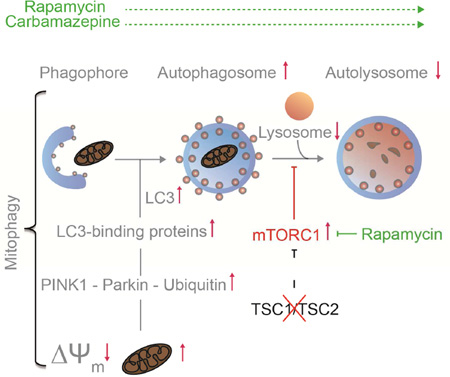
SUMMARY
Tuberous Sclerosis Complex (TSC) is a neurodevelopmental disease caused by TSC1 or TSC2 mutations and subsequent activation of the mTORC1 kinase. Upon mTORC1 activation, anabolic metabolism, which requires mitochondria, is induced, yet at the same time the principal pathway for mitochondrial turnover, autophagy, is compromised. How mTORC1 activation impacts mitochondrial turnover in neurons remains unknown. Here we demonstrate impaired mitochondrial homeostasis in neuronal in vitro and in vivo models of TSC. We find that Tsc1/2-deficient neurons accumulate mitochondria in cell bodies but are depleted of axonal mitochondria, including those supporting presynaptic sites. Axonal and global mitophagy of damaged mitochondria is impaired, suggesting that decreased turnover may act upstream of impaired mitochondrial metabolism. Importantly, blocking mTORC1 or inducing mTOR-independent autophagy restores mitochondrial homeostasis. Our study clarifies the complex relationship between the TSC-mTORC1 pathway, autophagy and mitophagy, and defines mitochondrial homeostasis as a therapeutic target for TSC and related diseases.
Keywords: mTOR, mTORC1, autophagy, mitochondria, autism, axonal transport, synapse, lysosome, rapamycin, carbamazepine
Graphical Abstract
INTRODUCTION
Tuberous Sclerosis Complex (TSC) is a neurodevelopmental disease caused by loss-of-function mutations in the TSC1 or TSC2 gene and subsequent activation of the mTORC1 kinase (DiMario et al., 2015; Lipton and Sahin, 2014). While mTORC1 inhibitors have emerged as a therapy for certain disease manifestations, their impact on complex neurological manifestations remains uncertain (https://clinicaltrials.gov:NCT01289912, NCT01730209, NCT01954693). It is imperative to understand the molecular mechanisms that result from loss-of-function mutations in TSC1/2 and that may promote intellectual disability, seizures or autism spectrum disorder in TSC.
Despite the fact that many TSC/mTORC1-related functions have been extensively studied in non-neuronal cell types (Albert and Hall, 2015), many remain unexplored in neuronal models of TSC. Constitutive activation of mTORC1 has been shown to promote anabolic metabolism, while simultaneously repressing catabolic processes such as autophagy. Mitochondria play a crucial role in supporting anabolic metabolism, yet at the same time, their function, maintenance and turnover are tightly regulated by autophagic flux. A central question thus remains how TSC1/2 deficiency impacts mitochondrial homeostasis in neurons.
In neurons, mitochondria form a highly dynamic reticular network and undergo constant remodeling and turnover. To support local demands for mitochondrial ATP supply and calcium buffering capacity, mitochondria are transported along microtubule tracks to remote axonal regions (Maday et al., 2014), and conversely dysfunctional mitochondria are returned to the cell body for degradation (Cai et al., 2012). Maintenance of a functional population of mitochondria is ensured through effective autophagic degradation of aged and damaged mitochondria, a process that has been termed mitophagy (Pickrell and Youle, 2015).
Neuropathological studies of mouse models of TSC and TSC patients document impaired autophagic flux, and an accumulation of organelles, including mitochondria (Di Nardo et al., 2014; Goto et al., 2011; McMahon et al., 2012; Tang et al., 2014; Yasin et al., 2013). The functional importance of the latter is corroborated by increased levels of oxidative stress in neuronal models of TSC (Di Nardo et al., 2009; Tsai et al., 2012). Despite these striking observations, however, relatively little is known about the impact of TSC/mTORC1 on the turnover of neuronal mitochondria.
Here, we investigate mitochondrial dynamics, metabolism and turnover in neuronal in vitro and in vivo models of TSC. We find that Tsc1/2-deficient neurons progressively accumulate mitochondria in cell bodies but are depleted of functional mitochondria in axons, including those that support presynaptic sites. The dynamics of mitophagy are impaired both locally in axons and globally across the neuron. Localizing the defect to the late stages of the mitophagy pathway, we find that autophagosome turnover is impaired. Finally, we find that either blocking mTORC1 or inducing mTOR-independent autophagy can reverse changes in mitochondrial homeostasis seen in Tsc1/2-deficient neurons. Collectively, these results provide evidence for a previously unappreciated role of impaired neuronal mitophagy in TSC. Addressing the role of mTORC1 in mitochondrial turnover holds the promise for a better understanding of TSC and the identification of therapeutic targets.
RESULTS
Mitochondria accumulate in Tsc2-deficient neurons but are depleted from axons
We hypothesized that Tsc2-deficiency induced mTORC1 activation might impact mitochondrial homeostasis. To address this, we employed a flow cytometry-based assay (Mauro-Lizcano et al., 2015) to quantitate mitochondrial content (Fig. 1A). We found that Tsc2-deficient neurons, with constitutive and significant mTORC1 activation (Fig. S1A, (Choi et al., 2008)), accumulate mitochondria and show an increase in mitochondrial mass to ∼2.5-fold the level of controls (Fig. 1B-C). This increase progresses even further as neurons mature in culture (Fig. S1B), cannot be explained by an increase in cell size alone (Fig. S1C), and mimics that observed in giant cells of Tsc1cc;Nes-rtTA+;TetOp-Cre+ (E16 doxy) mice (Goto et al., 2011). Increased mitochondrial mass can result from an increase in mitochondrial biogenesis, a decrease in mitochondrial turnover, or both. Importantly, the transcription of critical mitochondrial biogenesis genes is not upregulated in Tsc2-deficient neurons (Fig. S1D). In fact, we observed no change in Pgc1α expression and reduced expression levels of the Nrf1 and mtTFA in Tsc2-knockdown neurons (Fig. S1D). Levels of the mtDNA-encoded COX-II were also reduced, while levels of the nuclear DNA-encoded COX-IV were unchanged (Fig. S1D). These findings contrast with observations in non-neuronal cells (Cunningham et al., 2007; Morita et al., 2013) and argue that mitochondrial biogenesis is not increased in Tsc2-deficient neurons.
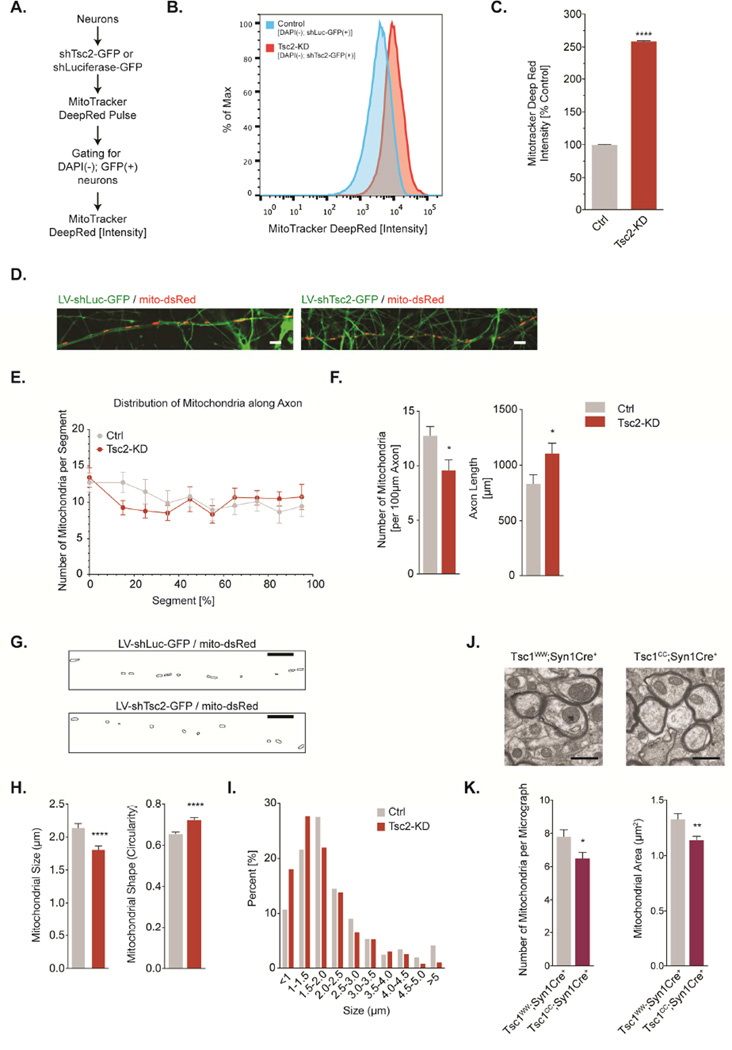
Figure 1. Tsc2-deficient neurons accumulate mitochondria in their cell bodies but are depleted of axonal mitochondria.
(A-C) Flow cytometry-based quantification of mitochondrial content using MitoTracker Deep Red in cortical neurons (DIV11). Note that this assay primarily quantifies the mitochondrial content in cell bodies since neurites may be lost during sample preparation (n=9×106 recorded events per condition from 9 independent samples).
(D) Live cell confocal imaging of individual mitochondria in single axons of hippocampal neurons transduced with shLuc-GFP or shTsc2-GFP (DIV7/8). Scale bar, 5 µm
(E) Distribution of mitochondria along the full length of axons in hippocampal neurons (DIV7/8, n=40 axons per condition from 8–10 experiments).
(F) Number of mitochondria per 100 µm of axon and the average length of axons in hippocampal neurons (DIV7/8, n=40 axons per condition from 8–10 experiments).
(G-I) Quantitative assessment of mitochondrial morphology (circularity and Feret’s diameter) in axons of hippocampal neurons (DIV7/8, n>400 mitochondria per condition from 8–10 experiments). Scale bar, 10 µm
(J&K) Transmission electron microscopy of callosal projection axons from Tsc1CC;Syn1Cre+ (n=2) and Tsc1WW;Syn1Cre+ littermates (n=3). Graphs show the number of mitochondria per micrograph and the average mitochondrial area (n>450 mitochondria per condition). Scale bar, 1 µm
Luc= Luciferase; *p<0.05, **p<0.01, ***p<0.001, ****<p.0001. See also Figure S1.
To examine the distribution and dynamics of mitochondria, we employed confocal live-cell imaging. Mitochondria in both conditions form a dense network in the cell body, while they sparsely populate axons (Fig. 1D&1E, S1E). Consistent with previous reports, we found that axon outgrowth is accelerated in Tsc2-deficient neurons with an overall increase in axon length (Fig. 1F) and multiple axons frequently occurring (Choi et al., 2008). Quantifying mitochondria along axons (Fig. 1E), we found that Tsc2-deficient axons contain fewer mitochondria per 100 µm (Fig. 1F), although their relative distribution follows a similar pattern to that of controls, with more mitochondria in the proximal axon (Fig. 1E). This finding suggests that Tsc2-deficient axons are relatively depleted of mitochondria with the remaining mitochondria re-distributing to cover the full length of the axon.
Mitochondrial morphology changes are intimately linked to mitochondrial dynamics and thus can serve as a surrogate marker to screen for underlying abnormalities (Pernas and Scorrano, 2016). Small and circular mitochondria often indicate mitochondrial membrane depolarization and increased production of reactive oxygen species. Axonal mitochondria in controls were mostly tubular in shape and variable in size (Fig. 1G-I). In contrast, Tsc2-deficient axons contained mitochondria that were significantly smaller and displayed a more circular shape, signifying mitochondrial fragmentation (Fig. 1G-H). The relative distribution of mitochondrial size revealed a left-shift in Tsc2-deficient neurons, indicating a greater proportion of small, fragmented mitochondria (Fig. 1I). Reintroducing TSC2 was able to reverse changes in the morphology and number of axonal mitochondria, suggesting that these are indeed a consequence of disturbed TSC-mTORC1 signaling (Fig. S1F). Further corroborating these findings in vivo using electron microscopy, we found that commissural axons of the corpus callosum of Tsc1cc;Syn1Cre+ mice (Meikle et al., 2007) showed both a reduced number of mitochondria and a decreased organelle size (Fig. 1J-K).
In summary, Tsc2-deficient neurons accumulate mitochondria in their cell body but are relatively depleted of axonal mitochondria with the remaining mitochondria showing an abnormal morphology indicative of disrupted mitochondrial dynamics.
Mitochondrial respiration is impaired and mitochondrial membrane potential (Δ Ψm) is diminished in Tsc2-deficient neurons and mutant TSC2 iPSC-derived neurons
To investigate the effect of Tsc2 deficiency on mitochondrial function, we measured oxygen consumption rates and the functional parameters that can be derived from it (Fig. S2A). In Tsc2-deficient neurons, we found impaired mitochondrial bioenergetics, evidenced by a reduction in baseline respiration, ATP turnover, and total respiratory capacity (Fig. 2A-B). This effect was specific to mitochondrial metabolism as non-mitochondrial respiration and proton leak did not change (Fig. 2B).
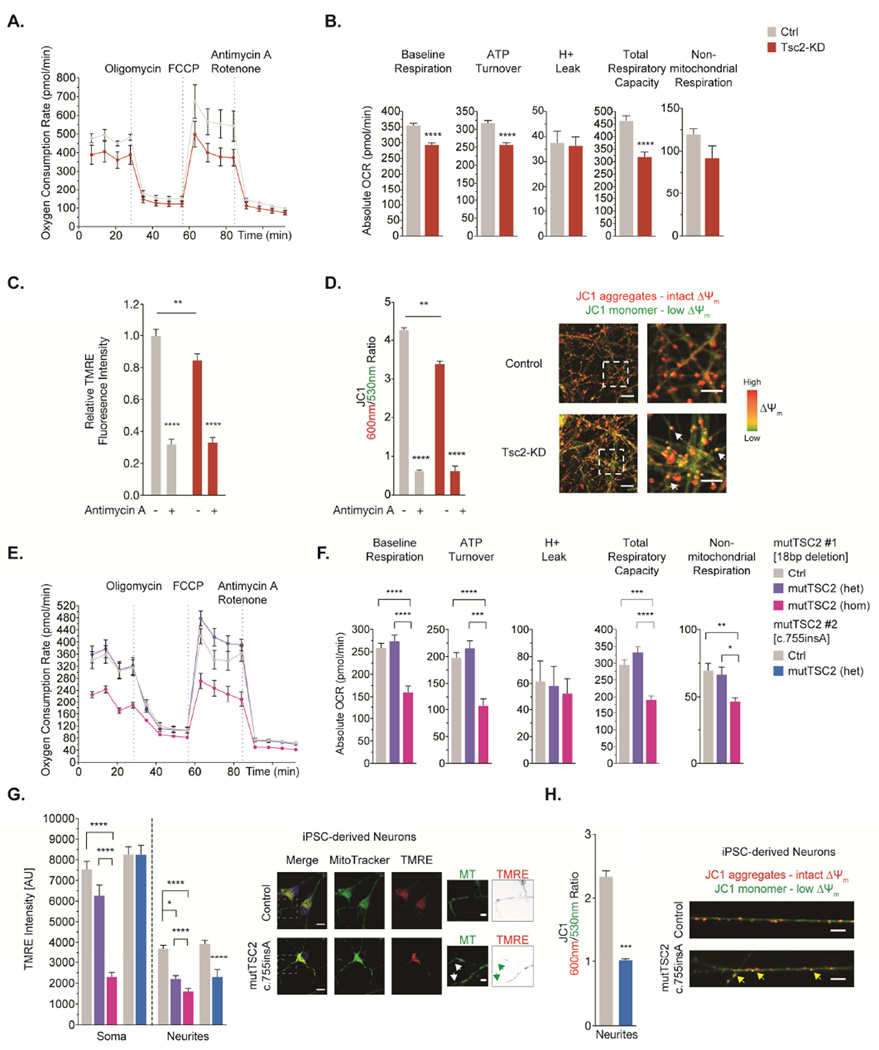
Figure 2. Mitochondrial respiration is impaired and mitochondrial membrane potential (Δ Ψm) is diminished in Tsc2-deficient neurons and neurites of human iPSC-derived cortical neurons from TSC patients.
(A&B) Mitochondrial oxygen consumption rates in hippocampal neurons (DIV8) measured using the Seahorse XF Extracellular Flux 96 analyzer. Graphs show different domains of mitochondrial function and non-mitochondrial respiration (n=10 measurements per condition).
(C) TMRE staining in hippocampal neurons (DIV7/8). Healthy mitochondria with an intact Δ Ψm accumulate TMRE, thus displaying high TMRE fluorescence, while dysfunctional mitochondria show reduced TMRE signal. Antimycin A (40 µM, 1hr), a complex III inhibitor, leads to a rapid breakdown of the Δ Ψm, confirming dynamic assay properties (n=8 measurements per condition).
(D) JC1 staining in hippocampal neurons (DIV7/8). JC-1 accumulates in mitochondria in a Δ Ψm-dependent manner, where it exists as monomers at low concentrations (emission 530nm) and forms aggregates at higher concentrations (emission 600nm). Representative confocal images of mitochondria in neurites. Arrows denote mitochondria with low Δ Ψm (n=6 experiments).
(E&F) Mitochondrial oxygen consumption rates in iPSC-derived cortical neurons from a TSC patient with a heterozygous TSC2 mutation [mutTSC2 (het)], the same cell line with a second, engineered TSC2 mutation leading to a complete loss of TSC2 [mutTSC2 (hom)], and a healthy related donor [Ctrl] (summarized in Table S1). Graphs show different domains of mitochondrial function and non-mitochondrial respiration (n=6 measurements per condition).
(G&H) TMRE and JC1 staining in iPSC-derived cortical neurons (summarized in Table S1). Arrows denote mitochondria with low Δ Ψm (TMRE: n>35 cells per condition from 3 experiments, JC1: n>85 mitochondria per condition from 3 experiments).
het=heterozygous, hom=homozygous, MT=MitoTracker. *p<0.05, **p<0.01, ***p<0.001, ****<p.0001. See also Figure S2.
An intact mitochondrial membrane potential (Δ Ψm) is a key requisite for effective oxidative phosphorylation. To approximate Δ Ψm, we used TMRE, a Δ Ψm-dependent dye. In Tsc2-deficient neurons, TMRE signal was reduced (Fig. 2C), reflecting a subset of mitochondria that exhibit a decreased Δ Ψm. To localize mitochondria with a diminished Δ Ψm, we employed the ratiometric Δ Ψm indicator, JC-1 (Fig. 2D). In control neurons, the vast majority of mitochondria show a high 600nm/530nm ratio, indicating an intact Δ Ψm (Fig. 2D). In Tsc2-deficient neurons, we found a significant shift towards a lower 600nm/530nm ratio (Fig. 2D), arguing for a reduced electrochemical status in a significant subset. Importantly, these mitochondria with diminished Δ Ψm were readily detectable in neurites of Tsc2-deficient neurons (Fig. 2D, white arrows). Expression analyses of electron-transport chain subunits revealed an increase of complex I (subunit NDUFB8), a major site for ROS production (Murphy, 2009), and a decrease of complex II (subunit 30kDa) in Tsc2-deficient neurons (Fig. S2B).
To test whether disease-specific mutations in patient-derived cells share similar changes in mitochondrial function, we generated iPSC-derived cortical neurons (Zhang et al., 2013) from individuals with TSC and controls (Table S1). We discovered that patient-derived neurons with a heterozygous TSC2 mutation did not display detectable deficits in mitochondrial respiration (Fig. 2E-F). However, upon introduction of a second loss-of-function mutation using TALEN, we found a marked reduction in mitochondrial function similar to that in rat Tsc2-KD cortical neurons (Fig. 2E-F). Next, we pulse-labeled mitochondria with TMRE and MTDR (Δ Ψm-independent) and quantified the TMRE intensity in the soma and neurites (Fig. 2G). TMRE signal in the soma was similar between TSC2+/− iPSC-derived neurons and controls, but reduced in homozygous mutants (Fig. 2G). The TMRE signal in neurites was overall weaker than that in the soma, likely reflecting the high density of mitochondria in the latter (Fig. 2G). Compared to controls, neurites of TSC2+/− and TSC2−/− iPSC-derived cortical neurons contained many mitochondria with a low TMRE fluorescence, although they retained their MTDR signal (Fig. 2G, arrows), suggesting an accumulation of depolarized mitochondria. This was confirmed using the JC1 assay (Fig. 2H, arrows). Notably, we detected mTORC1 activation in TSC2+/− iPSC-derived neurons (M-J.H. and M.S., unpublished), similar to recently published observations of mTORC1 hyperfunction in TSC2+/− human embryonic stem cell-derived neurons (Costa et al., 2016).
Taken together, these findings suggest that multiple domains of mitochondrial bioenergetics are compromised in both Tsc2-deficient rodent neurons and TSC patient-derived neurons in vitro.
Axonal mitochondria in Tsc2-deficient neurons are shuttled to the cell body leading to a depletion of presynaptic mitochondria
Neurons are unique in their architecture with critical compartments, such as axons and synapses, located far from the cell body. In Tsc2-deficient neurons, we observed increased mitochondrial mass in the cell body but mitochondrial depletion from axons (Fig. 1C & F), arguing for a redistribution of axonal mitochondria. To examine axonal transport in Tsc2-deficient neurons, we selected a mid-axonal segment, 500–800 µm from the cell body, to avoid regional differences in mitochondrial transport (Fig. 3A&S3A, Supp. Video 1&2). Consistent with previous reports in similar assays (Pekkurnaz et al., 2014; Wang and Schwarz, 2009), mitochondria in control neurons are stationary in ∼80–85% of the time and are moving bi-directionally ∼15–20% of the time (Fig. 3A-B). Strikingly, mitochondria in Tsc2-deficient neurons spend less time in a stationary position and instead display markedly enhanced retrograde transport (Fig. 3A-B).
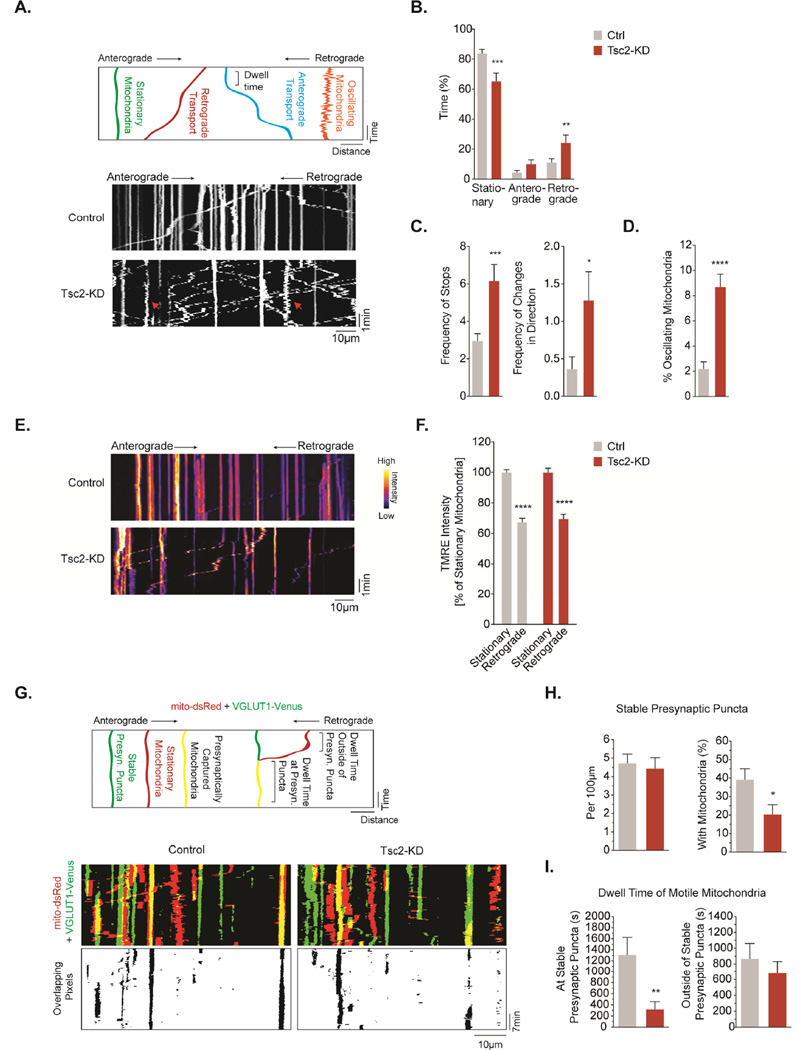
Figure 3. Axonal mitochondria in Tsc2-deficient neurons are shuttled to the cell body and presynaptic capturing of mitochondria is impaired.
(A) Mitochondrial transport quantified through a standardized live cell microscopy assay (see Fig. S3A).
(B&C) Mitochondrial transport in the mid axon of hippocampal neurons (DIV7/8). Graph shows the time that mitochondria spend in a stationary position, or moving in the retrograde or anterograde direction. The frequency of stops and changes in direction per 5min recording is calculated (n>25 axons per condition from 5–8 experiments).
(D) Percentage of axonal mitochondria with an oscillating movement pattern (n>25 axons per condition from 5–8 experiments).
(E&F) TMRE staining in stationary mitochondria vs. mitochondria transported in the retrograde direction (n>10 axons per condition from 3 experiments).
(G) Quantitative assessment of mitochondrial capturing at presynaptic sites and representative examples (DIV7/8).
(H) Number of stable presynaptic puncta per 100 µm axon and the percentage of stable presynaptic puncta supported by stationary mitochondria in hippocampal neurons (DIV7/8, n=14 axons per condition from 7 experiments).
(I) Average dwell time of motile mitochondria at and outside of stable presynaptic puncta in hippocampal neurons (DIV7/8, n=14 axons per condition from 7 experiments).
*p<0.05, **p<0.01, ***p<0.001, ****<p.0001. See also Figure S3.
Axonal mitochondria undergo dynamic transport, and motile mitochondria often pause and move again in response to physiological changes. We found that mitochondria in Tsc2-deficient axons exhibited more stops and more frequent changes in direction (Fig. 3C). Examining the kymographic morphology, we discovered that a subset of mitochondria in Tsc2-deficient axons showed a peculiar oscillating movement pattern, moving back and forth without any significant net displacement (Fig. 3A, red arrows). While ∼2% of mitochondria in control neurons showed this pattern, this rate was increased to ∼9% in Tsc2-deficient neurons (Fig. 3D). A similar oscillating pattern has been recently reported in axonal mitochondria of PINK1 or Parkin overexpressing D. melanogaster (Devireddy et al., 2015), potentially linking it to incipient mitophagy.
No change in the axonal transport pattern of other cargo, such as lysosomes, was observed, arguing that the effect of Tsc2-deficiency on mitochondria is relatively specific (Fig. S3B). Overexpression of the anchoring protein syntaphilin almost completely arrested mitochondrial transport, confirming that general mechanisms of axonal transport and anchoring are intact (Fig. S3C).
Since we observed an accumulation of mitochondria with low Δ Ψm in Tsc2-deficient neurites, we hypothesized that these potentially damaged mitochondria might be successively returned to the cell body, as previously suggested (Cai et al., 2012). Indeed, mitochondria transported in the retrograde direction displayed a reduced TMRE fluorescence in both control and Tsc2-deficient axons (Fig. 3E-F), indicating that dysfunctional mitochondria are preferentially returned to the cell body.
Stationary mitochondria are enriched at presynaptic sites where they supply ATP and buffer calcium, functions that are critical for synaptic vesicle release. Similarly, motile mitochondria passing through presynaptic boutons can dynamically influence synaptic transmission (Sun et al., 2013). We hypothesized that increased retrograde transport and depletion of axonal mitochondria in Tsc2-deficient neurons might influence the availability of mitochondria to synapses. To investigate the presynaptic capturing of mitochondria, we performed dual-channel time-lapse confocal microscopy in axons of neurons transfected with mito-DsRed and VGLUT1-Venus at DIV7/8 (Fig. 3G) and DIV14/15 (Fig. S3D) as described previously (Courchet et al., 2013). Tsc2-downregulation did not affect the density of stable VGLUT1-Venus puncta (Fig. 3H). However, quantifying the number of stable presynaptic sites that contain stationary mitochondria, we discovered a ∼50% reduction of mitochondria captured at presynaptic sites (Fig. 3H). We also observed that motile mitochondria frequently dwell over stable VGLUT1 puncta in control axons (Fig. 3I). In contrast, in Tsc2-deficient axons, we found a decrease in the time that motile mitochondria spend over stable VGLUT1-postive presynaptic sites, while the dwell-time outside the presynaptic compartment did not differ from controls (Fig. 3I). In more mature neurons at DIV14/15, most axonal mitochondria maintain a stationary position (Fig. S3D). Looking at the number of stationary mitochondria captured at presynaptic sites, we found that mature Tsc2-deficient axons contained fewer synapses that are supported by local stationary mitochondria (Fig. S3E). Hence, presynaptic capture of mitochondria is diminished in both developing and more mature Tsc2-deficient neurons in vitro.
Collectively, these experiments reveal enhanced retrograde transport of dysfunctional mitochondria as a potential mechanism that depletes axonal mitochondria in Tsc2-deficient neurons. Importantly, the availability of mitochondria to presynaptic sites is compromised arguing for insufficient mitochondrial capture.
Spatiotemporal dynamics of local mitophagy are impaired in axons of Tsc2-deficient neurons
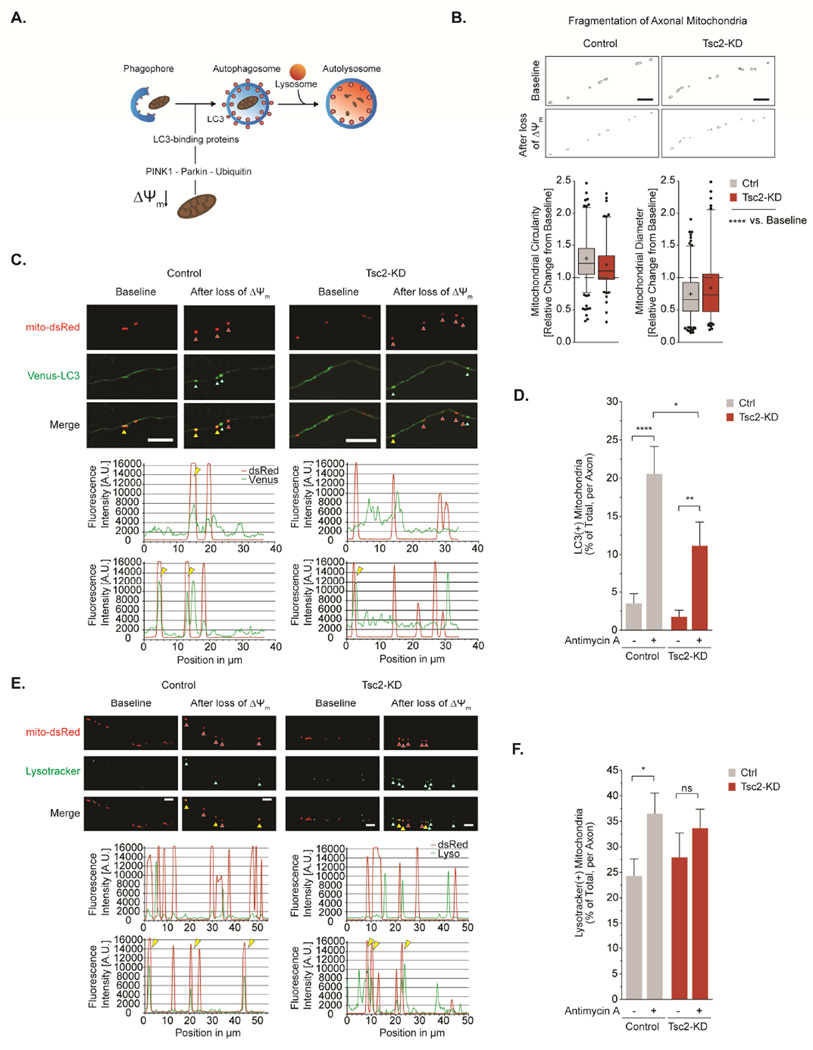
The above experiments indicate alterations in mitochondrial mass, dynamics and function that suggest impairment of mitochondrial turnover. Mitochondrial turnover via mitophagy occurs both locally in axons (Ashrafi et al., 2014) and in the soma (Cai et al., 2012). Might changes in mitophagy help explain decreased mitochondrial turnover? To address this question, we investigated both axonal mitophagy and global mitophagic flux.
To stimulate mitophagy in axons (Fig. 4A), we employed treatment with antimycin A which leads to a rapid loss of Δ Ψm (Fig. S4A), thus allowing us to investigate mitophagic events in a timeframe amenable to live cell imaging without impairing axonal integrity (Ashrafi et al., 2014) (Fig. S4B). Consistent with previous studies (Wang et al., 2011), we found that acute Δ Ψm depolarization renders most axonal mitochondria stationary (Fig. S4C, Supp. Video 3), and induces rapid fragmentation (Fig. 4B&S4D), which may precede mitophagy. We approximated the rates of early mitophagy in axons by quantifying the percentage of Venus-LC3 puncta that co-localize with mitochondria following Δ Ψm depolarization (Klionsky et al., 2016). At baseline, Venus-LC3 is mostly cytosolic and only a small fraction of Venus-LC3 vesicles co-localize with mitochondria (Fig. 4C & D). In response to Δ Ψm depolarization, however, we observed accumulation of Venus-LC3 on mitochondria with ∼20% of mitochondria becoming coated with LC3 in control neurons (Fig. 4D). In contrast, we observed a blunted mitophagic response in Tsc2-deficient neurons with only a small proportion of mitochondria co-localizing with Venus-LC3 vesicles (Fig. 4D). These data suggest that Tsc2 deficiency results in impaired spatiotemporal dynamics of local mitophagy in axons.
Figure 4. Spatiotemporal dynamics of axonal mitophagy are impaired in Tsc2-deficient neurons.
(A) Schematic showing the sequence of events during mitophagy.
(B) Morphology of axonal mitochondria before and after acute Δ Ψm-depolarization with antimycin A (40 µM, n>140 mitochondria per condition from 5 experiments). Scale bar, 5 µm
(C&D) Recruitment of autophagosomes to axonal mitochondria following acute Δ Ψm-depolarization with antimycin A (40 µM). Arrows denote mito-dsRed-labeled mitochondria (red), Venus-LC3-labeled autophagosomes (white), and their co-localization (yellow). Graph shows the maximal percentage of mitochondria co-localizing with autophagosomes in individual axons (n>40 axons per condition from 12 experiments). Scale bar, 10 µm
(E&F) Recruitment of lysosomes to axonal mitochondria following acute Δ Ψm-depolarization with antimycin A (40 µM). Arrows denote mito-dsRed-labeled mitochondria (red), LysoTracker Green-labeled lysosomes (white), and their co-localization (yellow). Graph shows the maximal percentage of mitochondria co-localizing with lysosomes in individual axons (n>25 axons per condition from 8 experiments). Scale bar, 5 µm
AU = arbitrary unit, *p<0.05, **p<0.01, ***p<0.001, ****<p.0001. See also Figure S4.
Autophagic degradation of mitochondria is a multistep pathway (Fig. 4A). To examine the late stages, we quantified the autophagic delivery of mitochondria to lysosomes. At baseline, we observed lysosomes in axons, a portion of which co-localized with mitochondria (Fig. 4E-F). Following mitophagy induction, we found a significant increase in the co-localization of lysosomes with mitochondria in control neurons. In Tsc2-deficient neurons, however, we could not detect a similar increase (Fig. 4E-F).
Taken together, these results reveal impaired recruitment of autophagosomes and lysosomes to damaged axonal mitochondria in Tsc2-deficient neurons, although some degree of early mitophagy is retained.
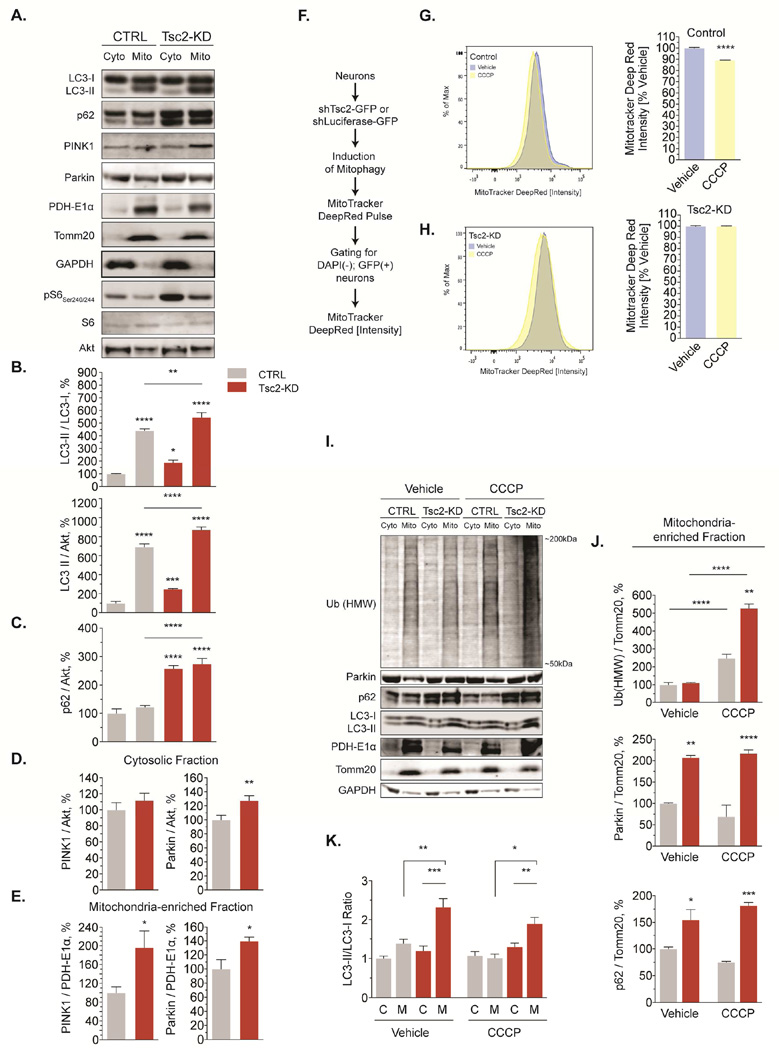
Global mitophagic flux is impaired in Tsc2-deficient neurons
Mitophagy has been well characterized in the cell body of neurons, where the majority of mature, acidic lysosomes reside (Johnson et al., 2016). To examine baseline steady-state levels of mitophagy, we purified mitochondria-enriched fractions from Tsc2-deficient neurons and controls as previously described (Fig. 5A&S5A, (Narendra et al., 2010)). Levels of the autophagy markers LC3-II and p62 (Fig. 5B-C) were robustly increased both in the cytosol and in mitochondria-enriched fractions from Tsc2-deficient neurons relative to controls, indicating an accumulation of autophagosomes and autophagy substrates. Measuring levels of PINK1 and Parkin, two proteins involved in mitophagy (Pickrell and Youle, 2015), we found that both were enriched in mitochondrial fractions of Tsc2-deficient neurons, potentially reflecting mitochondrial damage and early mitophagic events (Fig. 5D-E).
Figure 5. Global mitophagic flux is impaired in Tsc2-deficient neurons.
(A-E) Representative western blots and quantification of several proteins involved in mitophagy in cytosolic and mitochondria-enriched fractions from cortical neurons (DIV7/8, n=12 experiments)
(F-H) Mitophagic flux induced by CCCP (1 µM, 24hr) quantified using flow cytometry in cortical neurons (DIV11, n=6 × 106 recorded events from 6 experiments).
(I-K) Western blotting for several proteins involved in mitophagy in cytosolic and mitochondria-enriched fractions from cortical neurons (DIV7/8) treated with CCCP (1 µM, 24hr) or vehicle (n= 3–6 experiments).
C, Cyto=cytosolic fraction, HMW=high molecular weight, M, Mito=mitochondria-enriched fraction, mono=monomeric; *p<0.05, **p<0.01, ***p<0.001, ****<p.0001. See also Figure S5.
The above experiments allow us to investigate the early steps of mitophagy but do not assess the net flux through the pathway. To investigate mitophagic flux, we employed flow cytometry (Fig. 5F). We induced mitophagy by depolarizing mitochondria with a low dose of CCCP, a compound that dissipates the Δ Ψm (Mauro-Lizcano et al., 2015). Mitochondrial mass was determined by staining with the Δ Ψm-independent mitochondrial dye MTDR (Fig. 5F-H). In control neurons, 24hrs of CCCP led to a reduction of ∼10% of MTDR fluorescence relative to untreated cells (Fig. 5G), indicating that depolarized mitochondria are degraded efficiently. As expected, co-treatment with saturating concentrations of bafilomycin A1, a compound that blocks autophagosome-lysosome fusion (Klionsky et al., 2016), prevented this decrease (Fig. S5B). In Tsc2-deficient neurons treated with CCCP, no significant mitochondrial degradation was observed (Fig. 5H). Together, these findings indicate a block of global mitophagic flux in Tsc2-deficient neurons.
Upon mitochondrial damage the ubiquitin ligase Parkin is recruited to ubiquitinate mitochondrial proteins. This attracts ubiquitin-binding proteins such as p62, which are believed to facilitate the recruitment of LC3-positive autophagosomes (Fig. 4A). Clearance of damaged mitochondria also removes these modifications. Paralleling our studies of mitochondrial flux, we examined the presence of Parkin, ubiquitinated proteins and LC3 in mitochondrial fractions of Tsc2-deficient neurons and controls treated with CCCP (Fig. 5I-K). Polyubiquitinated proteins accumulated in mitochondrial fractions following CCCP treatment with a marked and sustained increase in Tsc2-deficient neurons (Fig. 5I-J). Levels of Parkin and p62 were similarly increased at baseline and remained high following CCCP indicating the continued presence of early mitophagy surrogates in Tsc2-deficient neurons (Fig. 5I-J). Measuring levels of lipidated LC3 (LC3-II), we discovered that it was enriched in mitochondrial fractions of Tsc2-deficient neurons both at baseline and after CCCP treatment, reflecting increased amounts of unprocessed autophagosomes (Fig. 5I & K).
Collectively these findings demonstrate impaired global mitophagic flux following mitochondrial damage in Tsc2-deficient neurons. Our findings point to a block downstream of autophagosome recruitment with proteins involved in early mitophagy and autophagosomes accumulating within mitochondrial fractions of Tsc2-deficient neurons.
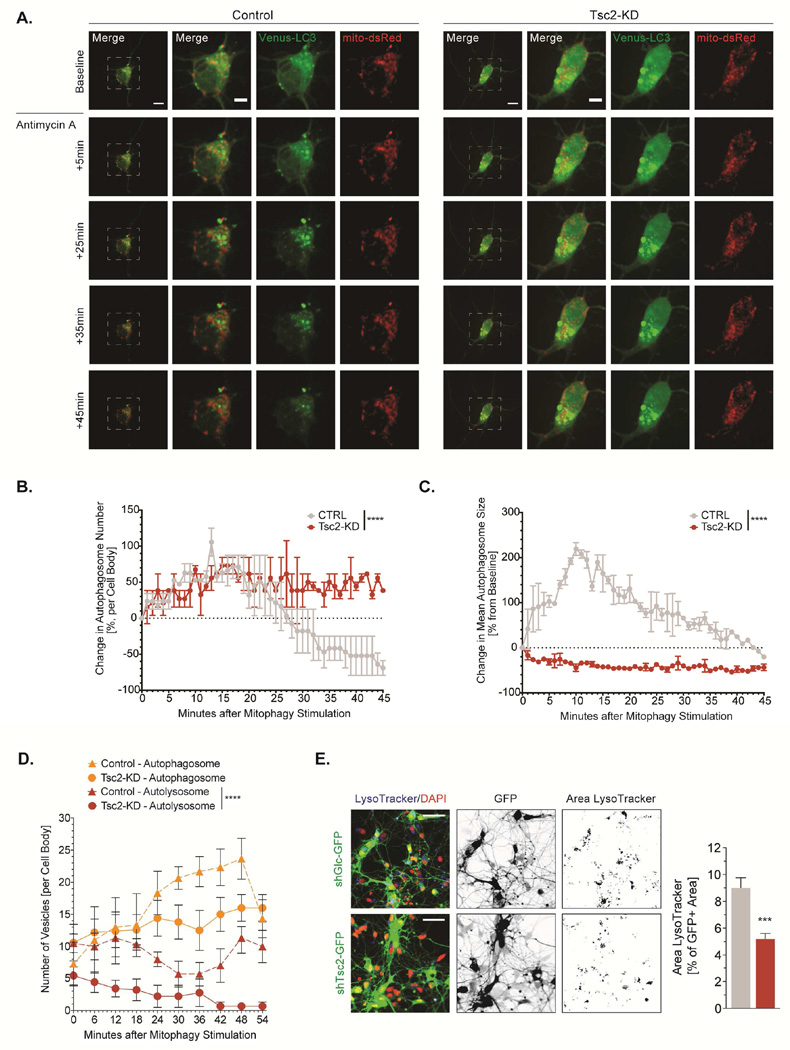
Deficits in lysosome-dependent stages of mitophagy
To better understand the block in mitophagic flux downstream of autophagosome recruitment, we analyzed the dynamics of autophagosome formation and recruitment in response to a stimulus that triggers mitophagy. We treated neurons with antimycin A and followed the formation and turnover of LC3-Venus-labeled autophagic vesicles using live cell confocal imaging (Fig. 6A, Supp. Video 4&5). At baseline, control cells carry several small LC3-Venus dots or crescent-shaped puncta representing nascent or maturing autophagosomes that are mostly outside of mitochondria-dense areas (Fig. 6A). In Tsc2-deficient neurons, LC3-Venus is present in fine dot-like vesicles that are observed to partly overlap with mitochondria but also in large vesicular structures that appear in no spatial relationship with mitochondria (Fig. 6A). Following induction of mitophagy, control cells rapidly form LC3-positive puncta and show autophagic turnover (Fig. 6A-C). In Tsc2-deficient neurons, new LC3-positive vesicles form, but stay relatively small and importantly do not seem to engage in fusion with preexisting large LC3-vesicles, therefore leading to no net increase in mean autophagosome size (Fig. 6A-C). Interestingly, no turnover of newly formed LC3 puncta is appreciated during the course of these live cell imaging experiments, indicating delayed and/or diminished autophagosome maturation or autophagosome-lysosome fusion in Tsc2-deficient cells (Fig. 6A-B).
Figure 6. Deficits in the lysosome-dependent stages of mitophagy in Tsc2-deficient neurons.
(A-C) Autophagosome turnover in hippocampal neurons (DIV7/8) acutely treated with antimycin A (40 µM). Graphs show the percent change in autophagosome number and size from baseline (n= 3 experiments). Scale bar, 10 µm, 5 µm (inset)
(D) Autophagosomes (GFP+ and mCherry+) and autolysosomes (RFP+only) in hippocampal neurons (DIV7/8) transfected with mCherry-EGFP-LC3 and acutely treated with antimycin A (40 µM, (n=4 experiments)).
(E) LysoTracker Deep Red stained lysosomes in hippocampal neurons (DIV7/8). Graph shows the lysosomal area as a percentage of the GFP stained cell area (n>25×103 cells per condition from 3 experiments). Scale bar, 40 µm
***p<0.001, ****<p.0001. See also Figure S5.
To probe autophagosome-lysosome fusion following mitophagy induction, we employed the mCherry-GFP-LC3 tandem assay (Kimura et al., 2007), that allows distinguishing autophagosomes before and after fusion with acidic lysosomes (Fig. S5C, (Klionsky et al., 2016)). Following mitophagy induction, we discovered that nascent autophagosomes mature and to a certain extend are converted to autolysosomes (Fig. 6D). In the Tsc2-deficient neurons, we did not observe such a recruitment of autolysosomes (Fig. 6D). Instead, these vesicles remained at the stage of autophagosomes, further underscoring a block in the late stages of mitochondrial-damage induced autophagy.
Reduced availability of mature lysosomes may be a rate-limiting factor for autophagosome-lysosome fusion. To quantify the pool of mature lysosomes available for fusion events, we employed an automated confocal microscopy approach to determine the amount of LysoTracker–stained lysosomes in a large number of Tsc2-deficient neurons and controls (Fig. 6E). Indeed, a smaller area was covered with lysosomal vesicles in Tsc2-deficient neurons (Fig. 6E), indicating fewer lysosomes available for fusion.
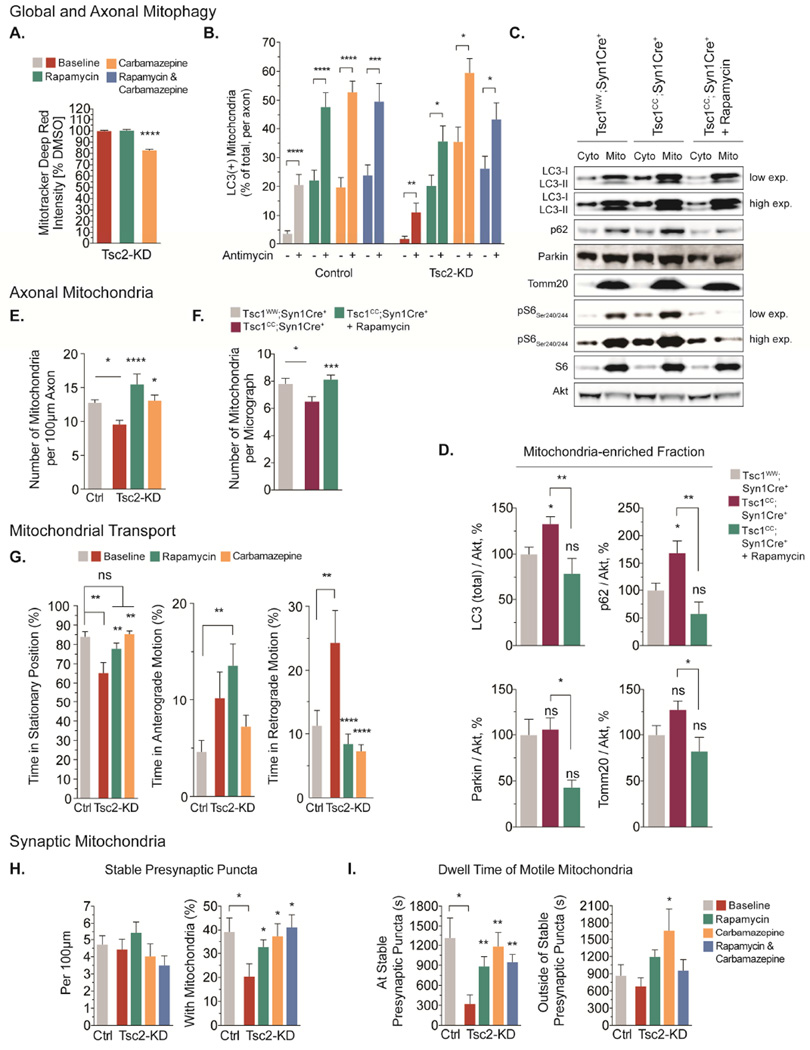
The impact of mTOR-dependent and mTOR-independent stimulation of autophagy on mitochondrial dynamics, turnover and function
The above experiments demonstrate that mitophagic flux in Tsc2-deficient neurons is impaired at the autophagosome recruitment stage and/or downstream lysosome-dependent stages. To test whether mTOR-dependent or mTOR-independent mechanisms are able to overcome or bypass this block in mitophagic flux, we treated neurons with rapamycin (mTORC1 inhibitor, mTOR-dependent enhancer of autophagy (Ravikumar et al., 2002)), and carbamazepine (mTOR-independent enhancer of autophagic flux, (Maetzel et al., 2014)). We found that both treatments engaged the formation of LC3-II and reduced levels of the autophagy substrate p62, confirming that autophagic flux is induced (Fig. S6A). Quantifying global mitophagic flux, we discovered that acute inhibition of mTORC1 with rapamycin did not induce a substantial turnover of mitochondria in Tsc2-deficient neurons (Fig. 7A). Carbamazepine, however, robustly reduced mitochondrial content in Tsc2-deficient neurons to about 85% of that of vehicle treated controls (Fig. 7A). Probing the recruitment of autophagosomes to axonal mitochondria, we found that both treatments stimulated the recruitment of LC3-positive autophagosomes to damaged mitochondria (Fig. 7B). Interestingly both treatments also increased co-localization of autophagosomes with mitochondria at baseline, without an additional mitophagic stimulus (Fig. 7B). This could indicate that existing damaged mitochondria are targeted when autophagic flux is stimulated. In line with the data on global mitophagic flux, carbamazepine showed a particularly strong effect in Tsc2-deficient axons (Fig. 7B). Combining rapamycin and carbamazepine had no additive effect, suggesting that each treatment alone may achieve saturating levels of autophagy induction in axons.
Figure 7. The impact of mTOR-dependent and mTOR-independent stimulation of autophagy on mitochondrial dynamics, turnover and function.
(A) Quantification of mitophagic flux using flow cytometry in cortical neurons (DIV11) treated with rapamycin (20nM, 24hr) or carbamazepine (100 µM, 24hr, n=3×106 recorded events per condition from 3–6 experiments).
(B) Recruitment of Venus-LC3 labeled autophagosomes to axonal mitochondria following acute Δ Ψm-depolarization with antimycin A (40 µM) in hippocampal neurons (DIV7/8) pretreated with rapamycin, carbamazepine or a combination of both drugs (n>35 axons per condition from 5–10 experiments).
(C&D) Western blotting for several proteins involved in mitophagy in cytosolic and mitochondria-enriched fractions from cortex of Tsc1cc;Syn1Cre+ mice (n=8), Tsc1CC;Syn1Cre+ mice treated with rapamycin (n=4), and Tsc1ww;Syn1Cre+ littermates (n=8, PND 21).
(E) Number of mitochondria per 100 µm axon in hippocampal neurons (DIV 7/8) pretreated with rapamycin or carbamazepine (n>20 axons per condition from >4 experiments).
(F) Number of mitochondria in callosal projection axons from Tsc1cc;Syn1Cre+ mice (n=2), Tsc1cc;Syn1Cre+ mice treated with rapamycin (n=3), and Tsc1ww;Syn1Cre+ littermate controls (n=3, PND 21) using transmission electron microscopy.
(G) Mitochondrial transport in the mid axon of hippocampal neurons (DIV7/8) pretreated with rapamycin or carbamazepine (n>15 axons per condition from 3–5 experiments).
(H) Number of stable presynaptic puncta per 100 µm axon and the percentage of stable presynaptic puncta supported by mitochondria in hippocampal neurons (DIV7/8) pretreated with rapamycin (20nM, 24hr), carbamazepine (100 µM, 24hr) or a combination of both drugs (n=14 axons per condition from 7 experiments).
(I) Dwell time of motile mitochondria at and outside of stable presynaptic puncta in hippocampal neurons (DIV7/8) pretreated with rapamycin, carbamazepine or a combination of both drugs (n=14 axons per condition from 7 experiments).
Cyto=cytosolic fraction, Mito=mitochondrial fraction, ns=not significant; *p<0.05, **p<0.01, ***p<0.001, ****<p.0001. See also Figure S6.
To test the relevance of these findings for neurons in vivo, we treated Tsc1cc;Syn1Cre+ mice with rapamycin and prepared mitochondria-enriched fractions from cortex. Mitochondrial fractions of Tsc1cc;Syn1Cre+ mice accumulate LC3 and p62, both of which were reduced in rapamycin-treated mice (Fig. 7C-D). Levels of Parkin and of the mitochondrial marker Tomm20 were also reduced following rapamycin treatment, suggesting that Tsc1cc;Syn1Cre+ mice can effectively prevent or clear mitochondria targeted for mitophagy once mTORC1 signaling is inhibited and autophagic flux is engaged as evidence by an increased cytosolic LC3II/LC3I ratio (Fig. 7C&Fig. S6B).
Does a correction of axonal mitophagy replenish axons with mitochondria? To address this question, we determined the number of mitochondria per axon area in cultured Tsc2-deficient neurons and callosal axons of Tsc1cc;Syn1Cre+ mice. In vitro, rapamycin and carbamazepine significantly increased the number of axonal mitochondria to levels that either did not differ from or surpassed controls (Fig. 7E). In vivo, chronic treatment of Tsc1cc;Syn1Cre+ mice with rapamycin also normalized the number of mitochondria in callosal axons to levels that are similar to those in control littermates (Fig. 7F). In addition to local turnover, influx of new mitochondria from the cell body and mechanisms that promote their peripheral anchoring determine the availability of mitochondria to distal axonal regions. Examining transport patterns of axonal mitochondria, we discovered that rapamycin and carbamazepine promoted the time that these spend in a stationary position at the expense of the increased retrograde transport observed in Tsc2-deficient neurons without treatment (Fig. 7G-I). Rapamycin particularly promoted their anterograde transport (Fig. 7G), perhaps leading to an even greater replenishment of axonal mitochondria (Fig. 7E).
To explore the functional consequences of correcting local mitochondrial turnover and transport, we quantified the capturing of mitochondria at presynaptic sites (Fig. 7H-I). While both treatments and their combination did not influence the overall number of presynaptic sites, they led to an increase in the proportion of presynaptic sites that are supported by stationary mitochondria (Fig. 7H). Likewise, the dwell time of motile mitochondria at presynaptic sites increased to levels that were similar to those in control axons (Fig. 7I). Arguing for an increased anchoring specifically at presynaptic sites, the dwell time outside of presynaptic compartments remained unchanged (Fig. 7I).
In summary, these data suggest that suppressing mTORC1 hyperactivity or inducing mTOR-independent autophagy can reverse many of the changes in mitochondrial dynamics and turnover seen in Tsc2-deficient neurons. Chronic treatment with rapamycin or carbamazepine for seven days also improved Δ Ψm (Fig. S6C), while short-term treatment did not (Fig. S6D). This dependence on chronic treatment argues that a complete restoration of mitochondrial function is only achieved when several stages of mitochondrial dynamics are intact for a prolonged time period.
DISCUSSION
Mitochondria are critical to many neuronal functions and their dynamics and turnover are tightly regulated. Autophagy is the principle pathway that clears damaged, aged and superfluous mitochondria (Pickrell and Youle, 2015). Previous work has shown that autophagy is significantly impaired in TSC deficient cells and tissues (Di Nardo et al., 2014; McMahon et al., 2012; Parkhitko et al., 2011; Tang et al., 2014; Yasin et al., 2013). Identifying specific deficits downstream of mTORC1 and autophagy dysfunction may reveal more precise targets for therapeutic interventions. Mitochondria are substrates of autophagy but also essential for mTOR-hyperactivity induced anabolism; thus, it is important to understand how TSC deficiency impacts mitochondrial homeostasis in neurons.
Here we address this question through a characterization of mitochondrial turnover and dynamics in neuronal in vitro and in vivo models of TSC. Using multiple approaches, we identified deficits in global and local mitophagy in Tsc2-deficient neurons and downstream accumulation of mitochondria, with at least a subset of organelles being defective. Importantly, we find that these changes in mitochondrial turnover affect the dynamics and integrity of axonal mitochondria leading to a depletion of functional mitochondria from presynaptic sites.
Mitophagy and Mitochondrial Fragmentation in TSC
The mitochondrial content in any given cell and their maintenance are subject to a complex interplay between mitochondrial biogenesis and degradation. In dividing, non-neuronal cells, loss of TSC2 leads to an increase in mitochondrial mass (Koyanagi et al., 2011; Morita et al., 2013) that is accompanied by an increase in the transcription of genes associated with mitochondrial biogenesis (Cunningham et al., 2007). In Tsc2-deficient neurons, we discovered an increased mitochondrial mass that seems to progress with maturation in vitro but were unable to identify an increase in mRNA levels of the core mitochondrial biogenesis genes, Pgc1α, Nrf-1 and mTFAM. Therefore, increased biogenesis is not likely to drive the increased mitochondrial mass observed in Tsc2-deficient neurons.
When mitochondria are excessively abundant, damaged or aged, mitophagy is engaged to restore homeostasis. Mitophagy undergoes several stages and relies on the general autophagy program to clear mitochondria via engulfment by autophagic vesicles and finally degradation in autolysosomes, the product of mature autophagosomes and lysosomes (Fig. 4A). In Tsc2-deficient neurons, we discovered a reduction in CCCP-induced mitophagic flux and an accumulation of proteins involved in the early steps of mitophagy, such as PINK1 and Parkin, in mitochondrial preparations. Similarly, we found abundant levels of p62, a stress-induced ubiquitin-binding protein, that, in addition to other functions, may be acting as a selective receptor for targeting damaged mitochondria to autophagosomes (Okatsu et al., 2010) and is commonly found in protein aggregates that result from autophagy impairment (Komatsu et al., 2007). We observed abundant levels of the autophagosome marker LC3-II in mitochondria-enriched fractions of Tsc2-deficient neurons, with no significant change after stimulation of mitophagy with CCCP. These results suggest that proteins involved in the early steps of mitophagy accumulate in Tsc2-deficient neurons. While mitophagy is engaged following the introduction of mitochondrial damage, it does not progress to the degradation of mitochondria and no net loss of mitochondria can be detected. Pointing to a block in the late stages of the autophagy pathway, autophagosome maturation into autolysosomes following mitophagy induction was impaired. Levels of lysosomes were reduced in Tsc2-deficient neurons, suggesting that the pool of mature lysosomes available for fusion with autophagosomes may be limited.
Mitochondrial morphology mirrors changes in turnover and function and can thus serve as a surrogate for both. Mitochondrial fragmentation is found in many forms of mtDNA diseases with reduced oxidative phosphorylation, where it is thought to segregate mitochondria containing deleterious mutations and may promote their degradation through autophagy (Pryde et al., 2016; Twig et al., 2008). In Tsc1/2-deficient neurons, we discovered a reduction of the average size of axonal mitochondria both in vitro and in vivo. We further found an increase in the circularity of axonal mitochondria, pointing to ongoing mitochondrial fragmentation reflecting dysfunction and perhaps ensuing turnover.
Axonal and presynaptic mitochondria in TSC
Intellectual disability, seizures and autism spectrum disorder in many patients with TSC may at least in part originate from synaptic dysfunction (Sahin and Sur, 2015). Underscoring the importance of axonal mitochondria in this process, removing mitochondria from axon terminals has been shown to result in aberrant synaptic transmission (Ma et al., 2009; Verstreken et al., 2005). Mitochondrial dysfunction, reactive oxygen species and a reduced availability of functional mitochondria to synapses, as a result of impaired mitophagy, may critically impair synaptic function (Haddad et al., 2013; Santini et al., 2015).
Axonal mitochondria are subject to active transportation (Sheng, 2014) and local turnover (Ashrafi et al., 2014). We explored both in Tsc2-deficient neurons. Mitochondria were relatively depleted from Tsc2-deficient axons, which may be partly explained by an increased retrograde transportation of mostly damaged mitochondria.
Damaged mitochondria were frequently observed and may be a result of impaired local turnover through mitophagy. In light of these findings, we speculate that reduced axonal mitophagy may lead to a build-up of damaged mitochondria over time. This build-up is met with increased retrograde transportation to clear dysfunctional and potentially harmful organelles from vulnerable, distal regions and perhaps leads to their clustering in the cell body where lysosomes are relatively enriched. The net effect of this is a depletion of axonal mitochondria.
Because of great regional differences in demand for ATP supply and calcium buffering, axonal mitochondria are transported bi-directionally and anchored as needed, particularly at active sites such as synapses (Rangaraju et al., 2014). To probe the availability of mitochondria to synapses, we investigated presynaptic capturing of mitochondria in developing and more mature axons. We discovered that presynaptic sites in Tsc2-deficient neurons were deprived of stationary mitochondria. In addition, we found that motile mitochondria, known to provide their function to presynaptic compartments en passant, spend less time dwelling over presynaptic sites. The reduced availability of mitochondria at synapses may bear importance to altered neuronal connectivity, intellectual disability and seizures in TSC.
Correcting deficits in mitochondrial homeostasis in TSC
mTOR-inhibitors have been shown to correct many molecular and behavioral deficits in neuronal models of TSC. This has paved the way for randomized placebo-controlled trials of mTOR inhibitors for neurocognitive deficits in children with TSC (Ebrahimi-Fakhari and Sahin, 2015). Autophagy can be activated in an mTORC1-dependent manner or by mechanisms that do not rely on mTOR kinase activity (Ebrahimi-Fakhari et al., 2016; Ebrahimi-Fakhari et al., 2014). To assess whether enhancing autophagy could correct mitochondrial deficits in Tsc2-deficient neurons, we treated them with rapamycin and carbamazepine. The latter is a widely used anticonvulsive drug that is thought to engage autophagy through reducing free inositol and myoinositiol-1,4,5-triphosphate levels (Sarkar et al., 2005; Schiebler et al., 2015). Both treatments corrected multiple deficits and importantly replenished axonal mitochondria, including those that support presynaptic sites. Our data thus indicate that induction of autophagy itself is beneficial and suggest that both compounds can restore the clearance of damaged mitochondria. This also implies that once autophagy deficits are bypassed, lysosomal proteolysis is functional in Tsc2-deficient neurons. These experiments also indicate that the deficits in lysosome-dependent stages of mitophagy still enable some increased substrate flux upon induction of autophagosome biogenesis, suggesting that the principal defect in mitochondrial turnover in Tsc2-deficient neurons results from a compromise of the autophagy-lysosomal pathway and is not primarily caused by impairment of early mitophagy events such as PINK1 or Parkin signaling. The fact that combining both treatments did not have an additive effect suggests that autophagy, rather than other downstream targets of mTORC1 signaling, is likely the main contributor to the restoration of mitochondrial phenotypes in Tsc2-deficient neurons, with either treatment alone achieving saturated levels of autophagy induction.
Our findings lend support to the concept that promoting clearance of damaged mitochondria through engaging autophagic flux by mTOR-dependent and independent mechanisms has potential therapeutic value for neurological diseases with mitochondrial dysfunction. Along these lines, rapamycin has been successfully used to drive selection against pathogenic mtDNA mutations in models of mtDNA diseases (Dai et al., 2014; Johnson et al., 2013). We acknowledge, however, that even though we probe mTORC1-dependent and independent mechanisms to bypass autophagy deficits, future studies will need to fully dissect the role of mTORC1-inhibition in restoring mitochondrial deficits beyond correction of mitochondrial turnover (Johnson et al., 2013; Zheng et al., 2016). Likewise, the mechanisms by which deficits in the lysosome-dependent stages of mitophagy are bypassed or corrected upon increased autophagic flux remain to be investigated in detail.
In summary, our results provide evidence for a role of impaired neuronal mitophagy in TSC, by showing that mTORC1 hyperactivity impairs the lysosome-dependent stages of mitochondrial turnover. Our study clarifies the complex relationship between the TSC-mTORC1 pathway, autophagy and mitophagy, and defines mitochondrial homeostasis as a therapeutic target for TSC and related diseases.
EXPERIMENTAL PROCEDURES
Please see the Supplemental Experimental Procedures for details.
Animals
Tsc1cc;Syn1Cre+ mice were described previously (Meikle et al., 2007). Rapamycin was administered i.p. at 6 mg/kg every other day starting at postnatal day 7.
Generation of Human iPSC & Cortical Neuron Differentiation
Human iPSCs were generated using episomal plasmids to introduce the reprogramming factors (Oct4, Sox2, Klf4, and L-Myc) into blood cells or fibroblasts (Table 1). Homozygous TSC2 mutant iPSC lines were generated using TALEN-mediated mutagenesis. Cortical neuronal differentiation was adapted from (Zhang et al., 2013).
Live Cell Confocal Imaging
Confocal images were obtained using a spinning disk confocal microscope (Perkin Elmer) with a Nikon Ti-Eclipse live cell imaging system and Volocity software (Perkin Elmer).
Mitochondrial Motility
A mid-axonal compartment (∼500–800 µm from the soma) was identified, and images were captured every 3s. Individual mitochondria were analyzed in a standardized manner using the custom-written Kymolizer macro in Image J (Pekkurnaz et al., 2014).
Axonal Mitophagy
LC3 and lysosome recruitment to mitochondria was quantified as described in (Ashrafi et al., 2014).
Presynaptic Capturing of Mitochondria
Mitochondrial capturing at synapses was measured as described in (Courchet et al., 2013).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6.0 (GraphPad Software, Inc.). Throughout the manuscript, the distribution of data points is expressed as mean ± standard error of the mean. Mann–Whitney U test, unpaired Student t-test or one-way ANOVA with post hoc Bonferroni’s multiple-comparisons test was used to determine significance. p<0.05 was considered significant.
Supplementary Material
Acknowledgments
The authors thank R. Kleiman, J. Micozzi, X. Yang, E. Ercan, E. Chadwick, S. Lammers, S. Schaeffer, C. Super (Boston Children’s Hospital, BCH) and D.J. Kwiatkowski (Brigham and Women’s Hospital) for helpful discussions and technical assistance. The authors are also grateful to A. Hill and the IDDRC Cellular Imaging Core (funded by NIH P30HD18655) at BCH for technical support and to G. Ashrafi, G. Pekkurnaz and T.L. Schwarz (BCH) for sharing reagents, software and advice. This work was supported by the Graduate Academy (D.E.-F.) and the Young Investigator Award Program at Heidelberg University (D.E.-F., L.W.), the Daimler & Benz Foundation (D.E.-F.), the Reinhard-Frank Foundation (D.E.-F.), the German National Academic Foundation (A.S.), the Nancy Lurie Marks Family Foundation and the Boston Children’s Hospital Translational Research Program (M.S.). Christopher Conrad and Jonathan M. Rothberg are affiliated with LAM Therapeutics. Other research projects in the Sahin laboratory are supported by Novartis and Pfizer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments:D.E.-F., A.S., L.W., M.S.; Performed the experiments:D.E.-F., A.S., L.W., A.D., D.T., C.C., J.R., M-J.H.; Analyzed the data:D.E.-F., A.S., L.W., A.D., C.C., J.R., M-J.H., M.S.; Contributed reagents/materials/analysis tools:S.K., G.F.H., T.L.L., J.O.L., F.P.; Drafted the article:D.E.-F., A.S., M.S. Revised the article: All authors.
REFERENCES
- Albert V, Hall MN. mTOR signaling in cellular and organismal energetics. Curr Opin Cell Biol. 2015;33:55–66. doi: 10.1016/j.ceb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Zakaria HM, Simone A, Sheng ZH. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biol. 2012;22:545–552. doi: 10.1016/j.cub.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, He X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V, Aigner S, Vukcevic M, Sauter E, Behr K, Ebeling M, Dunkley T, Friedlein A, Zoffmann S, Meyer CA, et al. mTORC1 Inhibition Corrects Neurodevelopmental and Synaptic Alterations in a Human Stem Cell Model of Tuberous Sclerosis. Cell Rep. 2016;15:86–95. doi: 10.1016/j.celrep.2016.02.090. [DOI] [PubMed] [Google Scholar]
- Courchet J, Lewis TL, Jr, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153:1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Dai Y, Zheng K, Clark J, Swerdlow RH, Pulst SM, Sutton JP, Shinobu LA, Simon DK. Rapamycin drives selection against a pathogenic heteroplasmic mitochondrial DNA mutation. Hum Mol Genet. 2014;23:637–647. doi: 10.1093/hmg/ddt450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy S, Liu A, Lampe T, Hollenbeck PJ. The Organization of Mitochondrial Quality Control and Life Cycle in the Nervous System In Vivo in the Absence of PINK1. J Neurosci. 2015;35:9391–9401. doi: 10.1523/JNEUROSCI.1198-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo A, Kramvis I, Cho N, Sadowski A, Meikle L, Kwiatkowski DJ, Sahin M. Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR-dependent manner. J Neurosci. 2009;29:5926–5937. doi: 10.1523/JNEUROSCI.0778-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo A, Wertz MH, Kwiatkowski E, Tsai PT, Leech JD, Greene-Colozzi E, Goto J, Dilsiz P, Talos DM, Clish CB, et al. Neuronal Tsc1/2 complex controls autophagy through AMPK-dependent regulation of ULK1. Hum Mol Genet. 2014;23:3865–3874. doi: 10.1093/hmg/ddu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMario FJ, Jr, Sahin M, Ebrahimi-Fakhari D. Tuberous sclerosis complex. Pediatr Clin North Am. 2015;62:633–648. doi: 10.1016/j.pcl.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Saffari A, Wahlster L, Lu J, Byrne S, Hoffmann GF, Jungbluth H, Sahin M. Congenital disorders of autophagy: an emerging novel class of inborn errors of neuro-metabolism. Brain. 2016;139:317–337. doi: 10.1093/brain/awv371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Sahin M. Autism and the synapse: emerging mechanisms and mechanism-based therapies. Curr Opin Neurol. 2015;28:91–102. doi: 10.1097/WCO.0000000000000186. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Wahlster L, Hoffmann GF, Kolker S. Emerging role of autophagy in pediatric neurodegenerative and neurometabolic diseases. Pediatr Res. 2014;75:217–226. doi: 10.1038/pr.2013.185. [DOI] [PubMed] [Google Scholar]
- Goto J, Talos DM, Klein P, Qin W, Chekaluk YI, Anderl S, Malinowska IA, Di Nardo A, Bronson RT, Chan JA, et al. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci U S A. 2011;108:E1070–E1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad DM, Vilain S, Vos M, Esposito G, Matta S, Kalscheuer VM, Craessaerts K, Leyssen M, Nascimento RM, Vianna-Morgante AM, et al. Mutations in the intellectual disability gene Ube2a cause neuronal dysfunction and impair parkin-dependent mitophagy. Mol Cell. 2013;50:831–843. doi: 10.1016/j.molcel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Ostrowski P, Jaumouille V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol. 2016;212:677–692. doi: 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013;342:1524–1528. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. (3rd) 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Asahara S, Matsuda T, Hashimoto N, Shigeyama Y, Shibutani Y, Kanno A, Fuchita M, Mikami T, Hosooka T, et al. Ablation of TSC2 enhances insulin secretion by increasing the number of mitochondria through activation of mTORC1. PLoS One. 2011;6:e23238. doi: 10.1371/journal.pone.0023238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Cai Q, Lu W, Sheng ZH, Mochida S. KIF5B motor adaptor syntabulin maintains synaptic transmission in sympathetic neurons. J Neurosci. 2009;29:13019–13029. doi: 10.1523/JNEUROSCI.2517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzel D, Sarkar S, Wang H, Abi-Mosleh L, Xu P, Cheng AW, Gao Q, Mitalipova M, Jaenisch R. Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick Type C patient-specific iPS cells. Stem Cell Reports. 2014;2:866–880. doi: 10.1016/j.stemcr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro-Lizcano M, Esteban-Martinez L, Seco E, Serrano-Puebla A, Garcia-Ledo L, Figueiredo-Pereira C, Vieira HL, Boya P. New method to assess mitophagy flux by flow cytometry. Autophagy. 2015;11:833–843. doi: 10.1080/15548627.2015.1034403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon J, Huang X, Yang J, Komatsu M, Yue Z, Qian J, Zhu X, Huang Y. Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci. 2012;32:15704–15714. doi: 10.1523/JNEUROSCI.2392-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Gravel SP, Chenard V, Sikstrom K, Zheng L, Alain T, Gandin V, Avizonis D, Arguello M, Zakaria C, et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013;18:698–711. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, Kimura M, Sato S, Hattori N, Komatsu M, et al. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells. 2010;15:887–900. doi: 10.1111/j.1365-2443.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhitko A, Myachina F, Morrison TA, Hindi KM, Auricchio N, Karbowniczek M, Wu JJ, Finkel T, Kwiatkowski DJ, Yu JJ, et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc Natl Acad Sci U S A. 2011;108:12455–12460. doi: 10.1073/pnas.1104361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158:54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas L, Scorrano L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu Rev Physiol. 2016;78:505–531. doi: 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde KR, Smith HL, Chau K-Y, Schapira AHV. PINK1 disables the anti-fission machinery to segregate damaged mitochondria for mitophagy. The Journal of Cell Biology. 2016 doi: 10.1083/jcb.201509003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju V, Calloway N, Ryan TA. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- Sahin M, Sur M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 2015:350. doi: 10.1126/science.aab3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Turner KL, Ramaraj AB, Murphy MP, Klann E, Kaphzan H. Mitochondrial Superoxide Contributes to Hippocampal Synaptic Dysfunction and Memory Deficits in Angelman Syndrome Model Mice. J Neurosci. 2015;35:16213–16220. doi: 10.1523/JNEUROSCI.2246-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebler M, Brown K, Hegyi K, Newton SM, Renna M, Hepburn L, Klapholz C, Coulter S, Obregon-Henao A, Henao Tamayo M, et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol Med. 2015;7:127–139. doi: 10.15252/emmm.201404137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH. Mitochondrial trafficking and anchoring in neurons: New insight and implications. J Cell Biol. 2014;204:1087–1098. doi: 10.1083/jcb.201312123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin SA, Ali AM, Tata M, Picker SR, Anderson GW, Latimer-Bowman E, Nicholson SL, Harkness W, Cross JH, Paine SM, et al. mTOR-dependent abnormalities in autophagy characterize human malformations of cortical development: evidence from focal cortical dysplasia and tuberous sclerosis. Acta Neuropathol. 2013;126:207–218. doi: 10.1007/s00401-013-1135-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Boyer L, Jin M, Kim Y, Fan W, Bardy C, Berggren T, Evans RM, Gage FH, Hunter T. Alleviation of neuronal energy deficiency by mTOR inhibition as a treatment for mitochondria-related neurodegeneration. Elife. 2016:5. doi: 10.7554/eLife.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.