; Zijlma R.; Neumann K. D.; Dierckx R. A. J. O.; DiMagno S. G.; Elsinga P. H. J. Nucl. Med. 2015, 56, 106–112. 10.2967/jnumed.114.145730. [DOI] [PubMed] [Google Scholar]
- A typical clinical scan uses 10 mCi of [18F]MPPF.
- Sanford M. S.; Scott P. J. H. ACS Cent. Sci. 2016, 2, 128–130. 10.1021/acscentsci.6b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M.; Le Bars D. In Radiochemical Syntheses; John Wiley & Sons, Inc., 2012; pp 87–94. [Google Scholar]
- Shao X.; Hoareau R.; Hockley B. G.; Tluczek L. J. M.; Henderson B. D.; Padgett H. C.; Scott P. J. H. J. Labelled Compd. Radiopharm. 2011, 54, 292–307. 10.1002/jlcr.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


