Green tea is derived from the plant Camellia sinesis by steaming or pan-frying, which inactivates enzymes and prevents oxidation of the tea polyphenols.1 Green tea polyphenols (GTP), also known as catechins, make up 30–42% dry weight of solid green tea.2 There are four catechins in green tea: epicatechin (EC), epigallocatechin (EGC), epcatechin-3-gallate (ECG), and epigallocatechin-3-gallate (EGCG). EGCG is the most abundant and most active of the GTP and is therefore the most widely studied.3
Epidemiology studies have shown that green tea consumption is linked to reduced risk of various types of cancers, including colorectal, lung, stomach, esophageal, breast and prostate cancer. Interestingly, the benefit of green tea in reducing the risk of breast and lung cancer appears to require a polymorphism in catechol-O-methyltransferase and 8-oxoguanine-DNA glycosylase, respectively.4 The benefit of GTP and tea extracts in chemoprevention has also been extensively documented in many animal tumor models. For example, green tea inhibited tumor cell proliferation and formation of adenomas in the lung and skin,5–7 decreased the incidence of tumor formation by 65% in the prostate, and eliminated metastasis to distal organs.8 In colon cancer, chemopreventive effects of GTP or green tea have been demonstrated in azoxymethane (AOM)-induced carcinogenesis in many studies; although, a few studies have shown a lack of a protective effect.9 Therefore, more studies are still needed to determine whether green tea has chemopreventive effects in colorectal cancer.
The mechanism by which green tea prevents or protects against carcinogenesis is largely unknown. Several mechanisms have been proposed, including inhibiting angiogenesis, metastasis, and cellular proliferation,1 and acting as an antioxidant to reduce cellular damage. GTP suppresses the expression of VEGF, MMP-2 and -9, and cdk2 and cdk4. One of the major constituents of green tea, EGCG, induces cell cycle arrest by inhibiting ERK1/2, MEK1/2 and IκB kinases, while inducing transcription of cyclin-dependent kinase (CDK) inhibitors p21, p16, p18 and p27.1
GTP can stimulate apoptosis, an important mechanism for tumor suppression.10,11 Some suggest that this is through the generation of reactive oxygen species (ROS). EGCG promoted hydrogen peroxide production and activated p53 in vitro.12 More studies suggested that GTP modulates the expression of Bcl-2 family of proteins to activate apoptosis via the intrinsic pathway of apoptosis (Fig. 1). For example, EGCG can directly bind and inhibit two Bcl-2 family members, Bcl-2 and Bcl-XL;13 therefore mimicking their endogenous inhibitors the BH3-only proteins such as PUMA, Bad and Bim.14 Furthermore, GTP caused decreased levels of Bcl-2 and Bcl-XL in many cell lines.15 EGCG can induce the expression of Bax and Bad.16 It is important to note that induction of apoptosis by GTPs is only seen at relatively high concentrations (>50 μM) in most in vitro studies.
Figure 1.
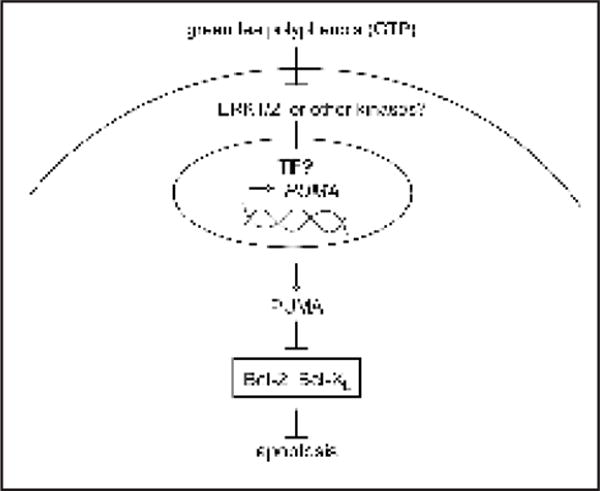
A model of PUMA in GTP-induced apoptosis of cancer cells.
In the paper, “The BH3-only Protein PUMA is Involved in Green Tea Polyphenol-induced Apoptosis in Colorectal Cancer Cell Lines” in this issue of Cancer Biology & Therapy, Wang et al., demonstrated that PUMA, a member of the BH3-only protein family, is induced by GTPs and contributed to GTP-induced apoptosis in colon cancer cells.17 PUMA was initially identified as a critical mediator of apoptosis p53-dependent apoptosis and promotes apoptosis through the mitochondrial pathway.18,19 p53 is required for PUMA induction by DNA damage but not by non-genotoxic stimuli.19 GTP treatment was found to induce PUMA in cell lines with a mutant p53 gene, suggesting that a p53-independent mechanism is involved.17
In this study, GTP inhibited cellular proliferation in two colorectal cancer cell lines within 24 hours. GTP-induced apoptosis occurring at 48 hours was found to be dose-dependent, and was associated with increased activity of caspase-3. GTP caused a robust induction of PUMA. Using stable PUMA knockdown cells established using lentivirus-expressed small hairpin RNA (shRNA), the authors further demonstrated that PUMA is required for GTP-induced apoptosis and suppression of colony formation.17
Next, the authors attempted to define a mechanism by which PUMA was induced by GTP. Consistent with previous reports that GTP inhibits MAPK activity,1 GTP caused a reduction in phosphorylated ERK1/2. A MAPK inhibitor (PD98059) was found to inhibit ERK1/2 and induce PUMA, suggesting that inactivation of ERK1/2 might account for PUMA induction.17 This finding is in agreement with several studies showing that inhibiting the activities of ERK1/2 induced PUMA expression.20–23
There are many questions that arise as a result of this article. First, ERK1/2 phosphorylates a large number of targets, many of which are transcription factors. PUMA expression has only been reported to be regulated exclusively at the level of transcription. What is the transcription factor responsible for PUMA transcription with GTP treatment? One possibility is that a transcriptional activator is negatively regulated by ERK1/2 and becomes activated when ERK1/2 activity is inhibited. Another possibility is that a transcriptional repressor is positively regulated by ERK1/2 and is removed from the PUMA promoter to allow active transcription. Second, do other kinases known to be affected by GTP, such as JNK, p38 and IKβ, contribute to PUMA induction? Inhibition of the PI3K/Akt pathway promoted PUMA transcription in murine T lymphocytes and embryonic fibroblasts, possibly through the forkhead transcription factor FoxO3a.24 Could it play a role here?
Despite the largely positive data in cancer prevention, GTP is unlikely to exhibit significant therapeutic effects. Based on this study, it would be interesting to determine if induction of PUMA and apoptosis occurs in vivo with GTP treatment, and whether tumors from PUMA-deficient mice show impaired apoptosis and responses to GTP. This is especially important, as GTP and green tea have anti-inflammatory properties. Such studies will provide a better understanding of the effects of GTP in the organismal context, and help explain some conflicting results obtained with mouse and human studies. While there have been extensive studies on the effects of green tea extracts or GTP on colon cancer and cells, little research has been conducted to analyze their effects on normal cells, especially the intestinal cells that undergo constant proliferation.
Another concern is that the high concentrations of EGCG and tea extracts used in many in vitro experiments may not be applicable in humans. Recently, “beverage” levels of green tea were found to inhibit the early stages of colon tumorigenesis in AOM-treated APC(Min/+) mice, providing a much needed support for a realistic and achievable effect in vivo.25 More studies will be needed to determine whether the use of EGCG might be more effective in chemoprevention than a mixture of catechins, and what would be the best way of administrating EGCG.26 To many, the key questions are: How much green tea one needs to drink and for how long to get any measurable protection against cancer? What would be the long term effects of drinking a large quantity of green tea on the incidence of other major diseases? What are the most important molecular targets of green tea in cancer chemoprevention? These are a set of questions that will likely be asked of any cancer chemopreventive agent.
References
- 1.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 2.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 3.Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm. 2007;4:819–25. doi: 10.1021/mp700075m. [DOI] [PubMed] [Google Scholar]
- 4.Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. 2007;17:395–402. doi: 10.1016/j.semcancer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Wang ZY, Kim S, Liao J, Seril DN, Chen X, Smith TJ, Yang CS. Characterization of early pulmonary hyperproliferation and tumor progression and their inhibition by black tea in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model with A/J mice. Cancer Res. 1997;57:1889–94. [PubMed] [Google Scholar]
- 6.Lu YP, Lou YR, Xie JG, Peng QY, Liao J, Yang CS, Huang MT, Conney AH. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc Natl Acad Sci USA. 2002;99:12455–60. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landau JM, Wang ZY, Yang GY, Ding W, Yang CS. Inhibition of spontaneous formation of lung tumors and rhabdomyosarcomas in A/J mice by black and green tea. Carcinogenesis. 1998;19:501–7. doi: 10.1093/carcin/19.3.501. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisburger JH, Rivenson A, Aliaga C, Reinhardt J, Kelloff GJ, Boone CW, Steele VE, Balentine DA, Pittman B, Zang E. Effect of tea extracts, polyphenols and epigallocatechin gallate on azoxymethane-induced colon cancer. Proc Soc Exp Biol Med. 1998;217:104–8. doi: 10.3181/00379727-217-44211. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–8. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 12.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–6. [PubMed] [Google Scholar]
- 13.Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–21. [PubMed] [Google Scholar]
- 14.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Shankar S, Ganapathy S, Srivastava RK. Green tea polyphenols: biology and therapeutic implications in cancer. Front Biosci. 2007;12:4881–99. doi: 10.2741/2435. [DOI] [PubMed] [Google Scholar]
- 16.Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, Li LC, Dahiya R. A component of green tea, (−)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res Commun. 2007;354:852–7. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Wang XW, Wang R, Hao MW, Dong K, Wei SH, Lin F, Ren JH, Zhang HZ. The BH3-only Protein PUMA is Involved in Green Tea Polyphenol-induced Apoptosis in Colorectal Cancer Cell Lines. Cancer Bio & Ther. 2008;7 doi: 10.4161/cbt.7.6.5911. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–82. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Zhang L. No PUMA, no death: implications for p53-dependent apoptosis. Cancer Cell. 2003;4:248–9. doi: 10.1016/s1535-6108(03)00249-6. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Li M, Wang J, Yeung CM, Zhang H, Kung HF, Jiang B, Lin MC. The BH3-only protein, PUMA, is involved in oxaliplatin-induced apoptosis in colon cancer cells. Biochem Pharmacol. 2006;71:1540–50. doi: 10.1016/j.bcp.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–42. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 23.Shankar S, Suthakar G, Srivastava RK. Epigallocatechin-3-gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Front Biosci. 2007;12:5039–51. doi: 10.2741/2446. [DOI] [PubMed] [Google Scholar]
- 24.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–63. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issa AY, Volate SR, Muga SJ, Nitcheva D, Smith T, Wargovich MJ. Green tea selectively targets initial stages of intestinal carcinogenesis in the AOM-ApcMin mouse model. Carcinogenesis. 2007;28:1978–84. doi: 10.1093/carcin/bgm161. [DOI] [PubMed] [Google Scholar]
- 26.Hao X, Sun Y, Yang CS, Bose M, Lambert JD, Ju J, Lu G, Lee MJ, Park S, Husain A, Wang S. Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr Cancer. 2007;59:62–9. doi: 10.1080/01635580701365050. [DOI] [PubMed] [Google Scholar]


