Abstract
Purpose
To determine the relationship between p16 status and the regional response of patients with node-positive oropharynx cancer treated on NRG Oncology RTOG 0129.
Methods and Materials
Patients with N1-N3 oropharynx cancer and known p16 status who underwent treatment on RTOG 0129 were analyzed. Pathologic complete response (pCR) rates in patients treated with a postchemoradiation neck dissection (with p16-positive or p16-negative cancer) were compared by Fisher exact test. Patients managed expectantly were compared with those treated with a neck dissection.
Results
Ninety-nine (34%) of 292 patients with node-positive oropharynx cancer and known p16 status underwent a posttreatment neck dissection (p16-positive: n = 69; p16-negative: n = 30). The remaining 193 patients with malignant lymphadenopathy at diagnosis were observed. Neck dissection was performed a median of 70 (range, 17-169) days after completion of chemoradiation. Neither the pretreatment nodal stage (P = .71) nor the postradiation, pre-neck dissection clinical/radiographic neck assessment (P = .42) differed by p16 status. A pCR was more common among p16-positive patients (78%) than p16-negative patients (53%, P = .02) and was associated with a reduced incidence of local–regional failure (hazard ratio 0.33, P = .003). On multivariate analysis of local–regional failure, a test for interaction between pCR and p16 status was not significant (P = .37). One-hundred ninety-three (66%) of 292 of initially node-positive patients were managed without a posttreatment neck dissection. Development of a clinical (cCR) was not significantly influenced by p16-status (P = .42). Observed patients with a clinical nodal CR had disease control outcomes similar to those in patients with a pCR neck dissection.
Conclusions
Patients with p16-positive tumors had significantly higher pCR and locoregional control rates than those with p16-negative tumors.
Introduction
More than 40,000 patients will be diagnosed with mouth and pharynx cancer in 2015 (1). Many of them will present with regionally advanced disease commonly managed with primary chemoradiation (2). Historically, patients with advanced neck disease (ie, N2-N3) were recommended a neck dissection after definitive (chemo) radiation treatment, owing to data suggesting improved regional control after the lymphadenectomy (3).
Despite gratifying regional control rates, practice patterns over the past 2 decades have eventuated in fewer posttreatment neck dissections. Many postradiation neck dissections do not disclose viable tumor (4). The neck dissection itself increases long-term side effects, including dysphagia (5, 6) and shoulder dysfunction (7). Patients with a complete imaging response by either computed tomography (CT) (8) or positron emission tomography (PET) (9) can be managed expectantly without an increase in the rate of regional recurrence. The success of neck observation was initially attributed to more intense therapy (accelerated fractionation (10) and concurrent chemotherapy (11)), but it may be due to the increasing incidence of human papillomavirus (HPV)-associated oropharynx cancer (OPC) (12), which is more responsive to nonsurgical management than is head and neck cancer caused by habitual tobacco and alcohol abuse (13).
Although posttreatment neck dissections are no longer undertaken as a matter of course, the determination of which patient may be safely observed can be difficult. Although both CT (8) and PET/CT threshold criteria (14) have been proposed, these are subject to considerable intraobserver variability. Human papillomavirus–associated OPC has a better prognosis than OPC caused by substance abuse (15), but the sometimes cystic nature of the involved lymph nodes (16) and the prolonged time that it takes to achieve a complete response (CR) can complicate the decision to operate (17). How HPV status is best incorporated into the decision-making process is unknown.
NRG Oncology RTOG 0129 accrued patients from 2002 to 2005—a time of considerable change in practice patterns surrounding posttreatment neck dissection. A majority of the oropharynx tumors treated on the protocol had a known HPV status. Thus, the results of the neck dissections performed as a component of treatment on NRG Oncology RTOG 0129 present a unique opportunity to examine the relationship between HPV status and pathologic response.
Methods and Materials
NRG Oncology RTOG 0129 was a phase 3 clinical trial designed to evaluate whether accelerated fractionation by concomitant boost in comparison with standard fractionation radiation therapy improves overall survival (OS) rates for head and neck cancer patients treated with concurrent high-dose cisplatin. The primary results of the trial have been published (18, 19). The utilization of a post-chemoradiation neck dissection, and its impact on outcomes, has not previously been addressed.
Before patient enrollment, each participating institution provided institutional review board approval, and each patient provided written, informed consent. Eligible patients had untreated, pathologically confirmed stage III to IV squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx (20); Zubrod performance status 0 to 1; age ≥18 years; and adequate bone marrow, hepatic, and renal function. Patients were stratified by tumor site (larynx vs non-larynx), nodal stage (N0 vs N1-N2b vs N2c-N3), and Zubrod performance status (0-1) and randomly assigned to receive cisplatin concurrent with either standard fractionation (70 Gy in 35 fractions [fx], 2 Gy/fx, over 7 weeks) or accelerated fractionation by concomitant boost (72 Gy delivered in 42 fx of 6 weeks, inclusive of twice-per-day irradiation for the last 12 treatment days). Computed tomography and/or magnetic resonance imaging of the neck 6 to 8 weeks after the completion of chemoradiation was required, and a planned neck dissection for patients with multiple neck nodes or with lymph nodes exceeding 3 cm in diameter (N2a, N2b, N3) was considered mandatory, regardless of the clinical/radiographic response.
History of cigarette smoking in pack-years was obtained at enrollment via interviewer-administered questionnaire. To assess disease status, follow-up examinations and imaging studies were performed 4 times per year for the first 2 years, twice per year through year 5, and annually thereafter.
Patients eligible for this secondary analysis were those with node-positive OPC enrolled on NRG Oncology RTOG 0129 with evaluable p16 expression (a surrogate of HPV status). Patients both with and without a posttreatment neck dissection were evaluated.
Laboratory analysis
Tumor p16 expression was evaluated by immunohistochemistry using a mouse monoclonal antibody (MTM Laboratories, Heidelberg, Germany) and was visualized with the Ventana XT autostainer using the 1-view secondary detection kit (Ventana, Tucson, AZ) (21). p16 expression was scored as positive if strong and diffuse nuclear and cytoplasmic staining was present in at least 70% of the tumor cells (22, 23). Testing and interpretation were centralized.
Statistical analysis
Clinical endpoints were pathologic CR (pCR), local–regional failure (LRF; recurrent disease above the clavicles or death due to study cancer or unknown causes as first event; distant metastases and death due to other causes were considered competing risks), progression-free survival (PFS; evidence of local, regional, or distant disease related to the index OPC, or death due to any causes), and OS. For patients who had a neck dissection, failure time was measured from date of neck dissection. For patients who did not have a neck dissection, failure time was measured from the date radiation therapy was completed. Local-regional failure rates were estimated by the cumulative incidence method. Progression-free survival and OS rates were estimated by the Kaplan-Meier method. Groups were compared by 2-sided log-rank test. Odds ratios were estimated by logistic regression and hazard ratios by Cox regression. Pathologic CR rates were compared by Fisher exact test. The recommendation for the timing of the posttreatment neck dissection was 6 to 10 weeks after the completion of chemoradiation. In view of the changing practice patterns (which not only limit the number of neck dissections but also increase the time until the neck dissections are performed), we arbitrarily chose 180 days from the completion of chemoradiation as a threshold for determining what constituted a planned neck dissection (≤180 days) and what neck dissection constituted treatment of a recurrence (> 180 days). Institutions were recommended to submit a postchemoradiation clinical/radiographic assessment of the neck. The mechanisms of assessment (eg, use of functional imaging or diagnostic imaging, or both) were not standardized.
Results
NRG Oncology RTOG 0129 randomized 721 patients. A total of 292 of those patients had node-positive OPC with a known p16 status. Ninety-nine of 292 (34%) had a posttreatment neck dissection, and 193 (66%) node-positive necks treated on NRG Oncology RTOG 0129 were observed after chemoradiation. Men (P = .02) and patients with increasing N stage (P = .01) were more likely to have a neck dissection. Although neck dissections were most commonly performed in the setting of an isolated incomplete neck response, 35% of neck dissections were performed among patients assessed to have a CR in the neck, and 19% of neck dissections were performed among patients with presumed persistent disease at the primary site after chemoradiation (and 3 of those patients had viable disease at the primary site resected at the time of neck dissection; Table 1). Among patients treated with a neck dissection, the mean time from latest assessment of the neck (clinical and/or radiographic) was similar among p16-positive (mean 37.7 days) and p16-negative (mean 31.1 days). At the time of neck dissection it is presumed that there was no distant disease, although information regarding evaluations below the clavicles was not captured, and distant metastasis surveillance was not required before the operation.
Table 1. Patient characteristics.
| Characteristic | Neck dissected (n=99) | Neck observed (n=193) | P |
|---|---|---|---|
| Assigned treatment | .80* | ||
| SFX + cisplatin | 53 (53.5) | 99 (51.3) | |
| AFX-C + cisplatin | 46 (46.5) | 94 (48.7) | |
| Age (y) | .12† | ||
| Median (range) | 55 (37-71) | 55 (31-82) | |
| Sex | .02* | ||
| Male | 90 (90.9) | 154 (79.8) | |
| Female | 9 (9.1) | 39 (20.2) | |
| Race | .72* | ||
| White | 84 (84.8) | 167 (86.5) | |
| Non-white | 15 (15.2) | 26 (13.5) | |
| Zubrod performance status | .80* | ||
| 0 | 64 (64.6) | 128 (66.3) | |
| 1 | 35 (35.4) | 65 (33.7) | |
| Smoking history: pack-years‡ | (n=81) | (n=160) | .96† |
| Median (range) | 20 (0-152) | 20 (0-92.5) | |
| Primary site | 1.00* | ||
| Oropharynx, NOS | 15 (15.2) | 17 (8.8) | |
| Faucial arch | 0 (0.0) | 1 (0.5) | |
| Tonsillar fossa, tonsil | 44 (44.4) | 81 (42.0) | |
| Base of tongue | 37 (37.4) | 78 (40.4) | |
| Pharyngeal oropharynx | 2 (2.0) | 8 (4.1) | |
| Soft palate | 1 (1.0) | 8 (4.1) | |
| p16 status | .79* | ||
| p16 positive | 69 (69.7) | 130 (67.4) | |
| p16 negative | 30 (30.3) | 63 (32.6) | |
| T stage | .14† | ||
| T2 | 40 (40.4) | 54 (28.0) | |
| T3 | 31 (31.3) | 81 (42.0) | |
| T4 | 28 (28.3) | 58 (30.1) | |
| N stage | .01† | ||
| N1 | 4 (4.0) | 42 (21.8) | |
| N2a | 21 (21.2) | 15 (7.8) | |
| N2b | 33 (33.3) | 72 (37.3) | |
| N2c | 21 (21.2) | 53 (27.5) | |
| N3 | 20 (20.2) | 11 (5.7) | |
| Primary disease status after chemoradiotherapy | .03§ | ||
| Absent | 74 (74.7) | 145 (75.1) | |
| Present | 19 (19.2) | 21 (10.9) | |
| Unknown | 6 (6.1) | 27 (14.0) | |
| Neck disease status after chemoradiotherapy | <.001§ | ||
| Absent | 35 (35.4) | 136 (70.5) | |
| Present | 54 (54.5) | 33 (17.1) | |
| Unknown | 10 (10.1) | 24 (12.4) |
Abbreviations: AFX-C = accelerated fractionation with concomitant boost radiation therapy; NOS = not otherwise specified; SFX = standard fractionation radiation therapy.
Values are number (percentage) unless otherwise noted.
Fisher exact test. Primary site is tested as tonsil or base of tongue versus others.
Wilcoxon rank-sum test.
A pack-year is defined as the equivalent of smoking one pack of cigarettes a day for 1 year.
Chi-squared test.
Pathologic response of neck dissection
A total of 99 neck dissections were performed within 180 days from the completion of chemoradiation, of which 70% (69 of 99) were p16-positive, similar to the larger NRG Oncology RTOG 0129 patient population. Again similar to the larger 0129 patient population, p16-negative patients generally were less likely to be of white race and had a high T-stage designation and more pack-years smoking. By contrast, the N-stage designation was similar, and p16 status was not associated with the clinical/radiographic interpretation of the postchemoradiation neck or timing of the neck dissection (Table 2). The negative predictive value of the posttreatment imaging was high for p16-positive patients (94%), but was otherwise not good at predicting pCR rates in the neck (Supplemental Table E1; available online at www.redjournal.org).
Table 2. Characteristics of patients who had a posttreatment neck dissection.
| Characteristic | p16 positive (n=69) | p16 negative (n=30) | P |
|---|---|---|---|
| Assigned treatment | .39* | ||
| SFX + cisplatin | 39 (56.5) | 14 (46.7) | |
| AFX-C + cisplatin | 30 (43.5) | 16 (53.3) | |
| Age (y) | .93† | ||
| Median (range) | 55 (38-66) | 55 (37-71) | |
| Sex | 1.00* | ||
| Male | 63 (91.3) | 27 (90.0) | |
| Female | 6 (8.7) | 3 (10.0) | |
| Race | .06* | ||
| White | 62 (89.9) | 22 (73.3) | |
| Non-white | 7 (10.1) | 8 (26.7) | |
| Zubrod performance status | .36* | ||
| 0 | 47 (68.1) | 17 (56.7) | |
| 1 | 22 (31.9) | 13 (43.3) | |
| Smoking history: pack-years‡ | (n=59) | (n=22) | <.001† |
| Median (range) | 10.8 (0-152) | 38.5 (0-100) | |
| Primary site | .40* | ||
| Oropharynx, NOS | 10 (14.5) | 5 (16.7) | |
| Tonsillar fossa, tonsil | 30 (43.5) | 14 (46.7) | |
| Base of tongue | 28 (40.6) | 9 (30.0) | |
| Pharyngeal oropharynx | 1 (1.4) | 1 (3.3) | |
| Soft palate | 0 (0.0) | 1 (3.3) | |
| T stage | .08† | ||
| T2 | 31 (44.9) | 9 (30.0) | |
| T3 | 22 (31.9) | 9 (30.0) | |
| T4 | 16 (23.2) | 12 (40.0) | |
| N stage | .71† | ||
| N1 | 2 (2.9) | 2 (6.7) | |
| N2a | 15 (21.7) | 6 (20.0) | |
| N2b | 26 (37.7) | 7 (23.3) | |
| N2c | 12 (17.4) | 9 (30.0) | |
| N3 | 14 (20.3) | 6 (20.0) | |
| Primary disease status before neck dissection | .09§ | ||
| Persistent abnormality | 9 (13.0) | 4 (13.3) | |
| No disease (cCR) | 50 (72.5) | 16 (53.3) | |
| Unknown/no assessment | 10 (14.5) | 10 (33.3) | |
| Neck disease status prior to neck dissection | .42§ | ||
| Persistent abnormality | 38 (55.1) | 13 (43.3) | |
| No disease (cCR) | 18 (26.1) | 8 (26.7) | |
| Unknown/no assessment | 13 (18.8) | 9 (30.0) | |
| Interval between chemoRT and neck dissection (d) | .95† | ||
| Median (range) | 71 (17-154) | 67 (35-169) |
Abbreviation: cCR = clinical complete response. Other abbreviations as in Table 1.
Values are number (percentage) unless otherwise noted.
Fisher exact test. Primary site is tested as tonsil or base of tongue versus others.
Wilcoxon rank-sum test.
A pack-year is defined as the equivalent of smoking one pack of cigarettes a day for 1 year.
Chi-squared test.
Patients with p16-positive disease were much more likely to achieve a pCR than p16-negative patients (78% vs 53%, P = .02). Patients with the most favorable pretreatment characteristics (24) (T2-3N1-2b, p16-positive, ≤10 pack-years smoking) had a pCR rate of 79% (15 of 19). Duration from completion of chemoradiation until neck dissection was not significant on univariate analysis (odds ratio [OR] 1.00 [95% confidence interval (CI) 0.99-1.01], P = .96). Pack-year smoking (>10 vs ≤10) approached significance (OR 0.36 [95% CI 0.13-1.05], P = .06). Among all patients, local-regional recurrence after neck dissection was significantly more common for those who did not achieve a pCR (Fig. 1A). However, when analyzed by p16 status, the worse local-regional control was more pronounced among p16-negative patients (HR 0.37, P = .05; Fig. 1C) than p16-positive patients (HR 0.63, P = .50; Fig. 1B). The multivariate model seemed to suggest a differential effect of pCR by p16 status, but a formal test of an interaction between pCR and p16 status did not prove significant (P = .37). As expected, p16-positive patients did better than p16-negative patients regardless of pathologic neck response status, and patients with bulkier (ie, N2b and higher) neck disease at diagnosis had a trend toward worse local-regional control (Table 3).
Fig. 1.
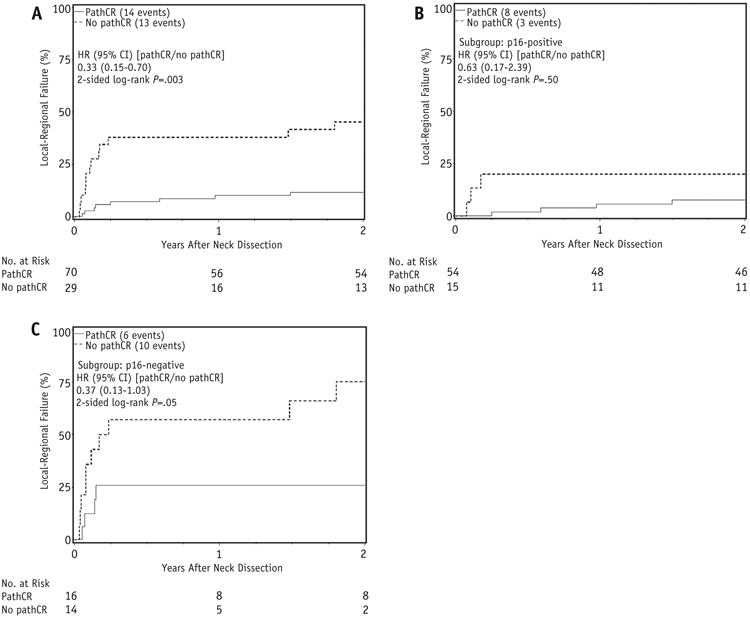
(A) Local-regional failure (LRF) after neck dissection by pathologic complete response (pCR), all patients; (B) LRF after neck dissection by pCR, p16-positive; (C) LRF after neck dissection by pCR, p16-negative. Abbreviations: CI = confidence interval; HR = hazard ratio.
Table 3. LRC, PFS, and OS, multivariate analysis.
| LRC (n=99; 27 events) | PFS (n=99; 42 events) | OS (n=99; 37 events) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Parameter | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Pathologic CR (yes vs no) If p16 negative | 0.31 (0.11-0.88) | .03 | 0.50 (0.21-1.19) | .12 | 0.58 (0.22-1.52) | .27 |
| Pathologic CR (yes vs no) If p16 positive | 0.68 (0.18-2.59) | .57 | 0.67 (0.24-1.86) | .44 | 1.03 (0.34-3.12) | .96 |
| p16 status (positive vs negative) If not pCR | 0.13 (0.03-0.48) | .003 | 0.22 (0.07-0.65) | .007 | 0.22 (0.07-0.74) | .01 |
| p16 status (positive vs negative) If pCR | 0.28 (0.10-0.80) | .02 | 0.29 (0.13-0.65) | .003 | 0.40 (0.17-0.94) | .04 |
| T stage (T4 vs T2/3) | 1.30 (0.56-2.97) | .54 | 2.17 (1.15-4.10) | .02 | 2.38 (1.21-4.67) | .01 |
| N stage (N2b-3 vs N1-2a) | 2.77 (0.94-8.18) | .07 | 1.65 (0.77-3.53) | .20 | 2.12 (0.90-4.96) | .08 |
| P for interaction between pCR and p16 status | .37 | .68 | .45 | |||
Abbreviations: HR = hazard ratio; LRC = locoregional control; OS = overall survival; pCR = pathologic complete response; PFS = progression-free survival.
Progression-free survival and OS were both worse among patients who did not achieve a pCR on univariate analysis (P = .008 and .09, respectively; Fig. 2). However, pCR did not maintain significance for either outcome on multivariate analysis, though p16 status and T stage at diagnosis were both significant variables (Table 3).
Fig. 2.
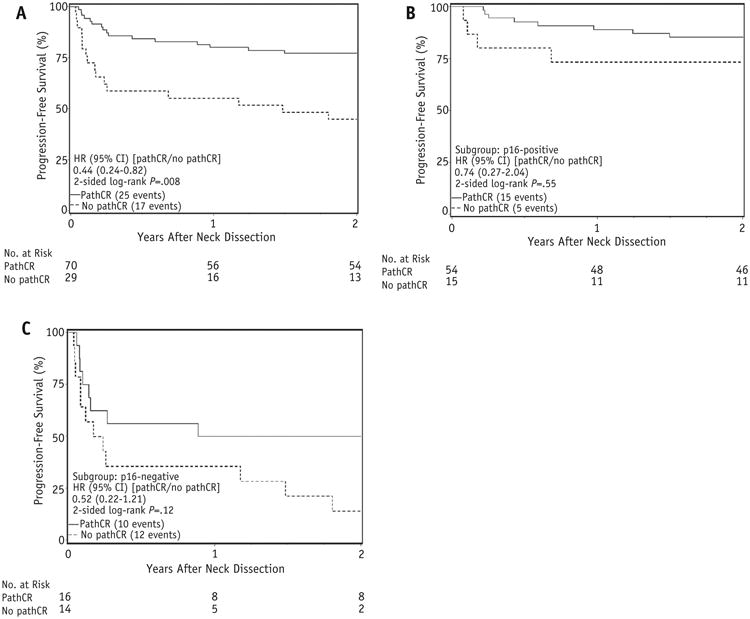
(A) Progression-free survival (PFS) after neck dissection by pathologic complete response (pCR), all patients; (B) PFS after neck dissection by pCR, p16-positive; (C) PFS after neck dissection by pCR, p16-negative. Abbreviations: CI = confidence interval; HR = hazard ratio.
Neck dissection versus neck observation
A total of 193 OPC patients with an initially involved neck and a known p16 status were observed on NRG Oncology RTOG 0129 (Table 1).
Local-regional failure, PFS, and OS 2 years after the completion of chemoradiation demonstrated that the LRF of patients with an observed neck was similar to that of patients with a pCR at neck dissection (Table 4), regardless of HPV status. The 2-year local-regional failure of p16-positive patients who fail to achieve a CR was similar to that of p16-negative patients who did achieve a CR.
Table 4. Two-year endpoints by p16 status and neck treatment after chemoradiation.
| 2-y Estimates (%) | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | Patients (n) | LRF | PFS | OS |
| p16 positive | ||||
| No neck dissection | 130 | 11.2 (6.4-17.5) | 75.3 (67.9-82.8) | 86.9 (81.1-92.7) |
| Neck dissection with pCR | 54 | 7.5 (2.4-16.7) | 85.2 (75.7-94.7) | 92.6 (85.6-99.6) |
| Neck dissection without pCR | 15 | 20.0 (4.5-43.3) | 73.3 (51.0-95.7) | 86.7 (69.5-100.0) |
| Neck dissection with/without pCR | 69 | 10.2 (4.5-18.8) | 82.6 (73.7-91.6) | 91.3 (84.7-98.0) |
| p16 negative | ||||
| No neck dissection | 63 | 31.1 (19.9-43.0) | 47.6 (35.3-60.0) | 57.1 (44.9-69.4) |
| Neck dissection with pCR | 16 | 26.0 (7.5-49.6) | 50.0 (25.5-74.5) | 62.5 (38.8-86.2) |
| Neck dissection without pCR | 14 | 75.0 (36.0-92.2) | 14.3 (0.0-32.6) | 50.0 (23.8-76.2) |
| Neck dissection with/without pCR | 30 | 49.1 (29.2-66.3) | 33.3 (16.5-50.2) | 56.7 (38.9-74.4) |
Abbreviations as in Table 3.
Discussion
The declining use of the posttreatment neck dissection (17) overlaps with other trends in head and neck cancer, including the increasing application of chemoradiation regimens (25) and the rising incidence of HPV-associated OPC (12). Before understanding the prognostic significance of HPV-associated OPC, it was widely assumed that increasingly intense chemoradiation was enhancing tumor control and rendering planned neck dissection unnecessary. These prospectively acquired data strongly suggest that the increase of pCR (and clinical CR [cCR]) in the neck is referable to HPV-associated disease, because p16 status was significantly associated with pCR and local-regional control, whereas treatment according to the investigational arm (accelerated fractionation chemoradiation) did not significantly affect the development of either a pCR or a cCR. Compared with those tumors not caused by HPV, patients with node-positive p16-positive OPC had significantly improved local-regional control, PFS, and OS regardless of the posttreatment management of the neck.
Viable tumor in the neck on a postchemoradiation neck dissection
This analysis suggests that the accepted poor prognostic association of viable disease in the postradiation neck (26, 27) is most likely referable to patients with p16-negative cancer. When evaluated as a whole, patients on NRG Oncology RTOG 0129 who did not achieve a pCR have a higher rate of LRF (P = .003) and a worse PFS (P = .007). When analyzed by p16 status, the hazard ratios for LRF and PFS of both p16-positive and p16-negative patients who do not achieve a pCR at neck dissection seem worse when compared with those who achieve a pCR, although neither of these relationships reach statistical significance. Despite this, the results suggest that the presence of viable disease at neck dissection is more impactful for p16-negative than p16-positive patients (Tables 3 and 4). Although there are limited data on p16-negative OPC patients who were submitted to neck dissection and did not achieve a pCR (n = 14), they seem to have an extraordinarily poor 2-year PFS of 14%. Thus, maneuvers to increase the CR rates in patients with p16-negative OPC seem justified. Although this trial did not suggest that a more intense chemoradiation regimen increased the rate of pCR, future endeavors might identify patients more likely to benefit from treatment intensification to the neck. Perhaps those with >10 pack-years smoking, who trended toward a lower rate of pCR (OR 0.36 [95% CI 0.13-1.05], P = .06), or bulkier neck disease at diagnosis, who trended toward a higher rate of LRF (P = .07), should be selected for intensification of treatment.
In contrast, patients with p16-positive OPC who do not demonstrate a pCR at neck dissection after chemoradiation have a 2-year LRF rate of 20% and PFS of 73%. Although the locoregional control hazard ratio for the development of a pCR suggests a potential differential effect—p16-positive OPC patients who fail to achieve a pCR seem to behave differently than p16-negative OPC patients who fail to achieve a pCR—the formal test for an interaction between pCR and p16 status was not significant (P = .37), perhaps due to limited sample size. This suggestion of a differential effect is in agreement with the different natural history of p16-positive OPC in both the definitive (18) and recurrent/metastatic (28) setting. However, it is unclear why the failure of chemoradiation to sterilize p16-positive neck nodes has a relatively minor impact on both LRC and PFS. Interestingly, the best-prognosis tumors (p16-positive, T1-3N1-2b, ≤10 pack-years smoking) had a similar pCR rate to that of all p16-positive patients (79% and 78%, respectively). Future studies with larger sample sizes are needed to confirm these results.
Neck dissections for p16-positive patients were performed at a median of 71 days after the completion of radiation. Imaging was protocol specified to occur 6 to 8 weeks after the completion of chemoradiation. Currently it is common to wait 12 weeks (9), if not longer (29), to image the neck and thus longer yet to perform the neck dissection. Although this series did not find that time between the completion of chemoradiation and neck dissection was predictive of pCR, the clinically assessed time for lymph node resolution suggests that enlarged nodes continue to regress for more than 6 months (17). It is therefore possible that some patients with apparently viable disease in the neck would eventually develop a pCR with a longer interval to resection. Conversely, it is also possible that our use of a 180-day threshold to delineate between a “post-chemoRT” neck dissection and a “salvage” neck dissection impacted our results. To this end we evaluated 2 additional time points: 140 days (the threshold currently used in NRG HN 002) and 90 days. Only 4 patients total received a neck dissection between 140 and 180 days after the completion of chemoradiotherapy; institution of a 140-day threshold did not meaningfully change any evaluated outcomes. By contrast, institution of a 90-day threshold did affect outcomes, particularly among the p16-negative patients without a pCR at neck dissection, for whom the 2-year PFS of p16-negative increased to 25%. Whether this finding based on a small sample of patients suggests that N2/N3 p16-negative patients should be submitted to earlier (and/or planned) neck dissections needs to be verified and validated by future research.
It has been proposed that patients with HPV-associated OPC with viable carcinoma on the neck dissection have a distinctly worse prognosis than patients with a pCR, owing largely to an increased rate of distant metastases (30). Exploratory analysis of this dataset demonstrates that pCR is not significantly associated with reduced incidence of distant metastasis (hazard ratio 0.56, P = .41). The number of events is too small (9 total patients with distant metastases) for multivariable analysis or analysis by p16 status. With a proposed different failure pattern of p16-positive OPC, it is unclear whether these patients will develop more distant metastases (31) with longer follow-up.
pCR on a postchemoradiation neck dissection
Although this study indicates that the poor prognostic outcome of viable disease in the neck is referable to p16-negative OPC, it also suggests that the accepted good outcome of a pCR (32) may be referable to p16-positive OPC. When evaluated as a whole, the 2-year LRF of patients with a pCR neck dissection was 12%, consistent with historical series in the literature (8). However, when analyzed by p16 status, the LRF of p16-positive patients with a pCR is 7.5%, whereas that of p16-negative patients is 26%. Thus, the historically accepted prognostic implications of a pCR neck dissection may not apply to p16-negative OPC. The PFS of a p16-negative patient with a pCR on a neck dissection is only 50% at 2 years. Thus, although p16-negative patients with a pCR have an improved outlook when compared with those with viable disease in the neck, disease progression is all too common. These patients should be considered for clinical trials to determine whether therapy intensification or the addition of novel targeted agents can improve their treatment outcomes.
cCR in an observed neck
p16-positive patients with a cCR can be safely observed with a low rate of LRF, and thus the use of pCR as an endpoint for future investigations does not seem warranted. A majority of p16-positive patients submitted to a neck dissection on this trial had no viable disease in the neck. NRG Oncology RTOG 0129 recorded whether imaging was used to render a postchemoradiation neck assessment (CT or magnetic resonance imaging), but the assessment was not standardized. In addition, it is likely that many patients were evaluated with functional imaging, although this information was not captured by the protocol. Thus, NRG Oncology RTOG 0129 cannot clearly define which patient is safe to observe. A recent prospective trial has demonstrated that PET/CT-guided active surveillance after chemoradiation for tumors of the upper aerodigestive tract results in considerably fewer neck dissections while maintaining similar survival and is cost-effective (33). In light of these data, it seems that future clinical trial planning should accommodate PET/CT-based determination of the suitability of a posttreatment neck dissection.
This analysis also demonstrates that even in the setting of a cCR, the 2-year PFS of p16-negative patients is unacceptably low (48%). Although only a small number of p16-negative patients (16 total) had a pCR neck dissection, the PFS of those patients was similar (50%), suggesting that a recommendation for routine neck dissection for p16-negative patients will not meaningfully limit failures. Even in the setting of a CR, p16-negative patients on NRG Oncology RTOG 0129 often developed a recurrence, suggesting that novel treatment approaches are needed for this patient group. However, a limitation of this study is that the primary endpoint (pCR neck dissection) is determined in part by the interval between completion of chemoradiation and neck dissection. In addition, p16-negative patients treated with a neck dissection—whose outcome may be most influenced by this interval—represent a small proportion of the OPC treated on study. Although 292 node-positive OPC patients with a known p16-status were treated on NRG Oncology RTOG 0129, only one-third of these patients received a posttreatment neck dissection within 180 days of chemoradiation completion, and thus the small sample size and limited number of events hamper opportunities for comparison of clinical endpoints, and the results of this analysis should be verified in future studies.
Conclusions
Patients with p16-positive OPC had significantly higher pCR and local–regional control rates than those with p16-negative tumors. Patients with p16-negative tumors had a poor PFS and OS regardless of neck response. Patients with a complete cCR were more likely to be managed without a neck dissection, and observed patients had a failure rate similar to that of patients with a pCR at neck dissection.
Supplementary Material
Summary.
This second analysis of NRG Oncology RTOG 0129 investigates the role of posttreatment neck dissection in the management of node-positive oropharynx cancer managed with primary chemoradiation. Patients treated on protocol were imaged 6 to 8 weeks after the completion of chemoradiation, and posttreatment neck dissection was recommended for those with advanced stage (N2-N3) at diagnosis. Tumors that are p16-positive are significantly more likely to develop a complete pathologic response. Many patients were ultimately observed, without increased regional failure.
Acknowledgments
This project was supported by grants U10CA21661 (RTOG-Ops-Stat), U10CA180822 (NRG Oncology SDMC), U10CA180868 (NRG Oncology Operations) from the National Cancer Institute.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Dansky Ullmann C, Harlan LC, Shavers VL, et al. A population-based study of therapy and survival for patients with head and neck cancer treated in the community. Cancer. 2012;118:4452–4461. doi: 10.1002/cncr.27419. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Villaret DB, Amdur RJ, et al. Planned neck dissection after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck. 2002;24:1012–1018. doi: 10.1002/hed.10187. [DOI] [PubMed] [Google Scholar]
- 4.Thariat J, Ang KK, Allen PK, et al. Prediction of neck dissection requirement after definitive radiotherapy for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:e367–e374. doi: 10.1016/j.ijrobp.2011.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lango MN, Egleston B, Ende K, et al. Impact of neck dissection on long-term feeding tube dependence in patients with head and neck cancer treated with primary radiation or chemoradiation. Head Neck. 2010;32:341–347. doi: 10.1002/hed.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watkins JP, Williams GB, Mascioli AA, et al. Shoulder function in patients undergoing selective neck dissection with or without radiation and chemotherapy. Head Neck. 2011;33:615–619. doi: 10.1002/hed.21503. [DOI] [PubMed] [Google Scholar]
- 8.Liauw SL, Mancuso AA, Amdur RJ, et al. Postradiotherapy neck dissection for lymph node-positive head and neck cancer: The use of computed tomography to manage the neck. J Clin Oncol. 2006;24:1421–1427. doi: 10.1200/JCO.2005.04.6052. [DOI] [PubMed] [Google Scholar]
- 9.Porceddu SV, Pryor DI, Burmeister E, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck. 2011;33:1675–1682. doi: 10.1002/hed.21655. [DOI] [PubMed] [Google Scholar]
- 10.Mendenhall WM, Amdur RJ, Stringer SP, et al. Radiation therapy for squamous cell carcinoma of the tonsillar region: A preferred alternative to surgery? J Clin Oncol. 2000;18:2219–2225. doi: 10.1200/JCO.2000.18.11.2219. [DOI] [PubMed] [Google Scholar]
- 11.Yao M, Smith RB, Graham MM, et al. The role of FDG PET in management of neck metastasis from head-and-neck cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2005;63:991–999. doi: 10.1016/j.ijrobp.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao M, Luo P, Hoffman HT, et al. Pathology and FDG PET correlation of residual lymph nodes in head and neck cancer after radiation treatment. Am J Clin Oncol. 2007;30:264–270. doi: 10.1097/01.coc.0000257611.65290.aa. [DOI] [PubMed] [Google Scholar]
- 15.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 17.Huang SH, O'Sullivan B, Xu W, et al. Temporal nodal regression and regional control after primary radiation therapy for N2-N3 head-and-neck cancer stratified by HPV status. Int J Radiat Oncol Biol Phys. 2013;87:1078–1085. doi: 10.1016/j.ijrobp.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long-term report of efficacy and toxicity. J Clin Oncol. 2014;32:3858–3866. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming ID, Cooper JS, Henson DE, et al. AJCC Cancer Staging Manual. 5th. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 21.Begum S, Gillison ML, Nicol TL, et al. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:1186–1191. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 22.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronhoj Larsen C, Gyldenlove M, Jensen DH, et al. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: A systematic review. Br J Cancer. 2014;110:1587–1594. doi: 10.1038/bjc.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 25.Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm–general principles. Nat Clin Pract Oncol. 2007;4:86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 26.Lango MN, Andrews GA, Ahmad S, et al. Postradiotherapy neck dissection for head and neck squamous cell carcinoma: Pattern of pathologic residual carcinoma and prognosis. Head Neck. 2009;31:328–337. doi: 10.1002/hed.20976. [DOI] [PubMed] [Google Scholar]
- 27.Ganly I, Bocker J, Carlson DL, et al. Viable tumor in post-chemoradiation neck dissection specimens as an indicator of poor outcome. Head Neck. 2011;33:1387–1393. doi: 10.1002/hed.21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zundel MT, Michel MA, Schultz CJ, et al. Comparison of physical examination and fluorodeoxyglucose positron emission tomography/computed tomography 4-6 months after radiotherapy to assess residual head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:e825–e832. doi: 10.1016/j.ijrobp.2010.11.072. [DOI] [PubMed] [Google Scholar]
- 30.Huang SH, Patel S, O'Sullivan B, et al. Longer survival in patients with human papillomavirus-related head and neck cancer after positive postradiation planned neck dissection. Head Neck. 2015;37:946–952. doi: 10.1002/hed.23690. [DOI] [PubMed] [Google Scholar]
- 31.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013;49:79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Clavel S, Charron MP, Belair M, et al. The role of computed tomography in the management of the neck after chemoradiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;82:567–573. doi: 10.1016/j.ijrobp.2010.11.066. [DOI] [PubMed] [Google Scholar]
- 33.Mehanna HW, Wong W, McConkey CC, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374:1444–1454. doi: 10.1056/NEJMoa1514493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


