Abstract
Background
Ventriculitis is a complication of temporary intraventricular drains. The limited penetration of meropenem into the cerebrospinal fluid (CSF) is well known. However, ventricular CSF pharmacokinetic data in patients with ventriculitis are lacking. The aim of this study was to evaluate meropenem pharmacokinetics in the serum and CSF of neurocritical care patients with proven or suspected ventriculitis.
Methods
We conducted an observational pharmacokinetic study of neurocritical care patients with proven or suspected ventriculitis receiving meropenem. Multiple blood and CSF samples were taken and were described using nonparametric pharmacokinetic modelling with Pmetrics.
Results
In total, 21 patients (median age 52 years, median weight 76 kg) were included. The median (range) of peak and trough concentrations in serum were 20.16 (4.40–69.00) mg/L and 2.54 (0.00–31.40) mg/L, respectively. The corresponding peak and trough concentrations in CSF were 1.20 (0.00–6.20) mg/L and 1.28 (0.00–4.10) mg/L, respectively, with a median CSF/serum ratio (range) of 0.09 (0.03–0.16). Median creatinine clearance ranged from 60.7 to 217.6 ml/minute (median 122.5 ml/minute). A three-compartment linear population pharmacokinetic model was most appropriate. No covariate relationships could be supported for any of the model parameters. Meropenem demonstrated poor penetration into CSF, with a median CSF/serum ratio of 9 % and high interindividual pharmacokinetic variability.
Conclusions
Administration of higher-than-standard doses of meropenem and therapeutic drug monitoring in both serum and CSF should be considered to individualise meropenem dosing in neurocritical care patients with ventriculitis.
Keywords: Meropenem, Cerebrospinal fluid, Pharmacokinetics, Ventriculitis, Neurocritical care patients
Background
Neurocritical care patients often require implantation of an intraventricular catheter (IVC) to manage hydrocephalus and monitor intracranial pressure [1, 2]. IVC-related ventriculitis and/or meningitis are the primary complications in these patients [3]. Infection rates are approximately 10 %, and they are associated with significant morbidity and mortality [1, 3]. Meropenem plus vancomycin is a frequently used antimicrobial combination for management of IVC-related infections because of its broad spectrum of antimicrobial activity [2, 4]. Nevertheless, relatively little is known about the pharmacokinetics (PK) of meropenem in the cerebrospinal fluid (CSF) of patients with ventriculitis [5, 6].
Meropenem exhibits time-dependent antimicrobial activity [7]. Its antibacterial effect is related primarily to the fraction of the dosing interval that the unbound concentration is above the minimum inhibitory concentration (fT>MIC) [7]. The bactericidal activity of meropenem in laboratory animal models requires 40–50 % fT>MIC in plasma [8, 9]. The relevance of this estimate for infections within the central nervous system (CNS) is not known. A significant challenge for critical care physicians is achieving and maintaining appropriate concentrations at the target site of infection (i.e., the CSF for neurocritical care patients). In randomised clinical trials, meropenem was as effective as cefotaxime and ceftriaxone for treating community-acquired bacterial meningitis in children and adults [10, 11]. The penetration of antibiotics into the CNS is dependent on several factors, such as the presence of meningeal inflammation [5, 6]. The meninges in ventriculitis are typically normal or only minimally inflamed [5, 6]. Thus, penetration into the CNS in patients with ventriculitis should not be extrapolated from other patient populations. While meropenem is recommended for the empirical treatment of meningitis and IVC-related infections [2, 4], there are no comparative efficacy trials for patients with minimally inflamed meninges with ventriculitis and no clear idea of optimal regimens for this patient group.
The aim of this study was to evaluate meropenem concentrations in the serum and CSF of neurocritical care patients with IVC and proven or suspected ventriculitis. This study provides a first critical step in identifying regimens of meropenem that can be used to treat patients with ventriculitis. These regimens can then be further studied in clinical trials and are a way in which clinical outcomes can potentially be improved.
Methods
Study design and population
This prospective, observational PK study was performed at the intensive care unit (ICU) of Munich University Hospital, Munich, Germany, between April 2014 and January 2016. The trial was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the university ethics committee (registration number 111-14). Written informed consent was obtained from all patients or their legally authorised representatives before enrolment. Patients were enrolled in the study if they were admitted to the ICU having an IVC and proven or suspected ventriculitis. Proven ventriculitis was defined as a positive CSF culture combined with clinical signs of infection [12]. Suspected ventriculitis was defined by abnormal CSF parameters, such as low CSF glucose levels (<50 % of serum glucose), high CSF protein (>50 mg/dl) or CSF pleocytosis, combined with clinical signs of infection and in the absence of a positive CSF culture [12]. Patients were excluded if they were under 18 years of age or death within 72 h was predicted.
Drug administration
Meropenem (Meropenem Hikma®; Hikma Pharma, Gräfelfing, Germany) was administered as a prolonged infusion over 4 h using a syringe pump. The dose was 2000 mg every 8 h for all patients, except for those with adverse drug effects or renal impairment (creatinine clearance [CrCL] ≤50 ml/minute), for whom the dose was reduced to 1000 mg every 8 h at the discretion of the attending physician.
Study procedures
Serial blood and CSF sampling occurred for initial dose and steady state (daily on days 1–3, followed by every second or third day). Blood samples (4 ml) were collected using the indwelling arterial catheter just before the start of the infusion (serum trough concentration [Cmin]) and after the end of the infusion (serum peak concentration [Cmax]). CSF samples (1 ml) were collected using the indwelling IVC nearest to the site of insertion (3-ml volume to sampling location) simultaneously with each blood sample just before the start of the infusion (cerebrospinal fluid concentration at serum trough concentration [Ctrough]) and after the end of the infusion (cerebrospinal fluid concentration 4 h after serum trough concentration [Cafter 4h]). Samples were centrifuged for 5 minutes at 4000 rpm immediately after sample collection and aliquoted into 2-ml propylene tubes (Eppendorf, Hamburg, Germany). Aliquots were stored at −80 °C within 45 minutes after sample collection for a maximum of 4 weeks until assay. Additional data were obtained from the medical record, including weight, height, serum creatinine, bilirubin, serum C-reactive protein (CRP), serum interleukin-6 (IL-6), serum procalcitonin (PCT), serum leucocytes, CSF cells, CSF erythrocytes, CSF IL-6, CSF glucose, CSF protein, CSF drain in 24 h, Simplified Acute Physiology Score II (SAPS II), Sepsis-related Organ Failure Assessment (SOFA) score, Glasgow Coma Scale (GCS) and dexamethasone therapy.
Bioanalytical methodology
Serum and CSF concentrations of meropenem were analysed using a validated high-performance liquid chromatography assay with ultraviolet detection. The analyses were performed in the laboratory of the pharmacy department of Heidenheim General Hospital [13]. The assay was linear from 1 to 30 mg/L in serum and from 0.5 to 5 mg/L in CSF with a relative SD for intra- and interday precision and accuracy <5 % at high, medium and low concentrations. The limits of quantification were 0.5 mg/L for serum samples and 0.2 mg/L for CSF samples.
Population pharmacokinetic analysis
The oncentration–time data for meropenem in serum and CSF were analysed using a non-parametric population methodology with the nonparametric adaptive grid program Pmetrics version 1.3.2 [14]. The structure of the PK mathematical model fitted to the study data was modified from a previously published meropenem model [15] and took the following form:
| 1 |
| 2 |
| 3 |
These three equations describe a three-compartment pharmacokinetic model with central, peripheral and CSF compartments denoted by the numbers 1, 2 and 3, respectively. R(t) in milligrams per hour represents the zero-order infusion of meropenem. Meropenem was cleared from the central compartment (clearance in litres per hour), which also has a volume (Vc; given in litres). K cp, K pc, K cb and K bc represent first-order transfer constants connecting the various compartments. The CSF compartment (X3) has an apparent CSF volume (VCSF; given in litres). Equation (1) describes the rate of change of the amount of meropenem (in milligrams) in the central compartment (X1). Equation (2) describes the rate of change of the amount of meropenem (in milligrams) in the peripheral compartment (X2). Equation (3) describes the rate of change of the amount of meropenem (in milligrams) in the CSF compartment (X3).
In Pmetrics, error can be separately attributed to assay variance and additional process noise such as errors in sampling time or dosing. The data were weighted using the inverse of the estimated assay variance. Additional process noise such as errors in sampling time or dosing was modelled using a fixed lambda as an additive error term in Pmetrics.
Population pharmacokinetic model diagnostics
The fit of the PK model to the data set was assessed in the following ways: (1) the log-likelihood value, (2) the coefficient of determination (r 2) of the linear regression and (3) visual inspection of diagnostic scatterplots, where model predictions were generated either by the median population parameter values or by the medians of each subject’s individual Bayesian posterior parameter value distributions.
Population pharmacokinetic covariate screening
The impact of weight, CrCL, bilirubin, serum CRP, serum IL-6, serum PCT, serum leucocytes, CSF cells, CSF erythrocytes, CSF IL-6, CSF glucose, CSF protein, CSF drain in 24 h, SAPS II, SOFA score and GCS as covariates was initially assessed by visual inspection. For that reason, graphical representation in Pmetrics of each covariate versus population parameter was performed to evaluate for inclusion in the final model.
Other pharmacokinetic calculations
Cmax and Cmin in serum and Cafter 4 h and Ctrough in CSF are the observed values. The average AUC for each patient was calculated using the Bayesian posterior parametric estimates from the final model using the trapezoidal rule in Pmetrics. We divided each subject’s cumulative AUC (AUCf) by the total time in hours and multiplied the result by 24 to estimate the daily average AUC (AUC0–24). Penetration of meropenem into CSF was described using the CSF/serum ratio, which was calculated by dividing the CSF AUCf by the serum AUCf. Half-life was calculated using transfer rate constants. CrCL was calculated using the Cockcroft-Gault equation [16]. All calculations were performed using IBM SPSS Statistics version 23.0 software (IBM, Armonk, NY, USA).
Assessment of meropenem concentration in CSF
Simulations of 1000 patients were performed using Pmetrics to compare different dosing regimens in this study population (2000 mg every 8 h, 4000 mg every 8 h, 4000 mg every 6 h, 5000 mg every 6 h; 4-h infusion). In addition, probability of target attainment (PTA) in CSF was analysed using Pmetrics to achieve meropenem concentrations in CSF of 1 mg/L, 2 mg/L and 4 mg/L. Linear regression was performed using Pmetrics. PTA presentation was performed using IBM SPSS software.
Results
In total, 209 blood samples and 199 CSF samples from 21 patients were included in the model. The demographic and general clinical characteristics of patients are shown in Table 1. Briefly, the study population was relatively young (median age 52 years, range 46–80 years) and had well-preserved renal function on the day of inclusion (median CrCL 120.1 ml/minute, range 52.3–217.6 ml/minute). The median (range) SAPS II score was 47 (13–62). All patients received vancomycin therapy in addition to meropenem. Vancomycin was replaced by linezolid in one patient (4.8 %), owing to an increase in serum creatinine level. Seven patients (33.3 %) received concomitant fosfomycin for 7 days, although one (4.8 %) of them also received rifampicin, which then was replaced by fosfomycin. Patient 1 additionally received dexamethasone during the first 2 days, and patient 14 additionally received dexamethasone during the first 5 days. The most frequent neurological disease was subarachnoid haemorrhage, observed in 17 (81.0 %) patients. In the remaining four patients, an IVC was placed for intracranial bleeding (4.8 %), tumour (9.5 %) or traumatic brain injury (4.8 %). A total of 20 patients (95.2 %) were CSF culture-negative, and one patient (4.8 %) had a positive culture for Pseudomonas aeruginosa that was susceptible to meropenem.
Table 1.
Patient characteristics
| Characteristic | Data |
|---|---|
| Age, years, median (range) | 52 (46–80) |
| Weight, kg, median (range) | 76 (55–105) |
| Body mass index, kg/m2, median (range) | 25.95 (20–33) |
| Sex, male/female | 52.4 %/47.6 % |
| CrCL on day of inclusion, ml/minute, median (range) | 120.1 (52.3–217.6) |
| CRP in serum on day of inclusion, mg/dl, median (range) | 3.1 (0.4–36.7) |
| Interleukin-6 in serum on day of inclusion, pg/ml, median (range) | 13.7 (2.4–274.0) |
| CSF drain in 24 h on day of inclusion, median (range) | 183 (21–360) |
| Interleukin-6 in CSF on day of inclusion, pg/ml, median (range) | 3398 (140–24,522) |
| Cells in CSF on day of inclusion, n/μl, median (range) | 503 (4–2894) |
| Protein in CSF on day of inclusion, mg/L, median (range) | 107 (13–303) |
| Glucose in CSF on day of inclusion, mg/dl, median (range) | 72 (47–126) |
| Glucose CSF/serum ratio on day of inclusion, median (range) | 0.55 (0.38–0.99) |
| SAPS II on day of inclusion | 47 (13–62) |
| SAPS II on day of exclusion, median (range) | 32 (13–61) |
| SOFA score on day of inclusion, median (range) | 6 (1–12) |
| SOFA score on day of exclusion, median (range) | 2.5 (0–8) |
| 30-day mortality | 0 |
Abbreviations: CrCL Estimated creatinine clearance (calculated using the Cockcroft-Gault equation [16]), CRP C-reactive protein, CSF Cerebrospinal fluid, SOFA Sepsis-related Organ Failure Assessment, SAPS II Simplified Acute Physiology Score II
In serum, the median Cmax (range) was 20.16 (4.40–69.00) mg/L and the median Cmin (range) was 2.54 (0.00–31.40) mg/L. In CSF, the median Cafter 4h (range) was 1.20 (0.00–6.20) mg/L and the median Ctrough (range) was 1.28 (0.00–4.10) mg/L. The median CrCL ranged from 60.7 to 217.6 ml/minute (median 122.5 ml/minute). Individual observed meropenem concentrations and median CrCL values are shown in Table 2. The median AUC0–24 in CSF was 26.56 mg∙h/L, and in serum it was 350.22 mg∙h/L. The values for the AUC0–24 in CSF and serum ranged from 7.44 to 85.53 mg∙h/L and from 112.95 to 768.63 mg∙h/L, respectively. The median CSF/serum ratio (range) was 0.09 (0.03–0.16). Individual AUC0–24 and penetration results are shown in Table 3.
Table 2.
Observed meropenem concentrations in serum and cerebrospinal fluid
| Meropenem dosing 1000 mg | Meropenem dosing 2000 mg | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient number | CrCL (ml/minute) | Cmin (mg/L) | Cmax (mg/L) | Ctrough (mg/L) | Cafter 4h (mg/L) | Cmin (mg/L) | Cmax (mg/L) | Ctrough (mg/L) | Cafter 4h (mg/L) |
| 1 | 60.7 | 3.80 | N/A | 2.00 | N/A | 10.90 | 48.60 | 3.00 | 4.00 |
| 2 | 156.8 | 6.90 | 11.00 | N/A | N/A | 4.60 | 26.35 | 2.75 | 2.60 |
| 3 | 83.8 | 4.80 | 24.10 | 2.60 | 1.90 | 13.80 | 45.30 | 2.25 | 3.45 |
| 4 | 162.9 | N/A | N/A | N/A | N/A | 1.10 | 16.40 | 0.60 | 0.60 |
| 5 | 217.6 | <0.5 | 5.80 | N/A | N/A | 2.90 | 19.40 | 0.31 | 0.53 |
| 6 | 124.5 | N/A | N/A | N/A | N/A | 0.86 | 19.20 | 0.76 | 0.86 |
| 7 | 120.1 | N/A | N/A | N/A | N/A | <0.5 | 15.20 | 0.43 | 0.78 |
| 8 | 73.1 | N/A | N/A | N/A | N/A | 11.00 | 64.80 | 3.35 | 3.00 |
| 9 | 92.1 | 0.81 | 12.30 | 1.08 | 0.97 | 3.05 | 19.65 | 1.40 | 1.97 |
| 10 | 96.3 | N/A | N/A | N/A | N/A | 1.31 | 33.30 | 1.62 | 1.04 |
| 11 | 142.9 | N/A | 12.82 | N/A | 1.14 | 1.02 | 19.33 | 0.62 | 0.74 |
| 12 | 174.1 | 0,62 | 5.62 | 0.39 | 0.32 | N/A | N/A | N/A | N/A |
| 13 | 100.3 | 1.38 | 10.65 | 1.47 | 1.15 | 0.79 | 19.80 | 0.87 | 0.80 |
| 14 | 96.2 | N/A | N/A | N/A | N/A | 9.83 | 32.94 | 2.27 | 2.19 |
| 15 | 108.8 | N/A | N/A | N/A | N/A | 1.13 | 17.51 | 1.18 | 0.88 |
| 16 | 125.0 | N/A | N/A | N/A | N/A | 2.99 | 27.01 | 0.92 | 1.17 |
| 17 | 144.7 | N/A | N/A | N/A | N/A | 2.44 | 18.15 | 0.74 | 0.58 |
| 18 | 90.5 | N/A | 20.10 | N/A | 2.28 | 7.63 | 33.80 | 2.39 | 1.84 |
| 19 | 131.3 | 2.50 | 17.47 | 0.89 | 0.83 | 6.22 | 26.94 | 1.79 | 1.82 |
| 20 | 174.6 | N/A | N/A | N/A | N/A | 2.63 | 23.23 | 1.52 | 1.58 |
| 21 | 122.5 | 1.69 | 10.57 | <0.2 | 0.25 | 3.65 | 16.74 | 0.31 | 0.44 |
| Median | 122.5 | 1.69 | 11.65 | 1.08 | 1.05 | 2.95 | 21.51 | 1.29 | 1.10 |
| Minimum | 60.7 | <0.5 | 4.40 | <0.2 | <0.2 | <0.5 | 10.70 | <0.2 | 0.24 |
| Maximum | 217.6 | 7.10 | 26.60 | 3.10 | 2.80 | 31.40 | 69.00 | 4.10 | 6.20 |
Abbreviations: C min Median observed serum trough concentration, C max Median observed serum peak concentration, C trough Median observed cerebrospinal fluid concentration at serum trough concentration, C after 4h Median observed cerebrospinal fluid concentration 4 h after serum trough concentration, N/A Not available (patient with only meropenem 1000 mg or only 2000 mg intravenously every 8 h), CrCL Estimated creatinine clearance (calculated using the Cockcroft-Gault equation [16])
Observed meropenem concentrations after 1000 mg or 2000 mg intravenously every 8 h (4-h infusion)
Table 3.
Pharmacokinetic properties of meropenem in serum and cerebrospinal fluid
| Patient number | CL (L/h) | Vc (L) | t 1/2 (h) | AUC0–24 serum (mg∙h/L) | AUC0–24 CSF (mg∙h/L) | CSF/serum ratio | t 1/2cb (h) | t 1/2bc (h) |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.63 | 6.45 | 0.59 | 768.63 | 85.53 | 0.11 | 3.30 | 4.62 |
| 2 | 17.88 | 14.45 | 0.56 | 327.09 | 53.00 | 0.16 | 17.33 | 9.90 |
| 3 | 8.62 | 14.95 | 1.20 | 428.94 | 48.59 | 0.11 | 4.62 | 3.47 |
| 4 | 20.38 | 5.05 | 0.17 | 300.14 | 19.37 | 0.06 | 13.86 | 17.33 |
| 5 | 22.87 | 14.95 | 0.45 | 237.60 | 8.93 | 0.04 | 17.33 | 2.57 |
| 6 | 14.89 | 5.05 | 0.24 | 401.45 | 18.63 | 0.05 | 34.66 | 69.31 |
| 7 | 23.37 | 14.95 | 0.44 | 258.79 | 15.02 | 0.06 | 34.66 | 5.78 |
| 8 | 7.88 | 5.05 | 0.44 | 760.20 | 79.10 | 0.10 | 8.66 | 9.90 |
| 9 | 17.13 | 14.95 | 0.60 | 280.81 | 26.56 | 0.09 | 23.10 | 17.33 |
| 10 | 11.88 | 13.75 | 0.80 | 489.44 | 27.25 | 0.06 | 69.31 | 34.66 |
| 11 | 20.63 | 12.75 | 0.43 | 287.15 | 15.59 | 0.05 | 34.66 | 17.33 |
| 12 | 29.87 | 9.95 | 0.23 | 112.95 | 10.56 | 0.09 | 11.55 | 9.90 |
| 13 | 15.07 | 5.05 | 0.23 | 234.65 | 22.10 | 0.09 | 23.10 | 69.31 |
| 14 | 9.63 | 14.95 | 1.08 | 569.59 | 48.90 | 0.09 | 13.86 | 5.78 |
| 15 | 14.88 | 5.05 | 0.24 | 413.23 | 25.62 | 0.06 | 34.66 | 69.31 |
| 16 | 17.13 | 14.95 | 0.60 | 337.21 | 30.99 | 0.09 | 23.10 | 17.33 |
| 17 | 14.98 | 5.08 | 0.24 | 415.22 | 17.31 | 0.04 | 34.66 | 69.31 |
| 18 | 10.63 | 5.05 | 0.33 | 563.84 | 42.95 | 0.08 | 5.78 | 8.66 |
| 19 | 13.12 | 14.95 | 0.79 | 394.45 | 33.99 | 0.09 | 34.66 | 13.86 |
| 20 | 17.13 | 14.95 | 0.60 | 350.22 | 33.71 | 0.10 | 23.10 | 17.33 |
| 21 | 18.88 | 14.15 | 0.52 | 242.60 | 7.44 | 0.03 | 17.33 | 1.73 |
| Median | 15.07 | 13.75 | 0.63 | 350.22 | 26.56 | 0.09 | 23.10 | 13.86 |
| Minimum | 7.63 | 5.05 | 0.17 | 112.95 | 7.44 | 0.03 | 3.30 | 1.73 |
| Maximum | 29.87 | 14.95 | 1.20 | 768.63 | 85.53 | 0.16 | 69.31 | 69.31 |
Abbreviations: CSF Cerebrospinal fluid, CL Median clearance, t 1/2 Median half-life, V c Median volume of distribution of the central compartment, AUC 0–24 Daily average area under the curve, CSF/serum ratio Cerebrospinal fluid penetration, t 1/2cb Median absorption half-life into cerebrospinal fluid, t 1/2bc Median elimination half-life of cerebrospinal fluid
Individual pharmacokinetic results in serum and CSF obtained using Pmetrics
Pharmacokinetic model building
The three-compartment model was adequately able to describe the observed concentrations for the full data set. The fit of the population PK model was acceptable according to visual inspection of the observed-versus-predicted plots and r 2 of the observed-versus-predicted values (r 2 = 0.926 in serum, r 2 = 0.694 in CSF) (Fig. 1). Individual PK results in serum and CSF obtained by Pmetrics for the PK model are shown in Table 3. The mean, median and SD for the population parameters identified by Pmetrics for the PK model are shown in Table 4. No covariate relationships could be supported for any of the model parameters.
Fig. 1.
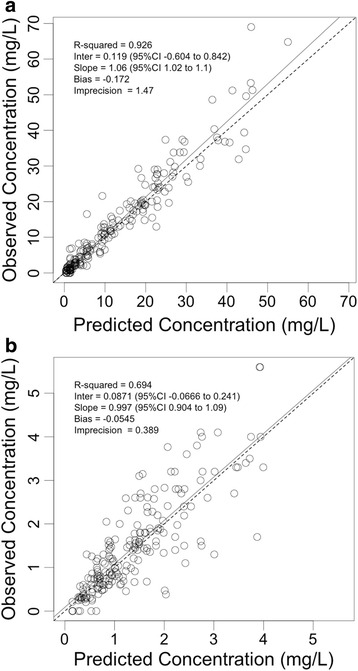
Diagnostic plots for the final population pharmacokinetics model. Individual predicted meropenem concentrations in serum versus observed serum concentrations (r 2 = 0.926) (a) and individual predicted meropenem concentrations in cerebrospinal fluid (CSF) versus observed CSF concentrations (r 2 = 0.694) (b), indicating that 92.6 % in serum and 69.4 % in CSF of the observed variability in meropenem concentrations were explained by the parametric distributions in the model. The solid black line shows the linear regression line of fit. The estimates of bias and imprecisions were also acceptable (−0.172 and 1.47 in serum and −0.0545 and 0.389 in CSF, respectively)
Table 4.
Population pharmacokinetic mean, median and SD parameters of meropenem obtained using Pmetrics
| Mean | Median | SD | |
|---|---|---|---|
| CL, L/h | 16.045 | 15.025 | ±5.575 |
| Vc, L | 10.949 | 13.736 | ±4.491 |
| K cp, L−1 | 1.562 | 1.248 | ±1.031 |
| K pc, L−1 | 1.686 | 1.898 | ±1.206 |
| K cb, L−1 | 0.052 | 0.026 | ±0.049 |
| K bc, L−1 | 0.092 | 0.054 | ±0.097 |
| VCSF, L | 82.932 | 93.902 | ±18.802 |
Abbreviations: CL Clearance, V c Volume of distribution of the central compartment, V CSF Volume of distribution of the cerebrospinal fluid compartment, K cp, K pc, K bc, K cb Linear transfer rate constants
Assessment of meropenem concentration in CSF
Simulated meropenem concentration–time profiles in serum and CSF of each regimen are shown in Fig. 2. The proportions of simulated patients who exceeded targeted meropenem concentrations in CSF of each regimen are shown in Fig. 3.
Fig. 2.
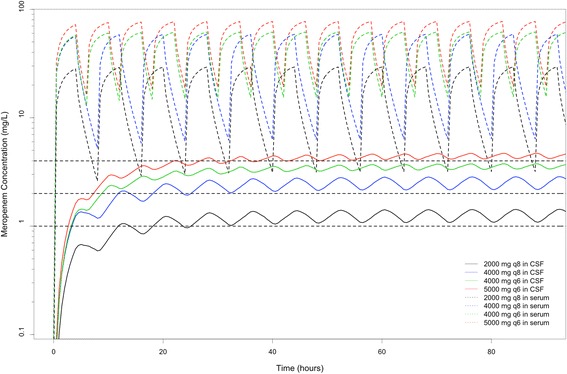
Comparison of different dosing regimens as prolonged infusions over 4 h using the pharmacokinetics model. Median time course of meropenem concentrations simulated in serum and cerebrospinal fluid (CSF) over 4 days. Targeted meropenem trough concentrations in CSF were 1 mg/L, 2 mg/L and 4 mg/L
Fig. 3.
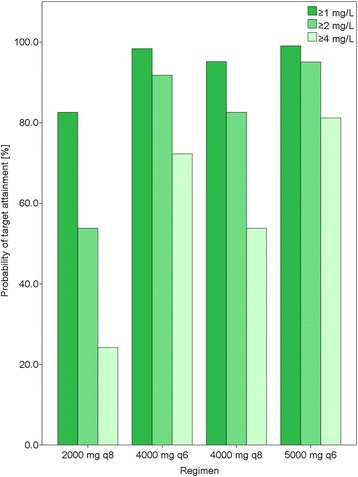
Probability of target attainment in cerebrospinal fluid (CSF) for different dosing regimens as prolonged infusions over 4 h. The proportions of simulated patients who exceeded meropenem trough concentrations in CSF greater than or equal to 1 mg/L, 2 mg/L and 4 mg/L for each regimen are shown
Discussion
To our knowledge, this is the first population PK study of meropenem concentrations in serum and CSF in neurocritical care patients with ventriculitis. Furthermore, it is also the largest study investigating the penetration of meropenem into CSF. We found that meropenem poorly penetrated into CSF, with a median penetration ratio of only 9 %. However, there was considerable interindividual variability in serum and CSF concentrations and resultant CSF/serum ratios. This variation is most likely due to the range of sickness severity and the consequent effect of altered physiology on meropenem exposure, as well as to the integrity of the blood–CSF barrier. These findings are concordant with those derived from previous studies in which researchers have also described large interindividual variability in meropenem concentrations in serum [17] and CSF [18]. However, PK variability was not explained by any covariates. Therefore, our study suggests the need for therapeutic drug monitoring of meropenem in both serum and CSF to avoid treatment failures due to underexposure or overdosing resulting in potential side effects, as suggested by a case report by Lonsdale et al. [19].
CSF is produced by the choroid plexus [20]. Drug penetration into CSF is indicative of the transport across the choroid plexus at the blood–CSF barrier [21]. The blood–CSF barrier is ‘leaky’ compared with the blood–brain barrier, and molecules enter the CSF by diffusion at a rate that is inversely proportional to their molecular weight [20, 21]. Our PK model suggests that meropenem penetration (median 23 h) is slower than CSF clearance (median 14 h). Approximately 24–48 h are required to achieve steady-state concentrations in the CSF (Fig. 1). In future studies, researchers could examine innovative ways to achieve effective CSF concentrations as quickly as possible. Robust estimates of early penetration would require optimal sampling in this early treatment period. Neither accumulation of drug nor significant intraindividual variability over the treatment course was observed. The apparently high volume of CSF reflects the relatively low CSF concentrations compared with serum. VCSF should not be viewed as the physiological CSF volume; it is merely a scalar that explains the concentration observed in the CSF.
A CSF penetration of meropenem of 20 % in normal or mildly infected meninges and 39 % in inflamed meninges is described elsewhere [6]. However, studies citing relatively higher CSF penetration (e.g., 21 % [22], 25 % [23], 39 % [10]) have been conducted in patients with bacterial meningitis [10, 23] or with CSF that was collected by lumbar drainage [22]. Nau et al. [18] observed a CSF penetration of meropenem of 4.6 % (range 1.9–8.9 %) in ten neurocritical care patients with extracerebral infections [18]. This is similar to our findings considering high interindividual variability in CSF/serum ratios. In this study, meropenem clearance in serum (16.0 L/h) was greater than that reported in other PK studies in critically ill patients (7.7–9.4 L/h [24], 9.3 L/h [25], 11.5 L/h [26], 13.6 L/h [27]). However, the patients in our study were generally young and without any measured renal dysfunction on the day of inclusion. Interestingly, in a previous study with healthy volunteers (16.3 L/h [28]), researchers described similar meropenem clearance. The fact that the clearance in our patients was similar to that observed in healthy volunteers may be due to the relatively preserved renal function of our patient population (median CrCL 122 ml/minute) in contrast to the renal function in other studies (mean CrCL 84 ml/minute [24], 78 ml/minute [25], 61 ml/minute [26], 100 ml/minute [27]). Greater than normal CrCL is common in neurocritical care patients [29], which may lead to sub-therapeutic concentrations of time-dependent antibiotics such as β-lactam agents. Augmented renal clearance has previously been shown to be an independent predictor of not achieving the pharmacokinetic/pharmacodynamic (PD) target for meropenem [19, 30, 31] as well as other β-lactams [30–33]. Nevertheless, patients’ renal function was not a covariate in our model. CrCL was the most important predictor for meropenem clearance with impaired renal function, although no correlation was observed between CrCL and meropenem clearance above CrCL of 100 ml/minute [31, 34].
The most dreaded pathogens in nosocomial CNS infection are aerobic Gram-negative pathogens (e.g., P. aeruginosa), Staphylococcus aureus and Staphylococcus epidermidis [2, 4]. From a PD point of view, CSF concentrations in our study population exceeded minimum inhibitory concentrations (MICs) for most members of the Enterobacteriaceae family (<0.125 mg/L), including Klebsiella pneumoniae and methicillin-sensitive S. aureus (0.25 mg/L) [9]. However, only 53.8 % of the simulated patients exceeded CSF trough concentrations of 2 mg/L with 2000 mg meropenem every 8 h, assuming all drug in the CSF is unbound (Fig. 3). In contrast, 95.1 % of simulated patients exceeded CSF trough concentrations of 2 mg/L with a regimen of 5000 mg meropenem every 6 h (Fig. 3). Therefore, in neurocritical care patients with CNS infections caused by pathogens with borderline susceptibility such as P. aeruginosa (2 mg/L) [9], the standard dosing regimen of meropenem 2000 mg every 8 h as a prolonged infusion is unlikely to achieve adequate CSF concentrations. More work is required to better understand PD targets at the site of infection for patients with ventriculitis.
There are several limitations of this study. First, the study was relatively small, which may have hampered robust estimates of the extent of PK variability and the identification of covariates that may have explained some of the observed variance. CrCL was estimated because the measurement is not routinely performed in routine clinical care. Second, we measured total drug concentrations because protein binding is not relevant for low to moderately protein-bound drugs (unbound fraction 91–98 %) [9]. Finally, all but one patient had suspected ventriculitis without a positive CSF culture. Therefore, we were unable to establish PK–PD relationships. Such an analysis would have been helpful to help establish drug exposure targets at the site of infection.
Conclusions
To our knowledge, this is the largest PK study of neurocritical care patients with proven or suspected ventriculitis. We found that meropenem showed relatively low penetration into CSF. Furthermore, high interindividual variability in serum and CSF concentrations was observed. Assuming MIC serum breakpoints, adequate CSF concentrations are not assured for pathogens with borderline susceptibility such as P. aeruginosa. To address this challenge, novel dosing strategies should be investigated in further clinical studies with high daily dosages and/or with administration by continuous infusion to avoid antibiotic underexposure in the context of augmented elimination or impaired target side penetration. The safety of these higher-dose regimens must be established. An alternative approach to optimising meropenem exposure is to use individualised dosing to achieve the desired drug exposure in both serum and CSF.
Key message
Currently recommended regimens for meropenem for proven or suspected ventriculitis may lead to insufficient drug concentrations in cerebrospinal fluid. Therefore, novel treatment strategies, including the possibility of therapeutic drug monitoring within serum and cerebrospinal fluid, should be investigated in further clinical studies.
Acknowledgements
This work was supported by the doctoral program in clinical pharmacy of Ludwig-Maximilians-University Munich, Munich, Germany.
Funding
This work was supported by the Dr. August and Dr. Anni Lesmüller Foundation, Munich, Germany.
Availability of data and materials
All data generated during this study are included in this article.
Authors’ contributions
UB participated in the design of the study, measured meropenem concentrations by HPLC, was responsible for acquisition of data, performed the pharmacokinetic analysis and drafted the manuscript. VH conceived of the study, participated in its design and coordination, and was responsible for acquisition of data. ORF participated in the design of the study, including interpretation of results, and measured meropenem concentrations by HPLC. CVK participated in the design of the study, including interpretation of results, and was responsible for acquisition of data. ACR measured meropenem concentrations by HPLC. WH performed the pharmacokinetic analysis and helped to draft the manuscript. NT made substantial contributions to the conception and design of the study and also interpreted the results. JB made substantial contributions to the conception and design of the study and also interpreted the results. All authors critically revised the manuscript for important intellectual content, and all authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethics approval was obtained from the university ethics committee (ethics committee at Ludwig-Maximilians-University Munich, registration number 111-14). Written informed consent was obtained from all patients or their legally authorised representatives before enrolment.
Abbreviations
- AUC0–24
Daily average area under the curve
- AUCf
Cumulative area under the curve
- Cafter 4h
Cerebrospinal fluid concentration 4 h after serum trough concentration
- CL
Clearance
- Cmax
Serum peak concentration
- Cmin
Serum trough concentration
- CNS
Central nervous system
- CrCL
Creatinine clearance
- CRP
C-reactive protein
- CSF
Cerebrospinal fluid
- Ctrough
Cerebrospinal fluid concentration at serum trough concentration
- fT>MIC
Fraction of the dosing interval that the unbound concentration is above the minimum inhibitory concentration
- GCS
Glasgow Coma Scale
- ICU
Intensive care unit
- IL
Interleukin
- IVC
Intraventricular catheter
- Kbc
Transfer constant from the cerebrospinal fluid compartment
- Kcb
Transfer constant to the cerebrospinal fluid compartment
- Kcp
Transfer constant to the peripheral compartment
- Kpc
Transfer constant from the peripheral compartment
- MIC
Minimum inhibitory concentration
- N/A
Not available
- PCT
Procalcitonin
- PD
Pharmacodynamics
- PK
Pharmacokinetics
- PTA
Probability of target attainment
- R(t)
Meropenem infusion rate
- SAPS II
Simplified Acute Physiology Score II
- SOFA
Sepsis-related Organ Failure Assessment
- t1/2
Half-life
- t1/2cb
Absorption half-life into cerebrospinal fluid, t 1/2bc, Elimination half-life of cerebrospinal fluid
- Vc
Volume of distribution of the central compartment
- VCSF
Volume of distribution of the cerebrospinal fluid compartment
- X1
Central compartment
- X2
Peripheral compartment
- X3
Cerebrospinal fluid compartment
Contributor Information
Ute Blassmann, Phone: +49 (0) 89 4400-76600, Email: ute.blassmann@med.uni-muenchen.de.
Anka C. Roehr, Email: anka.roehr@kliniken-heidenheim.de
Otto R. Frey, Email: otto.frey@kliniken-heidenheim.de
Cornelia Vetter-Kerkhoff, Email: cornelia.vetter@med.uni-muenchen.de.
Niklas Thon, Email: niklas.thon@med.uni-muenchen.de.
William Hope, Email: william.hope@liverpool.ac.uk.
Josef Briegel, Email: josef.briegel@med.uni-muenchen.de.
Volker Huge, Email: volker.huge@med.uni-muenchen.de.
References
- 1.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES., Jr Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51(1):170–81. doi: 10.1097/00006123-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol. 2008;255(11):1617–24. doi: 10.1007/s00415-008-0059-8. [DOI] [PubMed] [Google Scholar]
- 3.Kitchen WJ, Singh N, Hulme S, Galea J, Patel HC, King AT. External ventricular drain infection: improved technique can reduce infection rates. Br J Neurosurg. 2011;25(5):632–5. doi: 10.3109/02688697.2011.578770. [DOI] [PubMed] [Google Scholar]
- 4.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–84. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 5.Di Paolo A, Gori G, Tascini C, Danesi R, Del Tacca M. Clinical pharmacokinetics of antibacterials in cerebrospinal fluid. Clin Pharmacokinet. 2013;52(7):511–42. doi: 10.1007/s40262-013-0062-9. [DOI] [PubMed] [Google Scholar]
- 6.Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood–cerebrospinal fluid/blood–brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–83. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat Rev Microbiol. 2004;2(4):289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 8.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 9.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Meropenem: rationale for the EUCAST clinical breakpoints. Version 1.5. 1 June 2009. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Meropenem_EUCAST_Rationale_Document_1.5_090601.pdf. Accessed 14 Oct 2016.
- 10.Dagan R, Velghe L, Rodda J, Klugman K. Penetration of meropenem into the cerebrospinal fluid of patients with inflamed meninges. J Antimicrob Chemother. 1994;34(1):175–9. doi: 10.1093/jac/34.1.175. [DOI] [PubMed] [Google Scholar]
- 11.Schmutzhard E, Williams KJ, Vukmirovits G, Chmelik V, Pfausler B, Featherstone A. Meropenem Meningitis Study Group. A randomised comparison of meropenem with cefotaxime or ceftriaxone for the treatment of bacterial meningitis in adults. J Antimicrob Chemother. 1995;36(Suppl A):85–97. doi: 10.1093/jac/36.suppl_A.85. [DOI] [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Roehr AC, Frey OR, Koeberer A, Fuchs T, Roberts JA, Brinkmann A. Anti-infective drugs during continuous hemodialysis – using the bench to learn what to do at the bedside. Int J Artif Organs. 2015;38(1):17–22. doi: 10.5301/ijao.5000377. [DOI] [PubMed] [Google Scholar]
- 14.Neely M, van Guilder M, Yamada W, Schumitzky A, Jelliffe R. Accurate detection of outliers and subpopulations with Pmetrics, a non-parametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34(4):467–76. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodise TP, Nau R, Kinzig M, Drusano GL, Jones RN, Sorgel F. Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J Antimicrob Chemother. 2007;60(5):1038–44. doi: 10.1093/jac/dkm325. [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–83. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 18.Nau R, Lassek C, Kinzig-Schippers M, Thiel A, Prange HW, Sorgel F. Disposition and elimination of meropenem in cerebrospinal fluid of hydrocephalic patients with external ventriculostomy. Antimicrob Agents Chemother. 1998;42(8):2012–6. doi: 10.1128/aac.42.8.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonsdale DO, Udy AA, Roberts JA, Lipman J. Antibacterial therapeutic drug monitoring in cerebrospinal fluid: difficulty in achieving adequate drug concentrations. J Neurosurg. 2013;118(2):297–301. doi: 10.3171/2012.10.JNS12883. [DOI] [PubMed] [Google Scholar]
- 20.Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21(3):79–96. [PubMed] [Google Scholar]
- 21.Pardridge WM. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda T, Ikawa K, Ikeda K, Morikawa N, Tsumura R, Shibukawa M, et al. LC method for the determination of meropenem in cerebrospinal fluid: application to therapeutic drug monitoring. Chromatographia. 2009;69(9–10):1031–4. doi: 10.1365/s10337-009-1013-3. [DOI] [Google Scholar]
- 23.Chou YW, Yang YH, Chen JH, Kuo CC, Chen SH. Quantification of meropenem in plasma and cerebrospinal fluid by micellar electrokinetic capillary chromatography and application in bacterial meningitis patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856(1):294–301. doi: 10.1016/j.jchromb.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Thalhammer F, Traunmüller F, El Menyawi I, Frass M, Hollenstein UM, Locker GJ, et al. Continuous infusion versus intermittent administration of meropenem in critically ill patients. J Antimicrob Chemother. 1999;43(4):523–7. doi: 10.1093/jac/43.4.523. [DOI] [PubMed] [Google Scholar]
- 25.Kitzes-Cohen R, Farin D, Piva G, De Myttenaere-Bursztein SA. Pharmacokinetics and pharmacodynamics of meropenem in critically ill patients. Int J Antimicrob Agents. 2002;19(2):105–10. doi: 10.1016/S0924-8579(01)00474-5. [DOI] [PubMed] [Google Scholar]
- 26.Novelli A, Adembri C, Livi P, Vallani S, Mazzei T, De Gaudio AR. Pharmacokinetic evaluation of meropenem and imipenem in critically ill patients with sepsis. Clin Pharmacokinet. 2005;44(5):539–49. doi: 10.2165/00003088-200544050-00007. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother. 2009;64(1):142–50. doi: 10.1093/jac/dkp139. [DOI] [PubMed] [Google Scholar]
- 28.Krueger WA, Bulitta J, Kinzig-Schippers M, Landersdorfer C, Holzgrabe U, Naber KG, et al. Evaluation by Monte Carlo simulation of the pharmacokinetics of two doses of meropenem administered intermittently or as a continuous infusion in healthy volunteers. Antimicrob Agents Chemother. 2005;49(5):1881–9. doi: 10.1128/AAC.49.5.1881-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udy A, Boots R, Senthuran S, Stuart J, Deans R, Lassig-Smith M, et al. Augmented creatinine clearance in traumatic brain injury. Anesth Analg. 2010;111(6):1505–10. doi: 10.1213/ANE.0b013e3181f7107d. [DOI] [PubMed] [Google Scholar]
- 30.Carlier M, Carrette S, Roberts JA, Stove V, Verstraete A, Hoste E, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care. 2013;17(3):R84. doi: 10.1186/cc12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45(4):385–92. doi: 10.1016/j.ijantimicag.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Udy AA, Varghese JM, Altukroni M, Briscoe S, McWhinney BC, Ungerer JP, et al. Subtherapeutic initial β-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest. 2012;142(1):30–9. doi: 10.1378/chest.11-1671. [DOI] [PubMed] [Google Scholar]
- 33.Conil JM, Georges B, Mimoz O, Dieye E, Ruiz S, Cougot P, et al. Influence of renal function on trough serum concentrations of piperacillin in intensive care unit patients. Intensive Care Med. 2006;32(12):2063–6. doi: 10.1007/s00134-006-0421-1. [DOI] [PubMed] [Google Scholar]
- 34.Roehr AC, Frey OR, Köberer A, Fuchs T, Helbig S, Brinkmann A, et al. Creatinine-clearance as predictor for meropenem clearance [abstract EV0080]. Presented at the 26th European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark; 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in this article.


