SUMMARY
Aberrant hexanucleotide repeat expansions in C9orf72 are the most common genetic change underlying amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). RNA transcripts containing these expansions undergo repeat associated non-ATG (RAN) translation to form five dipeptide repeat proteins (DPRs). DPRs are found as aggregates throughout the CNS of C9orf72-ALS/FTD patients and some cause degeneration when expressed in vitro in neuronal cultures and in vivo in animal models. The spread of characteristic disease-related proteins drives the progression of pathology in many neurodegenerative diseases. While DPR toxic mechanisms continue to be investigated, the potential for DPRs to spread has yet to be determined. Utilizing different experimental cell culture platforms, including spinal motor neurons derived from induced pluripotent stem cells from C9orf72-ALS patients, we found evidence for cell-to-cell spreading of DPRs via exosome-dependent and independent pathways, which may potentially be relevant to disease.
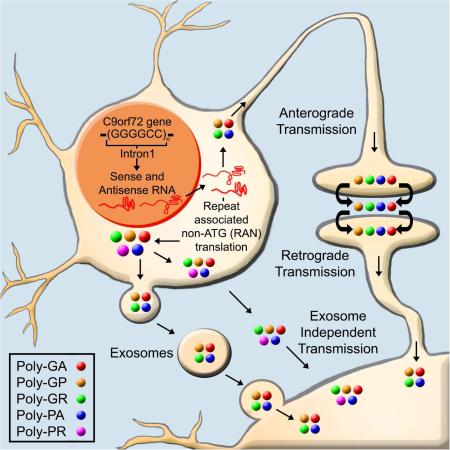
Graphical abstract
eTOC:
Westergard et al. examine the cell-to-cell spread of dipeptide repeat proteins related to C9orf72-ALS/FTD in vitro and in animal models. They suggest that transcellular transmission may explain the clustered expression pattern seen in human post-mortem CNS areas as well as the progressive neurodegeneration of these diseases.
INTRODUCTION
Abnormal intronic hexanucleotide (GGGGCC/CCGGGG) repeat expansions (HREs) in the C9orf72 gene are the most common genetic cause for both amyotrophic lateral sclerosis (ALS), a motor neuron degenerative disease, and frontotemporal dementia (FTD), a form of dementia characterized by selective deterioration of frontal and temporal lobes (DeJesus-Hernandez et al., 2011; Renton et al., 2011). The HREs result in three potential pathogenic hallmarks of disease. First, decreased C9orf72 mRNA expression levels in patients suggest a loss-of-function mechanism (Ciura et al., 2013; Therrien et al., 2013). Second, RNA transcripts from the HREs potentially gain a toxic function by sequestering RNA-binding proteins in foci (Gendron et al., 2014) and/or inhibiting transcription through formation of RNA-DNA hybrids (Gitler and Tsuiji, 2016). Lastly, both sense and antisense RNA transcripts can undergo non-canonical repeat-associated non-ATG translation (RAN-T) generating five potentially toxic dipeptide repeat protein species (DPRs): poly(glycine-alanine, GA), poly(glycine-proline, GP), poly(glycine-arginine, GR), poly(proline-alanine, PA), and poly(proline-arginine, PR)(Gitler and Tsuiji, 2016).
DPR inclusions were reported in different CNS areas of C9orf72 ALS/FTD patients (Ash et al., 2013; Gendron et al., 2013; Mori et al., 2013a; Mori et al., 2013b; Zu et al., 2013). Additionally, pervasive DPR pathology is found during presentation of initial symptoms of disease, preceding onset of other pathology such as TDP43 inclusions (Baborie et al., 2015; Proudfoot et al., 2014). DPRs alter cellular functions and induce toxicity in different ways in various models. In primary neurons and fly models, the arginine-rich DPRs display the highest toxicity. Poly(GR), which localizes in the cytoplasm and aggregates in the nucleus, and poly(PR), which exclusively aggregates in the nucleus, trigger nucleolar stress, nuclear transport defects, RNA processing alterations, and protein mislocalization (Gitler and Tsuiji, 2016). Poly(GA) is also toxic through proteasome impairment, aggregation of Unc119, and impairment of HR23 and nucleocytoplasmic transport proteins (May et al., 2014; Zhang et al., 2016; Zhang et al., 2014). In contrast, marginal or no toxicity has been associated with poly(GP) and poly(PA), respectively (Wen et al., 2014; Zu et al., 2013). These findings have solidified the importance of DPR pathology in C9orf72-ALS/FTD.
The progression of many neurodegenerative diseases, including ALS, is thought to be driven by cell-to-cell transmission of disease-related proteins, which leads to seeded aggregation and template directed misfolding. A growing body of evidence has uncovered a propensity for disease relevant proteins such as poly-glutamine, α-synuclein, β-amyloid, SOD1, and TDP43, to be transmitted from cell-to-cell and to seed template nucleation (Feiler et al., 2015; Gallegos et al., 2015; Kanouchi et al., 2012; Nath et al., 2012; Ren et al., 2009; Silverman et al., 2016). Mechanisms of transmission involve secretion of exosomes (Bellingham et al., 2012; Danzer et al., 2012; Pant et al., 2012; Saman et al., 2012), tunneling nanotubes, hemi-channels between cells, exocytosis and endocytosis of proteins, and phagocytosis of infected cells or cellular debris (Costanzo and Zurzolo, 2013; Gallegos et al., 2015). These protein spreading modalities are now commonly interpreted as a mechanism underlying the progressive nature of many neurodegenerative diseases.
While progress is currently being made on mechanisms behind DPR toxicity, their potential to transmit between cells is untested thus far. DPR transmission was recently hinted for a low repeat length, synthetic poly(GA) using N2a neuroblastoma cells (Chang et al., 2016). Poly(GP) has also been detected in patients’ CSF, suggesting active secretion (Su et al., 2014). Additionally, neuropathological analysis of C9orf72-ALS/FTD autopsy brains demonstrated two types of aggregation and localization patterns for cells containing DPRs: high-density clusters or isolated cells (Zu et al., 2013). This not only suggests specific foci where RAN-T occurs, but raises the hypothesis that DPRs produced in these foci could also spread to neighboring areas. Furthermore, DPRs are detected as insoluble aggregates in human tissue and most form aggregates in vitro, a common pathological hallmark of disease-relevant proteins found to spread (Brettschneider et al., 2015). Using live-cell microscopy, we observed that toxic poly(PR) aggregates persist in the dish long after the expressing neurons have broke up and died (Wen et al., 2014), again suggesting that these aggregates might be taken up by other cells. Based on these lines of evidence, we hypothesized that DPRs could undergo cell-to-cell transmission between CNS-resident cell types. Using a variety of cell culture platforms, we report here evidence for cell-to-cell transmission in an exosome-dependent and independent fashion for all C9-DPRs.
RESULTS
DPRs can be transmitted to neurons and glia cells
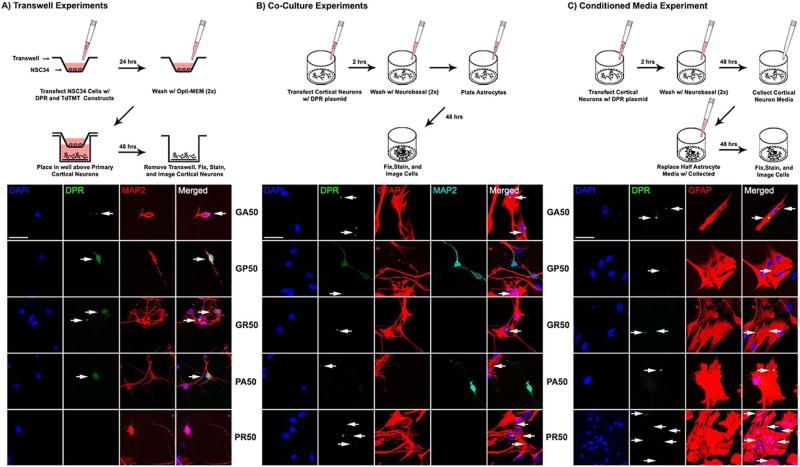
To test in vitro the hypothesis that DPRs are transmissible, we utilized constructs encoding GFP tagged poly(GA)50, poly(GP)50, poly(GR)50, poly(PA)50, and poly(PR)50 dipeptides (Wen et al., 2014). Our first approach was to use motor neuron-like NSC34 cells cultured in transwells on a mesh surface (0.4 μm pores; Figure 1A), and transfected with GFP or GFP-DPR encoding constructs. Twenty-four hours later, NSC34 cells were extensively washed and the transwells placed into wells plated with mature primary cortical neurons (DIV10). Forty-eight hours later, confocal microscopy analysis revealed the presence of DPRs in cortical neurons, suggesting that transmission occurred. Staining was absent from the GFP-only encoding group, indicating that the acquisition of DPRs was not due to unintended transfection from residual construct particles (Figure S1A). GA showed distinct cytoplasmic aggregates in recipient cortical neurons (~7% of total cortical neurons), whereas GR nuclear and cytoplasmic aggregates, in addition to diffuse localization, were detected in 6% of cortical neurons. GP and PA displayed both diffuse localization and cytoplasmic aggregates in recipient cortical neurons (~3% of total cortical neurons). Interestingly, there was no significant evidence of PR transmission to cortical neurons (Figure S1D).
Figure 1. In vitro transmission of DPRs from NSC34 cells to cortical neurons and from cortical neurons to astrocytes.
(A-C) Schematics of experimental workflow. (A) Lower panels: Transfected NSC34 cells transmitted DPRs to cortical neurons in a transwell system. All DPR species were detected in cortical neurons besides poly-PR. Nuclei were stained with DAPI (blue), green represents DPRs, red represents MAP2+ neurons. (B) Lower panels: Representative confocal images show transmission of all DPR species (GFP tagged) from cortical neurons to astrocytes. Cells were stained with DAPI (blue for nuclei), DPRs (GFP green), GFAP (red), and MAP2 (cyan). There was negligible MAP2 staining in GA, GR, and PR groups, suggestive of extensive neuronal death. (C) Lower panels: Cells were stained with DAPI (blue for nuclei), DPRs (GFP green), and GFAP (red) for astrocytes. Small or low fluorescent aggregates are marked by arrows. All bars=50 μm.
It was reported that neurodegenerative disease-relevant proteins, such as TDP43, propagate to both neuron and glial cells (Brettschneider et al., 2015). Additionally, DPRs have been observed in ependymal and subependymal glial cells (Schludi et al., 2015). Seeking additional evidence of DPR transmission, we tested whether transmission of the different DPRs could occur between neurons and glial cells. Cortical neurons were transfected with GFP or GFP-DPR constructs and thoroughly washed 24 hours later when untreated primary astrocytes were seeded in the culture plate. Confocal microscopy analysis performed 48 hours later revealed DPR localization within astrocytes (Figure 1B), with absent GFP-only staining in astrocytes (Figure S1B). Quantification of DPR transmission revealed that in the co-culture cell-contact model, the percentage of cell-containing DPRs was ~2-fold higher compared to the transwell experiments (Figure S1E). GA presented as cytoplasmic aggregates, whereas GR and PR displayed as both cytoplasmic and nuclear aggregates. GP and PA exhibited both cytoplasmic aggregates and diffuse localization in astrocytes.
Utilizing a different experimental approach, conditioned medium (CM) from GFP or GFP-DPR expressing cortical neurons was transferred to cultured astrocytes. Neuron-to-astrocyte transmission was again observed, with similar DPR localization patterns in the recipient cells as reported in co-cultures (Figure 1C), and absent GFP only transmission (Figure S1C). However, quantification of transmission was less (6-8% of astrocytes displaying GA, GR and PR-positive aggregates and ~4% of astrocytes displaying cytoplasmic aggregates and diffuse localization of GP and PA), as reported in the co-culture cell-contact model, but similar to the transwell model (Figure S1F). DPR transmission via CM transfer occurred also to other glia cell types, such as microglia and mature oligodendrocytes (Figure S2). These observations provide further evidence for DPR transmission between CNS cell types.
Anterograde and retrograde transmission of DPRs
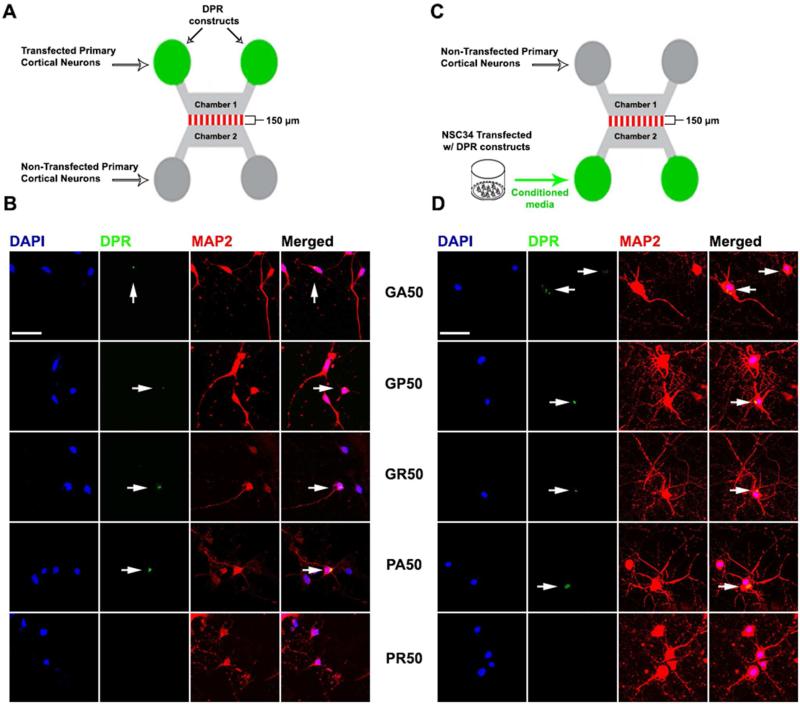
DPR aggregates are found throughout different CNS areas in high-density clusters and isolated cells (Zu et al., 2013). In a compartmentalized microfluidic culture system, both anterograde and retrograde transmission of TDP-43 was recently demonstrated. This could potentially explain the complex variable distribution of TDP-43 pathology in axonally connected distant brain regions (Feiler et al., 2015). We used a similar in vitro approach to test whether DPRs can potentially spread between neuronal networks. Cortical neurons were cultured in one of the microfluidic chambers (chamber #1) with their neurites made to extend across microgrooves to contact cortical neurons cultured in the opposite chamber (chamber #2) (Figure 2A). Axon projection directionality (chamber #1 to chamber #2) was driven by applying higher hydrostatic pressure in chamber #1. Once the microfluidic culture was established, higher hydrostatic pressure was applied to chamber #2 to impede anterograde transfer of solution and material, and the projecting cortical neurons in chamber #1 were transfected with DPR-encoding plasmids. Forty-eight hour post-transfection, presence of DPRs was detected in cortical neurons of chamber #2 (Figure 2B), indicating anterograde transfer of DPRs has occurred via the connecting neurites. GA, GP, and PA containing neurons displayed cytoplasmic aggregates, while GR containing neurons had both cytoplasmic and nuclear aggregates. We found no evidence for PR transmission, consistent with previous evidence with the transwell platform. Retrograde transmission was observed using a similar setup, but with the absence of cortical neurons in chamber #2 (Figure 2C). Upon establishment of the microfluidic culture, CM from DPR expressing NSC34 cells was transferred into chamber #2, while applying higher hydrostatic pressure in chamber #1 to block retrograde transfer of solution and material. Forty-eight hour later, presence of all DPRs except PR was detected in cortical neurons in chamber #1 (Figure 2D).
Figure 2. Anterograde and retrograde transmission of DPRs.
(A, C) Schematics of the experimental design. (B) Transfected cortical neurons in chamber #1 transmitted DPRs to cortical neurons in chamber #2. (D) Conditioned medium of DPR-expressing NSC34 cells added to chamber #2 transmitted DPRs to cortical neurons in chamber #1. All DPRs were detected in cortical neurons, except for PR. DAPI (blue), DPRs (green), MAP2 (red). DPR aggregates are marked by arrows. Bars=50 μm.
Exosome-dependent transmission of DPRs
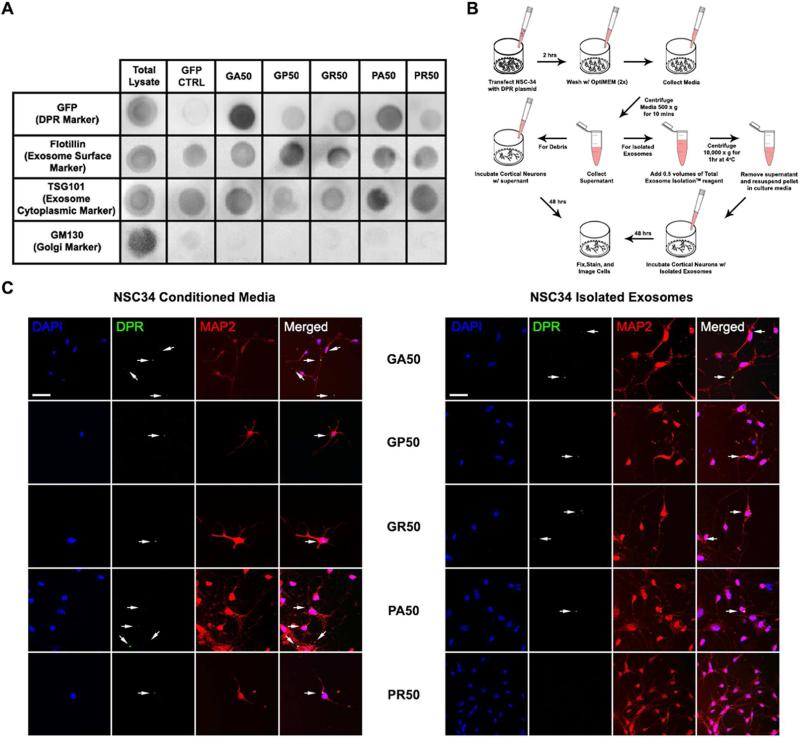
Cell-to-cell transmission of proteins may occur via different modalities, such as regulated secretion by exocytosis or release from dying cells (Costanzo and Zurzolo, 2013). Recently, packaging and release of microvesicles known as exosomes were implicated as a primary mode of transmission of proteins relevant in neurodegenerative disease (Bellingham et al., 2012; Danzer et al., 2012; Saman et al., 2012; Silverman et al., 2016). We demonstrated in the transwell and microfluidic systems that direct cell contact was not required for inter-cellular spreading of DPRs, suggesting that neuronal cells are also capable of releasing DPRs. To determine whether cell-contact independent DPRs transmission implicates release of DPR-containing exosomes, NSC34 cells were first transfected with DPR or GFP-encoding constructs and 48 hours later exosome particles were isolated and purified from the culture medium. Purity of the exosomal fraction was assessed by the enrichment of specific exosomal markers and absence of other vesicular types, such as those derived from the Golgi (GM130), endoplasmic reticulum (calnexin), and mitochondria (cytochrome C)(Figure 3A, Figure S3). Presence of DPRs in exosomes was assessed by dot blot analysis (Figure 3A, Figure S3). DPR immunopositive signals relative to markers of exosomes (i.e. flotillin, TSG101, and CD63)(Bellingham et al., 2012) revealed enrichment of GA, intermediate levels of PA and GR, low levels of GP, and very low to undetectable levels of PR.
Figure 3. Exosome-dependent and independent transmission of DPRs.
(A) Representative dot-blot of the exosomal fraction isolated from conditioned media of NSC34 cells transfected with different DPR-encoding constructs. DPRs were detected by GFP immunostaining. Flotillin and TSG101 were used as markers of the exosomal fraction. GM130 was used as marker of other membrane vesicle types. (B) Experimental workflow to compare DPR transmission from the exosomal fraction and total conditioned media. (C) Left: Representative confocal images show transmission of DPRs to cortical neurons through conditioned media. Right: Representative images show transmission of DPRs, except PR, to cortical neurons through isolated exosomes. DAPI (blue), DPRs (green), MAP2 (red). DPR aggregates are marked by arrows. Bars=50 μm.
To verify the transmission potential of DPR-containing exosomes, primary cortical neurons were incubated with DPR-enriched exosomal fractions isolated from CM of transfected NSC34 cells (Figure 3B). For comparison, a separate group of cortical neurons were incubated with unprocessed CM. In both conditions, GA, GP, GR, and PA were present with varying rates in the cortical neurons primarily as cytoplasmic aggregates indicating that neuronal uptake had occurred (Figure 3C, Figure S3B, Figure S3C). Interestingly, significant transmission of PR was only observed from the crude CM exposure, whereas incubation of neurons with the respective exosomal fraction showed no significant PR transmission.
Human iPSC-derived spinal motor neurons exhibit cell-to-cell transmission of DPRs
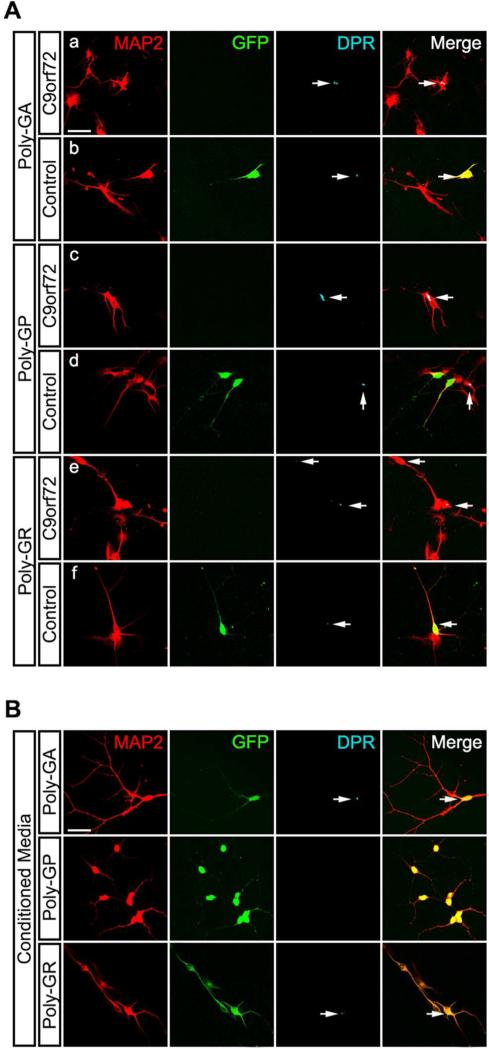
To establish whether DPR transmission also occurred in human neurons, the co-culture and CM experimental designs previously described was implemented by using human iPSC-derived spinal motor neurons (sMNs). C9orf72 (2 different iPSC lines, Target ALS) and control (1 iPSC line, Wicell) iPSC lines were differentiated into sMNs using a modified version of a protocol previously described (Maury et al., 2015) (Figure S4). The majority of differentiated neurons are ChAT and Hb9 positive. Control sMNs were transduced with a lentivirus expressing GFP under a CMV promoter for identification. We found evidence of expression of the sense strand DPRs (GA, GR and GP) in C9orf72 sMNs (Figure 4A, panel a, c, e). Expression of anti-sense DPRs (PA and PR) in sMNs were not detected under our culturing condition, hence we focused our analysis of GA, GR and GP. In the co-culture system, C9orf72 sMNs were co-cultured with GFP-expressing control sMNs and further matured to day 22. Staining for the sense strand DPRs, which have a higher concentration in vivo, revealed the presence of GA and GR aggregates in GFP labeled sMNs (control motor neurons normally negative for their presence), indicating transmission has occurred from the C9orf72 sMNs (Figure 4A, panel b, d, f). No detectable transmission occurred in the case of GP. Similarly, C9orf72 sMNs conditioned media applied to GFP-expressing control sMNs revealed the presence of GA and GR aggregates, but absent GP staining, in control sMNs (Figure 4B).
Figure 4. DPRs transmit in iPSC-derived spinal motor neurons.
(A) Representative confocal images show transmission of sense DPR species in co-cultures of control and C9orf72 iPSC-derived spinal motor neurons (sMNs). DPR production was verified in C9orf72 sMNs (a, c, e). Both C9orf72 iPSC lines provide similar results. Control sMNs, identified by GFP, showed presence of GA, GR, and GP, which is not normally present (b, d, f). MAP2 (red), DPRs (cyan). (B) Representative confocal images show transmission of sense DPR species through conditioned media of C9orf72 sMNs. Green represents transduced control sMNs, red represents MAP2+ neurons, cyan represents DPRs. Bars=50 μm.
DISCUSSION
The tendency for disease relevant proteins to spread across the CNS has begun to be reported in neurodegenerative diseases (Costanzo and Zurzolo, 2013), shaping our understanding of their progressive nature. In ALS, TDP43 and SOD1 spread inter-cellularly and promote seeding activity, which leads to the misfolding of native protein species within the recipient cells (Feiler et al., 2015; Kanouchi et al., 2012; Silverman et al., 2016). C9-RAN-T DPRs are key pathogenic mediators of C9orf72-linked ALS/FTD (Gitler and Tsuiji, 2016). In patients, DPRs have been reported throughout the CNS, including the spinal cord, frontal cortex, motor cortex, hippocampus, and cerebellum (Schipper et al., 2015). Recent studies showed toxicity and cellular dysfunctions resulting from some of these DPRs, however their ability to transmit between cells has yet to be described. Interestingly, neuropathological analysis of post-mortem tissues from C9orf72 ALS patients showed that the expression of DPR aggregates have a distinct clustered pattern (Zu et al., 2013). This might suggest the existence of focal areas in which RAN-T occurs, but also that once made, DPRs can progressively pollute adjacent cells and spread. Here, we have demonstrated intercellular transmittance of DPRs by employing various in vitro cell culture platforms, and have gained initial insights into possible modalities of this process.
We first explored DPR transmission between neurons and neuron-to-glia. Indeed, all of the DPR species transmitted from cell-to-cell, with disparate localization patterns and frequency of transmission. When NSC34 cells are transfected with equal amount of cDNA, the arginine-rich DPRs are normally less efficiently expressed than the other DPRs (Wen et al., 2014). This lesser expression is likely due to the arginine-rich DPR effect on protein translation (Gitler and Tsuiji, 2016). Quantification of frequency of transmission between different DPRs, therefore, does not accurately reflect differences in transmission efficiency intrinsic to the different DPR species, but it is meant only to compare transmission efficiency between different experimental paradigms. Interestingly, in the cases of GA, GR, and PR co-culture experiments, at the time when astrocytes were assessed for DPR presence, there were virtually no transfected and sparse non-transfected neurons remaining on the dish. Although circumstantial, this evidence suggests the establishment of a possible toxic environment produced as a result of changes in DPR-receiving astrocytes. Nevertheless, this evidence does not fully support the notion that transmission of DPRs from one cell to another is equivalent to propagation of pathology in vivo, which likely requires template nucleation. More follow-up experiments will be needed to establish a cause-effect relationship of the DPR transmission process. Furthermore, retrograde and anterograde transmission was observed for most DPRs, suggesting spread between connecting neurons in different CNS regions.
Ectopic expression of DPRs by lipofection of cells in culture can lead to their overabundance, potentially forcing a transmission event that otherwise would not occur. The pathophysiological relevance of this process was, however, confirmed by the observation of transmission of DPRs in iPS-derived spinal motor neurons from C9orf72 patients, which endogenously produce DPRs.
Cell-to-cell transmission can occur through various mechanisms such as phagocytosis, endocytosis and exocytosis, tunneling nanotubes (TNTs), hemichannels or through microvesicles release (Costanzo and Zurzolo, 2013). Direct cell-to-cell interaction was not a requirement for DPR transmission, though cellular contact was sufficient and did enhance transmission of some DPR species. We explored transmission through release of DPRs utilizing transwell and microfluidic culturing systems. In both, DPR transmission to cortical neurons was observed except for PR, which surprisingly displayed rare to absent transmission. Additionally, anterograde and retrograde transmission seen in microfluidic platforms suggests that it can occur through axon terminals, similarly to TDP43 (Feiler et al., 2015). Interestingly, in these systems GP and PA displayed cytoplasmic aggregates in the receiving neurons. In vitro, GP and PA display diffuse localization (Wen et al., 2014). However, GP and PA are present in aggregates in C9orf72 ALS/FTD human tissue (Ash et al., 2013; Gendron et al., 2013; Mori et al., 2013a; Mori et al., 2013b; Zu et al., 2013). This raises the possibility that GP and PA could undergo transmission in vivo and have a different toxic profile than their diffuse in vitro counterparts.
Exosomes have been implicated as a primary mode for cell-to-cell transmission in many neurodegenerative diseases (Danzer et al., 2012; Saman et al., 2012; Silverman et al., 2016). Exosomes are vesicles (50-150 nm in size) released by most mammalian cells that contain an assortment of macromolecules such as mRNAs, miRNAs, bioactive lipids, and proteins and play a role in health and disease (Pant et al., 2012; Raposo and Stoorvogel, 2013). We found that exosomes have the capability to incorporate DPRs, although PR accumulation was negligible compared to the others. Consequently, isolated exosomes transmit DPRs to cortical neurons. Interestingly, PR was primarily transmitted in an exosome-independent manner, likely because of its strong nuclear localization propensity and highly toxic profile. The role and modalities of an exosome-independent transmission for PR, however, still needs to be fully explored.
While the precise mechanisms involved in release and cellular uptake of DPRs still need to be investigated, we have begun to establish the involvement of exosomes in DPRs transmission. Important questions are currently being investigated. For example, can DPRs transfer across synapses in vivo, and can this account for the propagation of pathology along neural networks? As current studies will be extended and augmented by new findings, it would seem that stopping the spread of DPRs, and other disease relevant proteins, could provide an interesting and novel therapeutic target. Since DPRs initiate a cascade of toxic cellular dysfunctions, preventing this cell-to-cell transmission may salvage remaining healthy cells and halt disease progression.
EXPERIMENTAL PROCEDURES
Microfluidic chambers
Two-chamber microfluidic devices were assembled using Colorfrost Plus Slides as a support platform. Slides were UV-sterilized, coated with poly-D-lysine and placed into secondary containment dishes. Following rinse with 70% ethanol, standard Neuron Device (150 μm microgroove barrier; Xona Microfluidic cat.# SND150) were applied to the slides. 300 μL of neuronal growth medium was added to each set of plating wells connected by a channel, observing by eye that the channel became filled with media. Primary rat cortical neurons (20,000 cells/150 μL) were seeded into each well. The following day, total growth media was increased to 500 μL or 250 μL to establish hydrostatic pressure differential in the two chambers. This ensured unidirectional growth through the microgrooves during neurite and axon extension. At the time of treatment, hydrostatic pressure was reverted to prevent crossover of transfection reagents.
Exosome isolation
Exosomes from NSC34 cells were prepared using Total Exosome Isolation kit (Thermo Fisher #4478359) or through ultracentrifugation as described previously (Thery et al., 2006). Both techniques were used initially to ensure highest and more pure yield of exosomes. There was no significant difference between the yields, as determined via dot blot.
Supplementary Material
Highlights.
Cell-to-cell transmission of DPRs is evident in vitro;
C9orf72-ALS patient derived spinal motor neurons exhibit DPR transmission
Exosomes containing DPRs are one modality for transmission;
Exosome-independent transmission is the primary mechanism for poly(PR) spread;
ACKNOWLEDGMENTS
We thank Mr. Eric Kostuk (Thomas Jefferson University) for providing microglia cultures, and Dr. Judith Grinspan (CHOP) for providing antibodies against oligodendrocyte markers. The Stem Cell Center of the Vickie and Jack Farber Institute for Neuroscience is supported through the generosity of Kimberley Strauss and the Strauss Foundation. NIH (R21-NS090912 to D.T. and RO1-NS075839 to L.I.), Muscular Dystrophy Association (to D.T.) and Farber Family Foundation (to P.P. and B. K.J) grants supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
T.W. was involved in the design, execution and analysis of all the experiments. B.K.J. optimized microfluidic systems and oligodendrocyte cultures. X.W. performed dot blotting and imaging analysis. J.C. and E.K. were involved in the characterization of iPS-spinal motor neurons and data analysis. P.P. and L.I. participated to experimental design and data analysis. D.T. wrote the manuscript, oversaw project development, experimental design and data interpretation.
REFERENCES
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, 3rd, Rademakers R, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to C9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baborie A, Griffiths TD, Jaros E, Perry R, McKeith IG, Burn DJ, Masuda-Suzukake M, Hasegawa M, Rollinson S, Pickering-Brown S, et al. Accumulation of dipeptide repeat proteins predates that of TDP-43 in frontotemporal lobar degeneration associated with hexanucleotide repeat expansions in C9ORF72 gene. Neuropathol Appl Neurobiol. 2015;41:601–612. doi: 10.1111/nan.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol. 2012;3:124. doi: 10.3389/fphys.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci. 2015;16:109–120. doi: 10.1038/nrn3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Jeng US, Chiang YL, Hwang IS, Chen YR. The Glycine-Alanine Dipeptide Repeat from C9orf72 Hexanucleotide Expansions Forms Toxic Amyloids Possessing Cell-to-Cell Transmission Properties. J Biol Chem. 2016;291:4903–4911. doi: 10.1074/jbc.M115.694273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Lattante S, Le Ber I, Latouche M, Tostivint H, Brice A, Kabashi E. Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann Neurol. 2013;74:180–187. doi: 10.1002/ana.23946. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Zurzolo C. The cell biology of prion-like spread of protein aggregates: mechanisms and implication in neurodegeneration. Biochem J. 2013;452:1–17. doi: 10.1042/BJ20121898. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM, Li D, Thal DR, Walther P, Ludolph AC, et al. TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol. 2015;211:897–911. doi: 10.1083/jcb.201504057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos S, Pacheco C, Peters C, Opazo CM, Aguayo LG. Features of alpha-synuclein that could explain the progression and irreversibility of Parkinson's disease. Front Neurosci. 2015;9:59. doi: 10.3389/fnins.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron TF, Belzil VV, Zhang YJ, Petrucelli L. Mechanisms of toxicity in C9FTLD/ALS. Acta Neuropathol. 2014;127:359–376. doi: 10.1007/s00401-013-1237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron TF, Bieniek KF, Zhang YJ, Jansen-West K, Ash PE, Caulfield T, Daughrity L, Dunmore JH, Castanedes-Casey M, Chew J, et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013;126:829–844. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Tsuiji H. There has been an awakening: Emerging mechanisms of C9orf72 mutations in FTD/ALS. Brain Res. 2016;1647:19–29. doi: 10.1016/j.brainres.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanouchi T, Ohkubo T, Yokota T. Can regional spreading of amyotrophic lateral sclerosis motor symptoms be explained by prion-like propagation? J Neurol Neurosurg Psychiatry. 2012;83:739–745. doi: 10.1136/jnnp-2011-301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury Y, Come J, Piskorowski RA, Salah-Mohellibi N, Chevaleyre V, Peschanski M, Martinat C, Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nature biotechnology. 2015;33:89–96. doi: 10.1038/nbt.3049. [DOI] [PubMed] [Google Scholar]
- May S, Hornburg D, Schludi MH, Arzberger T, Rentzsch K, Schwenk BM, Grasser FA, Mori K, Kremmer E, Banzhaf-Strathmann J, et al. C9orf72 FTLD/ALS-associated Gly-Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration. Acta Neuropathol. 2014;128:485–503. doi: 10.1007/s00401-014-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Arzberger T, Grasser FA, Gijselinck I, May S, Rentzsch K, Weng SM, Schludi MH, van der Zee J, Cruts M, et al. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol. 2013a;126:881–893. doi: 10.1007/s00401-013-1189-3. [DOI] [PubMed] [Google Scholar]
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013b;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. J Neurosci. 2012;32:8767–8777. doi: 10.1523/JNEUROSCI.0615-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot M, Gutowski NJ, Edbauer D, Hilton DA, Stephens M, Rankin J, Mackenzie IR. Early dipeptide repeat pathology in a frontotemporal dementia kindred with C9ORF72 mutation and intellectual disability. Acta Neuropathol. 2014;127:451–458. doi: 10.1007/s00401-014-1245-7. [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper LJ, Raaphorst J, Aronica E, Baas F, de Haan R, de Visser M, Troost D. Prevalence of brain and spinal cord inclusions, including dipeptide repeat proteins, in patients with the C9ORF72 hexanucleotide repeat expansion: A systematic neuropathological review. Neuropathol Appl Neurobiol. 2015 doi: 10.1111/nan.12284. [DOI] [PubMed] [Google Scholar]
- Schludi MH, May S, Grasser FA, Rentzsch K, Kremmer E, Kupper C, Klopstock T, German Consortium for Frontotemporal Lobar, D. Bavarian Brain Banking A, Arzberger T, et al. Distribution of dipeptide repeat proteins in cellular models and C9orf72 mutation cases suggests link to transcriptional silencing. Acta Neuropathol. 2015;130:537–555. doi: 10.1007/s00401-015-1450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Fernando SM, Grad LI, Hill AF, Turner BJ, Yerbury JJ, Cashman NR. Disease Mechanisms in ALS: Misfolded SOD1 Transferred Through Exosome-Dependent and Exosome-Independent Pathways. Cell Mol Neurobiol. 2016 doi: 10.1007/s10571-015-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Zhang Y, Gendron TF, Bauer PO, Chew J, Yang WY, Fostvedt E, Jansen-West K, Belzil VV, Desaro P, et al. Discovery of a biomarker and lead small molecules to target r(GGGGCC)-associated defects in c9FTD/ALS. Neuron. 2014;83:1043–1050. doi: 10.1016/j.neuron.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien M, Rouleau GA, Dion PA, Parker JA. Deletion of C9ORF72 results in motor neuron degeneration and stress sensitivity in C. elegans. PLoS One. 2013;8:e83450. doi: 10.1371/journal.pone.0083450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3, Unit 3 22. [DOI] [PubMed] [Google Scholar]
- Vega C, Sachleben RL,J, Gozal D, Gozal E. Differential metabolic adaptation to acute and long-term hypoxia in rat primary cortical astrocytes. J Neurochem. 2006;97:872–883. doi: 10.1111/j.1471-4159.2006.03790.x. [DOI] [PubMed] [Google Scholar]
- Wen X, Tan W, Westergard T, Krishnamurthy K, Markandaiah SS, Shi Y, Lin S, Shneider NA, Monaghan J, Pandey UB, et al. Antisense proline-arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron. 2014;84:1213–1225. doi: 10.1016/j.neuron.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Gendron TF, Grima JC, Sasaguri H, Jansen-West K, Xu YF, Katzman RB, Gass J, Murray ME, Shinohara M, et al. C9ORF72 poly(GA) aggregates sequester and impair HR23 and nucleocytoplasmic transport proteins. Nat Neurosci. 2016 doi: 10.1038/nn.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Jansen-West K, Xu YF, Gendron TF, Bieniek KF, Lin WL, Sasaguri H, Caulfield T, Hubbard J, Daughrity L, et al. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol. 2014;128:505–524. doi: 10.1007/s00401-014-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Liu Y, Bañez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia.pdf. Proc Natl Acad Sci U S A. 2013;110:4968–4977. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.