Abstract
Background
Chemokines and chemokine receptors play important roles in autoimmune diseases; however, their role in immune thrombocytopenia (ITP) is unclear. High-dose dexamethasone (HD-DXM) may become a first-line therapy for adult patients with ITP, but the effect of HD-DXM on chemokines in ITP patients is unknown. Our aim was to investigate the mechanism of pulsed HD-DXM for management of ITP, specifically regarding the chemokine pathways.
Methods
Th1-/Th2-associated chemokine and chemokine receptor profiles in ITP patients before and after pulsed HD-DXM was studied. Plasma levels of CCL5 and CXCL11 (Th1-associated) and of CCL11 (Th2-associated) were determined by ELISA. Gene expression of these three chemokines and their corresponding receptors CCR5, CXCR3, and CCR3, in peripheral blood mononuclear cells (PBMCs) was determined by quantitative RT-PCR.
Results
Thirty-three of the thirty-eight ITP patients responded effectively to HD-DXM (oral, 40 mg/day, 4 days). In ITP patients, plasma CXCL11 levels increased, while CCL11 and CCL5 decreased compared to controls (P < 0.05). Similarly, gene expression of CXCL11 and its receptor CXCR3 increased, while CCL11 and CCR3 decreased (P < 0.05). CCL5 expression did not significantly change; however, expression of its receptor CCR5 increased (P < 0.05). Interestingly, in the patients who responded to pulsed HD-DXM, CXCL11 and CXCR3 expression was down-regulated, while CCL11 and CCR3 expression was up-regulated (P < 0.05). Meanwhile, CCL5 expression was up-regulated and CCR5 was down-regulated by HD-DXM (P < 0.05).
Conclusions
The abnormal profiles of Th1-/Th2-associated chemokines and chemokine receptors may play important roles in the pathogenesis of ITP. Importantly, regulating Th1 polarization by pulsed HD-DXM may represent a novel approach for immunoregulation in ITP.
Keywords: Immune thrombocytopenia, Chemokine, Chemokine receptor, Th1 polarization, High-dose dexamethasone
Background
Immune thrombocytopenia (ITP) is an acquired autoimmune-mediated bleeding disorder, mainly characterized by anti-platelet antibody-mediated thrombocytopenia in the reticuloendothelial system [1]. Interestingly, it has been shown that the destruction of platelets is associated with enhanced T-helper 1 (Th1) expression and an elevated Th1/Th2 ratio in ITP patients [2, 3]. However, expression of chemokines and chemokine receptors associated with the Th1/Th2 imbalance, particularly relating to immune regulation by modified application of glucocorticoids, has yet to be explored.
Chemokines and their receptors are involved in cellular migration and proliferation, molecule adhesion, regulation of apoptosis, T cell differentiation, leukocyte trafficking, and cytokine production [4]. For example, chemokine CCL5 is not only required for attracting CCR5+ T cells but is also essential for their activation [5]. CCR5 is specifically expressed on Th1 cells and has been reported as a critical chemokine receptor in several autoimmune diseases with polarization of Th1 cells such as rheumatoid arthritis, multiple sclerosis, Crohn’s disease and oral lichen planus [6–8]. CXCL10 expression is essential for Th1 cell differentiation [9, 10]. CXCL11 is overexpressed in Th1-dominated skin disorders, such as, psoriasis, lichen planus, and atopic dermatitis [11–13]. Moreover, CXCL11 is the most potent chemoattractant and has the highest receptor binding affinity for CXCR3 [14]. CXCR3, a marker for activated T lymphocytes, is characteristically expressed on Th1 and CD8+ T cells [15, 16] and is required for generation of interferon-γ (IFN-γ) -secreting Th1 cells in vivo [9]. In contrast, CCR3 is predominantly expressed on Th2 cells [17]. Recently, by characterizing Th1-associated chemokine receptors CXCR3 and CCR5, and Th2-associated chemokine receptor CCR3, we demonstrated that the abnormal expression of Th1/Th2 chemokine receptors may participate in the splenic immune dysregulation in ITP patients [18].
Glucocorticoid therapy is still the primary choice for treatment of ITP. Due to the poor long-term sustainability and possible adverse effects of long-term administration of corticosteroids [19], recent reports suggested that high-dose dexamethasone (HD-DXM) might be a promising regimen to replace the classical prednisone therapy [20–22]. Here, we investigated Th1- and Th2-associated chemokines and their receptors in peripheral blood of ITP patients. Specifically, we investigated whether HD-DXM could rectify the abnormal Th1-/Th2-associated chemokine and chemokine receptor profile in ITP.
Methods
Patients and controls
Between December 2014 and August 2016, 38 patients (12 males and 26 females, age range 12–71 years, median age 40.5 years) with active ITP in Shenzhen Baoan Hospital Affiliated to the Southern Medical University and the Second Hospital of Shandong University were enrolled. Patients’ platelet counts ranged from 2 to 34 × 109/L, with a median count of 10 × 109/L. All patients required treatment because of clinically significant bleeding. All of the cases met the diagnosis criteria of ITP as previously described [23]. None of them had received any corticosteroid or immunosuppressive therapy within the 3 months prior to sampling. Patients with diabetes, hypertension, cardiovascular diseases, pregnancy, active infection, or connective tissue diseases, such as systemic lupus erythematosus, were excluded. The clinical features of the patients are shown in Table 1.
Table 1.
Clinical characteristics of patients with ITP
| Patient | Gender | Age | Platelets (×109/L) | Bleeding symptoms | Major therapy | Platelets (×109/L) |
|---|---|---|---|---|---|---|
| Before treatment | After treatment | |||||
| 1 | F | 54 | 23 | EC | GC | 118 |
| 2 | F | 37 | 6 | EC, PT | GC | 154 |
| 3 | F | 23 | 15 | EC, EP | GC | 98 |
| 4 | F | 58 | 18 | EP, GUH | GC | 222 |
| 5 | F | 48 | 3 | EC, GUH | GC | 245 |
| 6 | M | 67 | 12 | PT | GC | 97 |
| 7 | F | 40 | 10 | PT | GC | 56 |
| 8 | M | 59 | 4 | EC | GC | 234 |
| 9 | F | 60 | 27 | EC, GUH | GC | 67 |
| 10 | M | 70 | 10 | EC, PT | GC | 21 |
| 11 | M | 49 | 9 | PT | GC | 180 |
| 12 | M | 12 | 34 | EC | GC | 78 |
| 13 | F | 13 | 21 | EC | GC | 203 |
| 14 | F | 26 | 10 | EC, GIH | GC | 82 |
| 15 | F | 28 | 19 | EC | GC | 168 |
| 16 | M | 65 | 20 | EC, GIH | GC | 166 |
| 17 | M | 42 | 11 | PT | GC | 267 |
| 18 | F | 17 | 9 | EC | GC | 80 |
| 19 | F | 56 | 2 | PT | GC | 9 |
| 20 | M | 61 | 27 | PT | GC | 174 |
| 21 | F | 63 | 10 | EC, GH | GC | 199 |
| 22 | F | 50 | 14 | EC, PT | GC | 143 |
| 23 | M | 31 | 11 | EC | GC | 20 |
| 24 | F | 26 | 25 | PT | GC | 156 |
| 25 | F | 71 | 21 | PT | GC | 151 |
| 26 | F | 51 | 28 | EC, GUH | GC | 32 |
| 27 | F | 28 | 8 | PE, PT, ET | GC | 103 |
| 28 | F | 24 | 9 | EC, EP | GC | 197 |
| 29 | F | 29 | 16 | PT | GC | 173 |
| 30 | F | 34 | 22 | PT | GC | 129 |
| 31 | F | 41 | 7 | PE, PT | GC | 301 |
| 32 | F | 20 | 5 | EC, GUH | GC | 24 |
| 33 | M | 25 | 23 | PT | GC | 71 |
| 34 | M | 37 | 3 | PT | GC | 96 |
| 35 | F | 19 | 2 | EC, PT | GC | 136 |
| 36 | M | 33 | 7 | PT | GC | 84 |
| 37 | F | 46 | 16 | PT | GC | 183 |
| 38 | F | 42 | 10 | PT | GC | 158 |
| Median | 40.5 | 10 | 148.5 | |||
| Range | 12–71 | 2–34 | 9–301 |
EC ecchymoses; GC glucocorticoid; PT petechiae; EP epistaxis; GUH genitourinary hemorrhage; GIH gastrointestinal hemorrhage; GH gingival hemorrhage
The control group consisted of 31 healthy adult volunteers (11 males and 20 females, age range 21–53 years, median 31.5 years), whose platelet counts ranged from 148 to 342 × 109/L, with the median count of 196 × 109/L.
Treatment regimen
Dexamethasone (DXM, 40 mg/day) was administered orally for four consecutive days. No maintenance or other treatment modality was used. Initial response was evaluated 2 weeks after treatment initiation. An effective response was defined as a platelet count greater than 30 × 109/L, at least a twofold increase compared to baseline, and an absence of bleeding [24].
Reagents and antibodies
Lymphoprep was obtained from Hao Yang Biological Manufacturer, Tianjin, China. Human CCL5, CXCL11, and CCL11 ELISA kits were purchased from R&D Systems, USA. TriZol reagent for total RNA was provided by Invitrogen, CA, USA. PrimeScript™ RT reagent Kit, SYBR Premix Ex Taq TM II were supplied by TaKaRa, Japan. Oligonucleotide primers for real-time PCR for CCL5, CXCL11, CCL11, CCR5, CXCR3, CCR3, and β-actin were synthesized by TaKaRa, Japan. Phosphate-buffered saline, fetal bovine serum, trypsin–EDTA and RPMI 1640 was provided by Gibco, USA. The microplate reader was supplied by Thermo Scientific, USA.
PBMCs and plasma preparation
Peripheral blood mononuclear cells (PBMCs) were purified from heparinized venous blood samples immediately following collection using Lymphoprep density gradient centrifugation. Plasma was isolated from fresh samples by centrifugation and stored at −80 °C until use.
Chemokine secretion
Plasma concentrations of CCL5, CXCL11, and CCL11 in active patients, patients who responded to HD-DXM, and controls were evaluated using a matched ELISA kit. All procedures were performed according to the manufacturer’s instructions. Each sample was tested in duplicate and optical densities were read using a microplate reader.
Assay of chemokine mRNA expression
Total RNA was extracted from PBMCs with TriZol according to the manufacturer’s instructions. cDNA was reversely transcribed from total RNA using an eppendorf Realplex2 PCR Detection System and the PrimeScript™ RT reagent kit. Following the manufacturer’s protocol, real-time PCR was performed to determine the expression of chemokine mRNA with SYBR Premix Ex Taq™ II. Reverse transcription was carried at 42 °C for 5 min. PCR was performed as follows: initial denaturation 30 s at 95 °C, and for 40 cycles of 5 s at 95 °C for denaturation and 30 s at 60 °C for transcription. All samples were measured in triplicate. The amount of chemokine mRNA was normalized with β-actin and expressed as relative quantification (RQ): RQ = 2−ΔΔct. The primer sequences for CCR5, CXCR3, CCR3, CCL5, CXCL11, CCL11, and β-actin are listed in Table 2.
Table 2.
Primer sequences
| Target gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| CCL5 | CAGAGAAGAAATGGGTTCGGGA | GAGCGGGTGGGGTAGGATAGTG |
| CXCL11 | GCCTTGGCTGTGATATTGTGTGC | CATCGTTGTCCTTTATTTTCTTT |
| CCL11 | TCAGCGACTAGAGAGCTACAGGA | GCTTTGGAGTTGGAGATTTTTGG |
| CCR3 | TACACAGGAATCATCAAAACGC | AGGAAGAGAGAAGGATAGCCAC |
| CXCR3 | CAACGCCACCCACTGCCAATAC | CAAAGGCCACCACGACCACCAC |
| CCR5 | GAAGAGCTGAGACATCCGTTCC | ACACCAG TGAGTAGAGCGGAG |
| β-actin | CACTCTTCCAGCCTTCCTTCC | AGGTCTTTGCGGATGTCCAC |
Statistical analysis
The Statistical Package for the Social Sciences version 19.0 (SPSS Inc., Chicago, IL) was used for statistical analysis of experimental data. Values are presented as mean ± SD. The date of plasma chemokine levels and mRNA levels among the active ITP groups and normal controls were normally distributed. The comparison between two groups was conducted by t test, measurement data were compared by ANOVA with Bonferroni test analysis among multiple groups. Briefly, ANOVA analysis was for comparison among active ITP, normal controls, and responders, and Bonferroni test was for comparison between any of two groups. Values of P < 0.05 were considered statistically significant.
Results
Therapeutic effect of HD-DXM
Out of all 38 patients, 33 cases (10 males and 23 females, median age 38.5 years, range 12–71 years) responded effectively to the HD-DXM therapy, according to the standard definition [19]. In these responders, platelet counts ranged from 56 to 301 × 109/L, with a median count of 155 × 109/L, as shown in Table 1. No bleeding or other complications were apparent, such as metabolized abnormality of multiple systems. Withdrawal symptoms were not observed throughout the treatment either.
Plasma levels of CCL5, CXCL11, and CCL11
Compared to normal controls, plasma CCL5 levels were reduced in the active ITP group (126.18 ± 19.84 vs 455.54 ± 68.29 pg/mL). Plasma CCL5 in ITP patients who responded well to HD-DXM (responders) improved (334.08 ± 53.63 pg/mL) but was still lower than control (Fig. 1a).
Fig. 1.
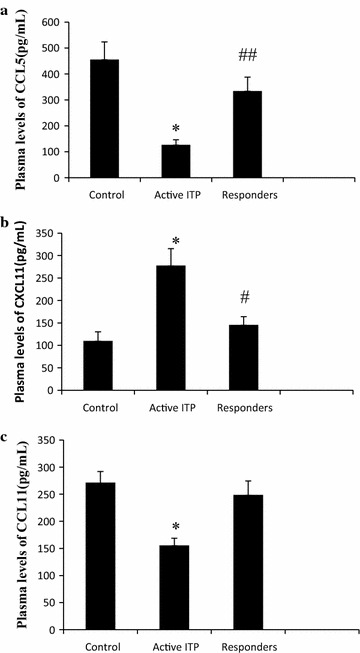
Plasma CCL5, CXCL11, and CCL11 levels. a Compared to normal controls, CCL5 levels were reduced in the active ITP. In responders (ITP patients who responded well to HD-DXM), CCL5 improved significantly but still lower than control (P = 0.000 < 0.05). b Compared to control, CXCL11 levels were increased in active ITP patients (P = 0.000 < 0.05). In responders, CXCL11 levels decreased, but were still higher than controls (P = 0.018). c Compared to control, CCL11 levels in active ITP patients were lower than in controls (P = 0.000 < 0.05). Responders had significantly higher CCL11 levels than active ITP patients that were not significantly different from control (P = 0.255 > 0.05). Asterisk represents P < 0.01 vs control and responders, # P < 0.05 and ## P < 0.01 vs control
Compared to control, CXCL11 levels were increased in active ITP patients (277.84 ± 37.78 vs 109.83 ± 20.30 pg/mL). In responders, CXCL11 levels decreased (145.53 ± 18.44 pg/mL), but were still higher than controls (Fig. 1b).
Compared to control, CCL11 levels in active ITP patients were lower than in controls (155.60 ± 13.22 vs 271.47 ± 20.48 pg/mL). Responders had significantly higher CCL11 levels (248.88 ± 25.53 pg/mL) than active ITP patients that were not significantly different from control (Fig. 1c).
CCL5, CXCL11, and CCL11 mRNA expression
Chemokine mRNA levels in PBMCs were determined by qRT-PCR. For CCL5 expression, no significant difference was found between any group (1.36 ± 0.31 vs 1.57 ± 0.20 vs 1.61 ± 0.25, in active ITP, control, and responders. Fig. 2a).
Fig. 2.
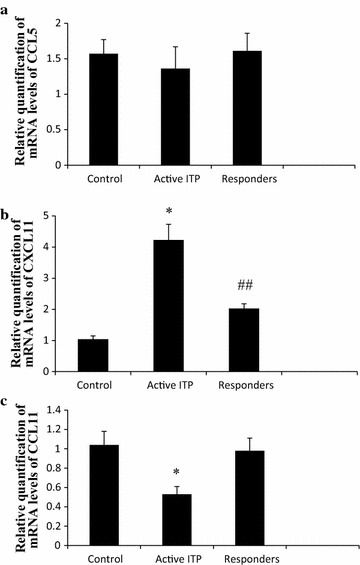
Relative quantification of mRNA levels of CCL5, CXCL11 and CCL11. a No significant difference was found between any groups (P = 0.382, 0.390, 1.000 > 0.05, respectively). b Compared to normal controls, CXCL11 mRNA levels were increased in active ITP patients (P = 0.000 < 0.05). CXCL11 expression in responders was significantly reduced compared to active ITP, but still higher than controls (P = 0.000 < 0.05, respectively). c Compared to control, CCL11 mRNA levels in active ITP patients was reduced (P = 0.000 < 0.05), however, CCL11 mRNA levels improved in responders and was not significantly different from control (P = 1.000 > 0.05). * P < 0.01 vs control and responders, ## P < 0.01 vs control
Compared to normal controls, CXCL11 mRNA levels were increased in active ITP patients (4.23 ± 0.50 vs 1.04 ± 0.11). CXCL11 expression in responders was significantly decreased (2.03 ± 0.15) compared to active ITP, but still higher than controls (Fig. 2b).
Compared to control, CCL11 mRNA levels in active ITP patients was reduced (0.53 ± 0.08 vs 1.04 ± 0.14). However, CCL11 mRNA levels improved in responders and was not significantly different from control (0.98 ± 0.13) (Fig. 2c).
CCR5, CXCR3, and CCR3 mRNA expression
Compared to control, as mRNA expression of the Th1-associated chemokine receptors, both of CCR5 and CXCR3 were elevated in active ITP patients (2.05 ± 0.20 vs 1.03 ± 0.06, 4.06 ± 0.23 vs 1.06 ± 0.08, respectively) (Fig. 3a, b). In responders, CCR5 expression returned to normal levels (1.06 ± 0.15, Fig. 3a). CXCR3 expression in responders decreased (2.43 ± 0.11), but was still higher than that in control (Fig. 3b).
Fig. 3.
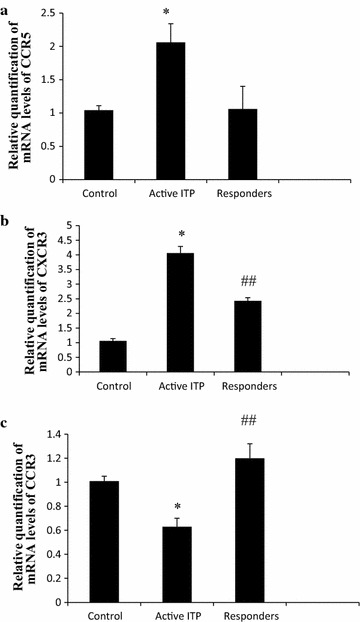
Relative quantification of mRNA levels of CCR5, CXCR3, and CCR3. a Compared to control, CCR5 mRNA levels in active ITP patients were elevated (P = 0.000 < 0.05). In responders, CCR5 expression returned to normal levels (P = 1.000 > 0.05). b Compared to control, CXCR3 mRNA levels in active ITP patients were elevated (P = 0.000 < 0.05). CXCR3 expression in responders decreased (P = 0.000 < 0.05), but was still higher than that in control (P = 0.000 < 0.05). c Compared to control, CCR3 mRNA levels in active ITP decreased (P = 0.000 < 0.05); after treatment with HD-DXM, CXCR3 expression in responders increased (P = 0.000 < 0.05). * P < 0.01 vs control and responders, ## P < 0.01 vs control
Compared to control, as one of the Th1-associated chemokine receptors, CCR3 mRNA levels in active ITP decreased (0.63 ± 0.07 vs 1.01 ± 0.04); after treatment with HD-DXM, CXCR3 expression in responders increased significantly (1.20 ± 0.12, Fig. 3c).
Discussion
ITP is an immune-mediated acquired disease characterized by a persistent or transient decrease of platelet count. In this study, we found an abnormal profile of Th1- and Th2-associated chemokines and their receptors in peripheral blood of ITP patients. Further, we demonstrated that HD-DXM can rectify the abnormal Th1-/Th2-associated chemokine and chemokine receptor profile in ITP.
Our findings showed that 85.71 % of ITP patients responded well to pulsed HD-DXM, confirming its impressive therapeutic effect. Previously, a high initial response was reported in a large study by Cheng et al. [20] after adult ITP patients were treated with a single dose of HD-DXM. The international consensus report and practice guidelines for ITP have now proposed HD-DXM therapy as one of the first-line therapeutic options for ITP patients [23, 24]. The latest clinical investigation showed that in addition to being generally better tolerated, HD-DXM resulted in a higher incidence of overall initial response and complete response compared to prednisone. These findings suggested that HD-DXM may be a preferred corticosteroid strategy for first-line management of adult primary ITP [25].
Although the etiology of ITP is multifactorial, many researchers have shown that ITP involves Th1 polarization, characterized by the oligoclonal accumulation of Th1 cells [3, 26]. Chemokines and chemokine receptors that are involved in T cell differentiation could influence the balance of Th1/Th2 [4]. These results prompted us to investigate the expression of chemokines and their receptors in ITP patients.
CXCL11 is one of the three ligands of CXCR3, the others being CXCL9 and CXCL10. CXCL11 and CXCL10 may play a role in the accumulation of Th1 cells in pulmonary sarcoidosis, a Th1-associated disease [27]. Of the three ligands, CXCL11 has the highest receptor binding affinity for CXCR3 through chemotactic migration and transient mobilization of intercellular calcium [28]. Here, we detected the status of CXCL11 and its receptor CXCR3 in ITP. We found that plasma CXCL11 levels in active ITP patients were higher than in controls, suggesting that CXCL11 may be an important chemoattractant for effector T cells involved in ITP. Moreover, CXCL11 acts as an antagonist for CCR3 but an agonist for CXCR3 [29]. In patients who responded well to pulsed HD-DXM (responders), plasma CXCL11 and mRNA levels of CXCL11 in PBMCs were decreased. Therefore, CXCL11 may be a potential therapeutic target for ITP, since the analogue is that Tizina et al. [30] successively modelled the structural determinants of these interactions of CXCL9/CXCR3, CXCL10/CXCR3 and CXCL11/CXCR3 complexes and their physico-chemical features in order to be used for drug design.
CCL5 is a CC chemokine that activates cells by binding to Th1-associated CCR1 and CCR5 [31]. CCL5/CCR5 interaction has been reported in a number of Th1-associated diseases, such as rheumatoid arthritis, multiple sclerosis [32], human immunodeficiency virus 1 (HIV-1) infection [33], Crohn’s disease [7], and oral lichen planus [8]. In this study, for mRNA levels of CCL5 in PBMCs, no significant difference was found between any group; however, plasma levels of CCL5 in active ITP patients was lower than in controls, perhaps because platelets are the principal source of CCL5 [34]. With the increase of platelet counts after pulsed HD-DXM treatment, there was an increase of CCL5 concentration in plasma. Thus, an important role for CCL5 in the pathogenesis of ITP is not evident; there are possibly other chemokines [35] binding to CCR5 to destroy platelets.
CCL11 is a specific and potent eosinophil chemoattractant that acts exclusively via CCR3 [36]. CCR3 is selectively expressed on Th2 subset, so CCL11 and CCR3 are effectors of the Th2 response. Adzemovic et al. [37] established a congenic rat strain with the Eae18b locus containing a chemokine cluster (CCL2, CCL7, CCL11, CCL12, and CCL1) from the experimental autoimmune encephalomyelitis (EAE)- resistant PVG rat strain. The group observed a milder disease and elevated CCL11 mRNA and protein levels in inguinal lymph nodes in the Eae18b congenic strain. Increased intrathecal production of CCL11 in congenic rats was accompanied by a tighter blood brain barrier, reflected by more occludin-positive blood vessels. In addition, the congenic strain showed a reduced antigen specific response and a predominant anti-inflammatory Th2 phenotype. Our results suggest that the reduced expression of CCR3 and the decline of CCL11 may be one of the causes of the Th1/Th2 imbalance in ITP. Correspondingly, the restoration of CCR3 and CCL11 after pulsed HD-DXM suggests the rebalancing of Th1/Th2. Thus, upregulation of CCL11 and CCR3 was associated with a Th2-like immune response, which could skew the immune response away from the autoimmune state.
Conclusions
Our data suggest that polarization of Th1-associated chemokines and chemokine receptors may play important roles in the pathogenesis of ITP. Further, rectifying the abnormal chemokine profile associated with the Th1/Th2 imbalance by treatment with pulsed HD-DXM provides us with new insight into the immunoregulatory mechanisms for the treatment of ITP.
Authors’ contributions
CSG, JP and MH conceived and designed the experiments. ZTL carried out the immunoassays. SFZ and JM performed the PCR experiments. ZTL and MYW analyzed the data. YS contributed reagents/materials/analysis tools. ZTL, CSG and MYW wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to Prof. Jingjie Zhao and Feng Kong (Central Molecular Biology Lab, the Second Hospital of Shandong University, Jinan, China) for their assistance in the laboratory. We appreciate Alexandra H. Marshall’s assistance (Marshall Medical Communications, Canada) in editing the manuscript. We specially appreciate statistician Xuedan Xing (Baiyang Pharmaceutical Group Co., Ltd, Tsingtao, China) for her help in statistical analysis.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within this article.
Ethics approval and consent to participate
PBMCs and plasma preparation for chemokine study were isolated from peripheral blood of ITP patients and healthy adult volunteers after written informed consent and ethics committee approval (BYLL10/2014). The patients gave written informed consent to research studies, and the study was approved by the local ethics committee (02832) and adhered to the tenets of the Declaration of Helsinki.
Funding
This work was supported by Grants from the National Natural Science Found of China (No. 81270577, No. 30971278, No. 81070411), the National Science Fund for Distinguished Young Scholars of China (No. 81125002), the Science and technology innovation Found of Shenzhen, China (No. JCYJ20160427191026117, No. JCYJ20160427190358849), and the Medical Science and technology Found of Guangdong Province, China (No. A2016296).
Abbreviations
- ITP
immune thrombocytopenia
- Th
T helper
- HD-DXM
high-dose dexamethasone
- PBMC
peripheral blood mononuclear cell
- IL
interleukin
- IFN
interferon
- EC
ecchymoses
- GC
glucocorticoid
- PT
petechiae
- EP
epistaxis
- GUH
genitourinary hemorrhage
- GIH
gastrointestinal hemorrhage
- GH
gingival hemorrhage
- ELISA
enzyme linked immunosorbent assay
- PCR
polymerase chain reaction
- PBMC
peripheral blood mononuclear cell
- CMV
cytomegalovirus
Footnotes
Zongtang Liu and Meiying Wang contributed equally to this work
Contributor Information
Zongtang Liu, Email: baby1728@163.com.
Meiying Wang, Email: wmy99wmy99@163.com.
Shufen Zhou, Email: xuanshmily@163.com.
Ji Ma, Email: phd.jima@gmail.com.
Yan Shi, Email: shiyansjj@163.com.
Jun Peng, Email: junpeng88@sina.com.
Ming Hou, Email: houming@medmail.com.cn.
Chengshan Guo, Phone: 86-755-2778-8311, Email: guochengshan1@163.com.
References
- 1.Bakchoul T, Sachs UJ. Platelet destruction in immune thrombocytopenia. Understanding the mechanisms. Hamostaseologie. 2016;36:187–194. doi: 10.5482/HAMO-14-09-0043. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Zhao H, Ren H, Guo J, Xu M, Yang R, et al. Type 1 and type 2 T-cell profiles in idiopathic thrombocytopenic purpura. Haematologica. 2005;90:914–923. [PubMed] [Google Scholar]
- 3.Cooper N, Bussel J. The pathogenesis of immune thrombocytopenic purpura. Br J Haematol. 2006;133:364–374. doi: 10.1111/j.1365-2141.2006.06024.x. [DOI] [PubMed] [Google Scholar]
- 4.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 5.Sapir Y, Vitenshtein A, Barsheshet Y, Zohar Y, Wildbaum G, Karin N. A fusion protein encoding the second extracellular domain of CCR5 arrests chemokine-induced cosignaling and effectively suppresses ongoing experimental autoimmune encephalomyelitis. J Immunol. 2010;185:2589–2599. doi: 10.4049/jimmunol.1000666. [DOI] [PubMed] [Google Scholar]
- 6.Szczucinski A, Losy J. CCL5, CXCL10 and CXCL11 chemokines in patients with active and stable relapsing-remitting multiple sclerosis. Neuroimmunomodulation. 2011;18:67–72. doi: 10.1159/000317394. [DOI] [PubMed] [Google Scholar]
- 7.Oki M, Ohtani H, Kinouchi Y, Sato E, Nakamura S, Matsumoto T, et al. Accumulation of CCR5 + T cells around RANTES + granulomas in Crohn’s disease: a pivotal site of Th1-shifted immune response? Lab Invest. 2005;85:137–145. doi: 10.1038/labinvest.3700189. [DOI] [PubMed] [Google Scholar]
- 8.Hu JY, Zhang J, Cui JL, Liang XY, Lu R, Du GF, et al. Increasing CCL5/CCR5 on CD4 + T cells in peripheral blood of oral lichen planus. Cytokine. 2013;62:141–145. doi: 10.1016/j.cyto.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, et al. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4(+) T helper 1 cell differentiation. Immunity. 2012;37:1091–1103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu D, Chen Z, Zhao H, Du W, Xue F, Ge J, et al. Th1 (CXCL10) and Th2 (CCL2) chemokine expression in patients with immune thrombocytopenia. Hum Immunol. 2010;71:586–591. doi: 10.1016/j.humimm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Penido C, Costa KA, Costa MF, Pereira Jde F, Siani AC, Henriques Md. Inhibition of allergen-induced eosinophil recruitment by natural tetranortriterpenoids is mediated by the suppression of IL-5, CCL11/eotaxin and NFkappaB activation. Int Immunopharmacol. 2006;6:109–121. doi: 10.1016/j.intimp.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Terlou A, Santegoets LA, van der Meijden WI, Heijmans-Antonissen C, Swagemakers SM, van der Spek PJ, et al. An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: a Th1 response and high levels of microRNA-155. J Invest Dermatol. 2012;132:658–666. doi: 10.1038/jid.2011.369. [DOI] [PubMed] [Google Scholar]
- 13.Mullol J, Roca-Ferrer J, Alobid I, Pujols L, Valero A, Xaubet A, et al. Effect of desloratadine on epithelial cell granulocyte-macrophage colony-stimulating factor secretion and eosinophil survival. Clin Exp Allergy. 2006;36:52–58. doi: 10.1111/j.1365-2222.2005.02403.x. [DOI] [PubMed] [Google Scholar]
- 14.Booth V, Clark-Lewis I, Sykes BD. NMR structure of CXCR3 binding chemokine CXCL11 (ITAC) Protein Sci. 2004;13:2022–2028. doi: 10.1110/ps.04791404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Lahiri A, Haines GK, Flavell RA, Abraham C. NOD2 regulates CXCR3-dependent CD8 + T cell accumulation in intestinal tissues with acute injury. J Immunol. 2014;192:3409–3418. doi: 10.4049/jimmunol.1302436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarski CA, Ford J, Katzman SD, Rosenberg AF, Fowell DJ. IL-4 attenuates Th1-associated chemokine expression and Th1 trafficking to inflamed tissues and limits pathogen clearance. PLoS ONE. 2013;8:e71949. doi: 10.1371/journal.pone.0071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong SK, Kim BS, Uhm TG, Lee W, Lee GR, Park CS, Lee CH, Chung IY. Different GATA factors dictate CCR3 transcription in allergic inflammatory cells in a cell type-specific manner. J Immunol. 2013;190:5747–5756. doi: 10.4049/jimmunol.1203542. [DOI] [PubMed] [Google Scholar]
- 18.Zhou SF, Ma J, Qu HT, Liu ZT, He WD, Wang JD, et al. Characterization of Th1- and Th2-associated chemokine receptor expression in spleens of patients with immune thrombocytopenia. J Clin Immunol. 2013;33:938–946. doi: 10.1007/s10875-013-9883-4. [DOI] [PubMed] [Google Scholar]
- 19.Guidry JA, George JN, Vesely SK, Kennison SM, Terrell DR. Corticosteroid side-effects and risk for bleeding in immune thrombocytopenic purpura: patient and hematologist perspectives. Eur J Haematol. 2009;83:175–182. doi: 10.1111/j.1600-0609.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Wong RS, Soo YO, Chui CH, Lau FY, Chan NP, et al. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med. 2003;349:831–836. doi: 10.1056/NEJMoa030254. [DOI] [PubMed] [Google Scholar]
- 21.Mazzucconi MG, Fazi P, Bernasconi S, De Rossi G, Leone G, Gugliotta L, et al. Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) thrombocytopenia working party. Therapy with highdose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood. 2007;109:1401–1407. doi: 10.1182/blood-2005-12-015222. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto K, Nakasone H, Tsurumi S, Sasaki K, Mitani K, Kida M, et al. Prednisone versus high-dose dexamethasone for untreated primary immune thrombocytopenia. A retrospective study of the Japan Hematology & Oncology Clinical Study Group. J Thromb Thrombolysis. 2014;37:279–286. doi: 10.1007/s11239-013-0939-3. [DOI] [PubMed] [Google Scholar]
- 23.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 24.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;2011(117):4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Ji XB, Wang YW, Wang JX, Yang EQ, Wang ZC, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127:296–302. doi: 10.1182/blood-2015-07-659656. [DOI] [PubMed] [Google Scholar]
- 26.Shao Q, Ning H, Lv J, Liu Y, Zhao X, Ren G, et al. Regulation of Th1/Th2 polarization by tissue inhibitor of metalloproteinase-3 via modulating dendritic cells. Blood. 2012;119:4636–4644. doi: 10.1182/blood-2011-08-376418. [DOI] [PubMed] [Google Scholar]
- 27.Agostini C, Cabrelle A, Calabrese F, Bortoli M, Scquizzato E, Carraro S, et al. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T-cell alveolitis in sarcoidosis. American journal of respiratory and critical care medicine. Am J Respir Crit Care Med. 2005;172:1290–1298. doi: 10.1164/rccm.200501-142OC. [DOI] [PubMed] [Google Scholar]
- 28.Heise CE, Pahuja A, Hudson SC, Mistry MS, Putnam AL, Gross MM, et al. Pharmacological characterization of CXC chemokine receptor 3 ligands and a small molecule antagonist. J Pharmacol Exp Ther. 2005;313:1263–1271. doi: 10.1124/jpet.105.083683. [DOI] [PubMed] [Google Scholar]
- 29.Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, et al. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem. 2001;276:2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- 30.Trotta T, Costantini S, Colonna G. Modelling of the membrane receptor CXCR3 and its complexes with CXCL9, CXCL10 and CXCL11 chemokines: putative target for new drug design. Mol Immunol. 2009;47:332–339. doi: 10.1016/j.molimm.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez C, Benítez A, Rojas L, Pujol M, Carvajal P, Díaz-Zúñiga J, et al. Differential expression of CC chemokines (CCLs) and receptors (CCRs) by human T lymphocytes in response to different Aggregatibacter actinomycetemcomitans serotypes. J Appl Oral Sci. 2015;23:580–590. doi: 10.1590/1678-775720150285. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Jalosinski M, Karolczak K, Mazurek A, Glabinski A. The effects of methylprednisolone and mitoxantrone on CCL5-induced migration of lymphocytes in multiple sclerosis. Acta Neurol Scand. 2008;118:120–125. doi: 10.1111/j.1600-0404.2008.00998.x. [DOI] [PubMed] [Google Scholar]
- 33.Duma L, Häussinger D, Rogowski M, Lusso P, Grzesiek S. Recognition of RANTES by extracellular parts of the CCR5 receptor. J Mol Biol. 2007;365:1063–1075. doi: 10.1016/j.jmb.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 34.Feng X, Scheinberg P, Samsel L, Rios O, Chen J, McCoy JP, Jr, et al. Decreased plasma cytokines are associated with low platelet counts in aplastic anemia and immune thrombocytopenic purpura. J Thromb Haemost. 2012;10:1616–1623. doi: 10.1111/j.1538-7836.2012.04757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norii M, Yamamura M, Iwahashi M, Ueno A, Yamana J, Makino H. Selective recruitment of CXCR3 + and CCR5 + CCR4 + T cells into synovial tissue in patients withrheumatoid arthritis. Acta Med Okayama. 2006;60:149–157. doi: 10.18926/AMO/30745. [DOI] [PubMed] [Google Scholar]
- 36.Millard CJ, Ludeman JP, Canals M, Bridgford JL, Hinds MG, Clayton DJ, et al. Structural basis of receptor sulfotyrosine recognition by a CC chemokine: the N-terminal region of CCR3 bound to CCL11/eotaxin-1. Structure. 2014;22:1571–1581. doi: 10.1016/j.str.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Adzemovic MZ, Öckinger J, Zeitelhofer M, Hochmeister S, Beyeen AD, Paulson A, et al. Expression of CCL11 associates with immune response modulation and protection against neuroinflammation in rats. PLoS ONE. 2012;7:e39794. doi: 10.1371/journal.pone.0039794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within this article.


