Abstract
Background
The cardio-ankle vascular index (CAVI) and brachial-ankle pulse wave velocity (baPWV) can reflect both central and peripheral arterial stiffness. Metabolic syndrome (MetS) and its components may increase arterial stiffness and the risk of cardiovascular diseases. However, the correlation of MetS and its components with arterial stiffness is still not clear. The primary aim of this study is thus the relationship using baPWV and CAVI in Caucasian adults with intermediate cardiovascular risk. The secondary aim is to analyze sex differences.
Methods
This study analyzed 2351 subjects aged 35–74 years (mean, 61.4 ± 7.7 years) comprising 61.7 % males and enrolled in the improving interMediAte Risk management (MARK) study. CAVI was measured using a VaSera VS-1500 ® device, and baPWV was calculated using a validated equation. MetS was defined based on the Joint Scientific Statement National Cholesterol Education Program III. Waist circumference, blood pressure, fasting plasma glucose, and lipid profile were measured.
Results
MetS was found in 51.9 % of the subjects. All MetS components except reduced HDL-cholesterol (p = 0.578) were associated with CAVI. High density lipoprotein cholesterol (p = 0.075) and waist circumference (p = 0.315) were associated with baPWV. The different MetS components that assess dyslipidemia using the stiffness measures show different associations according to patient sex. The high blood pressure component had a greater odds ratio (OR) for both baPWV ≥ 17.5 m/sec (OR = 6.90, 95 % CI 3.52–13.519) and CAVI ≥ 9 (OR = 2.20, 95 % CI 1.63–1.90).
Conclusions
MetS and all its components (except HDL-cholesterol with baPWV and CAVI and WC with baPWV) were associated with baPWV and CAVI. However, there were sex differences in the association of MetS and its components with baPWV and CAVI. Data from this study suggest a greater association of CAVI and baPWV values with MetS components in males than in females and indicate greater arterial stiffness in the event of simultaneously elevated blood pressure, fasting plasma glucose, and waist circumference.
Trial Registration Clinical Trials.gov Identifier: https://clinicaltrials.gov/ct2/show/ NCT01428934. Registered 2 September 2011. Last updated September 8, 2016
Electronic supplementary material
The online version of this article (doi:10.1186/s12933-016-0465-7) contains supplementary material, which is available to authorized users.
Keywords: Metabolic syndrome, Brachial-ankle pulse wave velocity, Cardio-ankle vascular index, Arterial stiffness
Background
Metabolic syndrome (MetS) is a cluster of multiple risk factors for atherosclerosis that include obesity, high blood pressure, elevated fasting plasma glucose (FPG), and atherogenic dyslipidemia [1]. MetS doubles the risk of morbidity and mortality from cardiovascular diseases and multiplies the risk of all-cause mortality by 1.5 [2–4]. However, the risk varies according to the associated components on which the MetS diagnosis is based [5]. For example, in the Framingham Heart Study cohort, the combination of central obesity, blood pressure (BP), and FPG increased the risk of mortality three-fold [6]. Similarly, different combinations of MetS components have different effects on arterial stiffness [7–9]. Several studies have also reported that greater arterial stiffness is associated with increased morbidity and mortality from cardiovascular diseases [10–12].
Arterial stiffness may be evaluated using the brachial-ankle pulse wave velocity (baPWV) [13] and the cardio-ankle vascular index (CAVI) [14]. baPWV has been demonstrated as an independent predictor of coronary artery disease and all-cause mortality in general populations and in subjects with diabetes [15]. CAVI is a measure [14] of overall arterial stiffness starting from the aorta all the way to the ankle [16]. It is associated with carotid and coronary atherosclerosis [10, 17–19] and is a predictor of cardiovascular events in the obese [10]. CAVI is a better predictor of coronary artery disease than baPWV [20, 21].
Studies have investigated the relationship of MetS and its components with baPWV [7, 22–24]. Most have reported increased baPWV in subjects with MetS or with a greater number of MetS components. Most of the studies have examined Asian populations, among whom MetS and its components affect arterial stiffness with greater severity in females than in males [23, 25]. It is likely that females with MetS develop more severe atherosclerosis, but this is not yet definitive [25]. Furthermore, the sex-dependent association of the specific cluster of MetS components with baPWV and CAVI in Caucasian adults with intermediate cardiovascular risk has not been previously.
Research on the relationship of MetS and its components with baPWV and CAVI is an important topic around the world. Unlike baPWV, the characteristics of CAVI as a physiological marker of arterial stiffness have not frequently been reported. The primary aim of this study is to investigate the relationship of MetS and its components with arterial stiffness measured by baPWV and CAVI in Caucasian adults with intermediate cardiovascular risk, with the secondary aim of analyzing sex differences.
Methods
Study design
This trial is a cross-sectional study of subjects recruited to the improving interMediAte RisK management (MARK) study (NCT01428934) [26], which is a longitudinal study designed to assess whether the ankle-brachial index, arterial stiffness (measured by CAVI), postprandial glucose, glycosylated hemoglobin, self-measured blood pressure, and the presence of comorbidities are independently associated with the occurrence of vascular events. It also investigates whether the predictive capacity of current risk equations can be improved in the intermediate risk population. The current study focuses on the baseline visit. The second step will be a 5- and 10 year follow-up trial to assess cardiovascular morbidity and mortality.
Study population
In this multicenter project, sample selection was performed by random sampling from among individuals who met the inclusion criteria and were seeing general practitioners at six primary care centers in three Spanish Autonomous Communities from July 2011 to June 2013. Subjects were recruited from those aged 35 to 74 years with intermediate cardiovascular risk defined as 10 year coronary risk ranging from 5–15 % according to the adapted Framingham risk equation [27]; 10 year vascular mortality risk ranging from 1–5 % according to the scoring risk in Europeans equation [28]; or moderate risk according to the European Society of Hypertension guidelines for the management of arterial hypertension [29]. The analysis examined 2351 of the 2495 subjects recruited to the MARK study. Exclusion criteria included end-stage disease or institutionalization at the time of the visit, or a history of atherosclerotic disease. Subjects were excluded for the following reasons: 88 had an altered ankle-brachial index, measured CAVI and/or baPWV values were not available for 23 subjects, and MetS components had not been measured for 33 subjects. The study was approved by the Research Ethics Committees of the Primary Care Research Institute of Jordi Gol, Health Care Area of Salamanca and Palma of Mallorca. All participants gave written informed consent according to the general recommendations of the Declaration of Helsinki [30].
Variables and measurement instruments
A detailed description of procedures for clinical data collection, anthropometric measurements, and laboratory tests has been published elsewhere [26].
Diagnostic criteria of MetS
According to the international consensus in the Joint Scientific Statement National Cholesterol Education Program III [1], MetS was defined as the presence of three or more of the following five components: abdominal obesity (waist circumference (WC) ≥88 cm in females and ≥102 cm in males); elevated triglycerides (TGC) ≥150 mg/dL (or drug treatment for elevated TGC); high-density lipoprotein (HDL) cholesterol <40 mg/dL in males or <50 mg/dL in females; high blood pressure (systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg, or antihypertensive drug treatment), and fasting plasma glucose (FPG) ≥100 mg/dL (or drug treatment for elevated glucose).
Analysis groups
In order to analyze the influence of the different combinations of MetS components upon arterial stiffness, the subjects were divided into three groups. The group “MetS-dyslipidemia” consisted of 367 subjects with components of high blood pressure and those that indicate dyslipidemia (low HDL-cholesterol and elevated triglycerides). The group “MetS-increased insulin resistance” consisted of 511 subjects with the components of high blood pressure and those that indicate increased insulin resistance (elevated FPG and abdominal obesity). The group “MetS-mixed” consisted of 342 subjects with MetS who were not included in either the dyslipidemia group or the increased insulin resistance group. The control group consisted of 175 subjects without MetS, arterial hypertension, FPG, or the use of antihypertensive, lipid-lowering, or antidiabetic drugs.
Diagnosis of cardiovascular risk factors
Subjects were considered hypertensive if previously diagnosed with hypertension, if they were taking antihypertensive drugs, or if they had blood pressure levels ≥140/90 mmHg. Diabetic subjects were those who had a previous diagnosed of the disease, were taking hypoglycemic drugs, or had fasting blood glucose levels ≥126 mg/dL or HbA1c ≥6.5 %. Dyslipidemia was defined as a prior diagnosis of the condition, use of lipid-lowering drugs, or fasting total cholesterol levels ≥250 mg/dL.
Cardio-ankle vascular index (CAVI) and brachial-ankle pulse wave velocity (baPWV)
CAVI was measured using a VaSera VS-1500 ® device (Fukuda Denshi) [31, 32]. CAVI values are calculated automatically by estimating the stiffness parameter β with the following equation: β = 2ρ × 1/(Ps−Pd) × ln (Ps/Pd) × PWV2, where ρ is blood density, Ps and Pd are SBP and DBP in mmHg, and PWV is measured between the aortic valve and the ankle [14]. The mean coefficient of variation of CAVI measurement is less than 5 %, which is small enough to allow for clinical use of the index and confirms that CAVI has a favorable reproducibility [32].
baPWV was estimated using the equation, baPWV = (0.5934 × height (cm) + 14.4724)/tba (tba is the time interval between the arm and ankle waves) [13]. Measurements were performed with the patient in supine position after resting for 10 min in a quiet room at a stable temperature. Subjects were instructed not to smoke or practice exercise in the hour prior to the test.
CAVI was classified as normal (CAVI < 8), borderline (8 ≤ CAVI < 9), or abnormal (CAVI ≥ 9). Abnormal CAVI represents subclinical atherosclerosis [ 14, 33–36 ]. A value of baPWV ≥17.5 m/sec was considered abnormal [37]. The average values of CAVI and baPWV were considered.
Office or clinical blood pressure
Office blood pressure measurement involved three measurements of SBP and DBP with a validated OMRON model M10-IT sphygmomanometer (Omron Health Care, Kyoto, Japan). The measurements followed the recommendations of the European Society of Hypertension [38], and the averages of the last two measurements used.
Anthropometric measurements
Body weight was measured twice with a certified electronic scale (Seca 770, Medical scale and measurement systems, Birmingham, United Kingdom) after adequate calibration (precision ±0.1 kg). Readings were rounded to 100 g. Height was measured with a stadiometer (Seca 222), and the average of two measurements was recorded. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Waist circumference was measured according to the 2007 recommendations of the Spanish Society for the Study of Obesity [39]. All measurements were performed with the subjects standing, wearing no shoes, and in light clothing. The researchers who performed the different tests were blinded to the clinical data of the subjects. All assessments were made within a period of 10 days.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation. Categorical variables are presented as frequency distributions and compared using the Chi-squared test or Fisher’s exact test when necessary. The difference in means between 2-category and quantitative variables was analyzed using the student’s t test for independent samples. ANCOVA models were used to test the differences in mean baPWV and CAVI values with the five components of MetS. Pairwise post hoc comparisons were examined using the Bonferroni test.
In the multivariate analysis, six multiple linear regression models were performed (ENTER method) using CAVI and baPWV as dependent variables and MetS and its components as independent variables of each model. Six logistic regression models were also developed in which baPWV (<17.5 = 0 and ≥17.5 = 1) and CAVI status (<9 = 0 and ≥9 = 1) were dependent variables, while the independent variables were the absence (0) or presence (1) of MetS and its components. All models included age, height, weight, antihypertensive drugs, lipid-lowering drugs, and antidiabetic drugs as adjusting variables. The exception was WC, which was adjusted for age and drug use because of collinearity problems. Analyses were performed for the subjects overall and according to gender. Data were analyzed using SPSS Statistics for Windows version 23.0 (IBM Corp, Armonk, NY). Values of p < 0.05 were considered statistically significant.
Results
Clinical characteristics of all subjects
Table 1 shows the characteristics (overall and by sex) of the 2351 subjects analyzed. Mean age was 61.4 ± 7.7 years. MetS was found in 51.9 % of the subjects (46.0 % of males and 61.4 % of females). The mean baPWV was 14.9 ± 2.6 m/sec (coefficient of variation: 0.172), and the mean CAVI was 8.8 ± 1.2 (coefficient of variation: 0.133). CAVI was higher among males than females (8.9 ± 1.2 versus 8.6 ± 1.1; p < 0.001). Mean baPWV values were similar among both sexes.
Table 1.
Characteristics of subjects global and stratified by gender
| Variables | Global (n = 2351) | Males (n = 1450) | Females (n = 901) | p value |
|---|---|---|---|---|
| Age, (years) | 61.4 ± 7.7 | 61.2 ± 8.1 | 61.8 ± 7.0 | 0.044 |
| Smoking, n (%) | 659 (28.1) | 455 (31.4) | 204 (22.7) | <0.001 |
| BMI, (kg/m2) | 29.2 ± 4.4 | 29.1 ± 3.9 | 29.5 ± 5.1 | 0.016 |
| BMI ≥ 30, n (%) | 850 (36.2) | 485 (33.4) | 365 (40.5) | 0.001 |
| WC, (cm) | 100.9 ± 11.6 | 102.9 ± 10.5 | 97.6 ± 12.5 | <0.001 |
| SBP, (mmHg) | 137.0 ± 17.4 | 138.9 ± 17.1 | 134.1 ± 17.5 | <0.001 |
| DBP, (mmHg) | 84.4 ± 10.2 | 85.5 ± 10.3 | 82.6 ± 9.6 | <0.001 |
| Hypertension, n (%) | 1842 (78.3) | 1163 (80.2) | 679 (75.4) | 0.006 |
| Antihypertensive drugs, n (%) | 1208 (51.4) | 733 (50.6) | 475 (52.7) | 0.309 |
| Total Cholesterol, (mg/dl) | 225.7 ± 41.0 | 220.6 ± 38.0 | 233.9 ± 42.8 | <0.001 |
| LDL-C, (mg/dl) | 140.4 ± 34.9 | 138.9 ± 34.2 | 142.7 ± 35.8 | 0.010 |
| HDL Cholesterol, (mg/dl) | 49.8 ± 12.9 | 47.9 ± 11.9 | 52.9 ± 13.8 | <0.001 |
| TGC, (mg/dl) | 145.7 ± 97.0 | 150.5 ± 106.7 | 138.0 ± 78.2 | 0.002 |
| Dyslipidemia, n (%) | 1583 (67.3) | 924 (63.7) | 659 (73.1) | <0.001 |
| Lipid lowering drugs, n (%) | 673 (28.6) | 392 (27.0) | 281 (31.2) | 0.031 |
| FPG, (mg/dl) | 107.7 ± 34.4 | 107.4 ± 33.5 | 108.0 ± 35.9 | 0.711 |
| HA1c, (%) | 6.1 ± 1.2 | 6.1 ± 1.1 | 6.2 ± 1.3 | 0.001 |
| Diabetes mellitus type 2, n (%) | 794 (33.8) | 464 (32.0) | 330 (36.6) | 0.022 |
| Antidiabetic drugs, n (%) | 477 (20.3) | 270 (18.6) | 207 (23.0) | 0.015 |
| Higher blood pressure, n (%) | 1986 (84.5) | 1253 (86.4) | 733 (81.4) | 0.001 |
| Higher FPG, n (%) | 1112 (47.3) | 693 (47.8) | 419 (46.5) | 0.552 |
| Lower HDL-C, n (%) | 791(33.6) | 357 (24.6) | 434 (48.2) | <0.001 |
| Higher TGC, n (%) | 839 (35.7) | 545 (37.6) | 294 (32.6) | 0.015 |
| Higher WC, n (%) | 1478 (62.9) | 762 (52.6) | 716 (79.5) | <0.001 |
| Metabolic syndrome, n (%) | 1220 (51.9) | 667 (46.0) | 553 (61.4) | <0.001 |
| CAVI | 8.80 ± 1.17 | 8.91 ± 1.19 | 8.65 ± 1.12 | <0.001 |
| CAVI ≥ 9, n (%) | 1061 (45.1) | 701 (49.6) | 360 (41.3) | 0.001 |
| baPWV, (m/s) | 14.87 ± 2.57 | 14.81 ± 2.56 | 14.98 ± 2.59 | 0.119 |
| baPWV ≥ 17.50 m/s, n (%) | 324 (13.8) | 192 (13.2) | 132 (13.9) | 0.356 |
Values are means (standard deviations (SD) for continuous data and number and proportions for categorical data
Metabolic syndrome: Three or more of the following criteria: (Abdominal obesity = Higher WC: waist circumference ≥88 in females and ≥102 in males. Higher blood pressure: SBP ≥130 mmHg and/or DBP ≥85 mmHg or antihypertensive drug treatment. Higher FPG: FPG >100 mg/dl or antidiabetic drug treatment. Lower HDL cholesterol: HDL cholesterol <40 mg/dl in males and <50 mg/dl in females. Higher triglycerides: TGC >150 mg/dl)
BMI body mass index; WC waist circumference; SBP systolic blood pressure; DBP diastolic blood pressure; LDL-C low density lipoprotein cholesterol; HDL-C high density lipoprotein cholesterol; TGC triglycerides; FPG fasting plasma glucose; HbA1c glycosylated hemoglobin; CAVI cardio-ankle vascular index; baPWV brachial-ankle pulse wave velocity
p value differences in male and females
Table 2 shows differences between subjects with and without MetS by sex. baPWV was higher in subjects with MetS, regardless of sex. Mean CAVI values were not different between subjects with and without MetS in either sex.
Table 2.
Characteristics of subjects stratified by gender and the presence/absence of metabolic syndrome
| Variables | Males subjects (n = 1450) | Females subjects (n = 901) | ||
|---|---|---|---|---|
| MetS + n = 667 | MetS − n = 783 | MetS + n = 553 | MetS − n = 348 | |
| Age, (years)* | 60.5 ± 8.1 | 61.8 ± 8.1 | 61.7 ± 7.1 | 62.1 ± 6.9 |
| SBP, (mmHg)*† | 141.4 ± 16.1 | 136.7 ± 17.5 | 136.3 ± 17.1 | 130.6 ± 17.7 |
| DBP. (mmHg)*† | 87.5 ± 10.3 | 83.7 ± 10.1 | 83.5 ± 9.2 | 81.2 ± 10.2 |
| Hypertension, n (%)*† | 600 (90.0) | 563 (71.9) | 469 (84.8) | 210 (60.3) |
| Antihypertensive drugs, n (%)*† | 404 (60.6) | 329 (42.0) | 352 (63.7) | 123 (35.3) |
| HDL-C, (mg/dl)*† | 43.2 ± 10.3 | 51.9 ± 11.8 | 48.3 ± 11.2 | 60.3 ± 14.2 |
| TGC, (mg/dl)*† | 184.0 ± 132.4 | 116.8 ± 54.0 | 160.8 ± 87.9 | 101.8 ± 37.5 |
| Dyslipidemia, n (%) | 436 (65.4) | 488 (62.3) | 403 (72.9) | 256 (73.6) |
| Lipid lowering drugs, n (%)*† | 207 (31.0) | 185 (23.6) | 197 (35.6) | 84 (24.1) |
| FPG (mg/dl)*† | 118.6 ± 37.9 | 98.0 ± 25.8 | 118.2 ± 39.3 | 91.8 ± 21.3 |
| Diabetes mellitus type 2, n (%)*† | 329 (49.3) | 135 (17.2) | 289 (52.3) | 41 (11.8) |
| Antidiabetic drugs, n (%)*† | 198 (29.7) | 72 (9.2) | 188 (34.0) | 19 (5.5) |
| WC, (cm)*† | 108.3 ± 10.1 | 98.3 ± 8.5 | 101.5 ± 11.3 | 91.4 ± 11.6 |
| Higher blood pressure, n (%)*† | 644 (96.6) | 609 (77.8) | 512 (92.6) | 221 (63.5) |
| Higher FPG, n (%)*† | 494 (74.1) | 199 (25.4) | 378 (68.4) | 41 (11.8) |
| Lower HDL-C, n (%)*† | 295 (44.2) | 62 (7.9) | 372 (67.3) | 62 (17.8) |
| Higher TGC, n (%)*† | 423 (63.4) | 122 (15.6) | 269 (48.6) | 25 (7.2) |
| Higher WC, n (%)*† | 539 (80.8) | 223 (28.5) | 516 (93.3) | 200 (57.5) |
| CAVI | 8.86 ± 1.24 | 8.94 ± 1.14 | 8.66 ± 1.19 | 8.61 ± 1.01 |
| CAVI ≥ 9, n (%) | 319 (49.3) | 382 (49.9) | 230 (42.8) | 130 (38.9) |
| baPWV, (m/s)*† | 14.98 ± 2.50 | 14.66 ± 2.61 | 15.23 ± 2.59 | 14.60 ± 2.36 |
| baPWV ≥ 17.5 m/s, n (%) | 94 (14.1) | 98 (12.5) | 97 (17.5) | 35 (10.1) |
Values are means (standard deviations (SD) for continuous data and number and proportions for categorical data
Metabolic syndrome: Three or more of the following criteria: (abdominal obesity = higher WC: waist circumference ≥88 in females and ≥102 in males. Higher blood pressure: SBP ≥130 mmHg and/or DBP ≥85 mmHg or antihypertensive drug treatment. Higher FPG: FPG >100 mg/dl or antidiabetic drug treatment. Lower HDL cholesterol: HDL cholesterol <40 mg/dl in males and <50 mg/dl in females. Higher triglycerides: TGC >150 mg/dl)
MetS metabolic syndrome; SBP systolic blood pressure; DBP diastolic blood pressure; HDL-C high density lipoprotein cholesterol; TGC triglycerides; FPG fasting plasma glucose; WC waist circumference; CAVI cardio-ankle vascular index; baPWV brachial-ankle pulse wave velocity
* p < 0.05 in males, † p < 0.05 in females
Association between the MetS and its components with arterial stiffness
After adjustment for potentially influencing variables, a multiple linear regression analysis showed that almost all MetS components were associated with baPWV and CAVI except for reduced HDL-cholesterol with respect to CAVI (p = 0.578) and baPWV (p = 0.075) and WC with respect to baPWV (p = 0.315). Among males, all MetS components (except HDL-cholesterol with respect to CAVI and WC with respect to baPWV) were associated with the two arterial stiffness measures. However, among females, SBP, DBP, and FPG were associated with baPWV and CAVI, and WC was associated with CAVI (Table 3).
Table 3.
Associations of MetS components with baPWV and CAVI values global and by gender
| Components MS | Global (n = 2351) | Males subjects (n = 1450) | Females subjects (n = 901) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β (95 % CI) | R2 | p value | β (95 % CI) | R2 | p value | β (95 % CI) | R2 | p value | |
| Dependent variable: baPWV | |||||||||
| SBP, (mmHg) | 0.062 (0.056 to 0.066) | 0.371 | <0.001 | 0.061 (0.055 to 0.067) | 0.352 | <0.001 | 0.061 (0.053 to 0.069) | 0.397 | <0.001 |
| DBP, (mmHg) | 0.082 (0.072 to 0.090) | 0.299 | <0.001 | 0.077 (0.066 to 0.089) | 0.281 | <0.001 | 0.085 (0.071 to 0.100) | 0.329 | <0.001 |
| HDL-C, (mg/dl) | 0.007 (−0.001 to 0.015) | 0.206 | 0.075 | 0.012 (0.002 to 0.023) | 0.196 | 0.003 | 0.012 (−0.008 to 0.014) | 0.233 | 0.561 |
| TGC, (mg/dl) | 0.002 (0.001 to 0.003) | 0.209 | 0.001 | 0.002 (0.001 to 0.003) | 0.198 | 0.002 | 0.001 (−0.001 to 0.003) | 0.234 | 0.182 |
| FPG, (mg/dl) | 0.007 (0.004 to 0.010) | 0.210 | <0.001 | 0.005 (0.001 to 0.009) | 0.198 | 0.031 | 0.010 (0.005 to 0.015) | 0.244 | 0.001 |
| WC, (cm) | −0.004 (−0.012 to 0.004) | 0.200 | 0.315 | −0.002 (−0.014 to 0.009) | 0.185 | 0.679 | −0.007 (−0.019 to 0.005) | 0.226 | 0.269 |
| Dependent variable: CAVI | |||||||||
| SBP, (mmHg) | 0.015 (0.013 to 0.017) | 0.398 | <0.001 | 0.016 (0.013 to 0.018) | 0.430 | <0.001 | 0.014 (0.011 to 0.018) | 0.327 | <0.001 |
| DBP, (mmHg) | 0.020 (0.017 to 0.024) | 0.377 | <0.001 | 0.022 (0.017 to 0.026) | 0.414 | <0.001 | 0.017 (0.011 to 0.024) | 0.302 | <0.001 |
| HDL-C, (mg/dl) | −0.001 (−0.004 to 0.002) | 0.348 | 0.578 | 0.002 (−0.003 to 0.006) | 0.382 | 0.486 | −0.002 (−0.007 to 0.002) | 0.282 | 0.301 |
| TGC, (mg/dl) | 0.001 (0.001 to 0.001) | 0.351 | 0.002 | 0.001 (0.001 to 0.001) | 0.286 | 0.002 | 0.001 (0.001 to 0.001) | 0.382 | 0.289 |
| FPG, (mg/dl) | 0.003 (0.002 to 0.005) | 0.355 | <0.001 | 0.003 (0.001 to 0.005) | 0.387 | 0.002 | 0.004 (0.002 to 0.006) | 0.289 | 0.001 |
| WC, (cm) | −0.013 (−0.016 to −0.009) | 0.290 | <0.001 | −0.017 (−0.021 to −0.012) | 0.354 | <0.001 | −0.017 (−0.023 to −0.012) | 0.236 | <0.001 |
Multiple linear regression models were used to analyze the associations of components of MetS to baPWV and CAVI values, globally and stratified by gender. Age, height, weight, antihypertensive drugs, lipid-lowering drugs and antidiabetic drugs were adjusted in the regression models. The exception was WC, which was adjusted for age and drug use because of collinearity problems
MetS metabolic syndrome; baPWV brachial-ankle pulse wave velocity; CAVI cardio-ankle vascular index; CI confidence interval; R 2 Coefficient of determination; SBP systolic blood pressure; DBP diastolic blood pressure; HDL-C high density lipoprotein cholesterol; TGC triglycerides; FPG fasting plasma glucose; WC waist circumference
The association persisted in the disaggregated analysis in subjects with and without antihypertensive, lipid-lowering, and antidiabetic treatments. The exceptions were the correlation between triglycerides and CAVI and baPWV, which was only seen in the treated subjects, and WC, which was only associated with baPWV in the untreated individuals and with CAVI in the drug treatment group (Additional file 1: Table S1).
The results of the multiple regression analysis of premenopausal and postmenopausal women and males over and under 50 years of age are shown in Additional file 1: Table 2S.
In a logistic regression analysis and after adjusting for potentially influencing variables, the component of MetS with the greatest odds ratio (OR) was high blood pressure for both baPWV ≥ 17.5 m/sec (OR = 6.90, 95 % CI 3.52–13.51) and CAVI ≥ 9 (OR = 2.20, 95 % CI 1.63–1.90) (Table 4).
Table 4.
Multiple logistic regression analysis of associations between MetS/components and baPWV and CAVI status in males and females
| Components MetS | Global (n = 2351) | Males subjects (n = 1450) | Females subjects (n = 901) | |||
|---|---|---|---|---|---|---|
| OR (95 % CI) | p value | OR (95 % CI) | p value | OR (95 % CI) | p value | |
| Dependent variable: baPWV | ||||||
| High BP | 6.899 (3.522 to 13.511) | <0.001 | 7.578 (2.975 to 19.345) | <0.001 | 5.272 (1.975 to 19.345) | <0.001 |
| High FPG | 1.527 (1.123 to 2.077) | 0.007 | 1.290 (0.875 to 1.902) | 0.199 | 2.035 (1.221 to 3.392) | 0.006 |
| High TGC | 0.745 (0.559 to 0.992) | 0.044 | 0.738 (0.480 to 1.135) | 1.116 | 0.802 (0.534 to 1.205) | 0.287 |
| Low HDL-C | 1.202 (0.915 to 1.579) | 0.186 | 1.159 (0.811 to 1.656) | 0.419 | 1.329 (0.863 to 2.248) | 0.197 |
| High WC | 0.965 (0.743 to 1.254) | 0.792 | 0.874 (0.633 to 1.207) | 0.414 | 1.149 (0.675 to 1.953) | 0.609 |
| MetS | 1.421 (1.062 to 1.902) | 0.018 | 1.512 (1.032 to 2.215) | 0.034 | 1.679 (1.019 to 2.765) | 0.042 |
| Dependent variable: CAVI | ||||||
| High BP | 2.204 (1.629 to 2.983) | <0.001 | 2.238 (1.499 to 3.339) | <0.001 | 2.115 (1.320 to 3.338) | 0.002 |
| High FPG | 1.368 (1.090 to 1.718) | 0.007 | 1.401 (1.052 to 1.866) | 0.021 | 1.256 (0.857 to 1.842) | 0.242 |
| High TGC | 0.931 (0.755 to 1.147) | 0.500 | 1.031 (0.768 to 1.384) | 0.839 | 0.888 (0.651 to 1.213) | 0.457 |
| Low HDL-C | 1.283 (1.043 to 1.579) | 0.018 | 1.374 (1.054 to 1.792) | 0.019 | 1.166 (0.832 to 1.633) | 0.372 |
| High WC | 0.686 (0.565 to 0.834) | <0.001 | 0.786 (0.617 to 1.001) | 0.051 | 0.741 (0.511 to 1.075) | 0.115 |
| MetS | 1.543 (1.235 to 1.927) | <0.001 | 1.723 (1.286 to 2.307) | <0.001 | 1.424 (0.990 to 2.048) | 0.056 |
Dependent variable: CAVI and baPWV (values of CAVI ≥9 and baPWV ≥17.5 m/s was considered abnormal)
Multiple logistic regression analysis was used to analyze the associations of MetS status and MetS components with baPWV and CAVI globally and stratified by gender. Age, height, weight, antihypertensive drugs, lipid-lowering drugs and antidiabetic drugs were adjusted in the regression models. The exception was WC, which was adjusted for age and drug use because of collinearity problems
MetS metabolic syndrome; baPWV brachial-ankle pulse wave velocity; CAVI cardio-ankle vascular index; OR odds ratio; CI confidence interval; BP blood pressure; FPG fasting plasma glucose; TGC triglycerides; HDL-C high density lipoprotein cholesterol; WC waist circumference
Results for subjects with MetS
Figure 1 shows the mean values corresponding to baPWV and CAVI according to sex and for each of the components of MetS. In the 1220 subjects with MetS, all the MetS components presented higher baPWV values in females except for waist circumference (p > 0.05). However, all the MetS components presented higher CAVI values in males, reaching significant differences in the case of the components related to increased blood pressure, fasting plasma glucose, and waist circumference.
Fig. 1.
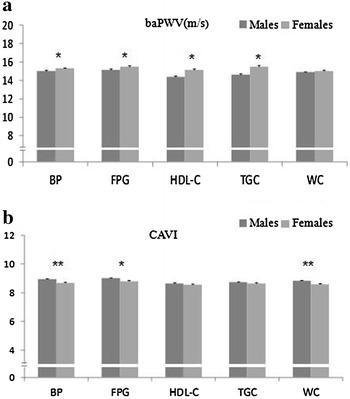
baPWV a and CAVI b according the MetS components in males and females. Data are given as mean ± standard error. baPWV and CAVI levels were compared using a Student’s t test. Mest criteria: abdominal obesity (n = 1055): WC ≥ 88 in females; ≥ 102 in males. BP (n = 1156): SBP ≥ 130 mmHg and/or DBP ≥ 85 mm Hg or antihypertensive drug treatment. Increase FPG (n = 872): FPG > 100 mg/dL or antidiabetic drug treatment. Reduced HDL-C (n = 667): HDL < 40 mg/dL in males and < 50 mg/dL in females. Increase TGC (n = 692): TGC > 150 mg/dL). baPWV brachial-ankle pulse wave velocity, CAVI cardio-ankle vascular index, BP blood pressure, FPG fasting plasma glucose, HDL-C high density lipoprotein cholesterol, TGC triglycerides, WC waist circumference. *p < 0.05 and **p < 0.01 between sexes
Figure 2 shows the change in mean baPWV and CAVI values after age adjustment for increasing number of MetS components. The mean baPWV values in females increased with the number of MetS components. However, in males, the mean baPWV values increased for only up to three MetS components. The mean CAVI values in females increased with two, three, and four MetS components. However, in males, CAVI values increased for up to three MetS components but decreased in groups with four or five components.
Fig. 2.
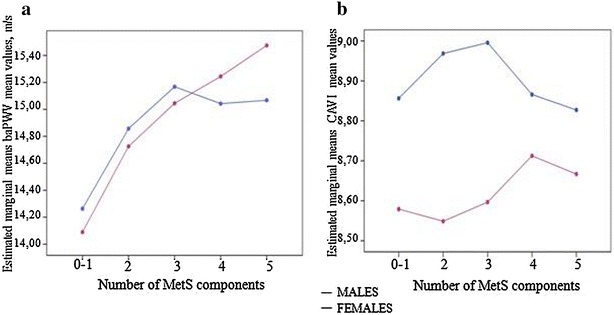
Multivariate analysis (ANCOVA). Brachial-ankle pulse wave velocity (baPWV) values in males and females (a) and cardio-ankle vascular index (CAVI) values in males and females (b). Values by number of MetS components. Adjusted by age. baPWV differences by number of MetS components in males between 0 and 1 components and 2, 3, and 4 components (p < 0.01); in females between 1 component and 3, 4, and 5 components (p < 0.01). Post-hoc contrasts were performed using a Bonferroni test. baPWV brachial-ankle pulse wave velocity, CAVI cardio-ankle vascular index, MetS metabolic syndrome
The results for the mean baPWV and CAVI values according to groups are shown in Additional file 2: Fig. S1 and Fig. 3. The MetS-increased insulin resistance group had the highest CAVI (8.96) and baPWV (15.55 m/sec) in both the global analysis and the sex-based analysis. In the global analysis, all groups of MetS components were associated with higher baPWV and CAVI values compared to the control group (p < 0.01), except for CAVI in the MetS-mixed group.
Fig. 3.
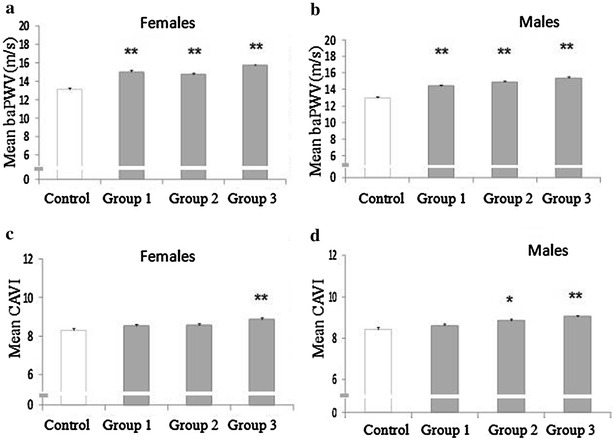
Impact of the specific groups of MetS components on brachial-ankle pulse wave velocity (baPWV) and cardio-ankle vascular index (CAVI) in the different groups. a Impact of the group in females on baPWV. b Impact of the group in males on baPWV. c Impact of the group in females on CAVI. d Impact of the group in males on CAVI. Data are given as mean ± standard error. baPWV and CAVI levels were compared using an ANOVA test, followed by post hoc analysis using a Bonferroni test. **p < 0.01 between the different groups and control; *p < 0.05 between the different groups and control. Group 1 Group MetS-mixed; Group 2 MetS-dyslipidemia; Group 3 Group MetS-increased insulin resistance; Group Control A group of 175 subjects without MetS, arterial hypertension, FPG or use of antihypertensive, lipid-lowering or antidiabetic drugs was used as control. baPWV brachial-ankle pulse wave velocity, CAVI cardio-ankle vascular index, MetS metabolic syndrome, FPG fasting plasma glucose
Discussion
The results show that in subjects with intermediate cardiovascular risk, MetS and its individual components (except HDL-cholesterol with the two measures and WC with baPWV) are associated with baPWV and CAVI. This association differs according to sex. In males, all MetS components (except HDL-cholesterol with CAVI and WC with baPWV) were associated with both arterial stiffness measures. In females only, SBP, DBP, and FPG were associated with both measures, and WC was associated with CAVI. The arterial stiffness values were highest when the MetS components of increased blood pressure, FPG, and WC occurred simultaneously.
FPG was associated with higher baPWV (15.50 m/sec) and CAVI (9.01) in females and males, respectively. Subjects in this study who had a combination of increased WC, FPG, and BP showed the highest values of both arterial stiffness measures. This finding is Similar to the results of the Framingham Heart Study cohort, where this combination increased the risk of mortality by three-fold [6]. These results suggest that analyzing arterial stiffness in these groups may be helpful for identifying subjects with greater cardiovascular risk [9].
A single study analyzed the relationship of CAVI to MetS and its components. Kawada et al. [24] found no significant association between MetS components and CAVI ≥ 9, with which only sex and age were significantly associated. However, our results suggest that the association of MetS and its components with CAVI is similar to the association with baPWV. These differences are probably due to the study size (144 subjects) and the different ethnic groups of the samples. On the other hand, in a Japanese population, CAVI was shown to be a predictor of cardiovascular events in obese subjects (MetS component) in the Japan Obesity and Metabolic Syndrome study [10]. It should not be forgotten, however, that this is the first study analyzing the association of each MetS component with two arterial stiffness measures and adjustment for different confounding factors in a large simple of subjects at intermediate cardiovascular risk.
The results do not coincide with those published by Satoh et al. [31] for 325 obese Japanese subjects enrolled in the multicenter Japan Obesity and Metabolic Syndrome Study. Their CAVI values were significantly higher in MetS than in non-MetS subjects, and CAVI was closely correlated with the severity of MetS. The discrepancies with our results may have several reasons: the subjects analyzed in this study were older (61.4 versus 49.4 years), and the most prevalent component of MetS in our group was blood pressure (a component less associated with CAVI than other stiffness measures). Other aspects are the different race, cardiovascular risk, and the drugs used for treatment of the different risk factors.
The subjects with arterial hypertension presented a six-fold higher risk of baPWV ≥ 17.5 m/sec, while the risk of CAVI ≥ 9 increased two-fold. These results support several studies [14, 40–42], according to which CAVI as an arterial stiffness measure is independent of blood pressure at the time of measurement. In relation to the rest of the MetS components, the odds ratios were similar for both stiffness measures, although increased waist circumference and low HDL-cholesterol only reached statistical significance with CAVI. This was probably due to the greater percentage of subjects with CAVI ≥ 9.
The MetS components BP and TGC were more common in males, and the WC and HDL cholesterol components were more common in females, while no difference was found in FPG. These results are similar to those reported for a Spanish population in the DARIOS study [43], but different from those reported in Asian populations [5, 7]. Prior studies support these results [7, 25]. Previous studies also analyzed the different behavior of arterial stiffness depending on sex [25, 44]. They found that stiffness was greater in females than males before puberty and increased after menopause. On the other hand, males arterial stiffness increases linearly from puberty, which suggests that women have intrinsically stiffer major arteries than men, but these effects are mitigated by sex steroids during reproductive life [45, 46]. Other factors that may influence these sex differences include height [47], body fat distribution [48], and inflammatory factors [49]. The tool used to assess arterial stiffness may also have an influence. The reason may be that CAVI reflects central and peripheral arterial stiffness [14, 50, 51] and is less influenced by BP values at the time of measurement [14, 40–42]. Arterial stiffness assessed with baPWV, however, is a measure of peripheral arterial stiffness.
The effects of MetS and its components on baPWV are not clear. Several studies on Eastern populations have shown that they are more evident in females than in males [7, 25]. Scuteri et al. [52] reported the impact of MetS on arterial stiffness to be similar in both sexes, and in this study, the number of MetS components associated with arterial stiffness measures was greater in males. However, baPWV and CAVI increased proportionally as the number of components increased in females, which occurred in other studies conducted on Asian populations [7, 8, 23, 25], but not in males. The different characteristics of the populations analyzed and the arterial stiffness measurement based on different parameters may explain these discrepancies.
Each component of MetS has a clear sex-dependent impact on baPWV [52]. As in other studies [7, 47], BP and especially SBP had the greatest association with baPWV and CAVI in our study, in contrast to all other components [25, 52]. Prior studies showed a positive correlation of HDL-C with baPWV in females only [7, 25]. In this study, HDL cholesterol and TGC levels correlated with baPWV in males only, and only TGC correlated with CAVI in males, which is in agreement with the results reported by Weng et al. [25]. However, low HDL cholesterol levels [53] and high TGC levels [54] are predictors of morbidity and mortality from cardiovascular diseases. Further studies analyzing the role of HDL cholesterol and TGC in arterial stiffness are therefore needed.
FPG induces many changes in vascular tissue cells, which may potentially accelerate the atherosclerotic process (mainly in females) [23, 55, 56]. This supports our results found in our study. Abdominal obesity is an essential element in MetS [57] and shows a negative association with CAVI in both sexes. Unlike our work, the association of baPWV with WC has been recorded in both sexes in Asian populations [23, 25]. These differences are related to the adjustment variables used.
The greater decrease in CAVI among the subjects with more MetS components could be due to the lesser influence of blood pressure on CAVI [14, 40–42]. Another possible explanation is the greater percentage of subjects receiving drug treatment in the groups with 4 or 5 MetS components. Thus, while 44 % of the subjects in the group with 0 or 1 component were receiving drugs for hypertension, diabetes, or dyslipidemia, the corresponding percentages were 77 and 88 % among the subjects with 4 and 5 MetS components, respectively.
Our results suggest that in Caucasian subjects with intermediate cardiovascular risk, arterial stiffness is associated with the MetS components, except HDL-cholesterol for baPWV and CAVI and WC for baPWV except for HDL-cholesterol with respect to both stiffness parameters and waist circumference with respect to baPWV. However, the association of triglycerides and HDL-cholesterol was only observed in males. These differences could have clinical relevance and may help to explain the discrepancies in cardiovascular risk between sexes. The results suggest that the treatment of hypertriglyceridemia could improve arterial stiffness, particularly in males with MetS.
The main limitation of our study is its cross-sectional design, which cannot establish causal relations or the direction of the impact of MetS on CAVI and baPWV. An additional limitation is the impact on arterial stiffness of drugs for treating specific MetS components such as blood glucose, dyslipidemia, and blood pressure. However, we did try to mitigate this effect by including them as adjustment variables in the regression analysis.
Conclusions
MetS and most of its individual components (except HDL-cholesterol for baPWV and CAVI and WC for baPWV) were associated with baPWV and CAVI. However, there were differences between sex in the association of MetS and its components with baPWV and CAVI. The data suggest a greater association of CAVI and baPWV values with MetS components in males than in females and indicate greater arterial stiffness upon simultaneously elevated blood pressure, fasting plasma glucose, and waist circumference. Therefore, the determination of arterial stiffness based on CAVI and baPWV may be useful for evaluating the cardiovascular risk of MetS and its components in Caucasian adults with intermediate cardiovascular risk.
Authors’ contributions
LG prepared the manuscript draft, participated in fundraising and interpretation of results, and corrected the final version of the manuscript. LG, MCP, and JAM performed all analyses, interpretation of results, and manuscript review. JIR, CA, RF, and ER participated in data collection and manuscript review. RR and RM participated in fundraising, interpretation of results, manuscript review, and data collection. MAG participated in protocol design, fundraising, analysis of results, and final review of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to all professionals participating in the MARK study. Lead author for this group: Rafel Ramos: Research Unit, Primary Health Care, Girona. Jordi Gol Institute for Primary Care Research (IDIAP Jordi Gol), Catalonia, Spain. E-mail: rramos.girona.ics@gencat.net. Coordinating Center: Rafel Ramos, Ruth Martí, Dídac Parramon, Anna Ponjoan, Miquel Quesada, Maria Garcia-Gil, Martina Sidera and Lourdes Camós. Research Unit, Primary Health Care. Jordi Gol Institute for Primary Care Research (IDIAP Jordi Gol). C/Maluquer Salvador, 11. 17002-Girona. Catalonia, Spain. Fernando Montesinos, Ignacio Montoya, Carlos López, Anna Agell, Núria Pagès of the Primary Care Services, Girona. Catalan Institute of Health (ICS), Catalonia, Spain. Irina Gil, Anna Maria-Castro of the Primary Care Services, Girona. Institut d’Assistència Sanitaria (IAS), Catalonia, Spain. Fernando Rigo, Guillermo Frontera, Antònia Rotger, Natalia Feuerbach, Susana Pons, Natividad Garcia, John Guillaumet, Micaela Llull and Mercedes Gutierrez of the San Agustín Primary Health Care Center. Ibsalut Balears, Spain. Cristina Agudo-Conde, Leticia Gómez-Sanchez, Carmen Castaño-Sanchez, Carmela Rodriguez-Martín, Benigna Sanchez-Salgado, Angela de Cabo-Laso, Gómez-Sánchez Marta, Emiliano Rodriguez-Sanchez, Jose Angel Maderuelo-Fernandez, Emilio Ramos-Delgado, Carmen Patino-Alonso, Jose I Recio-Rodriguez, Manuel A Gomez-Marcos and Luis Garcia-Ortiz. Primary Care Research Unit of Alamedilla, Salamanca, Spain. Castile and León Health Service–SACYL.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Consent for publication
All authors have read and approved the submission of the manuscript; the manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as an abstract.
Ethics approval and consent to participate
The study was approved by the Research Ethics Committees of the Primary Care Research Institute Jordi Gol, Health Care Area of Salamanca and Palma of Mallorca. All participants gave their written informed consent before data collection.
Funding
This work was supported by grants from the Spanish Ministry of Science and Innovation (MICINN), the Carlos III Health Institute/European Regional Development Fund (ERDF) (MICINN, ISCIII/FEDER) (Red IAPP RD12/0005, Research Groups: RD12/0005/0004, RD12/0005/0002, RD12/0005/0011), the Health Research Fund (PI10/01088, PI10/02077, PI10/02043), and the Regional Health Management of Castile and León (GRS 635/A/11; GRS 906/B/14).
Abbreviations
- baPWV
brachial-ankle pulse wave velocity
- BMI
body mass index
- BP
blood pressure
- CAVI
cardio-ankle vascular index
- DBP
diastolic blood pressure
- FPG
fasting plasma glucose
- HDL-C
high-density lipoprotein cholesterol
- MetS
metabolic syndrome
- MARK
MediAte RisK
- OR
odds ratio
- SBP
systolic blood pressure
- TGC
triglycerides
- WC
waist circumference
Additional files
Additional file 1: Table S1. Associations of MetS components with baPWV and CAVI values treatment in subjects with and without drug treatment. Table S2: Associations of MetS components with baPWV and CAVI values premenopausal and postmenopausal females in older males and younger than 50 years.
Additional file 2: Figure S1. Impact of the specific groups of MetS components on brachial-ankle pulse wave velocity (baPWV) and cardio-ankle vascular index (CAVI) in the different groups. a Impact of the group on baPWV. b Impact of the group i on baPWV. Data are given as mean ± standard error. baPWV and CAVI levels were compared using an ANOVA test, followed by post hoc analysis using a Bonferroni test. **p < 0.01 between the different groups and control; *p < 0.05 between the different groups and control. baPWV brachial-ankle pulse wave velocity; CAVI cardio-ankle vascular index; MetS metabolic syndrome. Group 1: Group MetS-mixed. Group 2: MetS-dyslipidemia. Group 3: Group MetS-increased insulin resistance. Group Control: A group of 175 subjects without MetS, arterial hypertension, fasting plasma glucose or use of antihypertensive, lipid-lowering or antidiabetic drugs was used as control.
Contributor Information
Leticia Gomez-Sanchez, Email: leticiagmzsnchz@gmail.com.
Luis Garcia-Ortiz, Email: lgarciao@usal.es.
M. Carmen Patino-Alonso, Email: carpatino@usal.es.
Jose I. Recio-Rodriguez, Email: donrecio@gmail.com
Rigo Fernando, Email: frigoc5@gmail.com.
Ruth Marti, Email: rmarti.girona.ics@gencat.cat.
Cristina Agudo-Conde, Email: cagudoconde@yahoo.es.
Emiliano Rodriguez-Sanchez, Email: Emiliano@usal.es.
Jose A. Maderuelo-Fernandez, Email: jmaderuelo@saludcastillayleon.es
Rafel Ramos, Email: rramos.girona.ics@gencat.net.
Manuel A. Gomez-Marcos, Email: magomez@usal.es
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 3.van Herpt TT, Dehghan A, van Hoek M, Ikram MA, Hofman A, Sijbrands EJ, Franco OH. The clinical value of metabolic syndrome and risks of cardiometabolic events and mortality in the elderly: the Rotterdam study. Cardiovasc Diabetol. 2016;15(1):69. doi: 10.1186/s12933-016-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotani K, Satoh-Asahara N, Nakakuki T, Yamakage H, Shimatsu A, Tsukahara T. Association between metabolic syndrome and multiple lesions of intracranial atherothrombotic stroke: a hospital-based study. Cardiovasc Diabetol. 2015;14:108. doi: 10.1186/s12933-015-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang B, Li B, Wang Y, Han B, Wang N, Li Q, Yang W, Huang G, Wang J, Chen Y, et al. The nine-year changes of the incidence and characteristics of metabolic syndrome in China: longitudinal comparisons of the two cross-sectional surveys in a newly formed urban community. Cardiovasc Diabetol. 2016;15(1):84. doi: 10.1186/s12933-016-0402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco OH, Massaro JM, Civil J, Cobain MR, O’Malley B, D’Agostino RB., Sr Trajectories of entering the metabolic syndrome: the framingham heart study. Circulation. 2009;120(20):1943–1950. doi: 10.1161/CIRCULATIONAHA.109.855817. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Zhu W, Mai L, Fang L, Ying K. The association of metabolic syndrome and its components with brachial-ankle pulse wave velocity in south China. Atherosclerosis. 2015;240(2):345–350. doi: 10.1016/j.atherosclerosis.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F, Zhang H, Yao W, Mei H, Xu D, Sheng Y, Yang R, Kong X, Wang L, Zou J, et al. Relationship between brachial-ankle pulse wave velocity and metabolic syndrome components in a Chinese population. J Biomed Res. 2014;28(4):262–268. doi: 10.7555/JBR.28.20130160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scuteri A, Cunha PG, Rosei EA, Badariere J, Bekaert S, Cockcroft JR, Cotter J, Cucca F, De Buyzere ML, De Meyer T, et al. Arterial stiffness and influences of the metabolic syndrome: a cross-countries study. Atherosclerosis. 2014;233(2):654–660. doi: 10.1016/j.atherosclerosis.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh-Asahara N, Kotani K, Yamakage H, Yamada T, Araki R, Okajima T, Adachi M, Oishi M, Shimatsu A. Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS) Atherosclerosis. 2015;242(2):461–468. doi: 10.1016/j.atherosclerosis.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 14.Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y, Saiki A, Takahashi M, Suzuki K, et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18(11):924–938. doi: 10.5551/jat.7716. [DOI] [PubMed] [Google Scholar]
- 15.Maeda Y, Inoguchi T, Etoh E, Kodama Y, Sasaki S, Sonoda N, Nawata H, Shimabukuro M, Takayanagi R. Brachial-ankle pulse wave velocity predicts all-cause mortality and cardiovascular events in patients with diabetes: the Kyushu Prevention Study of Atherosclerosis. Diabetes Care. 2014;37(8):2383–2390. doi: 10.2337/dc13-1886. [DOI] [PubMed] [Google Scholar]
- 16.Kanamoto M, Matsumoto N, Shiga T, Kunimoto F, Saito S. Relationship between coronary artery stenosis and cardio-ankle vascular index (CAVI) in patients undergoing cardiovascular surgery. J Cardiovasc Dis Res. 2013;4(1):15–19. doi: 10.1016/j.jcdr.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. 2008;72(4):598–604. doi: 10.1253/circj.72.598. [DOI] [PubMed] [Google Scholar]
- 18.Izuhara M, Shioji K, Kadota S, Baba O, Takeuchi Y, Uegaito T, Mutsuo S, Matsuda M. Relationship of cardio-ankle vascular index (CAVI) to carotid and coronary arteriosclerosis. Circ J. 2008;72(11):1762–1767. doi: 10.1253/circj.CJ-08-0152. [DOI] [PubMed] [Google Scholar]
- 19.Okura T, Watanabe S, Kurata M, Manabe S, Koresawa M, Irita J, Enomoto D, Miyoshi K, Fukuoka T, Higaki J. Relationship between cardio-ankle vascular index (CAVI) and carotid atherosclerosis in patients with essential hypertension. Hypertens Res. 2007;30(4):335–340. doi: 10.1291/hypres.30.335. [DOI] [PubMed] [Google Scholar]
- 20.Horinaka S, Yabe A, Yagi H, Ishimura K, Hara H, Iemua T, Matsuoka H. Comparison of atherosclerotic indicators between cardio ankle vascular index and brachial ankle pulse wave velocity. Angiology. 2009;60(4):468–476. doi: 10.1177/0003319708325443. [DOI] [PubMed] [Google Scholar]
- 21.Sairaku A, Eno S, Hondo T, Teragawa H, Nakano Y, Matsuda K, Kisaka T, Kihara Y. Head-to-head comparison of the cardio-ankle vascular index between patients with acute coronary syndrome and stable angina pectoris. Hypertens Res. 2010;33(11):1162–1166. doi: 10.1038/hr.2010.141. [DOI] [PubMed] [Google Scholar]
- 22.Lavalle FJ, Villarreal JZ, Montes J, Mancillas LG, Rodriguez SE, Gonzalez P, Lara R. Change in the prevalence of metabolic syndrome in a population of medical students: 6-year follow-up. J Diabetes Metab Disord. 2015;14:85. doi: 10.1186/s40200-015-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Lee J, Seo J, Chung W, Kim S, Zo J, Kim M. The effects of metabolic syndrome and its components on arterial stiffness in relation to gender. J Cardiol. 2015;65(3):243–249. doi: 10.1016/j.jjcc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Kawada T, Andou T, Fukumitsu M. Relationship between cardio-ankle vascular index and components of metabolic syndrome in combination with sex and age. Diabetes Metab Syndr. 2014;8(4):242–244. doi: 10.1016/j.dsx.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Weng C, Yuan H, Yang K, Tang X, Huang Z, Huang L, Chen W, Chen F, Chen Z, Yang P. Gender-specific association between the metabolic syndrome and arterial stiffness in 8,300 subjects. Am J Med Sci. 2013;346(4):289–294. doi: 10.1097/MAJ.0b013e3182732e97. [DOI] [PubMed] [Google Scholar]
- 26.Marti R, Parramon D, Garcia-Ortiz L, Rigo F, Gomez-Marcos MA, Sempere I, Garcia-Regalado N, Recio-Rodriguez JI, Agudo-Conde C, Feuerbach N, et al. Improving interMediAte risk management. MARK study. BMC Cardiovasc Disord. 2011;11:61. doi: 10.1186/1471-2261-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrugat J, D’Agostino R, Sullivan L, Elosua R, Wilson P, Ordovas J, Solanas P, Cordon F, Ramos R, Sala J, et al. An adaptation of the Framingham coronary heart disease risk function to European Mediterranean areas. J Epidemiol Community Health. 2003;57(8):634–638. doi: 10.1136/jech.57.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/S0195-668X(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 29.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 30.World Medical Association World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 31.Satoh N, Shimatsu A, Kato Y, Araki R, Koyama K, Okajima T, Tanabe M, Ooishi M, Kotani K, Ogawa Y. Evaluation of the cardio-ankle vascular index, a new indicator of arterial stiffness independent of blood pressure, in obesity and metabolic syndrome. Hypertens Res. 2008;31(10):1921–1930. doi: 10.1291/hypres.31.1921. [DOI] [PubMed] [Google Scholar]
- 32.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI) J Atheroscler Thromb. 2006;13(2):101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda-Denshi Company L T, Japan. http://www.fukuda.co.jp/english/products/special_features/vasera/cavi.html. Accessed Apr 16 2016.
- 34.Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Frontera G, Ramos R, Marti R, Agudo-Conde C, Rodriguez-Sanchez E, Maderuelo-Fernandez JA, et al. the association between the cardio-ankle vascular index and other parameters of vascular structure and function in caucasian adults: MARK study. J Atheroscler Thromb. 2015;22(9):901–911. doi: 10.5551/jat.28035. [DOI] [PubMed] [Google Scholar]
- 35.Sun CK. Cardio-ankle vascular index (CAVI) as an indicator of arterial stiffness. Integr Blood Press Control. 2013;6:27–38. doi: 10.2147/IBPC.S34423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu H, Cui H, Han W, Ye L, Qiu W, Yang H, Zhang C, Guo X, Mao G. A cutoff point for arterial stiffness using the cardio-ankle vascular index based on carotid arteriosclerosis. Hypertens Res. 2013;36(4):334–341. doi: 10.1038/hr.2012.192. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Maekawa Y, Rakugi H. Cut-off value of brachial-ankle pulse wave velocity to predict cardiovascular disease in hypertensive patients: a cohort study. J Atheroscler Thromb. 2013;20(4):391–400. doi: 10.5551/jat.15040. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23(4):697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 39.Salas-Salvado J, Rubio MA, Barbany M, Moreno B. SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med Clin (Barc) 2007;128(5):184–196. doi: 10.1016/S0025-7753(07)72531-9. [DOI] [PubMed] [Google Scholar]
- 40.Kubozono T, Miyata M, Ueyama K, Nagaki A, Otsuji Y, Kusano K, Kubozono O, Tei C. Clinical significance and reproducibility of new arterial distensibility index. Circ J. 2007;71(1):89–94. doi: 10.1253/circj.71.89. [DOI] [PubMed] [Google Scholar]
- 41.Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, Miyashita Y, Yamamura S, Takahashi M. Contradictory effects of β1- and α1- aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. J Atheroscler Thromb. 2011;18(1):49–55. doi: 10.5551/jat.3582. [DOI] [PubMed] [Google Scholar]
- 42.Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Hiratsuka A, Matsuzaki M. Cardio-ankle vascular index is superior to brachial-ankle pulse wave velocity as an index of arterial stiffness. Hypertens Res. 2008;31(7):1347–1355. doi: 10.1291/hypres.31.1347. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Berges D, Cabrera de Leon A, Sanz H, Elosua R, Guembe MJ, Alzamora M, Vega-Alonso T, Felix-Redondo FJ, Ortiz-Marron H, Rigo F, et al. Metabolic syndrome in Spain: prevalence and coronary risk associated with harmonized definition and WHO proposal. DARIOS study. Rev Esp Cardiol (Engl Ed) 2012;65(3):241–248. doi: 10.1016/j.recesp.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis. 2003;166(2):303–309. doi: 10.1016/S0021-9150(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 45.Marlatt KL, Kelly AS, Steinberger J, Dengel DR. The influence of gender on carotid artery compliance and distensibility in children and adults. J Clin Ultrasound. 2013;41(6):340–346. doi: 10.1002/jcu.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi P, Frances Y, Kingwell BA, Ahimastos AA. Gender differences in artery wall biomechanical properties throughout life. J Hypertens. 2011;29(6):1023–1033. doi: 10.1097/HJH.0b013e328344da5e. [DOI] [PubMed] [Google Scholar]
- 47.McEniery CM, Yasmin, Maki-Petaja KM, McDonnell BJ, Munnery M, Hickson SS, Franklin SS, Cockcroft JR, Wilkinson IB. The impact of cardiovascular risk factors on aortic stiffness and wave reflections depends on age: the Anglo-Cardiff Collaborative Trial (ACCT III) Hypertension. 2010;56(4):591–597. doi: 10.1161/HYPERTENSIONAHA.110.156950. [DOI] [PubMed] [Google Scholar]
- 48.Anoop S, Misra A, Bhardwaj S, Gulati S. High body fat and low muscle mass are associated with increased arterial stiffness in Asian Indians in North India. J Diabetes Complications. 2015;29(1):38–43. doi: 10.1016/j.jdiacomp.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Marcos MA, Recio-Rodriguez JI, Patino-Alonso MC, Agudo-Conde C, Gomez-Sanchez L, Rodriguez-Sanchez E, Gomez-Sanchez M, Martinez-Vizcaino V, Garcia-Ortiz L. Relationships between high-sensitive C-reactive protein and markers of arterial stiffness in hypertensive patients. Differences by sex. BMC Cardiovasc Disord. 2012;12:37. doi: 10.1186/1471-2261-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirai K. Analysis of vascular function using the cardio-ankle vascular index (CAVI) Hypertens Res. 2011;34(6):684–685. doi: 10.1038/hr.2011.40. [DOI] [PubMed] [Google Scholar]
- 51.Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Matsuda T, Hiratsuka A, et al. Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J. 2007;71(11):1710–1714. doi: 10.1253/circj.71.1710. [DOI] [PubMed] [Google Scholar]
- 52.Scuteri A, Najjar SS, Orru M, Usala G, Piras MG, Ferrucci L, Cao A, Schlessinger D, Uda M, Lakatta EG. The central arterial burden of the metabolic syndrome is similar in men and women: the SardiNIA Study. Eur Heart J. 2010;31(5):602–613. doi: 10.1093/eurheartj/ehp491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 54.Tenenbaum A, Klempfner R, Fisman EZ. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc Diabetol. 2014;13:159. doi: 10.1186/s12933-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez-Marcos MA, Recio-Rodriguez JI, Patino-Alonso MC, Agudo-Conde C, Gomez-Sanchez L, Gomez-Sanchez M, Rodriguez-Sanchez E, Maderuelo-Fernandez JA, Garcia-Ortiz L. Cardio-ankle vascular index is associated with cardiovascular target organ damage and vascular structure and function in patients with diabetes or metabolic syndrome, LOD-DIABETES study: a case series report. Cardiovasc Diabetol. 2015;14:7. doi: 10.1186/s12933-014-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hallberg V, Palomaki A, Lahtela J, Voutilainen S, Tarkka M, Kataja M. Associations of metabolic syndrome and diabetes mellitus with 16-year survival after CABG. Cardiovasc Diabetol. 2014;13:25. doi: 10.1186/1475-2840-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Daghri NM, Al-Attas OS, Wani K, Alnaami AM, Sabico S, Al-Ajlan A, Chrousos GP, Alokail MS. Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovasc Diabetol. 2015;14:101. doi: 10.1186/s12933-015-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


