Abstract
Background
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is an effective technique for providing emergency mechanical circulatory support for patients with cardiogenic shock. VA-ECMO enables a rapid restoration of global systemic organ perfusion, but it has not been found to always show a parallel improvement in the microcirculation. We hypothesized in this study that the response of the microcirculation to the initiation of VA-ECMO might identify patients with increased chances of intensive care unit (ICU) survival.
Methods
Twenty-four patients were included in this study. Sublingual microcirculation measurements were performed using the CytoCam-IDF (incident dark field) imaging device. Microcirculatory measurements were performed at baseline, after VA-ECMO insertion (T1), 48–72 h after initiation of VA-ECMO (T2), 5–6 days after (T3), 9–10 days after (T4), and within 24 h of VA-ECMO removal.
Results
Of the 24 patients included in the study population, 15 survived and 9 died while on VA-ECMO. There was no significant difference between the systemic global hemodynamic variables at initiation of VA-ECMO between the survivors and non-survivors. There was, however, a significant difference in the microcirculatory parameters of both small and large vessels at all time points between the survivors and non-survivors. Perfused vessel density (PVD) at baseline (survivor versus non-survivor, 19.21 versus 13.78 mm/mm2, p = 0.001) was able to predict ICU survival on initiation of VA-ECMO; the area under the receiver operating characteristic curve (ROC) was 0.908 (95 % confidence interval 0.772–1.0).
Conclusion
PVD of the sublingual microcirculation at initiation of VA-ECMO can be used to predict ICU mortality in patients with cardiogenic shock.
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-016-1519-7) contains supplementary material, which is available to authorized users.
Keywords: Microcirculation, VA-ECMO, Cardiogenic shock, Survival, ICU
Background
Cardiogenic shock (CS) has a high mortality rate and is defined as a state of tissue hypoperfusion induced by cardiac failure [1, 2]. Many conditions, such as acute myocardial infarction [3], end-stage dilated cardiomyopathy [4], myocarditis [5], complications following cardiac surgery [6], and cardiac arrest [7], can cause CS. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is an effective, portable, and rapidly deployable technique for providing emergency mechanical circulatory support for patients in CS [8–10], and it successfully reduces the time required for cardiac recovery [11]. Following this bridge therapy, some patients require implantation of a left ventricular-assist device (LVAD) or heart transplantation for adequate recovery and survival. Being able to identify hemodynamic variables related to survival could provide an important therapeutic window for optimizing VA-ECMO support. Until recently, lactate values have been suggested to predict outcomes of adult and pediatric patients on VA-ECMO [12–14]. However, these are downstream parameters and do not provide a direct hemodynamic endpoint.
Evaluation of systemic hemodynamic parameters, such as blood pressure, cardiac output, and mixed venous saturation, assist in diagnosing CS. VA-ECMO can quickly restore global hemodynamics. However, there is no guarantee that restoring these systemic hemodynamic variables will also improve microcirculatory and tissue perfusion, which is considered the ultimate success of resuscitation. Many studies have demonstrated that altered microcirculatory functional parameters can be a good predictor of mortality, despite normalized systemic hemodynamic variables [15–18]. The use of an intra-aortic balloon pump (IABP) did not affect the microcirculation parameters in these patients with or without VA-ECMO while restoring the macrocirculation [19, 20]. However, to date, no study has evaluated the sublingual microcirculatory response of patients with CS on VA-ECMO in relation to intensive care unit (ICU) outcomes after VA-ECMO. In this study, we tested the hypothesis that assessment of the microcirculation at initiation of VA-ECMO may identify patients with increased chances of ICU survival. To test this hypothesis we measured sublingual microcirculation in CS patients eligible for VA-ECMO therapy using hand-held microscopy.
Methods
Study setting and population
This study was conducted between September 2014 and October 2015 at the ICU of the Erasmus Medical Center Rotterdam, The Netherlands. All consecutive patients requiring VA-ECMO for CS were included in the study with the exception of two patients who died within 24 h for whom it was not possible to measure microcirculation due to continuous resuscitation in an overcrowded ICU box and for four patients who did not consent to inclusion in the study. Survival was defined as discharge from the ICU without a need for re-admission or re-implantation of the VA-ECMO after explantation. Additionally, bridged patients with durable LVADs or cardiac transplantations were documented as survivors. Non-survivors were those who died in the ICU during VA-ECMO support or within a couple of hours of explantation of VA-ECMO.
The following data were recorded at ICU admission: age, gender, body mass index, Sequential Organ Failure Assessment (SOFA) score [21], Acute Physiology and Chronic Health Evaluation (APACHE) II score, indications for VA-ECMO, heart rate, mean arterial pressure, lactate, hemoglobin (Hb), hematocrit (Htc), platelet count, lactate dehydrogenase (LDH), free hemoglobin (free-Hb), N-terminal of the prohormone brain natriuretic peptide (NT-proBNP), high sensitive troponin T (HsTnT), creatine kinase (CK), MB fraction of creatine kinase (CK-MB), and echocardiographic parameters.
VA-ECMO circuit and placement, and patient management under VA-ECMO
VA-ECMO consisted of polyvinyl chloride tubing, a membrane oxygenator (Quadrox Bioline; Jostra-Maquet, Orleans, France), a centrifugal pump (Rotaflow; Jostra-Maquet), and either percutaneous arterial and venous femoral, or central right atrial and aortic cannulae (Biomedicus Carmeda; Medtronic, Boulogne-Billancourt, France). An oxygen-air blender (Sechrist Industries, Anaheim, CA, USA) ventilated the membrane oxygenator. Because of the percutaneous femoral VA-ECMO, an additional 7-F cannula was inserted distally into the femoral artery to prevent severe leg ischemia. Patients were kept on VA-ECMO for at least 48 h. The circuit was checked daily for significant fibrin deposition or clots accumulated on the membrane during hemolysis, thrombocythemia, or a sharp and systematic decline after blood oxygenation by experienced perfusionists and changed according to protocol.
Microcirculatory measurements
Microcirculation measurements were performed using a CytoCam imaging device (CytoCam; Braedius Medical, Huizen, The Netherlands), which is a third-generation hand-held microscope [22]. The CytoCam device has a computer-controlled, high-resolution image sensor. Additionally, a CytoCam-IDF (incident dark field) device has a specifically designed microscopic lens that produces high-resolution images, showing approximately 30 % more capillaries than previous-generation devices [22–24]. The newly introduced lightweight (140 g versus previous devices weighing approximately 450 g) Cytocam-IDF device (with improved optics, a computer-controlled image sensor and illumination, and a more precise focusing mechanism) used in the present study allows for a faster and more stable and precise measurement to be made. In addition to these improvements, the device also has a faster measurement acquisition time, requiring only 3 to 5 s to assess the quality of the microcirculation. The sufficiency of this time was agreed upon in the second consensus conference held recently in November 2015 in Amsterdam and in March 2016 in Brussels, where most of the practitioners used the new Cytocam-IDF technology evaluated in the present study. Without applying pressure, the tip of the light guide is gently placed on the mucosal surface of the sublingual area. A 3- to 5-s video recording of predefined (left, right, and midline sublingual cavity) sites was obtained in each patient. Recordings were then blinded and analyzed to obtain the following microcirculatory parameters: total vessel density (TVD; mm/mm2), perfused vessel density (PVD; mm/mm2), proportion of perfused vessels (PPV; %), and microvascular flow index (MFI; arbitrary units (AU); a semiquantitative measure of microcirculatory flow [25]). Microcirculatory measurements were repeated at the following time points: baseline, after VA-ECMO insertion within 24 h (T1), 2–3 days after initiation of VA-ECMO (T2), 5–6 days after initiation of VA-ECMO (T3), 9–10 days after initiation of VA-ECMO (T4), and within 24 h of explantation of VA-ECMO. The timing between VA-ECMO insertion and the first microcirculatory and echocardiography measurements are shown in Table 1. The first echocardiography measurement was performed after a median of 9 (range 1–23) h and the microcirculatory measurement was performed after a median of 15 (range 1–22) h. Image clips were included which confirmed a quality score defined by Massey et al. [26]. Thus, 154 clips of 335 were excluded, and 181 clips were analyzed. Details of the excluded and included clips and the number of patients at each time point are shown in Additional file 1: Table S5. The most important reason for the poor quality of the clips was patient-related clinical conditions (such as bleedings, saliva, difficulty opening the mouth, difficulty inserting the device appropriately due to an endotracheal or gastric tube, and the lack of patient cooperation), especially in explanted patients. In addition, device-related conditions such as difficulties with focusing, brightness, and pressure artefacts resulted in poor-quality clips. Image clips were randomized and blinded to the three investigators (AK, SA, WdW) who performed the analysis using software to calculate the various functional microcirculatory parameters [27]. Microcirculatory parameters were divided into two categories: small vessels, defined as having a diameter of 25 μm or less (for example, TVD small vessel, PVD small vessel); and all vessels, defined as having small vessels and large vessels (>25–100 μm diameter; TVD all vessel, PVD all vessel) according to the consensus on microcirculatory assessment by De Backer et al. [25].
Table 1.
Patient baseline characteristics
| Demographics | Total (n = 24) | Survivors (n = 15) | Non-survivors (n = 9) | P valuea |
|---|---|---|---|---|
| Age (years) | 56 (20–70) | 51 (23–70) | 51 (20–67) | 0.244 |
| Male gender (n (%)) | 16 (67) | 12 (80) | 4 (44) | 0.08 |
| BMI (kg/m2) | 24.5 (19–37) | 25.0 (19–37) | 24.0 (20–37) | 0.904 |
| SOFA score on admission day | 11 (2–20) | 10 (4–20) | 12 (2–15) | 0.309 |
| APACHE II | 32 (19–45) | 31 (19–45) | 34 (29–44) | 0.244 |
| VA-ECMO indications | ||||
| Dilated cardiomyopathy | 1 | 0 | 1 | ns |
| Ischemic cardiomyopathy | 2 | 1 | 1 | ns |
| Fulminant myocarditis | 1 | 0 | 1 | ns |
| Intoxication | 1 | 1 | 0 | ns |
| Pulmonary embolism | 1 | 1 | 1 | ns |
| Drowning | 1 | 1 | 0 | ns |
| Post-cardiectomy | 5 | 4 | 1 | ns |
| Cardiac arrest (n (%)) | 12 (50) | 8 | 4 | 0.680 |
| IHCA | 4 | 3 | 1 | |
| OHCA | 8 | 5 | 3 | |
| Global hemodynamics at admission | ||||
| Heart rate (beats/min) | 93 (50–185) | 91 (50–128) | 95 (64–185) | 0.858 |
| MAP (mmHg) | 67 (15–103) | 71 (49–98) | 66 (15–103) | 0.269 |
| Lactate (mmol/L) | 4.1 (1.1–26) | 4.0 (1.20–26) | 6.2 (1.1–18) | 0.743 |
| Hb (mmol/L) | 6.0 (4.7–11.3) | 6.2 (4.7–11.30) | 5.7 (5.0–7.9) | 0.152 |
| Htc (L/L) | 0.32 (0.23–0.50) | 0.30 (0.27–0.50) | 0.28 (0.23–0.39) | 0.100 |
| Platelet count (1000/mm3) | 136 (18–336) | 136 (64–336) | 148 (18–227) | 0.835 |
| LDH (U/L) | 888 (264–16,779) | 810 (264–16,779) | 973 (360–3863) | 0.493 |
| Free-Hb (mg/dl) | 4 (1–640) | 4 (1.0–640) | 4 (1.0–17) | 0.516 |
| HsTnT (ng/L) | 4657 (45–89,641) | 1683 (45–42,813) | 13,369 (215–89,641) | 0.030 |
| CK (U/L) | 2771 (27–18,526) | 1082 (223–7286) | 2637 (27–18,526) | 0.114 |
| CKMB (μg/L) | 45.8 (1.2–859) | 36.9 (2.5–412.1) | 226 (1.2–859) | 0.233 |
| VA-ECMO flow (L/min) | 4.14 (2.40–6.30) | 4.0 (2.40–6.30) | 4.1 (3.30–4.70) | 0.929 |
| Timing between VA-ECMO insertion and first microcirculation measurement (h) | 15 (1–22) | 15 (2–22) | 13 (1–21) | 0.857 |
| Timing between VA-ECMO insertion and first echocardiography measurement (h) | 9 (1–23) | 7 (1–23) | 11 (3–21) | 0.426 |
| Echocardiographic parameter at first 24 h after VA-ECMO implantation | ||||
| Aortic VTI (cm) | 7 (5–22) | 8,5 (5–22) | 6 (5–12) | 0.220 |
| LVEF (%) | 15 (7–55) | 20 (8–55) | 10 (7–20) | 0.010 |
| TDSab (cm/s) | 5 (5–7) | 5 (5–7) | 5.5 (5–6) | 1 |
| TAPSE (cm) | 10 (8–20) | 10 (8–15) | 8 (8–20) | 0.845 |
| Global hemodynamic parameters at first 24 h after VA-ECMO implantation | ||||
| COc (L/min) | 2.1 (0.75–5.73) | 2.46 (0.75–5.73) | 1.37 (0.97–4.0) | 0.035 |
| CId (L/min/m2) | 1.1 (0.39–2.81) | 1.23 (0.39–2.81) | 0.74 (0.48–1.69) | 0.069 |
| DO2 e (ml O2/min) | 289 (122–790) | 327 (150–790) | 174 (122–510) | 0.006 |
| Fluid balance | 1.35 (–1.90 to 4.00) | 1.50 (–1.90 to 3.80) | 1.0 (–1.10 to 4.0) | 0.590 |
| CVP (mmHg) | 12 (1–32) | 12 (1–32) | 12 (5–17) | 0.726 |
| Microcirculation at first 24 h after VA-ECMO implantation | ||||
| All | ||||
| TVD (mm/mm2) | 17.13 (11.88–30.39) | 20.07 (15.71–30.39) | 14.92 (11.88–23.99) | 0.008 |
| PVD (mm/mm2) | 16.32 (11.01–29.60) | 19.21 (12.99–29.60) | 13.78 (11.01–18.47) | 0.001 |
| PPV (%) | 96.48 (76.99–100) | 98.06 (76.99–100) | 89.20 (77–100) | 0.044 |
| MFI (AU) | 2.97 (1.75–3.0) | 3.0 (2.50–3.0) | 2.87 (1.75–3.0) | 0.191 |
| Small | ||||
| TVD (mm/mm2) | 15.70 (10.91–29.62) | 18.85 (12.80–29.62) | 11.84 (10.91–20.32) | 0.009 |
| PVD (mm/mm2) | 14.16 (9.80–28.23) | 18.61 (9.80–28.83) | 11.01 (10.13–16.89) | 0.003 |
| PPV (%) | 96.45 (43.59–100) | 97.97 (76.46–100) | 89.77 (82.93–100) | 0.038 |
| MFI (AU) | 3 (0.75–3.0) | 3.0 (2.25–3.0) | 3.0 (0.75–3.0) | 0.084 |
| Outcome | ||||
| Total days on VA-ECMO (days) | 5.5 (2–36) | 6 (2–21) | 5 (2–36) | 0.588 |
| ICU length of stay (days) | 13.5 (2–65) | 18 (6–65) | 5 (2–36) | 0.03 |
| VA-ECMO-free days on ICU (days) | 2.5 (0–52) | 10 (0–52) | 0 | 0.0000 |
| Hospital length of stay (days) | 17 (2–72) | 22 (6–72) | 5 (2–36) | 0.01 |
| ICU mortality (n (%)) | 9 (37.5) | 0 | 9 | |
| Hospital mortality (n (%)) | 11 (45.8) | 2 | 9 | |
Categorical variables are presented as frequencies and percentages (n (%)) where stated; all other (continuous) variables are presented as median (range)
APACHE Acute Physiology and Chronic Health Evaluation, AU arbitrary units, BMI body mass index, BSA body surface area, CI cardiac index, CK creatine kinase, CK-MB myoglobin fraction of creatine kinase, CO cardiac output, CVP central venous pressure, DO 2 oxygen delivery, free-Hb free hemoglobin, Hb hemoglobin, HsTnT high sensitive troponin T, Htc haematocrit, ICU intensive care unit, IHCA in-hospital cardiac arrest, LDH lactate dehydrogenase, LVEF left ventricular ejection fraction, LVOT left ventricular outflow tract, MAP mean arterial pressure, MFI microvascular flow index, OHCA out-of-hospital cardiac arrest, PaO 2 arterial oxygen tension, PPV portion of perfused vessels, PVD perfused vessel density, SaO 2 arterial oxygen saturation, SOFA Sequential Organ Failure Assessment, TAPSE Tricuspid annular plane systolic excursion, TDSa spectral tissue Doppler imaging mitral annulus peak systolic velocity, TVD total vessel density, VA-ECMO veno-arterial extracorporeal membrane oxygenation, VTI velocity time integral
aA Mann Whitney U test was used for comparison between the survivor and non-survivor groups; significant results are shown in bold text
bTDSa was only retrospectively found in 7 of the 24 patients’ echocardiography
cCO = heart rate × stroke volume, where stroke volume = LVOT area × LVOT VTI, and LVOT area was calculated by two-dimensional echocardiography using the following formula: (π × (LVOT diameter/2)2 (45)
dCI = CO/BSA, where BSA (in m2) was calculated according to the formula (by DuBois and DuBois) BSA = 0.20247 × height (m)0.725 × weight (kg)0.425
eDO2 = CO × CaO2 × 10, where CaO2 = (Hb × 1.34 × SaO2) + (PaO2 × 0.003); 0.003 is the solubility coefficient of oxygen in human plasma; each gram of hemoglobin is capable of carrying 1.34 mL of oxygen, and the amount of oxygen carried on the haemoglobin is Hb × 1.34 × SaO2
Statistical analyses
Categorical variables are presented as frequencies and percentages. Continuous variables are presented as medians and range. Continuous variables were compared using a Mann–Whitney U test. For comparisons of microcirculatory parameters of patients in the same group at consecutive time points, a Friedman test was used. Baseline PVDs of all vessels, lactate HsTnT, and left ventricular ejection fraction (LVEF) were assessed as prognostic tests of survival using receiver operating characteristic curves (ROC) and their corresponding area under the curve. Cut-off points were calculated by obtaining the best Youden index (sensitivity + specificity – 1). Sensitivity, specificity, and positive and negative predictive values were calculated and compared to each other. With the cut-off values, an association between microcirculatory impairment and survival was explored. Statistical significance was defined as p < 0.05. Statistical analyses were performed using SPSS version 21.0.0.1 (SPSS, IBM, Armonk, NY, USA).
Results
Thirty consecutive patients requiring VA-ECMO for CS were recruited to the study. Six patients were excluded: two patients who died within 24 h for whom it was not possible to measure microcirculation due to continuous resuscitation in an overcrowded ICU box, and four patients who did not consent to inclusion in the study. In total, 24 patients were included in the study.
Of the 24 patients who comprised the study population, 15 survived and 9 died on VA-ECMO. Eight patients were also inserted with an IABP in addition to VA-ECMO (two patients in the survival group (IABP was placed before ECMO), and six patients in the non-survival group received IABP (4 patients before ECMO and 2 patients during ECMO support)). The baseline characteristics, baseline global hemodynamics, and microcirculatory parameters are shown in Table 1. The median ages of the patients in the survivor and non-survivor groups were 51 (range 23–70) and 51 (20–67), respectively. The number of males was higher in the survivor group (n = 12; 80 %) compared to the non-survivor group (n = 4; 44 %). The APACHE II scores (31 (19–45) and 34 (29–44), respectively), SOFA scores (10 (4–20) and 12 (2–15), respectively), and systemic hemodynamics were not significantly different between the groups. The use of vasopressors and inotropic drug doses and blood transfusions did not statistically differ between groups on the first day of VA-ECMO or during VA-ECMO (Additional file 2: Table S6). Systemic hemodynamic variables, such as the lactate and hemoglobin values at the microcirculatory measurement time points, did not differ between survivors and non-survivors (Additional file 2: Table S6 and Additional file 3: cardiac output ROC, curve 5). While the cardiac output at baseline was statistically significantly different between the groups, the predictive value for ICU survival is less significant than the PVD measured sublingually. The cardiac function assessed by echocardiography was comparable between the groups, except for the LVEF 20 (8–55) and 10 (7–20); p = 0.010). The heart function biomarkers also showed no differences between the survivors and non-survivors. However, the baseline troponin levels at the initiation of VA-ECMO (1683 (45–42,813) ng/L and 13,369 (215–89,641) ng/L; p = 0.030) were significantly higher in the non-survivors. The ICU and hospital durations of stay were significantly different between the groups (18 (6–65) and 5 (2–36); and 22 (6–72) and 5 (2–36) days for survivors and non-survivors, respectively). The total number of days on VA-ECMO was similar for the survivor and non-survivor groups.
As shown in Fig. 1, the microcirculatory parameters at the initiation of VA-ECMO were significantly lower in the patients who subsequently did not survive compared to those who survived. The comparisons of measured microcirculatory parameters are shown for all time points in Additional file 4: Table S2. The microcirculatory parameters at T1 (i.e., the TVD, PVD, and PPV values) were significantly different between the survivor and non-survivor groups (all vessels p = 0.008, p = 0.001, and p = 0.044, respectively; small vessels p = 0.009, p = 0.003, and p = 0.038, respectively). The microcirculatory values decreased in both groups 3 days after the initiation of VA-ECMO at T2 in parallel with a reduction in arterial Hb, which is associated with hemodilution. Although the microcirculatory parameters of the non-survivor group somewhat recovered at day 6 (T3), they remained significantly lower than those in the survivor group (Fig. 2; Additional file 5: Table S3). All the non-survivors passed away while on VA-ECMO, whereas additional microcirculation was observed in the survivors following weaning from VA-ECMO. The microcirculatory parameters of the survivors then became slightly lower upon removal of VA-ECMO compared to their values while still on VA-ECMO (Fig. 2). Notably, the microcirculatory parameter values did not significantly change in time when on VA-ECMO. This effect was observed in both the survivors and non-survivors (Additional file 5: Table S3) and is shown in Fig. 3, where no difference in the VA-ECMO blood flow was observed between the initiation of VA-ECMO and all the other time points between the both groups. None of the surviving patients were bridged to a cardiac transplantation or LVAD in this period.
Fig. 1.
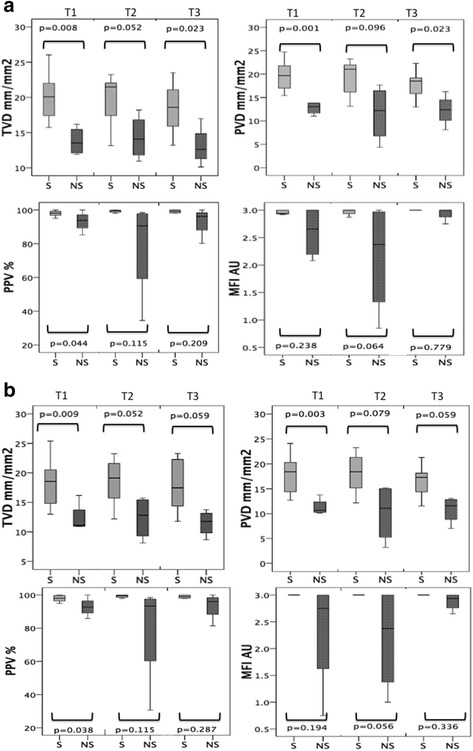
a Microcirculatory measurements showing the median and interquartile range between the survivors (S) and non-survivors (NS) for a all vessels and b small vessels at the initiation of the VA-ECMO insertion (T1), 48–72 h after VA-ECMO initiation (T2), and 5–6 days after VA-ECMO initiation (T3). AU arbitrary units, MFI microvascular flow index, PPV proportion of perfused vessels, PVD perfused vessel density, TVD total vessel density
Fig. 2.
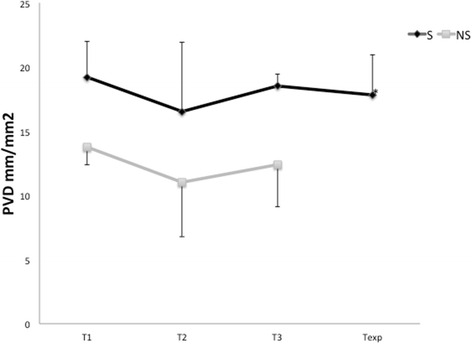
Example of change in the perfused vessel density (PVD) parameters in the survivor (S) and non-survivor (NS) groups at the following time points: initiation of the VA-ECMO insertion (T1), 48–72 h after VA-ECMO initiation (T2), 5–6 days after VA-ECMO initiation (T3), and after VA-ECMO explantation (Texp). The medians are depicted
Fig. 3.
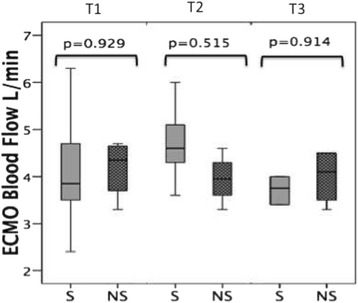
VA-ECMO blood flow showing the median and interquartile range between the survivors (S) and non-survivors (NS) at the initiation of the VA-ECMO insertion (T1), 48–72 h after VA-ECMO initiation (T2), and 5–6 days after VA-ECMO initiation (T3). ECMO extracorporeal membrane oxygenation
To compare the sensitivity and specificity of the microcirculatory parameters for PVD in all vessels, LVEF, HsTnT, and lactate at the moment of initiation of VA-ECMO and at T1 were evaluated with ROC curve analysis to differentiate these values between the survivors and non-survivors (Fig. 4). The areas under the ROC curves (and the 95 % confidence interval) were 0.908 (0.772–1.0), 0.847 (0.672–1.0), 0.755 (0.524–0.986), and 0.556 (0.300–0.813) in the survivors and non-survivors, respectively. According to the ROC curve analysis, the threshold value of PVD for all the vessels was 15.2 (mm/mm2) and was found to predict ICU survival (sensitivity 88.9 %, specificity 86.7 %, positive predictive value 80 %, negative predictive value 92.9 %, and Youden index 0.75; Additional file 6: Table S4, Additional file 7, Additional file 8).
Fig. 4.
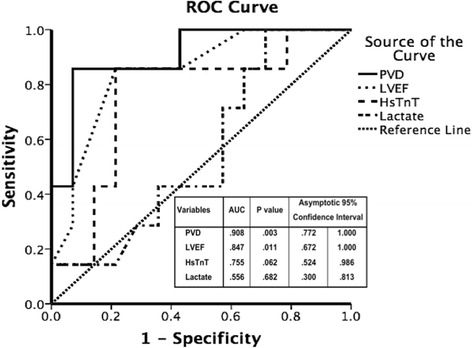
Receiver operating characteristics (ROC) curves showing the relationship between sensitivity and 1 – specificity in determining the perfused vessel density (PVD) all vessels, high sensitive troponin T (HsTnT), lactate, and left ventricular ejection fraction (LVEF) to predict ICU survival
Additional file 7.
Clip 1 A surviving patient’s microcirculatory image clip recorded at the initiation of the VA-ECMO insertion (T1). (MOV 4513 kb)
Additional file 8.
Clip 2 A non-surviving patient’s microcirculatory image clip recorded at the initiation of the VA-ECMO insertion (T1). (MOV 3850 kb)
Discussion
This prospective, observational, single-center study on patients with cardiogenic shock requiring VA-ECMO circulatory support showed that ICU survival could be predicted by observation of poor microcirculatory function at the initiation of VA-ECMO. Our study furthermore showed that VA-ECMO in itself did not change the physiological condition of the microcirculation throughout the course of VA-ECMO. These results suggest that assessment of sublingual microcirculation at initiation of VA-ECMO can be used to predict survival. While most of the differences are observed at baseline, changes over time seem less relevant. This seems to reflect more what occurred before, rather than during, ECMO. It also characterizes VA-ECMO as a bridge-to-treat procedure not contributing to resolving cardiac dysfunction following CS. From these considerations it could be argued that our results lay the foundation for introducing new adjunct therapeutic modalities aimed at improving microcirculatory function during the course of VA-ECMO, which may improve the chances of successful weaning from VA-ECMO and survival. Finally, our study suggests that microcirculatory monitoring could provide an easy tool to help the intensivist make the clinical decision to initiate or escalate further treatment options in patients with cardiogenic shock.
To date, several studies have evaluated predictors of outcome at the time of VA-ECMO initiation for CS. Schmidt et al. [28] described the value of pre-VA-ECMO implantation factors for predicting survival in cardiogenic shock patients receiving VA-ECMO on which they formulated a survival after VA-ECMO (SAVE) score. They found that this SAVE score could predict survival in these patients. Van Genderen et al. [29] investigated sublingual microcirculatory and peripheral tissue perfusion parameters in relation to systemic hemodynamics during and after therapeutic hypothermia following out-of-hospital cardiac arrest. They found that microcirculatory parameters were significantly lower in non-survivors at hospital admission and after rewarming than survivors. Microcirculatory alterations were also associated with organ failure and death, independent of changes in systemic hemodynamic parameters [29]. Aissaoui et al. [30] investigated predictors of successful VA-ECMO weaning after assistance for refractory CS. They described a number of echo criteria predictive for successful weaning. However, their study did not describe any predictors for patient survival. In this study, we found that baseline perfused vessel density is the best microcirculatory parameter predictive of ICU survival. Observation of low PVD measurements at initiation of VA-ECMO could lead to earlier initiation of advanced therapies such as LVAD or heart transplantations.
Another study we performed in pediatric ECMO patients [31] showed that patients who responded to ECMO with improved microcirculation went on to survive, whereas those that did not failed to survive.
A surrogate biomarker for left ventricular function was measured in our study. HsTnT was also found to be a good predictor for survival in the ROC analysis we performed. The study by Luyt et al. [32] assessed the predicative value of biomarkers for the prediction of cardiac recovery in patients on VA-ECMO who had not been investigated for HsTnT. However, their study investigated troponin Ic, which may have been the reason why they had found no relationship between cardiac biomarkers and cardiac recovery in VA-ECMO. Apart from HsTnT, LVEF was found to be the second best predictor for survival in the ROC analysis we performed. However, after initiating VA-ECMO, the heart is artificially bypassed and the cardiac output and ejection fraction are influenced by VA-ECMO. Thus, the echocardiography is affected by several factors such as aortic valve regurgitation, the aortic valve opening ratio, afterload change, and the VA-ECMO flow. In addition, transthoracic echocardiography is poor in quality, and transesophageal echocardiography is a less reliable ejection fraction measurement during VA-ECMO, which affects the measurement of the LVEF. However, even though the above parameters were related to the outcome to various degrees, our ROC analysis identified microcirculatory alterations measured on day 1 as having the highest specificity and sensitivity of all parameters measured for predicting mortality in the ICU.
In our observational study, volume status was mainly assessed by echocardiography and general hemodynamic parameters, such as the central venous pressure (CVP). We found no statistical significance between the two groups regarding the associations between fluid balance and CVP in the first 24 h and the outcome. It is worth pointing out, however, that even though the assessment of volume in non-VA-ECMO critically ill patients has extensively been studied and included in guidelines, accurate assessments of volume status in patients on ECMO with altered circulatory conditions (e.g., the presence of extracorporeal circulation and by-passing different parts of the endogenous circulation) is a subject in need of further investigation.
Our findings highlight the fact that ECMO is not a therapy that improves patient outcomes but rather it is a bridge to treatment. Our findings therefore identify a mean of 5 days as a window for treatment. It also means that additional therapeutic modalities targeting the microcirculation need to be developed for inclusion in the ECMO procedure, which may then elevate ECMO from the therapeutic modality status as a bridge to a treatment.
In the course of VA-ECMO, systemic perfusion is switched to an extracorporeal circulation, the blood is exposed to non-biocompatible polymers that can activate blood cells and the coagulation system, and it changes from a pulsatile to a non-pulsatile flow [33, 34]. While IABP may provide a pulsatile flow, many studies have shown that this was of no benefit to the microcirculation [20, 35, 36]. All of these factors might have detrimental effects on the microcirculation and may explain why the microcirculatory parameters decreased from T1 to T2 in both groups. At the same time, even though the VA-ECMO flows were similar in both groups, microcirculatory parameters were absolutely different at all time points. This means that there was no hemodynamic coherence between macrohemodynamics and the microcirculation and that microcirculatory alterations manifest themselves independently of systemic hemodynamics [37]. Furthermore, differences in microcirculatory measurements between the groups were observed at all time points, while persistent microcirculatory derangement was observed in the non-survivor group. In addition, our study showed that microcirculation remains high after VA-ECMO explantation in the survivor group.
Our study, however, has some limitations. First, it was single-center study and concerned a relatively small number of patients. However, to the best of our knowledge, this is the largest series of patients investigated on this topic to date. Second, we included patients who presented with different kinds of VA-ECMO cardiac injury. Therefore, these patients may have had different microcirculation properties prior to presentation. Another limitation was the exclusion of patients dying within 24 h. Fourth, apart from global hemodynamics, VA-ECMO can adversely affect the microcirculation for many reasons such as hemodilution, hemolysis, coagulopathy, non-pulsatile flow, and hypothermia [38]. T1 measurements were performed within 24 h after the initiation of VA-ECMO, which can be considered a long time period.
The last, but still controversial, limitation is the length of recording sequences used in our study. The use of a new generation hand-held microscopy device may have advantages, but also may have disadvantages. The advantage of the Cytocam IDF camera used in this study is that images are able to observe more capillaries (30 %) than the previous-generation sidestream dark field (SDF) cameras due to improved optics [22, 24]. In combination with a larger field of view (1.55 × 1.16 mm = about three times larger than the field of view of previous devices) of the new generation camera, it could be argued that this camera provides more accurate information concerning microcirculation alterations then previous-generation SDF camera. On the other hand, default Cytocam measurements take between 3- and 5-s recordings which have been found to be of sufficient length in a large number of recent studies [22, 39–42]. A recent review on microcirculation measurements by Massey and Shapiro [43] also recommended that lengths of between 3 and 5 s are sufficient. However, this length may be considered too short when comparing it to video sequences of 20 s recommended for SDF devices in the consensus of 2006 [25], although this recommendation was based on opinion and has never been validated in the literature. Even for SDF images, it has suggested in a recent experimental study by Kildal et al. [44] in pigs that measurements up to 10 min provide extra information. The only way to establish the sufficient length of the video sequences is to demonstrate statistical significance between the groups, which we did for this study. Whether such a length is sufficient for other applications, however, has to be investigated.
Conclusion
In our study, altered baseline perfused sublingual microcirculatory vessel density was found to be related to ICU survival in cardiogenic shock patients treated with VA-ECMO. To our knowledge, this is the first study to describe such an application in this group of patients.
We conclude that monitoring sublingual microcirculation could be used for earlier identification of patients who will survive, as well as for exploring the usefulness of earlier consideration of the utilization of advanced therapies. Our expectation is that such assessment may be of value in the early identification of patients at a high risk of death, or those with poor recovery, and to potentially develop microcirculatory-targeted recovery strategies. Further studies with a larger number of patients and the same etiology of CS are needed.
Acknowledgements
We would like to thank Dr. R.T. van Domburg, PhD (Department of Cardiology, Erasmus MC Rotterdam), for his statistical advice.
Authors’ contributions
AK contributed to the writing this manuscript, carried out the analysis of the microcirculatory measurements at the bedside, and drafted the manuscript. He analyzed the clips independently. He performed the statistical analysis under supervision of Dr. R.T. van Domburg, and designed the tables and the figures. SA contributed to the writing of this manuscript, analyzed the echocardiograms and the data from laboratory and global hemodynamics, included the patients into the study for subsequent measurements at the bedside, and revised the manuscript. He helped design the tables and figures after statistical analysis was performed under supervision of Dr. R.T. van Domburg. DdRM participated in the style and writing the discussion part of the manuscript, the interpretation of the results at the beside, and revising the manuscript. AS participated in the design of the study, contributed to interpretation of the data, and revised the manuscript. KC participated in the design of the study, interpretations of the echocardiography data, and participated in the writing of the manuscript. RJvT participated in the design of the study, the interpretation of the data, and revision of the manuscript. EAD participated in coordination during inclusion of the patients in the ICU, and revision of the manuscript. WdW participated in analysis of the clips and microcirculation measurements at the bedside. FZ helped to draft the manuscript and revised the manuscript critically for important intellectual content. DG made contributions to the conception of the study, and participated in its design and coordination, and helped to draft the manuscript. CI conceived the study design and contributed during inclusion, analyzing, discussion, interpretations of the microcirculatory data, and writing and revising this manuscript. All authors read and approved the final manuscript.
Competing interests
CI has developed SDF imaging and is listed as an inventor for related patents commercialized by Micro Vision Medical (MVM) under a license from the Academic Medical Center (AMC). He has been a consultant for MVM in the past. Although he has not been involved with the company for the last 5 years, he is still a shareholder in the company. Braedius Medical, a company owned by a relative of CI, has developed and designed a hand-held microscope called CytoCam-IDF imaging. CI has no financial relationship with Braedius Medical and has never owned shares or received consultancy or speaker fees from Braedius Medical. All other authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from either patients or patient representatives.
Ethical approval and consent to participate
The protocol was approved by the Medical Ethical Committee of our institution (NL459115.078.13).
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- AU
Arbitrary units
- CK
Creatine kinase
- CS
Cardiogenic shock
- CVP
Central venous pressure
- ECMO
extracorporeal membrane oxygenation
- free-Hb
Free hemoglobin
- Hb
Hemoglobin
- HsTnT
High sensitive troponin T
- Htc
Hematocrit
- IABP
Intra-aortic balloon pump
- ICU
Intensive care unit
- IDF
Incident dark field
- LDH
Lactate dehydrogenase
- LVAD
Left ventricular-assist device
- LVEF
Left ventricular ejection fraction
- MFI
Microvascular flow index
- PPV
Proportion of perfused vessels
- PVD
Perfused vessel density
- ROC
Receiver operating characteristic curve
- SAVE
Survival after VA-ECMO
- SDF
Sidestream dark field
- SOFA
Sequential Organ Failure Assessment
- TVD
Total vessel density
- VA-ECMO
Veno-arterial extracorporeal membrane oxygenation
Additional files
Numbers of video clips and numbers of patients included at each time point. (DOCX 16 kb)
The median values of administered drugs, blood transfusions, and laboratory parameters at VA-ECMO insertion within 24 h (T1), 2–3 days after initiation of VA-ECMO (T2), and 5–6 days after initiation of VA-ECMO (T3). (DOCX 15 kb)
Cardiac output ROC curve 5. (TIFF 1521 kb)
Comparison of the measured microcirculatory parameters TVD all vessel, TVD small vessel, PVD all vessel, PVD small vessel, PPV all vessels, PPV small vessel, MFI all vessel, and MFI small vessel at the initiation of the VA-ECMO insertion (T1), 48–72 h after VA-ECMO initiation (T2), and 5–6 days after (T3) between the survivors and non-survivors. (DOCX 16 kb)
Changes in the microcirculatory parameters in the survivor and non-survivor groups at the following time points: initiation of the VA-ECMO insertion (T1); 48–72 h after VA-ECMO initiation (T2); and 5–6 days after (T3). (DOCX 15 kb)
Sensitivity, specificity, positive predictive value, and negative predictive value for PVD all vessels, HsTnT, and LVEF to predict the ICU survival. (DOCX 14 kb)
Contributor Information
Atila Kara, Phone: +31622467111, Email: atila.kara@hacettepe.edu.tr, Email: a.kara@erasmusmc.nl.
Sakir Akin, Email: s.akin@erasmusmc.nl.
Dinis dos Reis Miranda, Email: d.dosreismiranda@gmail.com.
Ard Struijs, Email: a.struijs@planet.nl.
Kadir Caliskan, Email: k.caliskan@erasmusmc.nl.
Robert J. van Thiel, Email: r.vanthiel@erasmusmc.nl
Eric A. Dubois, Email: e.dubois@erasmusmc.nl
Wouter de Wilde, Email: dewildeww@gmail.com.
Felix Zijlstra, Email: f.zijlstra.1@erasmusmc.nl.
Diederik Gommers, Email: d.gommers@erasmusmc.nl.
Can Ince, Email: c.ince@erasmusmc.nl.
References
- 1.Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26(4):384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 3.Chen JS, Ko WJ, Yu HY, Lai LP, Huang SC, Chi NH, et al. Analysis of the outcome for patients experiencing myocardial infarction and cardiopulmonary resuscitation refractory to conventional therapies necessitating extracorporeal life support rescue. Crit Care Med. 2006;34(4):950–7. doi: 10.1097/01.CCM.0000206103.35460.1F. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz B, Mair P, Margreiter J, Pomaroli A, Hoermann C, Bonatti J, et al. Experience with percutaneous venoarterial cardiopulmonary bypass for emergency circulatory support. Crit Care Med. 2003;31(3):758–64. doi: 10.1097/01.CCM.0000053522.55711.E3. [DOI] [PubMed] [Google Scholar]
- 5.Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005;26(20):2185–92. doi: 10.1093/eurheartj/ehi411. [DOI] [PubMed] [Google Scholar]
- 6.Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010;139(2):302–11. 11 e1. doi: 10.1016/j.jtcvs.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 7.Massetti M, Tasle M, Le Page O, Deredec R, Babatasi G, Buklas D, et al. Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg. 2005;79(1):178–83. doi: 10.1016/j.athoracsur.2004.06.095. [DOI] [PubMed] [Google Scholar]
- 8.Chen YS, Chao A, Yu HY, Ko WJ, Wu IH, Chen RJ, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41(2):197–203. doi: 10.1016/S0735-1097(02)02716-X. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell BG, Powers AJ, Sheikh AY, Lee PH, Lobato RL, Wong JK. Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the Nationwide Inpatient Sample 1998–2009. J Thorac Cardiovasc Surg. 2014;148(2):416–21. e1. doi: 10.1016/j.jtcvs.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64(14):1407–15. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 11.Brugts JJ, Caliskan K. Short-term mechanical circulatory support by veno-arterial extracorporeal membrane oxygenation in the management of cardiogenic shock and end-stage heart failure. Expert Rev Cardiovasc Ther. 2014;12(2):145–53. doi: 10.1586/14779072.2014.880051. [DOI] [PubMed] [Google Scholar]
- 12.Buijs EA, Houmes RJ, Rizopoulos D, Wildschut ED, Reiss IK, Ince C, et al. Arterial lactate for predicting mortality in children requiring extracorporeal membrane oxygenation. Minerva Anestesiol. 2014;80(12):1282–93. [PubMed] [Google Scholar]
- 13.Park SJ, Kim SP, Kim JB, Jung SH, Choo SJ, Chung CH, et al. Blood lactate level during extracorporeal life support as a surrogate marker for survival. J Thorac Cardiovasc Surg. 2014;148(2):714–20. doi: 10.1016/j.jtcvs.2014.02.078. [DOI] [PubMed] [Google Scholar]
- 14.Li CL, Wang H, Jia M, Ma N, Meng X, Hou XT. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: a retrospective observational study. J Thorac Cardiovasc Surg. 2015;149(5):1445–50. doi: 10.1016/j.jtcvs.2014.11.052. [DOI] [PubMed] [Google Scholar]
- 15.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–31. doi: 10.1097/01.CCM.0000138558.16257.3F. [DOI] [PubMed] [Google Scholar]
- 16.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166(1):98–104. doi: 10.1164/rccm.200109-016OC. [DOI] [PubMed] [Google Scholar]
- 17.Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49(1):88–98. e1-2. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 18.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147(1):91–9. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 19.den Uil CA, Lagrand WK, van der Ent M, Jewbali LS, Brugts JJ, Spronk PE, et al. The effects of intra-aortic balloon pump support on macrocirculation and tissue microcirculation in patients with cardiogenic shock. Cardiology. 2009;114(1):42–6. doi: 10.1159/000212060. [DOI] [PubMed] [Google Scholar]
- 20.Petroni T, Harrois A, Amour J, Lebreton G, Brechot N, Tanaka S, et al. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Crit Care Med. 2014;42(9):2075–82. doi: 10.1097/CCM.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 21.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365(20):1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 22.Aykut G, Veenstra G, Scorcella C, Ince C, Boerma C. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp. 2015;3(1):40. doi: 10.1186/s40635-015-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert-Kawai E, Coppel J, Bountziouka V, Ince C, Martin D, Caudwell Xtreme E, et al. A comparison of the quality of image acquisition between the incident dark field and sidestream dark field video-microscopes. BMC Med Imaging. 2016;16(1):10. doi: 10.1186/s12880-015-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Elteren HA, Ince C, Tibboel D, Reiss IK, de Jonge RC. Cutaneous microcirculation in preterm neonates: comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging. J Clin Monit Comput. 2015;29(5):543–8. doi: 10.1007/s10877-015-9708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Backer D, Hollenberg S, Boerma C, Goedhart P, Buchele G, Ospina-Tascon G, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11(5):R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massey MJ, Larochelle E, Najarro G, Karmacharla A, Arnold R, Trzeciak S, et al. The microcirculation image quality score: development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J Crit Care. 2013;28(6):913–7. doi: 10.1016/j.jcrc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Dobbe JG, Streekstra GJ, Atasever B, van Zijderveld R, Ince C. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput. 2008;46(7):659–70. doi: 10.1007/s11517-008-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36(33):2246–56. doi: 10.1093/eurheartj/ehv194. [DOI] [PubMed] [Google Scholar]
- 29.van Genderen ME, Lima A, Akkerhuis M, Bakker J, van Bommel J. Persistent peripheral and microcirculatory perfusion alterations after out-of-hospital cardiac arrest are associated with poor survival. Crit Care Med. 2012;40(8):2287–94. doi: 10.1097/CCM.0b013e31825333b2. [DOI] [PubMed] [Google Scholar]
- 30.Aissaoui N, Luyt CE, Leprince P, Trouillet JL, Leger P, Pavie A, et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2011;37(11):1738–45. doi: 10.1007/s00134-011-2358-2. [DOI] [PubMed] [Google Scholar]
- 31.Top AP, Ince C, van Dijk M, Tibboel D. Changes in buccal microcirculation following extracorporeal membrane oxygenation in term neonates with severe respiratory failure. Crit Care Med. 2009;37(3):1121–4. doi: 10.1097/CCM.0b013e3181962a5f. [DOI] [PubMed] [Google Scholar]
- 32.Luyt CE, Landivier A, Leprince P, Bernard M, Pavie A, Chastre J, et al. Usefulness of cardiac biomarkers to predict cardiac recovery in patients on extracorporeal membrane oxygenation support for refractory cardiogenic shock. J Crit Care. 2012;27(5):524 e7–14. doi: 10.1016/j.jcrc.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Kolackova M, Krejsek J, Svitek V, Kunes P, Mandak J, Holubcova Z, et al. The effect of conventional and mini-invasive cardiopulmonary bypass on neutrophil activation in patients undergoing coronary artery bypass grafting. Mediators Inflamm. 2012;2012:152895. doi: 10.1155/2012/152895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neil MP, Fleming JC, Badhwar A, Guo LR. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: microcirculatory and systemic effects. Ann Thorac Surg. 2012;94(6):2046–53. doi: 10.1016/j.athoracsur.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 35.Lundemoen S, Kvalheim VL, Mongstad A, Andersen KS, Grong K, Husby P. Microvascular fluid exchange during pulsatile cardiopulmonary bypass perfusion with the combined use of a nonpulsatile pump and intra-aortic balloon pump. J Thorac Cardiovasc Surg. 2013;146(5):1275–82. doi: 10.1016/j.jtcvs.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Jung C, Fuernau G, de Waha S, Eitel I, Desch S, Schuler G, et al. Intraaortic balloon counterpulsation and microcirculation in cardiogenic shock complicating myocardial infarction: an IABP-SHOCK II substudy. Clin Res Cardiol. 2015;104(8):679–87. doi: 10.1007/s00392-015-0833-4. [DOI] [PubMed] [Google Scholar]
- 37.Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(Suppl 3):S8. doi: 10.1186/cc14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kara A, Akin S, Ince C. The response of the microcirculation to cardiac surgery. Curr Opin Anaesthesiol. 2016;29(1):85–93. doi: 10.1097/ACO.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 39.Hutchings S, Watts S, Kirkman E. The Cytocam video microscope. A new method for visualising the microcirculation using incident dark field technology. Clin Hemorheol Microcirc. 2016;62(3):261–71. doi: 10.3233/CH-152013. [DOI] [PubMed] [Google Scholar]
- 40.van Elteren HA, Ince C, Tibboel D, Reiss IK, de Jonge RC. Cutaneous microcirculation in preterm neonates: comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging. J Clin Monit Comput. 2015;29(5):543–48. [DOI] [PMC free article] [PubMed]
- 41.van Elteren HA, de Jonge RC, van Rosmalen J, Ince C, Reiss IK. Adaptation of the cutaneous microcirculation in preterm neonates. Microcirculation. 2016;23(6):468–74. doi: 10.1111/micc.12295. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka S, Harrois A, Nicolai C, Flores M, Hamada S, Vicaut E, et al. Qualitative real-time analysis by nurses of sublingual microcirculation in intensive care unit: the MICRONURSE study. Crit Care. 2015;19:388. doi: 10.1186/s13054-015-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massey MJ, Shapiro NI. A guide to human in vivo microcirculatory flow image analysis. Crit Care. 2016;20:35. doi: 10.1186/s13054-016-1213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kildal AB, Stenberg TA, Sanden E, Myrmel T, How OJ. Prolonged observation time reveals temporal fluctuations in the sublingual microcirculation in pigs given arginine vasopressin. J Appl Physiol (1985) 2015;118(8):965–70. doi: 10.1152/japplphysiol.00900.2014. [DOI] [PubMed] [Google Scholar]


