Abstract
Hypertension affects over 25 % of the population with the incidence continuing to rise, due in part to the growing obesity epidemic. Chronic elevations in sympathetic nerve activity (SNA) are a hallmark of the disease and contribute to elevations in blood pressure through influences on the vasculature, kidney, and heart (i.e., neurogenic hypertension). In this regard, a number of central nervous system mechanisms and neural pathways have emerged as crucial in chronically elevating SNA. However, it is important to consider that “sympathetic signatures” are present, with differential increases in SNA to regional organs that are dependent upon the disease progression. Here, we discuss recent findings on the central nervous system mechanisms and autonomic regulatory networks involved in neurogenic hypertension, in both non-obesity- and obesity-associated hypertension, with an emphasis on angiotensin-II, salt, oxidative and endoplasmic reticulum stress, inflammation, and the adipokine leptin.
Keywords: Sympathetic nerve activity, Autonomic, Blood pressure, Central nervous system, Brain
Introduction
Hypertension remains a leading worldwide cause of mortality and morbidity [1, 2]. While the underlying pathological mechanisms continue to emerge, overactivation of the sympathetic nervous system is a hallmark characteristic of the disease. Once thought to be an innocent byproduct, it is now well accepted that chronic elevations in sympathetic nerve activity (SNA) play a causative role in the development, progression, and pathogenesis of hypertensive conditions [3–5]. In addition to the vascular actions of SNA, sympathetic outflow is intricately involved in the regulation of the cardiovascular system through direct influences on the kidney and heart, and can indirectly influence cardiovascular control through efferent outflow to metabolic organs such as adipose tissue. This widespread influence of the sympathetic nervous system highlights the potential for adverse cardiovascular consequences that contribute to and are associated with hypertensive conditions [5], as highlighted in Table 1. Importantly, accumulating evidence indicates that sympathetic nervous system activation is not a global response, and differential increases in SNA to individual organ systems occur during the development of hypertension [6]. Inherent to the development of effective and safe therapeutic strategies for hypertension is an understanding of the underlying mechanisms involved in the regional control of the sympathetic nervous system. This short review will focus on recent and emerging findings on the central control of SNA, with a specific emphasis on regional sympathetic outflow to “non-vasomotor” organs in non-obesity- and obesity-induced hypertension.
Table 1.
Deleterious consequences of chronically elevated sympathetic nerve activity
| Kidney |
|
| Heart |
|
| Vascular |
|
| Venous |
|
| Immune |
|
| Metabolic |
|
Central Organization of Sympathetic Outflow
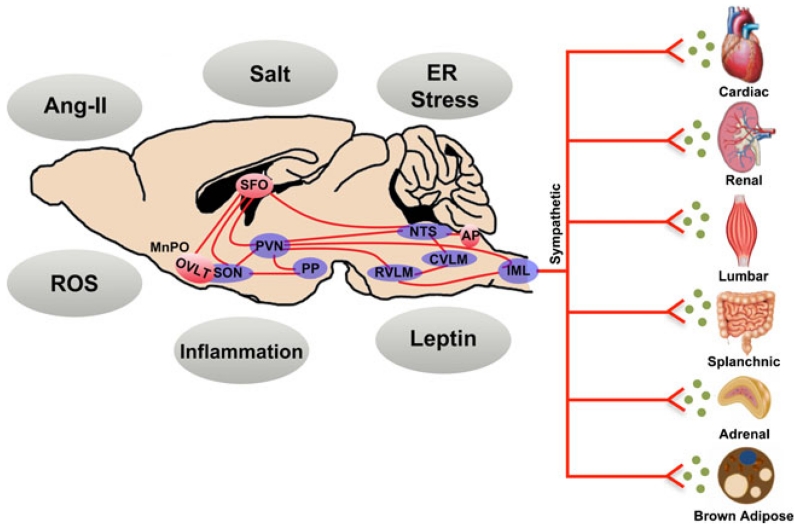
The central nervous system (CNS) control of SNA occurs through the integration of neural signals from autonomic brain networks, input from circulating factors, and reflex influences such as the baroreflex and chemoreflex [7–9] (Fig. 1). In brief, sympathetic preganglionic neurons in the intermediolateral column (IML), a region of gray matter of the spinal cord, receive excitatory drive from many CNS areas including the hypothalamus, medulla oblongata, pons, and amygdala [7]. Within the setting of hypertension, a major focus has been on hypothalamic and hindbrain regions involved in the regulation of SNA, including the paraventricular nucleus of the hypothalamus (PVN) and rostral ventrolateral medulla (RVLM), respectively [10]. The circumventricular organs, unique CNS regions that lack a blood-brain barrier and project to hypothalamic and hindbrain autonomic areas, have also garnered significant attention as they provide a means by which circulating factors can modulate central sympathetic outflow [11]. Within these CNS areas, a balance between a wide number of excitatory and inhibitory neurotransmitters ultimately contribute to neural activation [10]. Adding to this complexity is a topographical organization of neuron populations within certain autonomic nuclei (e.g. RVLM) that preferentially control sympathetic outflow to individual organs [10, 12]. Thus, in simplistic terms, regional-specific regulation of SNA is collectively dependent on a complex interaction between inputs, neural areas, cell populations, and neurotransmitters involved. Several key and emerging mechanisms involved in these processes are described below. The influence of sympathetic outflow to vascular beds has been extensively described, and therefore, we have emphasized recent investigations that have used direct recordings of regional sympathetic nerve fibers to “non-vasomotor” organs including the kidney, heart, and metabolic organs.
Fig. 1.
The control of regional sympathetic outflow to target organs is dependent upon the integration of signaling mechanisms and factors within integrated neural networks. These CNS pathways include circumventricular organs situated outside of the blood brain barrier (red), along with hypothalamic and hindbrain autonomic nuclei. Not shown are afferent reflex inputs, which also modulate activity within these networks. See text for additional details. Ang-II angiotensin II, AP area postrema, CVLM caudal ventral lateral medulla, ER endoplasmic reticulum, IML intermediolateral nucleus, MnPO median preoptic nucleus, NTS, nucleus tractus solitarii, OVLT organum vasculosum lamina terminalis, PP posterior pituitary, PVN paraventricular nucleus of the hypothalamus, ROS reactive oxygen species, RVLM rostral ventral lateral medulla, SFO subfornical organ, SON supraoptic nucleus
Non-obesity Hypertension
Angiotensin-II and Salt
In addition to peripheral cardiovascular influences, angiotensin-II (Ang-II) acting within the CNS is well recognized to increase sympathetic outflow to cardiovascular organs and consequently elevate arterial blood pressure. As recently reviewed [13], a variety of animal models that evoke either elevations in circulating or brain-specific components of the renin-angiotensin system (RAS) have provided insight into the sympathoexcitatory actions of Ang-II, including peripheral or central Ang-II infusion, Ang-II infusion plus a salt diet, deoxycorticosterone acetate (DOCA), and genetic models. To date, the majority of findings have been limited to the recording of SNA at a single timepoint, typically after the development of hypertension. However, a recent elegant study by Osborn and colleagues utilized longitudinal measurements of renal SNA (RSNA) and lumbar SNA (LSNA) during ~2-week infusion of Ang-II in rats on a high salt diet. Interestingly, RSNA transiently decreased and then returned to basal levels, whereas LSNA did not change throughout the course of the study [14•]. Previous findings have also demonstrated a decrease in RSNA in response to chronic Ang-II infusion in rabbits and dogs [15, 16]. These intriguing findings suggest that hypertension due to Ang-II plus high salt and/or Ang-II alone may not be due to elevations in sympathetic outflow to the kidney or hindlimb vasculature. Moreover, they raise important questions such as: Which regional SNA is contributing to elevations in arterial blood pressure if RSNA and LSNA are not elevated? What are the underlying mechanisms in the CNS that drive these differential sympathetic responses to Ang-II or synergistically to Ang-II plus salt?
In this context, several recent findings have advanced our understanding of a role for salt in the central regulation of sympathetic outflow. This is particularly relevant as sympathetic overactivity and central hypernatremia have been reported in salt-sensitive humans [17]. Animal models have established that sympathoexcitation in response to salt loading is dependent upon efferent projections from osmosensitive circumventricular organs to the hypothalamus, as well as spinal and RVLM projecting pathways from the PVN [17]. In a recent tour de force, Stocker et al. used simultaneous recordings of LSNA, RSNA, splanchnic SNA (SSNA), and adrenal SNA to provide additional insight into RVLM mechanisms that mediate salt-induced regional sympathoexcitation [18•]. In response to intracerebroventricular (ICV) infusion of NaCl, a dose-dependent increase in arterial blood pressure, LSNA and adrenal SNA was noted, with no changes in SSNA and a decrease in RSNA. These sympathoexcitatory responses were attenuated following removal of circumventricular organ input (i.e., anteroventral third ventricle lesions), as well as blockade of ionotropic glutamatergic but not Ang-II type 1 receptor (AT1R) signaling in the RVLM. Interestingly, RVLM neuron populations were excited, inhibited, or did not change firing discharge in response to NaCl, suggesting a potential cellular basis for differential control of SNA during salt loading.
With or without salt on board, the CNS hypertensive actions of Ang-II primarily occur through binding to AT1R, which is densely expressed in autonomic regulatory regions. Indeed, AT1R and angiotensin converting enzyme (ACE) expression is elevated in the SFO, PVN, and RVLM in a wide variety of animal models of hypertension [19–23], and pharmacological blockade of AT1R or ACE inhibition in the entire brain or within discrete neural areas decreases hypertension-associated elevations in SNA [21, 24•, 25]. More recent findings have focused on the influence of the brain Ang-II type 2 receptor (AT2R), which often exhibits counter-regulatory actions to AT1R (e.g., neuronal inhibition versus excitation), despite binding of Ang-II to both receptor types [26, 27]. In general, overexpression or activation of AT2R within the CNS regions results in a lowering of blood pressure and catecholamine levels, although limited investigations have directly measured SNA [28]. In support of a sympathoinhibitory action, Gao et al. revealed that RVLM-targeted microinjection of Ang-II in rats elicited an increase in RSNA that was further elevated following pharmacological blockade of AT2R [29] Furthermore, activation of RVLM AT2R alone induced a decrease in RSNA. However, in the context of hypertension, the extent to which AT2R, as well as additional RAS pathways including ACE2 and Ang-(1-7) [30], play in regional activation of sympathetic nerves remains unclear and warrants additional investigation.
Reactive Oxygen Species
It is now well accepted that elevations in reactive oxygen species (ROS) (i.e., oxidative stress) are involved in the pathogenesis of hypertension, both in peripheral cardiovascular organs, as well as within the central cardiovascular nuclei. In line with this ever-growing body of evidence, several recent reports have extended our understanding of CNS oxidative stress in the control of SNA. Within the brain, Ang-II stimulates an increase in ROS primarily via nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase. Activation of the sympathetic nervous system is an important factor in renovascular hypertension, and modeling this disease in rodents using a 2-kidney 1-clip (2K1C) model results in upregulation of AT1R, NAD(P)H oxidase subunits, and ROS in the PVN and RVLM [21, 31–35]. Interestingly, oral administration of the AT1R antagonist losartan in 2K1C hypertensive rats partially reduces RVLM ROS levels and renal sympathetic outflow, concomitant with a lowering of arterial blood pressure and improved baroreflex control of RSNA [25]. Oral dosing of 2K1C animals with a vitamin C antioxidant also elicits reductions in RSNA, blood pressure and messenger RNA (mRNA) levels of NAD(P)H oxidase subunits in the RVLM and PVN [31, 36]. While the peripheral administration of these agents limits interpretation of the site of action (peripheral vs. central), these findings are in line with previous work demonstrating that acute microinjection of losartan, vitamin C, or the antioxidant tempol into the RVLM all result in a decrease in blood pressure and RSNA in 2K1C animals [32, 33]. Similarly, although direct recordings of SNA were not obtained, de Oliveira-Sales et al. and Burmeister et al. demonstrated that viral overexpression of cytoplasmic superoxide dismutase (CuZnSOD) to scavenge superoxide in the RVLM or PVN, respectively, blunted the development of 2K1C-induced hypertension [34, 35].
In addition to ROS-induced elevations in sympathetic outflow to the kidney, emerging evidence implicates similar mechanisms in the control of cardiac SNA (CSNA), which can be technically challenging to evaluate. Yuan et al. utilized an intriguing approach to measure CSNA, in which the cardiac nerve was cut distal to the recording electrode and the decrease in SNA was evaluated in response to topically applied lidocaine upstream (to block conduction) of the recording electrode [37•]. Interestingly, relative to normotensive counterparts, spontaneously hypertensive rats (SHR) demonstrated a greater fall in CSNA in response to lidocaine (i.e., elevated basal SNA), which was restored to normal levels following targeted scavenging of superoxide in the PVN with CuZnSOD [37•]. Perhaps even more intriguing was the finding that the cardiac sympathetic afferent reflex (CSAR), a sympathoexcitatory reflex mediated by metabolites in the heart, was elevated in SHR in a PVN superoxide-related manner [37•]. That is, stimulation of the CSAR resulted in exaggerated increases in RSNA in SHR animals; a response that was blunted following PVN-targeted CuZnSOD. Additional work from the same group has recently demonstrated an exaggerated CSAR in renovascular hypertension that is restored following scavenging of hydrogen peroxide in the PVN [38]. Thus, these findings suggest that afferent cardiac input may elevate ROS in the PVN, which subsequently drives increases in RSNA and hypertension development.
In rodent models of hypertension, increased excitatory glutamatergic input to presympathetic neurons in the PVN, which subsequently project to the RVLM and IML, have been described to play a role in hypertension development [39–41]. Interestingly, in 2K1C rats, chronic inhibition of ACE in the PVN has been shown to reduce ROS in the RVLM in parallel with a reduction in RSNA [21]. These findings suggest a pathway in which PVN angiotensinergic signaling drives downstream oxidative stress in the RVLM to contribute to hypertension, although the cell types and neurotransmitter pathways involved remain unclear. In this context, ROS are known to affect neuronal activity by directly altering the function of Ca2+ and K+ channels, as well as modulating transcription factor-dependent processes [42]. Recent work from Nishihara et al. indicates that modulation of synaptic transmission in central autonomic nuclei by oxidative stress also contributes to hypertension [43]. In brief, using RVLM-targeted overexpression of mitochondrial SOD (manganese, MnSOD) and RSNA recordings in SHR animals, their findings demonstrate that hypertension-associated elevations in ROS in the RVLM enhance glutamatergic excitatory inputs and attenuate GABAergic inhibitory inputs to the RVLM. Furthermore, elevations in RSNA in response to glutamate activation in the PVN were blunted following RVLM-specific scavenging of superoxide, suggesting that ROS in the RVLM modulate excitatory input from the PVN. These findings are interesting in light of the fact that mitochondrial ROS have been shown to enhance (versus attenuate) GABAergic signaling in cerebellar cells, highlighting potential physiological versus pathophysiological roles of brain ROS, along with regional specificity in the responses [44]. Collectively, these recent studies support previous work demonstrating a clear role for ROS in neural cardioregulatory regions in elevating renal and cardiac sympathetic outflow (as well as SNA to vascular beds) in non-obese hypertensive conditions. However, our understanding of the ROS-induced cellular signaling mechanisms, neurotransmitter relationships, and precise neural networks involved remain in its infancy.
Inflammation
The immune system, in particular the adaptive arm, is involved in the pathogenesis of hypertension [45, 46]. In addition to inflammatory-mediated events in peripheral cardiovascular organs, circulating proinflammatory cytokines can influence cardiovascular and autonomic regulation through actions at circumventricular organs, afferent-mediated signaling, or via entry into the brain due to the blood-brain barrier disruption, such as occurs during hypertension. Local production of cytokines within the CNS represents an additional means linking inflammation to hypertension development [47]. Despite the rapid expansion of this field of investigation, there are relatively few reports that have directly examined immune-mediated control of SNA to regional organs. In line with studies from over 20 years ago revealing cytokine-induced elevations in adrenal, splenic, and RSNA [48, 49], Felder and colleagues found that peripheral administration of the proinflammatory cytokine tumor necrosis factor-α (TNF-α) promoted firing of PVN and RVLM cells with simultaneous elevations in RSNA and arterial blood pressure [50]. In a recent follow-up study, they further demonstrated that direct microinjection of TNF-α and interleukin-1β (IL-1β) into the SFO mimicked the renal sympathoexcitatory and pressor responses seen with peripheral cytokine administration; a response that was dependent on angiotensinergic and cyclooxygenase-2 mechanisms [24]. Interestingly, SFO-targeted proinflammatory cytokine delivery resulted in upregulation of angiotensinergic and cyclooxygenase-2 components in both the SFO and downstream PVN. In the setting of chronic hypertension, SHR demonstrate elevated TNF-α and IL-1β in the PVN and RVLM, relative to normotensive controls [23, 51], and PVN-targeted microinjection of these cytokines results in exaggerated increases in RSNA and arterial blood pressure in hypertensive animals [52]. Collectively, these findings are consistent with the critical role of the SFO in sensing blood-borne factors and subsequently influencing sympathetic outflow through efferent hypothalamic and hindbrain projections, but also suggest that inflammatory mechanisms within the PVN and RVLM may also contribute to hypertension development. Although beyond the scope of this review, the influence of SNA on the peripheral immune system should be considered, including a recently revealed brain to bone marrow sympathetic connection [53, 54]. There are several excellent reviews on this topic [55, 56].
Endoplasmic Reticulum Stress
The endoplasmic reticulum (ER) is a specialized organelle involved in protein folding, maturation, and processing, and is thus intimately involved in cellular homeostasis in response to internal and external stimuli. In situations where the protein load exceeds the ER folding capacity, a collection of conserved signaling pathways, termed the unfolded protein response (UPR), are activated as a conserved mechanism to preserve ER function. While beneficial in the short-term, chronic UPR activation (i.e., ER stress) is now recognized as a pathological mechanism in a variety of diseases. Due to the close relationship between the ER, ROS, and inflammatory signaling, we recently postulated that ER stress in the CNS may represent a central integrating factor in hypertension development. Using a mouse model of Ang-II-mediated hypertension, global pharmacological blockade of brain ER stress, or selectively reducing ER stress in the SFO with a molecular chaperone, was found to prevent hypertension. In line with this, Chao et al. revealed activation of the UPR in the RVLM of SHR prior to hypertension development, and intracisternal infusion (to target medullary regions) of an ER stress inhibitor resulted in a decrease in arterial blood pressure [57]. Given that these models of hypertension have a clear neurogenic component, it has been presumed that ER stress in cardiovascular control areas elevates SNA. We and Purkansantha et al. have demonstrated that acute induction of brain ER stress, with ICV administration of the chemical ER stress inducer thapsigargin, results in robust elevations in RSNA [58, 59]. The extent to which these acute SNA findings can be translated to the setting of chronic hypertension, as well as the central nuclei involved and the influence of ER stress on regional sympathetic outflow to other organs, is an open area of investigation.
Obesity Hypertension
Obesity affects more than a third of the population in Western societies and significantly increases the risk of cardiovascular diseases including hypertension. Similar to “non-obese” forms of hypertension, activation of SNA to cardiovascular organs is implicated. Several key CNS factors that may play a role in the control of regional SNA in obesity-induced hypertension are presented below, highlighting critical missing points that need to be addressed when appropriate.
Ang-II
Similar to humans, diet-induced obesity in laboratory animals promotes the development of hypertension, and this is associated with upregulation of angiotensingergic signaling in the central autonomic nuclei including the lamina terminalis and PVN [60–62]. Consistent with the importance of brain Ang-II in hypertension development, selective removal of the AT1R from the PVN blunts obesity-induced hypertension, despite elevations in adiposity [60]. Similarly, we have found that short-term ICVadministration of losartan rescues high fat diet (HFD)-mediated hypertension, independent of an effect on body weight, adiposity or food intake (unpublished observations). However, the extent to which the sympathetic nervous system is involved in these responses remains unclear. Interestingly, a series of studies by Head and colleagues utilizing chronic SNA recordings in rabbits demonstrate that RSNA is elevated and baroreflex control of RSNA is impaired within 1 week after the start of a HFD and these changes persist for up to 3 weeks [63, 64•]. Recent findings also indicate fairly rapid upregulation of RAS components in the lamina terminalis in response to diet-induced obesity (3 weeks HFD) [61]. Thus, although the precise role of Ang-II in mediating sympathoexcitation in obesity-induced hypertension is yet to be determined, the pieces are emerging to implicate this central pathway. However, extensive work is required, particularly CNS spatiotemporal evaluation of the brain RAS, in conjunction with examination of Ang-II-mediated control of regional SNA during obesity-mediated hypertension.
ROS, Inflammation, ER Stress
Despite a plethora of evidence implicating brain oxidative stress in the development of virtually every other form of hypertension, limited evidence exists for this pathway in obesity-induced hypertension. In response to HFD feeding, Nagae et al. found upregulation of NAD(P)H oxidase subunits along with enhanced NAD(P)H oxidase activity in the whole hypothalamus, suggestive of increased ROS production [65]. Furthermore, ICV infusion of the antioxidants tempol or apocynin, or the NAD(P)H oxidase inhibitor diphenyleneiodonium, lowered RSNA and arterial blood pressure to a greater extent in obese hypertensive animals, relative to lean normotensive controls. Subsequent work has demonstrated elevations in ROS markers in the RVLM of obesity-prone rats, relative to obesity-resistant animals, along with an exaggerated depressor response to RVLM-specific tempol administration [66]. These findings are consistent with previous work implicating the RVLM in obesity-induced hypertension and suggest that oxidative stress in the hypothalamus and RVLM may contribute [67, 68]. However, a role for oxidative stress in other neural regions and regional sympathetic control cannot be excluded.
As mentioned above, emerging evidence indicates activation of the RAS in neural cardioregulatory regions, and interestingly this appears to occur in parallel with CNS inflammation. Indeed, Xue et al. found elevations in proinflammatory cytokines in the lamina terminalis following 3 weeks of HFD feeding in rats and ICV administration of an AT1R antagonist prevented obesity-induced lamina terminalis inflammation [61]. Following longer-term obesity (8 weeks HFD) parallel RAS, inflammatory gene, microglia, and astrocyte activation in the SFO, PVN and mediobasal hypothalamus have also been noted [62]. Moreover, selective removal of the AT1R from the PVN was shown to blunt the HFD-mediated inflammatory events in this region. Thus, evidence exists for obesity-induced activation of inflammatory cascades in central autonomic regions that is due, at least in part, to the RAS. Cai and colleagues have demonstrated that blockade of nuclear factor-κ-B (NF-κB), a key transcription factor involved in inflammatory processes, in the mediobasal hypothalamus lowers arterial blood pressure in obese mice [69]. In line with the intimate relationship between inflammation and ER stress, their results also illustrate obesity-associated elevations in ER stress in the mediobasal hypothalamus and show that short-term pharmacological rescue of brain ER stress reduces obesity-induced hypertension [58]. We have recently confirmed these findings and have further found activation of the UPR in CNS autonomic control areas including the SFO, PVN, and RVLM following the development of obesity-induced hypertension [70, 71]. Given that inflammation and ER stress contribute to elevations in sympathetic outflow [24•, 50, 58, 59], it is likely that these hypertensive mediators contribute to obesity-associated elevations in SNA, although this remains to be explored.
Leptin
The adipokine leptin is produced and secreted in proportion to white adipose tissue mass, thus, making hyperleptinemia a key characteristic of obesity. In addition to pleiotropic influences on energy homeostasis, fat mass and feeding behavior, leptin also increases SNA to a range of tissues including vascular, renal, adrenal, adipose tissue, liver, and bone [72, 73•]. Importantly, in the setting of obesity, the beneficial anorexic and metabolic SNA actions of leptin are lost while the cardiovascular SNA influences are maintained (i.e., selective leptin resistance) [72, 73•]. In this regard, leptin has been suggested to be a major player in obesity-induced hypertension, and there is a strong positive association between plasma leptin levels and renal norepinephrine spillover in human subjects with different levels of adiposity [74]. The role of leptin in the control of SNA and blood pressure in obesity has been recently detailed in depth, including the nuances involved when translating findings with leptin into the setting of human obesity [72, 73•]. For this reason, here, we briefly touch upon several recent papers related to the underlying neurocircuitry and signaling mechanisms involved in leptin-mediated control of SNA.
As mentioned above, a series of studies using conscious longitudinal sympathetic recordings in rabbits have demonstrated that RSNA is increased within 1 week after starting HFD, and interestingly this occurs in parallel with elevations in plasma leptin [64•]. In line with this, ICV leptin was found to cause dose-dependent increases in RSNA to a greater extent in obese hypertensive rabbits relative to lean normotensive controls [75], consistent with previous work in rodent models of obesity [76, 77]. Furthermore, the elevations in RSNA and arterial blood pressure in HFD-fed rabbits were reversed by ICV administration of a leptin antagonist, but not an insulin antagonist [63]. These recent investigations build upon a large body of evidence supporting a role for leptin in sympathetic activation [73•]. However, it is important to consider that the majority of investigations have been performed in male animals. Recent findings from Shi and Brooks indicate that ICV leptin administration increases RSNA (and LSNA) in female rats, but only during proestrous (i.e., high gonadal hormones) or in ovarectomized females treated with 17β-estradiol [78]. Moreover, ICV leptin was shown to increase arterial blood pressure in male animals, but not females. Whether these intriguing findings translate to obesity is unknown, but suggest that sex and estrogen-dependent sympathetic responses to leptin may exist, which warrant consideration.
Since the realization that leptin increases SNA to a variety of tissues, a primary investigative focus has been on the underlying CNS intracellular mechanisms. This is important in the context of obesity as distinct transduction pathways could modulate metabolic and cardiovascular related SNA, thus providing a molecular explanation for selective leptin resistance [72, 73•]. For example, phosphoinositol-3 kinase and downstream mammalian target of rapamycin signaling have been shown to be necessary for leptin-induced elevations in RSNA [79, 80], whereas the signal transducer and activator of transcription 3 and MAPK/ERK pathways may not be involved [81, 82]. The reader is referred to several excellent reviews that have extensively covered this topic [72, 73•]. An expanding body of literature also indicates an underlying neuroanatomical organization in leptin-mediated sympathoexcitation [83]. Hypothalamic regions have garnered the majority of attention, and indeed direct microinjection of leptin into the arcuate nucleus, dorsomedial, and ventromedial portions of the hypothalamus have been shown to elevate RSNA and arterial blood pressure [84, 85]. Building upon this, Harlan et al. recently utilized Cre-LoxP technology to selectively ablate leptin receptors from the arcuate nucleus [86]. In doing so, leptin administration did not increase RSNA and brown adipose tissue SNA in control animals. Moreover, the elevations in RSNA and blood pressure in response to leptin in obese animals were abolished, suggesting that leptin receptors in the arcuate nucleus contribute to obesity-induced renal sympathoexcitation and hypertension.
Importantly, leptin receptors are distributed throughout the CNS, and there is also evidence for extra-hypothalamic nuclei involvement [87]. In support of hindbrain control, direct microinjection of leptin into the brainstem nucleus tractus solitarii increases RSNA [88], and recent observations demonstrate that RVLM-targeted leptin administration also increases RSNA and blood pressure [89]. Moreover, retrograde tracing techniques have illustrated direct RVLM leptin expressing projections to the kidney. We have also begun to explore the role of leptin signaling in the SFO in the control of SNA. The unique location of this neural region outside of the blood-brain barrier highlights the potential for the SFO to play a key role in integrating metabolic and cardiovascular signals [90]. Using an approach similar to Harlan et al. [86], we found that removal of the leptin receptor from the SFO abolished leptin-induced elevations in RSNA, but did not influence the body weight, food intake, or the brown adipose tissue SNA effects of leptin [91]. While the extent to which our findings translate to obesity remain unclear, the selectivity in the responsiveness of the SFO may be important in explaining differential control of SNA in the setting of obesity. For example, in obesity, the arcuate nucleus becomes leptin resistant [92], which would potentially explain impaired leptin-mediated metabolic effects. In contrast, if the SFO remains leptin-sensitive, this could potentially contribute to the preserved elevations in RSNA in response to leptin, although dissection of these intricate relationships requires further investigation. Overall, these recent findings illustrate a distributed and interconnected network, from the forebrain to hindbrain, that are critical in mediating the SNA responses to leptin.
In addition to the emergence of novel sites of action and leptin signaling pathways, the interaction of other receptors with leptin is becoming evident. Specifically, a unique leptin-angiotensinergic action in the control of SNA has recently been identified. Hilzendeger and colleagues demonstrated that AT1aR knockout mice and brain-specific RAS blockade attenuated leptin-induced increases in RSNA and brown adipose tissue SNA [93]. Interestingly, we recently found that selective genetic removal of the AT1aR from the SFO prevented leptin-mediated elevations in SNA to brown adipose tissue, suggesting that it is AT1aR, not the leptin receptor in the SFO, that plays an important role in leptin-brown adipose tissue SNA regulation [94]. Together, these findings suggest that changes in the circulating or brain RAS (i.e., obesity) could modulate the cardiovascular and metabolic sympathetic effects of leptin.
Conclusions
An abundance of evidence indicates that chronic elevations in SNA contribute to the development of hypertension. However, activation of the sympathetic nervous system is not a global response and “sympathetic signatures” exist with differential increases in SNA to target organs depending on the stage of hypertension development [95]. Integrating findings on non-vasomotor sympathetic outflow, along with the vascular actions of SNA, are key to deciphering the relative contribution of region-specific SNA to hypertension development. Furthermore, while a number of putative CNS sympathetic control mechanisms have been highlighted, the intricate interplay between these factors, particularly in the contribution to hypertension, is only beginning to emerge. Moving forward, consideration of multiple CNS pathological mechanisms in an integrative fashion, from the cellular to neural network to whole systems level, will be necessary to advance our understanding and treatment of sympathetic overactivity in hypertensive states. This may be particularly relevant for the growing epidemic of obesity-induced hypertension as it is becoming evident that CNS pathways/factors that are classically involved in sympathetic cardiovascular control also modulate sympathetic outflow to metabolic organs [94]. Consideration for the mechanisms discussed above, along with additional factors (e.g., insulin, resistin) [96, 97] and reflex pathways (e.g., adipose tissue afferent reflexes) [98] not discussed in this review, will be crucial in this regard. Lastly, it is important to consider regional sympathetic outflow in both hypertensive human and animal models. For instance, norepinephrine spillover to the kidney (i.e., RSNA) is elevated in hypertensive humans [99], which is opposite to the depressed or lack of change in RSNA observed in animals following exposure to Ang-II infusion [14•, 15, 16], Ang-II plus salt [14•], or acute NaCl administration [18•]. While animal models are essential to investigate CNS control of sympathetic outflow, considering the requisite advantages and disadvantages will be essential when translating findings from animal studies to the clinical setting of human hypertension.
Footnotes
Conflict of Interest The authors declare a grant from the National Institutes of Health (HL116776).
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. doi:10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Nickey WA, Lenfant C, Chobanian AV, Roccella EJ. The National High Blood Pressure Education Program: longtime partners with new strategies. J Am Osteopath Assoc. 2003;103(6):297–9. [PubMed] [Google Scholar]
- 3.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285(23):17271–6. doi: 10.1074/jbc.R110.113175. doi:10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116(6):976–90. doi: 10.1161/CIRCRESAHA.116.303604. doi:10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci. 2009;148(1-2):5–15. doi: 10.1016/j.autneu.2009.02.003. doi:10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp Physiol. 2010;95(1):61–8. doi: 10.1113/expphysiol.2008.046326. doi:10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74(2):323–64. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 8.Thrasher TN. Arterial baroreceptor input contributes to long-term control of blood pressure. Curr Hypertens Rep. 2006;8(3):249–54. doi: 10.1007/s11906-006-0058-z. [DOI] [PubMed] [Google Scholar]
- 9.Loewy AD, Spyer KM. Central autonomic pathways. Oxford University Press; New York: 1990. pp. 88–103. [Google Scholar]
- 10.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–46. doi: 10.1038/nrn1902. doi:10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 11.Simpson JB. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology. 1981;32(4):248–56. doi: 10.1159/000123167. [DOI] [PubMed] [Google Scholar]
- 12.Pyner S, Coote JH. Rostroventrolateral medulla neurons preferentially project to target-specified sympathetic preganglionic neurons. Neuroscience. 1998;83(2):617–31. doi: 10.1016/s0306-4522(97)00355-2. [DOI] [PubMed] [Google Scholar]
- 13.Young CN, Davisson RL. Angiotensin-II, the brain, and hypertension: an update. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.115.03624. doi:10.1161/HYPERTENSIONAHA.115.03624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension. 2010;55(3):644–51. doi: 10.1161/HYPERTENSIONAHA.109.145110. doi:10.1161/HYPERTENSIONAHA.109.145110. This paper utilized longitudinal measurements of RSNA and LSNA in conscious rats during the development of Ang-II salt hypertension. Findings from this study indicate that RSNA decreased within a few days of Ang-II + high salt diet and returned to baseline after ~10 days, whereas LSNA did not change over the course of the study.
- 15.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res. 2003;92(12):1330–6. doi: 10.1161/01.RES.0000078346.60663.A0. doi:10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- 16.Carroll RG, Lohmeier TE, Brown AJ. Chronic angiotensin II infusion decreases renal norepinephrine overflow in conscious dogs. Hypertension. 1984;6(5):675–81. doi: 10.1161/01.hyp.6.5.675. [DOI] [PubMed] [Google Scholar]
- 17.Stocker SD, Monahan KD, Browning KN. Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr Hypertens Rep. 2013;15(6):538–46. doi: 10.1007/s11906-013-0385-9. doi:10.1007/s11906-013-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Stocker SD, Lang SM, Simmonds SS, Wenner MM, Farquhar WB. Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension. 2015;66(6):1184–90. doi: 10.1161/HYPERTENSIONAHA.115.05936. doi:10.1161/HYPERTENSIONAHA.115.05936. This paper reported simultaneous recordings of LSNA, RSNA, SSNA, and adrenal SNA in response to ICV administration of NaCl. Overall, the findings provide insight into RVLM mechanisms that mediate salt-induced regional sympathoexcitation.
- 19.Zhang M, Qin DN, Suo YP, Su Q, Li HB, Miao YW, et al. Endogenous hydrogen peroxide in the hypothalamic paraventricular nucleus regulates neurohormonal excitation in high salt-induced hypertension. Toxicol Lett. 2015;235(3):206–15. doi: 10.1016/j.toxlet.2015.04.008. doi:10.1016/j.toxlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, et al. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension. 2013;61(3):716–22. doi: 10.1161/HYPERTENSIONAHA.111.00356. doi:10.1161/HYPERTENSIONAHA.111.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HB, Qin DN, Ma L, Miao YW, Zhang DM, Lu Y, et al. Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicol Appl Pharmacol. 2014;279(2):141–9. doi: 10.1016/j.taap.2014.06.004. doi:10.1016/j.taap.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Upregulation of AT1R and iNOS in the rostral ventrolateral medulla (RVLM) is essential for the sympathetic hyperactivity and hypertension in the 2K-1C Wistar rat model. Am J Hypertens. 2010;23(7):708–15. doi: 10.1038/ajh.2010.64. doi:10.1038/ajh.2010.64. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol. 2011;106(6):1069–85. doi: 10.1007/s00395-011-0231-7. doi:10.1007/s00395-011-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126–33. doi: 10.1161/HYPERTENSIONAHA.114.05112. doi:10.1161/HYPERTENSIONAHA.114.05112. This study demonstrates that proinflammatory cytokines in the SFO lead to increases in RSNA and blood pressure via angiotensinergic and cyclooxygenase-2 mechanisms. The authors also report that proinflammatory cytokines in the SFO upregulate angiotensinergic and cyclooxygenase-2 pathways in downstream PVN areas.
- 25.Nishi EE, Bergamaschi CT, Oliveira-Sales EB, Simon KA, Campos RR. Losartan reduces oxidative stress within the rostral ventrolateral medulla of rats with renovascular hypertension. Am J Hypertens. 2013;26(7):858–65. doi: 10.1093/ajh/hpt037. doi:10.1093/ajh/hpt037. [DOI] [PubMed] [Google Scholar]
- 26.Zhu M, Natarajan R, Nadler JL, Moore JM, Gelband CH, Sumners C. Angiotensin II increases neuronal delayed rectifier K(+) current: role of 12-lipoxygenase metabolites of arachidonic acid. J Neurophysiol. 2000;84(5):2494–501. doi: 10.1152/jn.2000.84.5.2494. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura T, Kumagai H, Onimaru H, Kawai A, Iigaya K, Onami T, et al. Electrophysiological properties of rostral ventrolateral medulla neurons in angiotensin II 1a receptor knockout mice. Hypertension. 2005;46(2):349–54. doi: 10.1161/01.HYP.0000173421.97463.ac. doi:10.1161/01.HYP.0000173421.97463.ac. [DOI] [PubMed] [Google Scholar]
- 28.Gao L, Zucker IH. AT2 receptor signaling and sympathetic regulation. Curr Opin Pharmacol. 2011;11(2):124–30. doi: 10.1016/j.coph.2010.11.004. doi:10.1016/j.coph.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52(4):708–14. doi: 10.1161/HYPERTENSIONAHA.108.116228. doi:10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Zhang F, Zhou YB, Cui BP, Han Y. Superoxide anions modulate the effects of angiotensin-(1-7) in the rostral ventrolateral medulla on cardiac sympathetic afferent reflex and sympathetic activity in rats. Neuroscience. 2012;223:388–98. doi: 10.1016/j.neuroscience.2012.07.048. doi:10.1016/j.neuroscience.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 31.Campos RR, Oliveira-Sales EB, Nishi EE, Paton JF, Bergamaschi CT. Mechanisms of renal sympathetic activation in renovascular hypertension. Exp Physiol. 2015;100(5):496–501. doi: 10.1113/expphysiol.2014.079855. doi:10.1113/expphysiol.2014.079855. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira-Sales EB, Nishi EE, Carillo BA, Boim MA, Dolnikoff MS, Bergamaschi CT, et al. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am J Hypertens. 2009;22(5):484–92. doi: 10.1038/ajh.2009.17. doi:10.1038/ajh.2009.17. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira-Sales EB, Dugaich AP, Carillo BA, Abreu NP, Boim MA, Martins PJ, et al. Oxidative stress contributes to renovascular hypertension. Am J Hypertens. 2008;21(1):98–104. doi: 10.1038/ajh.2007.12. doi:10.1038/ajh.2007.12. [DOI] [PubMed] [Google Scholar]
- 34.Burmeister MA, Young CN, Braga VA, Butler SD, Sharma RV, Davisson RL. In vivo bioluminescence imaging reveals redox-regulated activator protein-1 activation in paraventricular nucleus of mice with renovascular hypertension. Hypertension. 2011;57(2):289–97. doi: 10.1161/HYPERTENSIONAHA.110.160564. doi:10.1161/HYPERTENSIONAHA.110.160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira-Sales EB, Colombari DS, Davisson RL, Kasparov S, Hirata AE, Campos RR, et al. Kidney-induced hypertension depends on superoxide signaling in the rostral ventrolateral medulla. Hypertension. 2010;56(2):290–6. doi: 10.1161/HYPERTENSIONAHA.110.150425. doi:10.1161/HYPERTENSIONAHA.110.150425. [DOI] [PubMed] [Google Scholar]
- 36.Nishi EE, Oliveira-Sales EB, Bergamaschi CT, Oliveira TG, Boim MA, Campos RR. Chronic antioxidant treatment improves arterial renovascular hypertension and oxidative stress markers in the kidney in Wistar rats. Am J Hypertens. 2010;23(5):473–80. doi: 10.1038/ajh.2010.11. doi:10.1038/ajh.2010.11. [DOI] [PubMed] [Google Scholar]
- 37•.Yuan N, Zhang F, Zhang LL, Gao J, Zhou YB, Han Y, et al. SOD1 gene transfer into paraventricular nucleus attenuates hypertension and sympathetic activity in spontaneously hypertensive rats. Pflugers Arch - Eur J Physiol. 2013;465(2):261–70. doi: 10.1007/s00424-012-1173-0. doi:10.1007/s00424-012-1173-0. This paper performed technically challenging direct recordings of CSNA in hypertensive rats. The findings indicate that CSNA is elevated in SHR animals, relative to normotensive controls, and that the cardiac sympathetic afferent reflex may contribute to sympathoexcitation and hypertension via PVN oxidative stress mechanisms.
- 38.Xu Y, Gao Q, Gan XB, Chen L, Zhang L, Zhu GQ, et al. Endogenous hydrogen peroxide in paraventricular nucleus mediates sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol. 2011;96(12):1282–92. doi: 10.1113/expphysiol.2011.059733. doi:10.1113/expphysiol.2011.059733. [DOI] [PubMed] [Google Scholar]
- 39.Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007;320(2):615–26. doi: 10.1124/jpet.106.109538. doi:10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- 40.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586(6):1637–47. doi: 10.1113/jphysiol.2007.149732. doi:10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li DP, Zhou JJ, Pan HL. Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Physiol. 2015;593(19):4439–52. doi: 10.1113/JP270831. doi:10.1113/JP270831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerman MC. Angiotensin II and angiotensin-1-7 redox signaling in the central nervous system. Curr Opin Pharmacol. 2011;11(2):138–43. doi: 10.1016/j.coph.2011.01.001. doi:10.1016/j.coph.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishihara M, Hirooka Y, Matsukawa R, Kishi T, Sunagawa K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens. 2012;30(1):97–106. doi: 10.1097/HJH.0b013e32834e1df4. doi:10.1097/HJH.0b013e32834e1df4. [DOI] [PubMed] [Google Scholar]
- 44.Accardi MV, Daniels BA, Brown PM, Fritschy JM, Tyagarajan SK, Bowie D. Mitochondrial reactive oxygen species regulate the strength of inhibitory GABA-mediated synaptic transmission. Nat Commun. 2014;5:3168. doi: 10.1038/ncomms4168. doi:10.1038/ncomms4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ. 2014;38(1):20–4. doi: 10.1152/advan.00063.2013. doi:10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116(6):1022–33. doi: 10.1161/CIRCRESAHA.116.303697. doi:10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol. 2010;37(2):e52–7. doi: 10.1111/j.1440-1681.2009.05234.x. doi:10.1111/j.1440-1681.2009.05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niijima A, Hori T, Aou S, Oomura Y. The effects of interleukin-1 beta on the activity of adrenal, splenic and renal sympathetic nerves in the rat. J Auton Nerv Syst. 1991;36(3):183–92. doi: 10.1016/0165-1838(91)90042-2. [DOI] [PubMed] [Google Scholar]
- 49.Kannan H, Tanaka Y, Kunitake T, Ueta Y, Hayashida Y, Yamashita H. Activation of sympathetic outflow by recombinant human interleukin-1 beta in conscious rats. Am J Physiol. 1996;270(2 Pt 2):R479–85. doi: 10.1152/ajpregu.1996.270.2.R479. [DOI] [PubMed] [Google Scholar]
- 50.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;284(4):R916–27. doi: 10.1152/ajpregu.00406.2002. doi:10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- 51.Masson GS, Nair AR, Silva Soares PP, Michelini LC, Francis J. Aerobic training normalizes autonomic dysfunction, HMGB1 content, microglia activation and inflammation in hypothalamic paraventricular nucleus of SHR. Am J Physiol Heart Circ Physiol. 2015;309(7):H1115–22. doi: 10.1152/ajpheart.00349.2015. doi:10.1152/ajpheart.00349.2015. [DOI] [PubMed] [Google Scholar]
- 52.Shi Z, Jiang SJ, Wang GH, Xu AL, Guo L. Pro-inflammatory cytokines in paraventricular nucleus mediate the cardiac sympatheticafferent reflex in hypertension. Auton Neurosci. 2014;186:54–61. doi: 10.1016/j.autneu.2014.10.001. doi:10.1016/j.autneu.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117(2):178–91. doi: 10.1161/CIRCRESAHA.117.305853. doi:10.1161/CIRCRESAHA.117.305853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63(3):542–50. doi: 10.1161/HYPERTENSIONAHA.113.02722. doi:10.1161/HYPERTENSIONAHA.113.02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zubcevic J, Santisteban MM, Pitts T, Baekey DM, Perez PD, Bolser DC, et al. Functional neural-bone marrow pathways: implications in hypertension and cardiovascular disease. Hypertension. 2014;63(6):e129–39. doi: 10.1161/HYPERTENSIONAHA.114.02440. doi:10.1161/HYPERTENSIONAHA.114.02440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Case AJ, Zimmerman MC. Sympathetic-mediated activation versus suppression of the immune system: consequences for hypertension. J Physiol. 2016;594(3):527–36. doi: 10.1113/JP271516. doi:10.1113/JP271516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chao YM, Lai MD, Chan JY. Redox-sensitive endoplasmic reticulum stress and autophagy at rostral ventrolateral medulla contribute to hypertension in spontaneously hypertensive rats. Hypertension. 2013;61(6):1270–80. doi: 10.1161/HYPERTENSIONAHA.111.00469. doi:10.1161/HYPERTENSIONAHA.111.00469. [DOI] [PubMed] [Google Scholar]
- 58.Purkayastha S, Zhang H, Zhang G, Ahmed Z, Wang Y, Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci U S A. 2011;108(7):2939–44. doi: 10.1073/pnas.1006875108. doi:10.1073/pnas.1006875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, et al. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122(11):3960–4. doi: 10.1172/JCI64583. doi:10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, et al. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33(11):4825–33. doi: 10.1523/JNEUROSCI.3806-12.2013. doi:10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, et al. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II-elicited hypertension. Hypertension. 2016;67(1):163–70. doi: 10.1161/HYPERTENSIONAHA.115.06263. doi:10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, et al. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav. 2014;136:31–8. doi: 10.1016/j.physbeh.2014.01.016. doi:10.1016/j.physbeh.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61(3):628–34. doi: 10.1161/HYPERTENSIONAHA.111.00705. doi:10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 64•.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, et al. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60(1):163–71. doi: 10.1161/HYPERTENSIONAHA.111.190413. doi:10.1161/HYPERTENSIONAHA.111.190413. The authors demonstrate that RSNA and arterial blood pressure increases, whereas baroreflex control of RSNA is blunted, in rabbits within 1 week after starting a high fat diet.
- 65.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation. 2009;119(7):978–86. doi: 10.1161/CIRCULATIONAHA.108.824730. doi:10.1161/CIRCULATIONAHA.108.824730. [DOI] [PubMed] [Google Scholar]
- 66.Kishi T, Hirooka Y, Ogawa K, Konno S, Sunagawa K. Calorie restriction inhibits sympathetic nerve activity via anti-oxidant effect in the rostral ventrolateral medulla of obesity-induced hypertensive rats. Clin Exp Hypertens. 2011;33(4):240–5. doi: 10.3109/10641963.2011.583969. doi:10.3109/10641963.2011.583969. [DOI] [PubMed] [Google Scholar]
- 67.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension. 2007;49(3):640–6. doi: 10.1161/01.HYP.0000254828.71253.dc. doi:10.1161/01.HYP.0000254828.71253.dc. [DOI] [PubMed] [Google Scholar]
- 68.Suhaimi FW, Yusoff NH, Dewa A, Yusof AP. Role of excitatory amino acid input in rostral ventrolateral medulla neurons in rats with obesity-induced hypertension. Acta Neurol Belg. 2010;110(1):57–64. [PubMed] [Google Scholar]
- 69.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17(7):883–7. doi: 10.1038/nm.2372. doi:10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mark AL, Young CN, Butler SD, Guruju M, Davisson RL. Inhibition of brain endoplasmic reticulum stress attenuates hypertension in diet-induced obesity. FASEB J. 2012;26 705.1. [Google Scholar]
- 71.Guruju M, Ho DJ, Young CN, Butler SD, Mark AL, Davisson RL. Diet-induced obesity (DIO) activates the PERK branch of the endoplasmic reticulum (ER) stress response in cardio-regulatory nuclei of the brain. Hypertension. 2011;58:e33–183. [Google Scholar]
- 72.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. doi:10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305(6):R566–81. doi: 10.1152/ajpregu.00180.2013. doi:10.1152/ajpregu.00180.2013. This is a comprehensive review on the mechanisms of selective leptin action in the control of SNA in obesity.
- 74.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41(5):1072–9. doi: 10.1161/01.HYP.0000066289.17754.49. doi:10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 75.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, et al. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55(4):862–8. doi: 10.1161/HYPERTENSIONAHA.109.141119. doi:10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 76.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension. 2002;39(2 Pt 2):486–90. doi: 10.1161/hy0202.102836. [DOI] [PubMed] [Google Scholar]
- 77.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54(7):2012–8. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 78.Shi Z, Brooks VL. Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: role of oestrogen. J Physiol. 2015;593(7):1633–47. doi: 10.1113/jphysiol.2014.284638. doi:10.1113/jphysiol.2014.284638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 2013;17(4):599–606. doi: 10.1016/j.cmet.2013.02.017. doi:10.1016/j.cmet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41(3 Pt 2):763–7. doi: 10.1161/01.HYP.0000048342.54392.40. doi:10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 81.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58(3):536–42. doi: 10.2337/db08-0822. doi:10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harlan SM, Morgan DA, Dellsperger DJ, Myers MG, Jr, Mark AL, Rahmouni K. Cardiovascular and sympathetic effects of disrupting tyrosine 985 of the leptin receptor. Hypertension. 2011;57(3):627–32. doi: 10.1161/HYPERTENSIONAHA.110.166538. doi:10.1161/HYPERTENSIONAHA.110.166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harlan SM, Rahmouni K. Neuroanatomical determinants of the sympathetic nerve responses evoked by leptin. Clin Auton Res. 2013;23(1):1–7. doi: 10.1007/s10286-012-0168-4. doi:10.1007/s10286-012-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49(3):647–52. doi: 10.1161/01.HYP.0000254827.59792.b2. doi:10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 85.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42(4):488–93. doi: 10.1161/01.HYP.0000090097.22678.0A. doi:10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 86.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108(7):808–12. doi: 10.1161/CIRCRESAHA.111.240226. doi:10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9(2):117–23. doi: 10.1016/j.cmet.2008.12.001. doi:10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53(2):375–80. doi: 10.1161/HYPERTENSIONAHA.108.124255. doi:10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barnes MJ, McDougal DH. Leptin into the rostral ventral lateral medulla (RVLM) augments renal sympathetic nerve activity and blood pressure. Front Neurosci. 2014;8:232. doi: 10.3389/fnins.2014.00232. doi:10.3389/fnins.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mimee A, Smith PM, Ferguson AV. Circumventricular organs: targets for integration of circulating fluid and energy balance signals? Physiol Behav. 2013;121:96–102. doi: 10.1016/j.physbeh.2013.02.012. doi:10.1016/j.physbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 91.Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61(3):737–44. doi: 10.1161/HYPERTENSIONAHA.111.00405. doi:10.1161/HYPERTENSIONAHA.111.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munzberg H. Differential leptin access into the brain—a hierarchical organization of hypothalamic leptin target sites? Physiol Behav. 2008;94(5):664–9. doi: 10.1016/j.physbeh.2008.04.020. doi:10.1016/j.physbeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 93.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, et al. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303(2):H197–206. doi: 10.1152/ajpheart.00974.2011. doi:10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young CN, Morgan DA, Butler SD, Rahmouni K, Gurley SB, Coffman TM, et al. Angiotensin type 1a receptors in the forebrain subfornical organ facilitate leptin-induced weight loss through brown adipose tissue thermogenesis. Mol Metab. 2015;4(4):337–43. doi: 10.1016/j.molmet.2015.01.007. doi:10.1016/j.molmet.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Osborn JW, Kuroki MT. Sympathetic signatures of cardiovascular disease: a blueprint for development of targeted sympathetic ablation therapies. Hypertension. 2012;59(3):545–7. doi: 10.1161/HYPERTENSIONAHA.111.182899. doi:10.1161/HYPERTENSIONAHA.111.182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kosari S, Rathner JA, Chen F, Kosari S, Badoer E. Centrally administered resistin enhances sympathetic nerve activity to the hindlimb but attenuates the activity to brown adipose tissue. Endocrinology. 2011;152(7):2626–33. doi: 10.1210/en.2010-1492. doi:10.1210/en.2010-1492. [DOI] [PubMed] [Google Scholar]
- 97.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol. 2013;304(11):H1538–46. doi: 10.1152/ajpheart.00081.2013. doi:10.1152/ajpheart.00081.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding L, Zhang LL, Gao R, Chen D, Wang JJ, Gao XY, et al. Superoxide anions in paraventricular nucleus modulate adipose afferent reflex and sympathetic activity in rats. PLoS One. 2013;8(12):e83771. doi: 10.1371/journal.pone.0083771. doi:10.1371/journal.pone.0083771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, et al. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11(1):3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]