Abstract
Background
The mechanisms of atrial fibrillation (AF) are highly divergent. The prevalence of AF increases significantly with age, and underling mechanisms might vary with age. Endothelial dysfunction may be associated with AF and atrial arrhythmia recurrence after catheter ablation. We tested the hypothesis that the impact of endothelial dysfunction on arrhythmia recurrence following catheter ablation is age dependent.
Methods and Results
This study enrolled 92 participants with AF undergoing catheter ablation. Endothelial function was assessed by peripheral arterial tonometry before ablation, and the natural logarithmic transformation of reactive hyperemia index was calculated. Endothelial dysfunction was defined as a natural logarithmic transformation of reactive hyperemia index <0.618 (median). Participants were followed for atrial tachycardia, flutter, and fibrillation recurrence for a median of 14 months. The mean age was 57±10 years. There was significant interaction between age and endothelial dysfunction in association with recurrence of AF (P=0.029) and any atrial arrhythmia (P=0.015), and the risk associated with endothelial dysfunction for arrhythmia recurrence was higher in younger versus older participants. Participants were divided into 2 age groups at a threshold of 60 years. Among participants aged ≤60 years, multivariate Cox proportional hazards analysis revealed the independent association between endothelial dysfunction and increased risk of arrhythmia recurrence (hazard ratio for AF 4.18 [95% CI 1.33–15.82], P=0.014, and for any atrial arrhythmia 3.62 [95% CI 1.29–11.81], P=0.014). Kaplan–Meier analysis showed that participants with endothelial dysfunction had significantly higher rates of recurrence of AF (P=0.01) and any atrial arrhythmia (P=0.002).
Conclusions
The risk associated with endothelial dysfunction for arrhythmia recurrence following catheter ablation was age dependent and was higher in younger participants.
Keywords: catheter ablation, endothelium, fibrillation, follow‐up study
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Atrial Fibrillation, Endothelium/Vascular Type/Nitric Oxide
Introduction
Atrial fibrillation (AF) is the most common type of sustained cardiac arrhythmia encountered in clinical practice and is associated with an increased risk of all‐cause mortality and morbidity including stroke, heart failure, dementia, embolic events, hospitalization, and impaired quality of life.1 Catheter ablation for AF has been established as an effective therapeutic option for drug‐resistant AF, and maintaining sinus rhythm after catheter ablation has been shown to be associated with a lower rate of cardiovascular mortality.2 Nevertheless, despite continuous improvements in outcomes with advances in ablation techniques, recurrences of atrial arrhythmias remain a common clinical problem following ablation. A recent meta‐analysis reported that after left atrial ablation, only 53.1% of patients were free from atrial arrhythmia at long‐term follow‐up.3 The pathogenesis of AF is multifactorial and involves a complex interaction between substrates (vulnerable tissue allowing AF to be induced and, in some instances, sustained), triggers (initiating electric stimulus, mostly from pulmonary veins), and reentry.4 Accordingly, preventing the development of AF substrates in addition to electrical therapy by catheter ablation is necessary for decreasing atrial arrhythmia recurrence. Furthermore, AF substrates have a substantial role in cardiovascular outcomes in AF patients and might be at least part of the reason why previous randomized controlled trials could not demonstrate the survival benefit of a rhythm control strategy with antiarrhythmic drugs and cardioversion over rate control.5, 6
The mechanisms underlying both initiation and perpetuation of AF are not well established, and AF manifests as a result of multiple heterogeneous groups of disorders. The prevalence of AF is low in those aged <60 years and increases significantly with age in those aged >60 years.7 As used in the definition of “lone AF,” older age (>60 years) is an important risk factor for AF.8, 9 Furthermore, it was reported that the incidence of AF recurrence after catheter ablation is higher in AF patients aged ≥60 years.10 Electrophysiological and structural remodeling of the atrium has been observed with advancing age and could play an important role in triggering AF.11 Dominant underlying mechanisms might be different for those aged <60 and >60 years. Emerging evidence has demonstrated an association between endothelial dysfunction and AF. Shin et al reported that baseline endothelial function was an important predictor of AF recurrence after catheter ablation.12 It remains unclear whether the impact of endothelial dysfunction on AF recurrence differs by age.
The aim of the present study was to test the hypothesis that the impact of endothelial function on AF and atrial arrhythmia recurrence following catheter ablation is age dependent.
Methods
Study Population
Between January 2008 and December 2009, a double‐blind, placebo‐controlled trial was conducted to evaluate the efficacy of statins in preventing AF recurrence following left atrial ablation. The Mayo Clinic institutional review board approved the study protocol, and all study participants gave written informed consent. Details of the study methodology were described previously.13 Briefly, 125 eligible participants who had no statin indication and who were undergoing catheter ablation for drug‐refractory AF were randomly assigned at a 1:1 ratio to receive either daily 80 mg atorvastatin (n=62) or matching placebo (n=63) for 3 months. Major exclusion criteria were known malignancy; known inflammatory disease; surgery, trauma, or myocardial infarction in the previous month; known contraindication to statin therapy; elevated liver enzymes above 2 times the upper limit of normal; or use of statin, niacin, or fibrates at the time of randomization. The ablation procedure, based on electrical isolation of the pulmonary veins with additional left atrial linear ablation, has been described previously.14, 15
In the current substudy, 92 participants (46 in the atorvastatin group and 46 in the placebo group) who underwent noninvasive endothelial function testing by peripheral arterial tonometry before ablation were included (Figure 1).
Figure 1.
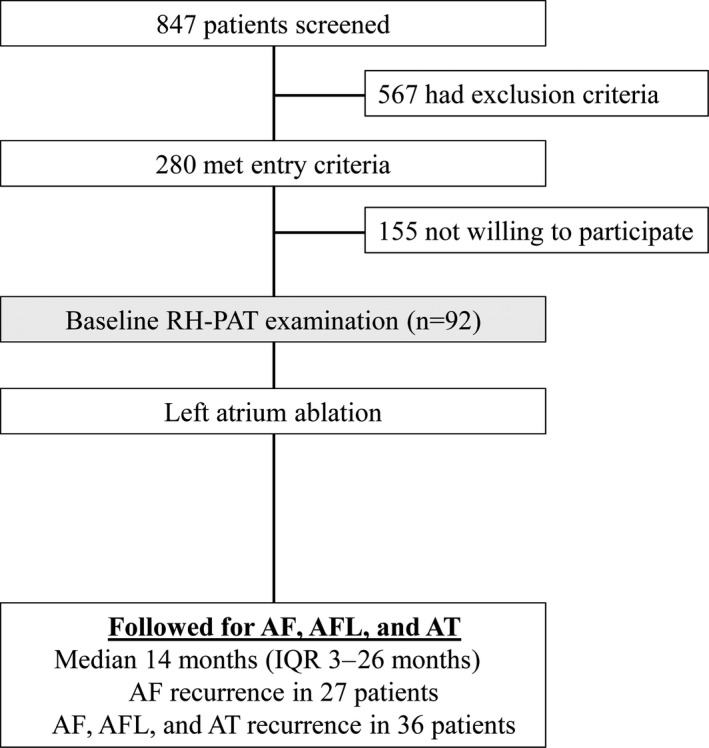
Study design. AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; IQR, interquartile range; RH‐PAT, reactive hyperemia–peripheral arterial tonometry.
Endothelial Function Assessment
Endothelial function was prospectively collected and blinded to the participants and physicians. All studies were performed in a quiet, temperature‐controlled room. Participants fasted for 4 hours before the study and abstained from coffee or tobacco use on the day of the examination. Vasoactive medications were discontinued at least 24 hours prior to testing.
Peripheral arterial tonometry signals were obtained in all participants prior to the ablation procedure and in 71 participants at 3‐month follow‐up using an EndoPAT 2000 device (Itamar Medical Inc). This method has been validated and described previously in other populations16, 17, 18, 19, 20 and in patients with AF.21 After a 5‐minute baseline measurement, the blood pressure cuff on the test arm was inflated to 60 mm Hg above baseline systolic blood pressure or a maximum of 200 mm Hg for 5 minutes. After 5‐minute occlusion, the cuff was deflated, and the peripheral arterial tonometry tracing recorded for another 5 minutes. Endothelial function was measured by reactive hyperemia index, and a natural logarithmic transformation of reactive hyperemia index (Ln_RHI) was used because of its skewed distribution. Endothelial dysfunction was defined as Ln_RHI <0.618 (median).
Follow‐up and End Points
All enrolled participants were prospectively followed up for arrhythmia recurrence periodically or sooner, as dictated by symptoms, at a hospital near the participants' primary physicians. Electrocardiograms and 72‐hour Holter monitors were obtained at the follow‐up visit 3 months after ablation in all participants and sooner for those with symptoms suggestive of arrhythmia recurrence. Although all participants were followed mandatorily for 3 months after ablation, follow‐up after 3 months was based on the primary physicians' discretion. Consequently, event data were analyzed for 3 months and throughout the follow‐up period. The primary end point was freedom from symptomatic AF during the follow‐up period. Documented AF recurrence was considered symptomatic if the participants complained of typical symptoms of palpitations. The secondary end point was freedom from any atrial arrhythmia (AF, atrial tachycardia, and atrial flutter) recurrence, regardless of symptoms during the follow‐up period. Any episode of atrial tachyarrhythmias >30 seconds detected by electrocardiograms or Holter monitors was considered an atrial arrhythmia recurrence.
Statistical Analysis
Descriptive statistics were analyzed to describe the baseline characteristics according to the median value of baseline Ln_RHI (0.618) and age 60 years. Data are expressed as mean±SD, median (interquartile range), or frequency (percentage), as appropriate, and variables were compared by the Fisher exact test, an unpaired t test, or the Wilcoxon rank sum test. To investigate the existence of a linear association of endothelial function with arrhythmia recurrence according to age, participants were divided into 3 groups by age (≤50, 51–60, and ≥61 years), as shown in Figure 2. In other analyses, participants were divided into 2 groups by age with a threshold of 60 years. The cumulative probability of events was estimated by the Kaplan–Meier method, and curves were compared with the log‐rank test. To account for confounding variables, the propensity score was calculated for each participant using a logistic regression model in which the dependent variable was high Ln_RHI (greater than the median), and the independent variables were age, sex, body mass index, hypertension, sleep apnea, paroxysmal AF, AF duration, AF rhythm at EndoPAT examination, left ventricular ejection fraction, valvular heart disease, left atrium length, ablation procedures (left atrium isthmus, roof line, and cavotricuspid isthmus line), antiarrhythmic drugs on admission and discharge, and randomization group (atorvastatin or placebo). Univariate and multivariable time‐to‐event analyses were performed using the Cox proportional hazards model. A 2‐sided P value <0.05 was considered statistically significant. Data were analyzed using JMP version 9.0.0 (SAS Institute Inc).
Figure 2.

HRs of endothelial dysfunction for incident AF and atrial arrhythmia recurrence by age groups. The HRs were calculated for Ln_RHI <0.618. The vertical lines through the HRs represent 95% CIs in predicting AF recurrence (A) and any atrial arrhythmia recurrence (B). There is significant interaction between age and endothelial dysfunction in association with arrhythmia recurrence. The risk associated with endothelial dysfunction for arrhythmia recurrence is higher in younger versus older participants. AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; HR, hazard ratio; Ln_RHI, natural logarithmic transformation of reactive hyperemia index.
Results
Baseline Characteristics
In total, 72 participants (78%) were men aged 57±10 years. Median duration of AF was 4 years (interquartile range 2–8 years), and 74% participants had paroxysmal AF. The median value of baseline Ln_RHI was 0.618 (first and third quartiles 0.368 and 0.791, respectively). Baseline characteristics of the study population according to the median value of baseline Ln_RHI and age 60 years are shown in Tables 1 and 2. Between the groups with lower and upper medians of Ln_RHI, participant characteristics were comparable with respect to demographic, echocardiographic, and biological parameters. Participants aged >60 years were more likely to have hypertension and to use anticoagulant therapy compared with those aged ≤60 years.
Table 1.
Baseline Characteristics According to Median Value of Baseline Ln_RHI and Age 60 Years
| Age | P Value | Ln_RHI | P Value | |||
|---|---|---|---|---|---|---|
| ≤60 Years (n=58) | >60 Years (n=34) | ≥0.618 (n=46) | <0.618 (n=46) | |||
| Age, y, mean±SD | 51.7±7.5 | 67.0±4.5 | <0.001 | 57.6±8.9 | 57.2±10.8 | 0.84 |
| Male sex, n (%) | 48 (82.8) | 24 (70.6) | 0.20 | 35 (76.1) | 37 (80.4) | 0.80 |
| Body mass index, kg/m2, mean±SD | 29.2±5.5 | 28.8±4.1 | 0.71 | 29.3±5.4 | 28.8±4.6 | 0.60 |
| Body mass index ≥30 | 19 (32.8) | 13 (38.2) | 0.65 | 15 (32.6) | 17 (37.0) | 0.83 |
| Hypertension, n (%) | 12 (20.7) | 16 (47.1) | 0.01 | 13 (28.3) | 15 (32.6) | 0.82 |
| Diabetes mellitus, n (%) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Coronary artery disease, n (%) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Congestive heart failure, n (%) | 1 (1.7) | 1 (2.9) | >0.99 | 1 (2.2) | 1 (2.2) | >0.99 |
| Valvular heart disease, n (%) | 7 (12.1) | 3 (8.8) | 0.74 | 4 (8.7) | 6 (13.0) | 0.74 |
| History of CVA or TIA, n (%) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Chronic kidney disease, n (%) | 1 (1.7) | 1 (2.9) | >0.99 | 1 (2.2) | 1 (2.2) | >0.99 |
| Chronic lung disease, n (%) | 2 (3.5) | 1 (2.9) | >0.99 | 0 (0) | 3 (6.5) | 0.24 |
| Sleep apnea, n (%) | 9 (15.5) | 7 (20.6) | 0.58 | 9 (19.6) | 7 (15.2) | 0.58 |
| Paroxysmal AF, n (%) | 42 (72.4) | 26 (76.5) | 0.81 | 38 (82.6) | 30 (65.2) | 0.095 |
| Years of AF, median (IQR) | 4.0 (1.8–8.0) | 3.5 (2.0–7.8) | 0.93 | 4.0 (2.0–7.0) | 4.0 (1.3–9.0) | 0.84 |
| Redo procedure, n (%) | 17 (29.3) | 11 (32.4) | 0.82 | 15 (32.6) | 13 (28.3) | 0.82 |
| CRP, mg/L, median (IQR) | 1.8 (0.7–3.8) | 2.1 (1.1–3.5) | 0.80 | 1.6 (0.7–3.6) | 2.3 (0.9–3.8) | 0.50 |
| Ln_RHI, y, mean±SD | 0.61±0.29 | 0.59±0.30 | 0.79 | 0.838±0.186 | 0.367±0.151 | NA |
| Medication on admission | ||||||
| Aspirin, n (%) | 27 (46.6) | 15 (44.1) | 0.83 | 22 (47.8) | 20 (43.5) | 0.83 |
| Oral anticoagulation, n (%) | 32 (55.2) | 32 (94.1) | <0.001 | 30 (65.2) | 34 (73.9) | 0.50 |
| β‐blocker, n (%) | 32 (55.2) | 15 (44.1) | 0.39 | 25 (54.4) | 22 (47.8) | 0.68 |
| ACEI/ARB, n (%) | 5 (8.6) | 4 (11.8) | 0.72 | 4 (8.7) | 5 (10.9) | >0.99 |
| Atorvastatin, n (%) | 27 (46.6) | 19 (55.9) | 0.52 | 23 (50) | 23 (50) | >0.99 |
| Antiarrhythmic drugs, n (%) | 30 (51.7) | 19 (55.9) | 0.83 | 25 (54.4) | 24 (52.2) | >0.99 |
| Sotalol/dofetilide | 10 (17.2) | 7 (20.6) | 10 (21.7) | 7 (15.2) | ||
| Amiodarone | 2 (3.4) | 2 (5.9) | 3 (6.5) | 1 (2.2) | ||
| Propafenone/flecainide | 19 (32.8) | 10 (29.4) | 13 (28.3) | 16 (34.8) | ||
| Medication on discharge | ||||||
| Antiarrhythmic drugs, n (%) | 11 (19.0) | 6 (17.6) | 0.74 | 11 (23.9) | 6 (13.0) | 0.14 |
| Sotalol/dofetilide | 4 (6.9) | 3 (8.8) | 4 (8.7) | 3 (6.5) | ||
| Amiodarone | 3 (5.2) | 1 (2.9) | 4 (8.7) | 0 (0) | ||
| Propafenone/flecainide | 4 (6.9) | 2 (5.9) | 3 (6.5) | 3 (6.5) | ||
| Echocardiographic parameters | ||||||
| LA diameter, mm, mean±SD | 57.6±7.6 | 58.7±7.8 | 0.53 | 57.4±8.0 | 58.6±7.3 | 0.46 |
| LA volume index, mL/m2, mean±SD | 40.6±16.9 | 40.3±10.9 | 0.94 | 38.7±12.1 | 42.3±17.4 | 0.26 |
| LVEF, %, mean±SD | 58.0±9.6 | 60.6±6.8 | 0.18 | 60.0±6.3 | 57.9±10.6 | 0.25 |
| Procedure | ||||||
| LA isthmus, n (%) | 6 (10.3) | 5 (14.7) | 0.53 | 4 (8.7) | 7 (15.2) | 0.52 |
| Roof line, n (%) | 12 (20.7) | 5 (14.7) | 0.58 | 5 (10.9) | 12 (26.1) | 0.11 |
| Cavotricuspid isthmus line, n (%) | 46 (79.3) | 28 (82.4) | 0.79 | 34 (73.9) | 40 (87.0) | 0.19 |
| Complications, n (%) | 1 (1.7) | 1 (2.9) | >0.99 | 0 (0.0) | 2 (4.4) | 0.49 |
Data are mean±SD, median (IQR), or n (%). Significance was assessed by unpaired t test, Mann–Whitney U test, or Fisher exact test. ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CRP, C‐reactive protein; CVA, cerebrovascular accident; IQR, interquartile range; LA, left atrium; Ln_RHI, natural logarithmic transformation of reactive hyperemia index; LVEF, left ventricular ejection fraction; NA, not applied; TIA, transient ischemic attack.
Table 2.
Baseline Characteristics According to Median Value of Baseline Ln_RHI Stratified by Age 60 Years
| Age ≤60 Years | P Value | Age >60 Years | P Value | |||
|---|---|---|---|---|---|---|
| Ln_RHI ≥0.618 (n=30) | Ln_RHI <0.618 (n=28) | Ln_RHI ≥0.618 (n=16) | Ln_RHI <0.618 (n=18) | |||
| Age, y, mean±SD | 52.6±6.3 | 50.8±8.6 | 0.35 | 66.9±4.6 | 67.1±4.4 | 0.88 |
| Male sex, n (%) | 26 (86.7) | 22 (78.6) | 0.50 | 9 (56.3) | 15 (83.3) | 0.13 |
| Body mass index, kg/m2, mean±SD | 29.4±6.2 | 28.9±4.7 | 0.72 | 29.1±3.6 | 28.5±4.6 | 0.69 |
| Body mass index ≥30 | 10 (33.3) | 9 (32.1) | >0.99 | 5 (31.3) | 8 (44.4) | 0.50 |
| Hypertension, n (%) | 7 (23.3) | 5 (17.9) | 0.75 | 6 (37.5) | 10 (55.6) | 0.33 |
| Diabetes mellitus, n (%) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Coronary artery disease, n (%) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Congestive heart failure, n (%) | 1 (3.3) | 0 (0) | >0.99 | 0 (0) | 1 (5.6) | >0.99 |
| Valvular heart disease, n (%) | 3 (10.0) | 4 (14.3) | 0.70 | 1 (6.3) | 2 (11.1) | >0.99 |
| History of CVA or TIA, n (%) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Chronic kidney disease, n (%) | 1 (3.3) | 0 (0) | >0.99 | 0 (0) | 1 (5.6) | >0.99 |
| Chronic lung disease, n (%) | 0 (0) | 2 (7.1) | 0.23 | 0 (0) | 1 (5.6) | >0.99 |
| Sleep apnea, n (%) | 6 (20.0) | 3 (10.7) | 0.47 | 3 (18.8) | 4 (22.2) | >0.99 |
| Paroxysmal AF, n (%) | 24 (80.0) | 18 (64.3) | 0.24 | 14 (87.5) | 12 (66.7) | 0.23 |
| Years of AF, median (IQR) | 4.5 (2.0–7.3) | 3.5 (1.0–8.8) | 0.60 | 2.5 (1.3–6.5) | 4.5 (2.0–9.0) | 0.25 |
| Redo procedure, n (%) | 9 (30.0) | 8 (28.6) | >0.99 | 6 (37.5) | 5 (27.8) | 0.72 |
| CRP, mg/L, median (IQR) | 1.6 (0.7–3.4) | 2.4 (1.0–4.1) | 0.25 | 1.9 (1.2–4.0) | 2.1 (0.7–3.4) | 0.56 |
| Ln_RHI, y, mean±SD | 0.83±0.18 | 0.37±0.15 | NA | 0.85±0.20 | 0.36±0.16 | NA |
| Medication on admission | ||||||
| Aspirin, n (%) | 17 (56.7) | 10 (35.7) | 0.12 | 20 (43.5) | 22 (47.8) | 0.83 |
| Oral anticoagulation, n (%) | 15 (50.0) | 17 (60.7) | 0.44 | 34 (73.9) | 30 (65.2) | 0.50 |
| β‐blocker, n (%) | 16 (53.3) | 16 (57.1) | 0.80 | 22 (47.8) | 25 (54.4) | 0.68 |
| ACEI/ARB, n (%) | 3 (10.0) | 2 (7.1) | >0.99 | 5 (10.9) | 4 (8.7) | >0.99 |
| Atorvastatin, n (%) | 12 (40.0) | 15 (53.6) | 0.43 | 23 (50) | 23 (50) | >0.99 |
| Antiarrhythmic drugs, n (%) | 16 (53.3) | 15 (53.6) | >0.99 | 10 (62.5) | 9 (50.0) | 0.19 |
| Sotalol/dofetilide | 5 (16.7) | 5 (17.9) | 5 (31.3) | 2 (11.1) | ||
| Amiodarone | 1 (3.3) | 1 (3.6) | 2 (12.5) | 0 (0) | ||
| Propafenone/flecainide | 10 (33.3) | 9 (32.1) | 3 (18.8) | 7 (38.9) | ||
| Medication on discharge | ||||||
| Antiarrhythmic drugs, n (%) | 7 (23.3) | 4 (14.3) | 0.20 | 4 (25.0) | 2 (11.1) | 0.22 |
| Sotalol/dofetilide | 3 (10.0) | 1 (3.6) | 1 (6.3) | 2 (11.1) | ||
| Amiodarone | 3 (10.0) | 0 (0) | 1 (6.3) | 0 (0) | ||
| Propafenone/flecainide | 1 (3.3) | 3 (10.7) | 2 (12.5) | 0 (0) | ||
| Echocardiographic parameters | ||||||
| LA diameter, mm, mean±SD | 56.8±8.4 | 58.5±6.6 | 0.40 | 58.6±7.3 | 58.8±8.5 | 0.94 |
| LA volume index, mL/m2, mean±SD | 37.8±12.5 | 43.6±20.6 | 0.20 | 40.4±11.5 | 40.3±10.7 | 0.98 |
| LVEF, mean±SD, % | 59.7±6.1 | 56.2±12.1 | 0.16 | 60.6±6.7 | 60.6±7.1 | >0.99 |
| Procedure | ||||||
| LA isthmus, n (%) | 3 (10.0) | 3 (10.7) | >0.99 | 1 (6.3) | 4 (22.2) | 0.34 |
| Roof line, n (%) | 4 (13.3) | 8 (28.6) | 0.20 | 1 (6.3) | 4 (22.2) | 0.34 |
| Cavotricuspid isthmus line, n (%) | 22 (73.3) | 24 (85.7) | 0.34 | 12 (75.0) | 16 (88.9) | 0.39 |
| Complications, n (%) | 0 (0) | 1 (3.5) | 0.48 | 0 (0) | 1 (5.6) | >0.99 |
Data are mean±SD, median (IQR), or n (%). Significance was assessed by unpaired t test, Mann–Whitney U test, or Fisher exact test. ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CRP, C‐reactive protein; CVA, cerebrovascular accident; IQR, interquartile range; LA, left atrium; Ln_RHI, natural logarithmic transformation of reactive hyperemia index; LVEF, left ventricular ejection fraction; NA, not applied; TIA, transient ischemic attack.
Ablation Outcomes
Pulmonary vein isolation was achieved in all participants. Bidirectional cavotricuspid isthmus block was successfully performed in 74 participants (80%). Mitral isthmus ablation was performed in 5 participants (5%), most of whom required additional pulses from within the coronary sinus. In addition, left atrial roofline ablation was performed in 17 participants (18%). No serious procedure‐related complications (eg, pericardial tamponade, pulmonary vein stenosis requiring balloon dilatation and stent placement) were noted. Procedures and complications of catheter ablation did not differ by age (Table 1).
Predictive Value of Baseline Endothelial Function for Arrhythmia Recurrence
During the follow‐up period (median 14 months [interquartile range 3–26 months]), 27 participants experienced symptomatic AF recurrence, and 36 experienced atrial arrhythmia recurrence during follow‐up. Event rates were not significantly different between participants aged ≤60 and >60 years (AF: 16 [28%] versus 11 [32%], log‐rank P=0.59; any atrial arrhythmia: 22 [38%] versus 14 [41%], log‐rank P=0.59). Baseline Ln_RHI levels tended to be associated with symptomatic AF (hazard ratio [HR] 1.99 [95% CI 0.92–4.51], P=0.079) and atrial arrhythmia recurrence (HR 1.93 [95% CI 0.99–3.92], P=0.054) in Cox proportional hazards analyses (Table 3). Importantly, there was significant interaction between age and endothelial dysfunction in association with recurrence of AF (P=0.029) and any atrial arrhythmia (P=0.015), and the risk associated with impaired endothelial function for arrhythmia recurrence was higher in younger participants (Figure 2). Participants were divided into 2 groups at a threshold of age 60 years; in the group aged ≤60 years, attenuated endothelial function was significantly associated with the risk of the recurrence of symptomatic AF (HR 4.01 [95% CI 1.39–14.38], P=0.009) and any atrial arrhythmia (HR 4.35 [95% CI 1.72–13.25], P=0.002), but there was no significant association in participants aged >60 years (Table 3). This association of endothelial dysfunction with arrhythmia recurrence in participants aged ≤60 years remained significant even after adjustment for age and sex or for propensity score (HR for AF 4.18 [95% CI 1.33–15.82], P=0.014, and for any atrial arrhythmia 3.62 [95% CI 1.29–11.81], P=0.014) (Table 3). Figure 3 shows Kaplan–Meier estimates of the probability of arrhythmia recurrence according to baseline Ln_RHI levels in the subgroup of participants aged ≤60 years. Those with endothelial dysfunction had significantly higher rates of recurrence of AF (P=0.010) and any atrial arrhythmia (P=0.002). When limited to the events within 3 months after ablation, the numbers of events were quite small (6 AF events and 16 events with any atrial arrhythmia recurrence). The analyses with only events within 3 months showed a similar tendency in the association between baseline endothelial function and arrhythmia recurrence and its age dependence (Table 4, Figures 4 and 5).
Table 3.
Multivariate Cox Proportional Hazards Analyses for Arrhythmia Recurrence During the Follow‐up Period
| Univariate | Age and Sex Adjusted | Propensity Score Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Entire study population (n=92) | |||||||||
| Ln_RHI <0.618 | |||||||||
| For AF recurrence | 1.988 | 0.924–4.513 | 0.079 | 1.948 | 0.902–4.434 | 0.090 | 1.970 | 0.804–5.011 | 0.139 |
| For AF, AFL, and AT recurrence | 1.927 | 0.989–3.921 | 0.054 | 1.898 | 0.966–3.886 | 0.063 | 1.549 | 0.695–3.551 | 0.286 |
| Participants aged ≤60 years (n=58) | |||||||||
| Ln_RHI <0.618 | |||||||||
| For AF recurrence | 4.008 | 1.390–14.38 | 0.009 | 4.136 | 1.432–14.86 | 0.008 | 4.178 | 1.331–15.82 | 0.014 |
| For AF, AFL, and AT recurrence | 4.347 | 1.715–13.25 | 0.002 | 4.456 | 1.756–13.59 | 0.001 | 3.616 | 1.288–11.81 | 0.014 |
| Participants aged >60 years (n=34) | |||||||||
| Ln_RHI <0.618 | |||||||||
| For AF recurrence | 0.848 | 0.244–2.823 | 0.785 | 0.750 | 0.207–2.624 | 0.648 | 0.311 | 0.049–1.843 | 0.200 |
| For AF, AFL, and AT recurrence | 0.711 | 0.488–4.279 | 0.526 | 0.616 | 0.195–1.864 | 0.388 | 0.192 | 0.032–1.064 | 0.059 |
The propensity score was calculated for each participant using a logistic regression model in which the dependent variable was high Ln_RHI (greater than the median) and the independent variables were age, sex, body mass index, hypertension, sleep apnea, paroxysmal AF, AF duration, AF rhythm at EndoPAT examination, left ventricular ejection fraction, valvular heart disease, left atrium length, ablation procedures (left atrium isthmus, roof line, and cavotricuspid isthmus line), antiarrhythmic drugs on admission and discharge, and randomization group (atorvastatin or placebo). AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; HR, hazard ratio; Ln_RHI, natural logarithmic transformation of reactive hyperemia index.
Figure 3.

Kaplan–Meier analysis for the probability of AF and atrial arrhythmia recurrence according to baseline endothelial function in participants aged ≤60 years in predicting AF recurrence (A) and any atrial arrhythmia recurrence (B). AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; Ln_RHI, natural logarithmic transformation of reactive hyperemia index.
Table 4.
Multivariate Cox Proportional Hazards Analyses for Arrhythmia Recurrence Within 3 Months After Ablation
| Univariate | Age and Sex Adjusted | Propensity Score Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Entire study population (n=92) | |||||||||
| Ln_RHI <0.618 | |||||||||
| For AF recurrence | 2.282 | 0.444–16.51 | 0.33 | 2.243 | 0.432–16.35 | 0.34 | 2.969 | 0.466–24.41 | 0.25 |
| For AF, AFL, and AT recurrence | 1.966 | 0.729–5.788 | 0.18 | 1.959 | 0.724–5.781 | 0.19 | 1.433 | 0.468–4.678 | 0.53 |
| Participants aged ≤60 years (n=58) | |||||||||
| Ln_RHI <0.618 | |||||||||
| For AF recurrence | >999 | 1.848–>999 | 0.014 | >999 | 2.027–>999 | 0.011 | >999 | 1.873–>999 | 0.015 |
| For AF, AFL, and AT recurrence | 3.815 | 1.134–17.23 | 0.030 | 4.090 | 1.211–18.51 | 0.022 | 2.782 | 0.773–13.07 | 0.121 |
| Participants aged >60 years (n=34) | |||||||||
| Ln_RHI <0.618 | |||||||||
| For AF recurrence | <0.001 | <0.001–1.823 | 0.111 | <0.001 | <0.001–1.284 | 0.071 | <0.001 | <0.001–3.271 | 0.133 |
| For AF, AFL, and AT recurrence | 0.348 | 0.017–2.729 | 0.33 | 0.319 | 0.015–2.622 | 0.30 | 0.164 | 0.004–3.524 | 0.26 |
The propensity score was calculated for each participant using a logistic regression model in which the dependent variable was high Ln_RHI (greater than the median) and the independent variables were age, sex, body mass index, hypertension, sleep apnea, paroxysmal AF, AF duration, AF rhythm at EndoPAT examination, left ventricular ejection fraction, valvular heart disease, left atrium length, ablation procedures (left atrium isthmus, roof line, and cavotricuspid isthmus line), antiarrhythmic drugs on admission and discharge, and randomization group (atorvastatin or placebo). AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; HR, hazard ratio; Ln_RHI, natural logarithmic transformation of reactive hyperemia index.
Figure 4.

Hazard ratios of endothelial dysfunction for incident AF and atrial arrhythmia recurrence within 3 months after ablation by age group (as in Figure 2). The HRs were calculated for Ln_RHI <0.618. The vertical lines through the HRs represent 95% CIs in predicting AF recurrence (A) and any atrial arrhythmia recurrence (B). AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; HR, hazard ratio; Ln_RHI, natural logarithmic transformation of reactive hyperemia index.
Figure 5.

Kaplan–Meier analysis for the probability of AF and atrial arrhythmia recurrence within 3 months after ablation according to baseline endothelial function in participants aged ≤60 years (as in Figure 3) in predicting AF recurrence (A) and any atrial arrhythmia recurrence (B). AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; Ln_RHI, natural logarithmic transformation of reactive hyperemia index.
Endothelial Function at 3‐Month Follow‐up
Among 92 enrolled participants, follow‐up of endothelial function at 3 months was available for 71 participants. Among these, endothelial function remained unchanged after atrial ablation (Ln_RHI from 0.60±0.29 to 0.65±0.25, P=0.41). Changes in endothelial function were slightly higher in the atorvastatin group than in the placebo group, but the difference was not statistically significant (ΔLn_RHI; the atorvastatin group 0.07±0.30 versus the placebo group −0.01±0.30, P=0.31). We assessed the impact of atorvastatin in the subgroup of participants aged ≤60 years because baseline endothelial dysfunction was significantly associated with arrhythmia recurrence in this group. Nevertheless, even in this group, 3‐month atorvastatin treatment neither improved endothelial function nor prevented arrhythmia recurrence during follow‐up.
In the subgroup with normal endothelial function at baseline (baseline Ln_RHI ≥0.618; n=37), endothelial function worsened to Ln_RHI <0.618 at 3‐month follow‐up in 12 participants, and worsening endothelial function was significantly associated with atrial arrhythmia recurrence between 3 months after ablation and end of follow‐up (HR 10.69 [95% CI 1.713–205.4], P=0.009). In contrast, in the subgroup with endothelial dysfunction at baseline (baseline Ln_RHI <0.618; n=34), only 7 participants showed improvement in endothelial function (Ln_RHI ≥0.618 at 3‐month follow‐up). Although the number of participants is quite small to perform subgroup analysis, participants with persistent endothelial dysfunction (both baseline Ln_RHI and Ln_RHI at 3‐month follow‐up <0.618) showed a slightly higher risk for atrial arrhythmia recurrence between 3 months after ablation and end of follow‐up compared with participants with improvement in endothelial dysfunction (HR 1.386 [95% CI 0.405–6.339], P=0.621).
Discussion
The current study demonstrated that the risk associated with endothelial dysfunction for AF and any atrial arrhythmia recurrence after catheter ablation was higher in younger patients. In participants aged ≤60 years, baseline endothelial dysfunction was significantly associated with increased risk of AF and atrial arrhythmia recurrence. Consequently, endothelial dysfunction might have an important role in the development of vulnerable substrate for AF occurrence in patients aged <60 years.
The endothelium lines the entire circulatory system, including the heart and the smallest capillaries. It plays an active and critical role in the physiological regulation of vascular tone, cellular adhesion, vascular smooth muscle migration and resistance to thrombosis, inflammation, and oxidative stress.22 The phenotypic features of endothelial dysfunction include decreased myocardial perfusion, increased inflammation, and oxidative stress, all of which might contribute to arrhythmogenesis. Furthermore, it was reported that endothelial dysfunction might contribute to heart failure with preserved ejection fraction, which causes elevated atrial pressure.23 Endothelial dysfunction may have an important role in the development of vulnerable substances for AF and thus is a potential treatment target. Interestingly, Pathak et al recently reported that aggressive risk factor modification (blood pressure control, weight management, lipid management, glycemic control, sleep‐disordered breathing management, and smoking and alcohol management) improved the long‐term success of AF ablation.24 Improvement in endothelial function by risk factor modification might mediate this beneficial effect. Based on the results of the current study, endothelial function should be assessed in persons aged ≤60 years. Furthermore, we suggest modifying therapies and searching for other risk factors, including nontraditional risks, in those who present with endothelial dysfunction, even after implementation of optimal therapies. Nevertheless, prospective randomized studies are needed to elucidate whether endothelial function–guided therapies provide benefits in improving outcomes in patients undergoing catheter ablation for AF.
The mechanisms of AF are highly divergent, and aging plays an important role in AF genesis. Because the prevalence of AF increases significantly with age, especially in patients aged >60 years,7 dominant underlying mechanisms might be different according to age. It is suggested that in older patients, an increase of spatial variability in repolarization in older atria may contribute to the initiation of AF, and atrial fibrosis and slowed conduction of premature beats may be important for both initiation and stabilization of AF.25 Tuan et al reported that left atrium mean voltage was significantly reduced in older AF patients compared with younger patients, and that may reflect age‐related development of atrial fibrosis.26 In contrast, in younger patients, AF may be largely a disease of the pulmonary veins, blood vessels that may be subject to endothelial dysfunction.
Generally, the majority of patients with AF have diabetes mellitus, hypertension (usually with left ventricular hypertrophy), or some other form of structural heart disease (mostly valvular). In addition, obesity, metabolic disorder, and obstructive sleep apnea syndrome have been found to independently increase the risk of AF.27 A variety of factors may damage endothelial cells, including physical injuries, biochemical injuries, and immune‐mediated damage. Increased systemic inflammatory factors and oxidative stress might be partly responsible for endothelial dysfunction and subsequent AF among obese patients. In patients with sleep apnea, intermittent hypoxia, directly or through reactive oxygen species formation, causes endothelial dysfunction.28 Sleep apnea and metabolic disorders, which increase inflammation, oxidative stress, and endothelial dysfunction,29, 30, 31 might have important roles in younger patients, whereas electrophysiological and structural remodeling including arterial fibrosis may dominate in older patients. The prevalence of diabetes mellitus, hypertension, and valvular heart diseases in this study—the common causes of AF—were relatively low, at 0%, 30%, and 11%, respectively. Furthermore, in participants aged ≤60 years, frequency of hypertension was significantly less than in those aged >60 years. Formal tests for obstructive sleep apnea were not performed, and the history of obstructive sleep apnea syndrome was established based on inquiries from participants. On that account, obstructive sleep apnea syndrome might have been underestimated in this study. In addition, because participants with a clinical indication for statins were excluded, only a small proportion of participants had comorbidities. Our findings imply that endothelial dysfunction might not only result from AF risk factors including hypertension but also directly cause AF.
Our previous13 and current studies demonstrated that atorvastatin treatment following catheter ablation for AF did not have a beneficial effect on atrial arrhythmia recurrence in participants with no standard indication for statin therapy. The mechanisms behind the antiarrhythmic effect of statins remain unclear but are possibly attributable to improvement in inflammation, oxidative stress, and endothelial dysfunction.32, 33, 34 Nonimprovement in endothelial function by atorvastatin treatment in this study likely affected the lack of benefit for arrhythmia recurrence. Participants who had no statin indication under the current clinical guidelines were enrolled in this study, and an atherosclerotic cardiovascular disease prevention benefit of statins is unclear in this population.35 Our findings roughly imply that statin therapy for this population might not have a significant impact on endothelial function. More tailored patient selection might be required.
Strengths and Limitations
Strengths of this study include prospective collection of data on endothelial function with blinding of the participants and physicians. The number of participants in the subgroup analyses with follow‐up endothelial function results was quite small. Because of the small number of participants in each group, the results of this study could be biased or confounded; therefore, the statistical power is not high enough. This study was conducted in selected participants because of the exclusion of participants with a preexisting statin indication. Overall, 55% of participants were not willing to participate and were excluded. Consequently, generalizability to other populations is limited. The heterogeneity of the study population has to be considered in interpreting the results. The current study was not designed to determine an optimal cutoff value. Asymptomatic AF can be underestimated. All participants were followed until 3 months after ablation; however, follow‐up after 3 months was based on the primary physicians' discretion. This might cause an informative or dependent censoring bias. Consequently, further studies with larger populations and fixed long‐term follow‐up are necessary to confirm our hypothesis.
Conclusion
Among participants aged ≤60 years, endothelial dysfunction prior to left atrial ablation for AF was significantly associated with increased risk of AF and atrial arrhythmia recurrence. Consequently, endothelial dysfunction might be a marker of and play an important role in the likelihood of AF occurrence in patients aged <60 years.
Sources of Funding
This work was supported by the National Institutes of Health (NIH Grants HL‐92954 and AG‐31750), the Mayo Foundation, and unrestricted grant from Pfizer.
Disclosures
Amir Lerman declares consulting for Itamar Medical. The remaining authors have no disclosures to report.
Acknowledgments
Role of the Sponsors: The National Institute of Health, the Mayo Foundation, and Pfizer had no role in the process of designing, implementing, and reporting of the study apart from its financial contribution.
(J Am Heart Assoc. 2016;5:e003183 doi: 10.1161/JAHA.115.003183)
References
- 1. January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghanbari H, Baser K, Jongnarangsin K, Chugh A, Nallamothu BK, Gillespie BW, Duygu Baser H, Swangasool A, Crawford T, Latchamsetty R, Good E, Pelosi F Jr, Bogun F, Morady F, Oral H. Mortality and cerebrovascular events after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2014;11:1503–1511. [DOI] [PubMed] [Google Scholar]
- 3. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts‐Thomson KC, Sanders P. Long‐term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004549 doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157:243–252. [DOI] [PubMed] [Google Scholar]
- 5. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD; Atrial Fibrillation Follow‐up Investigation of Rhythm Management I . A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 6. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study G . A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. [DOI] [PubMed] [Google Scholar]
- 7. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 8. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC Jr, Priori SG, Estes NA III, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson WG, Tarkington LG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task F . 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. [DOI] [PubMed] [Google Scholar]
- 9. Wyse DG, Van Gelder IC, Ellinor PT, Go AS, Kalman JM, Narayan SM, Nattel S, Schotten U, Rienstra M. Lone atrial fibrillation: does it exist? J Am Coll Cardiol. 2014;63:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saad EB, d'Avila A, Costa IP, Aryana A, Slater C, Costa RE, Inacio LA Jr, Maldonado P, Neto DM, Camiletti A, Camanho LE, Polanczyk CA. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score </=3: a long‐term outcome study. Circ Arrhythm Electrophysiol. 2011;4:615–621. [DOI] [PubMed] [Google Scholar]
- 11. Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–116. [DOI] [PubMed] [Google Scholar]
- 12. Shin SY, Na JO, Lim HE, Choi CU, Choi JI, Kim SH, Kim EJ, Park SW, Rha SW, Park CG, Seo HS, Oh DJ, Kim YH. Improved endothelial function in patients with atrial fibrillation through maintenance of sinus rhythm by successful catheter ablation. J Cardiovasc Electrophysiol. 2011;22:376–382. [DOI] [PubMed] [Google Scholar]
- 13. Suleiman M, Koestler C, Lerman A, Lopez‐Jimenez F, Herges R, Hodge D, Bradley D, Cha YM, Brady PA, Munger TM, Asirvatham SJ, Packer DL, Friedman PA. Atorvastatin for prevention of atrial fibrillation recurrence following pulmonary vein isolation: a double‐blind, placebo‐controlled, randomized trial. Heart Rhythm. 2012;9:172–178. [DOI] [PubMed] [Google Scholar]
- 14. Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ; Heart Rhythm S, European Heart Rhythm A, European Cardiac Arrhythmia S, American College of C, American Heart A and Society of Thoracic S . HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007;9:335–379. [DOI] [PubMed] [Google Scholar]
- 15. O'Neill MD, Jais P, Hocini M, Sacher F, Klein GJ, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation. Circulation. 2007;116:1515–1523. [DOI] [PubMed] [Google Scholar]
- 16. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. [DOI] [PubMed] [Google Scholar]
- 17. Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. [DOI] [PubMed] [Google Scholar]
- 18. Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 19. Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, Nagayoshi Y, Yamamuro M, Izumiya Y, Iwashita S, Matsui K, Jinnouchi H, Kimura K, Umemura S, Ogawa H. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. [DOI] [PubMed] [Google Scholar]
- 20. Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, Matsubara J, Kurokawa H, Fujisue K, Konishi M, Akiyama E, Suzuki H, Nagayoshi Y, Yamamuro M, Sakamoto K, Iwashita S, Jinnouchi H, Taguri M, Morita S, Matsui K, Kimura K, Umemura S, Ogawa H. Peripheral endothelial function and cardiovascular events in high‐risk patients. J Am Heart Assoc. 2013;2:e000426 doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshino S, Yoshikawa A, Hamasaki S, Ishida S, Oketani N, Saihara K, Okui H, Kuwahata S, Fujita S, Ichiki H, Ueya N, Iriki Y, Maenosono R, Miyata M, Tei C. Atrial fibrillation‐induced endothelial dysfunction improves after restoration of sinus rhythm. Int J Cardiol. 2013;168:1280–1285. [DOI] [PubMed] [Google Scholar]
- 22. Reriani MK, Flammer AJ, Jama A, Lerman LO, Lerman A. Novel functional risk factors for the prediction of cardiovascular events in vulnerable patients following acute coronary syndrome. Circ J. 2012;76:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST‐AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 25. Anyukhovsky EP, Sosunov EA, Chandra P, Rosen TS, Boyden PA, Danilo P Jr, Rosen MR. Age‐associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66:353–363. [DOI] [PubMed] [Google Scholar]
- 26. Tuan TC, Chang SL, Tsao HM, Tai CT, Lin YJ, Hu YF, Lo LW, Udyavar AR, Chang CJ, Tsai WC, Tang WH, Suenari K, Huang SY, Lee PC, Chen SA. The impact of age on the electroanatomical characteristics and outcome of catheter ablation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:966–972. [DOI] [PubMed] [Google Scholar]
- 27. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 28. Gopalakrishnan P, Tak T. Obstructive sleep apnea and cardiovascular disease. Cardiol Rev. 2011;19:279–290. [DOI] [PubMed] [Google Scholar]
- 29. Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. [DOI] [PubMed] [Google Scholar]
- 31. Negi S, Sovari AA, Dudley SC Jr. Atrial fibrillation: the emerging role of inflammation and oxidative stress. Cardiovasc Hematol Disord Drug Targets. 2010;10:262–268. [DOI] [PubMed] [Google Scholar]
- 32. Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–431. [DOI] [PubMed] [Google Scholar]
- 33. Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta‐analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–835. [DOI] [PubMed] [Google Scholar]
- 34. Savelieva I, Kourliouros A, Camm J. Primary and secondary prevention of atrial fibrillation with statins and polyunsaturated fatty acids: review of evidence and clinical relevance. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:1–13. [DOI] [PubMed] [Google Scholar]
- 35. Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]


