Abstract
Background
The natural history of coronary artery aneurysms (CAA) after intravenous immunoglobulin (IVIG) treatment in the United States is not well described. We describe the natural history of CAA in US Kawasaki disease (KD) patients and identify factors associated with major adverse cardiac events (MACE) and CAA regression.
Methods and Results
We evaluated all KD patients with CAA at 2 centers from 1979 to 2014. Factors associated with CAA regression, maximum CA z‐score over time (zMax), and MACE were analyzed. We performed a matched analysis of treatment effect on likelihood of CAA regression. Of 2860 KD patients, 500 (17%) had CAA, including 90 with CAA z‐score >10. Most (91%) received IVIG within 10 days of illness, 32% received >1 IVIG, and 27% received adjunctive anti‐inflammatory medications. CAA regression occurred in 75%. Lack of CAA regression and higher CAA zMax were associated with earlier era, larger CAA z‐score at diagnosis, and bilateral CAA in univariate and multivariable analyses. MACE occurred in 24 (5%) patients and was associated with higher CAA z‐score at diagnosis and lack of IVIG treatment. In a subset of patients (n=132) matched by age at KD and baseline CAA z‐score, those receiving IVIG plus adjunctive medication had a CAA regression rate of 91% compared with 68% for the 3 other groups (IVIG alone, IVIG ≥2 doses, or IVIG ≥2 doses plus adjunctive medication).
Conclusions
CAA regression occurred in 75% of patients. CAA z‐score at diagnosis was highly predictive of outcomes, which may be improved by early IVIG treatment and adjunctive therapies.
Keywords: cardiovascular outcomes, coronary aneurysm, Kawasaki disease
Subject Categories: Quality and Outcomes, Mortality/Survival, Pediatrics, Imaging
Introduction
Kawasaki disease (KD) is an acute vasculitis that preferentially affects medium‐sized arteries, particularly the coronary arteries (CA).1, 2, 3 CA involvement can range from transient mild dilatation or ectasia, occurring in up to 40% of patients, to giant coronary artery aneurysms (CAA).4, 5 In the pre–intravenous immunoglobulin (IVIG) era, CAA occurred in 20% to 25% of KD patients.6 With IVIG therapy, persistent CAAs are considerably less common but still occur in 4% to 6% of patients, with ≈1% developing giant CAA7, 8, 9 using 1984 Japanese Ministry of Health criteria (absolute CA dimension ≥8 mm).10 The incidence of coronary abnormalities is greater using z‐score criteria.4, 8, 11, 12 A recent, 2‐center US study found that 2.6% of patients met the z‐score definition for giant aneurysms (any CA segment with z≥10).8 Patients with large or giant CAAs are at risk for cardiac events including CA thrombosis or stenosis, myocardial infarction (MI), ventricular tachycardia, and death.6, 13, 14, 15 Large case series of Japanese patients with giant CAA have shown good overall survival but high cardiac event rates.6, 13, 15
The long‐term natural history of CAA after treatment with IVIG in the US population is not well described. Many patients have regression of CAA to normal internal lumen diameter as a result of luminal myofibroblastic proliferation and layering of thrombus in larger CAA.16 A large Japanese study from the pre‐IVIG era analyzed serial angiograms in KD patients with CAA and showed that 55% to 60% have CAA regression, typically within 1 to 2 years of the acute illness.6 In the US population, only small cohorts of patients with large and giant CAA have been reported,14, 17, 18 limiting the ability to assess risk factors associated with persistent CAA and major adverse cardiac events (MACE). The aims of this study were to describe the natural history of CAA in a contemporary cohort of US KD patients and to identify factors associated with MACE and CAA regression.
Methods
Subjects
In this 2‐center retrospective study, we reviewed all patients with KD managed at Boston Children's Hospital and Rady Children's Hospital, San Diego, between 1979 and 2014. We included all patients with CAA at any time in their illness. We defined CAA as left anterior descending coronary artery (LAD) and/or right coronary artery (RCA) z‐score >3 or original Japanese Ministry of Health and Welfare criteria for CAA in CA segments for which z‐scores are not available (CA dimension >3 mm for patients <5 years of age and >4 mm in patients ≥5 years of age).2 Left main CA (LMCA) z‐score was not used for inclusion due to previously reported variability in LMCA anatomy and measurement.2, 11, 19 Of the 500 patients included, 498 were included based on z‐score criteria, and 2 by Japanese Ministry of Health criteria. There were no patients in the data base with isolated LMCA CAA. Second episodes of KD were excluded, defined as repeat episode of complete or incomplete KD after complete resolution of the previous episode, or presence of congenital heart disease, except for bicommissural aortic valve, mitral valve prolapse, and hemodynamically insignificant ventricular septal defects.
Demographic, clinical, and cardiac imaging data were collected from medical records. Clinical data included demographics, date of KD onset, treatment center, KD diagnostic criteria met, and KD treatment. First‐line treatment for KD at both participating institutions since 1986 has been IVIG (2 g/kg) and aspirin. IVIG treatment was considered delayed if given after day 10 of illness. For patients who were IVIG‐resistant, had CAA at diagnosis (CA z‐score >3 or Japanese Ministry of Health Criteria), and/or were deemed to be at high risk by clinical criteria,20, 21 second‐line therapy has varied over time and by site and has included repeat IVIG (2 g/kg), oral or intravenous steroids (varying dosing and duration regimens), infliximab, cyclosporine, and/or cyclophosphamide.22, 23, 24, 25, 26, 27 Patients with giant CAA (CA z‐score ≥10 or absolute dimension >8 mm) were chronically anticoagulated with either warfarin or low‐molecular‐weight heparin. This study was conducted with approval from the Committee for Clinical Investigation at Children's Hospital Boston and the Institutional Review Board at Children's Hospital Boston and Rady Children's Hospital with requirement for consent waived.
Imaging Data
Echocardiographic data were collected from reports produced at the time of the study. We collected CA measurements for the following segments: LMCA, proximal and distal LAD, circumflex, proximal and distal RCA, and posterior descending CA. Using the reported measurements, we calculated z‐scores for the proximal LAD and proximal RCA using z‐score equations derived from previously described normative data.4 Prior studies from our institution have shown high inter‐ and intraobserver reliability for proximal LAD and RCA measurement.11, 19 In cases where additional imaging modalities were performed, including cardiac magnetic resonance imaging, cardiac computed tomography, or cardiac catheterization, CA dimensions were collected from reports produced at the time of the study. The z‐scores for the proximal RCA and proximal LAD were recalculated for all subjects using the echo‐derived CA z‐score formula for the respective CA segment. We classified CAA as small (z‐score=3.0‐4.9), moderate (z‐score=5‐9.9), or giant (z‐score ≥10).12 The zMax was defined as the higher value between RCA and LAD z‐scores on each echocardiogram. Analysis included CA size and z‐score at initial echocardiogram, maximum RCA or LAD z‐score over follow‐up (highest zMax), and most recent echocardiogram.
Patient Outcomes
All patients who met inclusion/exclusion criteria were included in analysis of MACE (Figure 1). We reviewed all available clinical information including echocardiogram, catheterization reports, nuclear perfusion studies, stress echocardiograms, internal and external clinic notes, and operative notes. Patients were classified as having MACE if they had any of the following at any time point: complete proximal CA occlusion, clinical or imaging evidence of MI, coronary artery bypass graft (CABG), percutaneous CA intervention (PCI), cardiac death, ventricular tachycardia, or orthotopic heart transplant (OHT). Asymptomatic CA stenosis was not included as MACE. The primary MACE analysis was performed on the entire cohort of CAA patients. A subgroup analysis for MACE was performed using only patients treated with IVIG within 10 days of fever onset.
Figure 1.
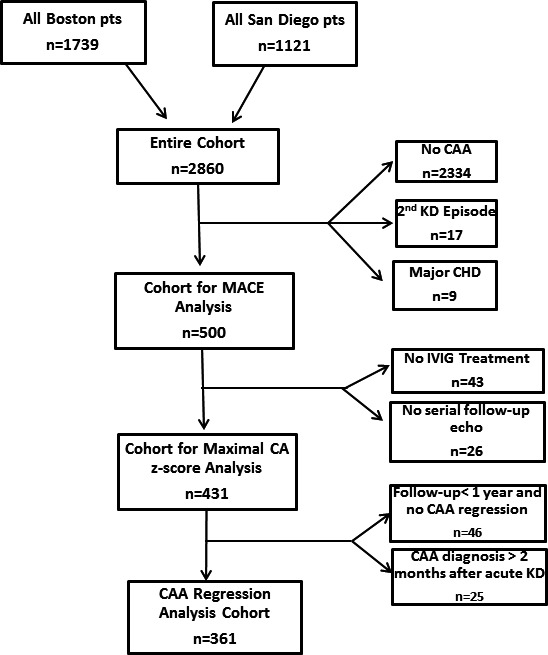
Patient selection. CAA indicates coronary artery aneurysm; CHD, congenital heart disease; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; MACE, major adverse cardiac events.
Additional analyses were restricted to patients who were treated with IVIG at one of the participating institutions and had serial follow‐up echocardiograms (≥1 study with CA measurements after the initial diagnosis of CAA) (Figure 1). The follow‐up cohort was used to analyze factors associated with the highest zMax over follow‐up and for analysis of time to CAA regression. To avoid selection bias in analyses of time to regression of CAA, we excluded patients who had CAA diagnosed >2 months after acute KD or who had <1 year of follow‐up and no CAA regression. Regression of CAA was defined on a patient basis (rather than individual CA segment). We considered CAA regression to have occurred when echocardiograms showed z‐score <2.5 for proximal LAD and proximal RCA as well as CA dimensions below the thresholds for the Japanese Ministry of Health criteria for CA segments for which z‐scores are not available.
Statistics
Demographic and clinical data are presented as count (percentage) or median with (interquartile range) or (range) as specified. Factors associated with MACE were analyzed using logistic regression. Model discrimination for MACE was assessed using the area under the receiver‐operator characteristic curve (c statistic). Associations with highest zMax over follow‐up were evaluated using median regression. Time to CAA regression was estimated using the Kaplan‐Meier method, with follow‐up censored at 2 years after diagnosis of CAA. Factors associated with CAA regression were explored using the log‐rank test and Cox proportional hazards regression. All multivariable analyses were performed using forward selection. A matched subgroup analysis was performed across 4 treatment groups: single dose of IVIG, repeat IVIG (≥2 IVIG doses), single IVIG plus adjunctive anti‐inflammatory (infliximab, steroids, cyclosporine, etc), or both repeat IVIG (≥2 IVIG doses) and adjunctive anti‐inflammatory medication. Patients were matched on CAA z‐score at diagnosis (small, moderate, and large/giant) and age at diagnosis (<1 year of age or ≥1 year of age). Comparisons across groups were performed using the Fisher exact test or the Kruskal‐Wallis test. Time to CAA was compared using Cox regression, with patients with only a single dose of IVIG serving as the reference group. Analyses were performed with SAS version 9.2 (SAS Institute, Inc, Cary, NC).
Results
Demographic and clinical data for the entire cohort (n=500) and for the subgroup of patients who were treated with IVIG and had follow‐up imaging data (follow‐up cohort, n=431) are in Table 1. There were more patients from the more recent era (after 2000) than earlier time periods. Patients younger than 1 year of age comprised one‐third of both cohorts. In the follow‐up cohort, the median CA z‐score at diagnosis was 4.6 (IQR 3.6–8.9), with 15% having large/giant CAA. In the entire cohort, 94% of patients were treated with IVIG. The percentage of patients treated with IVIG increased over time with 44% treated in 1977–1989, 87% treated in 1990–1999, and 97% treated in 2000–2014 (P<0.001). Re‐treatment with IVIG (32%) and use of adjunctive anti‐inflammatory medications (1 or more adjunctive medications given in 27% of patients) were common. Adjunctive anti‐inflammatory medication usage consisted of steroids in 91 patients (18%), infliximab in 69 (14%), cyclosporine in 6 (1.2%), cyclophosphamide in 1 (0.2%), and anakinra in 1 (0.2%) patient. Comparisons of patient characteristics between the 2 sites are shown in Table S1.
Table 1.
Summary of Cohorts
| MACE Analysis (n=500) | Follow‐Up (n=431) | |
|---|---|---|
| Decade of KD episode | ||
| 1977–1989, n (%) | 32 (6%) | 13 (3%) |
| 1990–1999 | 111 (22%) | 87 (20%) |
| 2000–2009 | 254 (51%) | 234 (54%) |
| 2010–2014 | 103 (21%) | 97 (23%) |
| Site | ||
| Boston | 316 (63%) | 265 (61%) |
| San Diego | 184 (37%) | 166 (39%) |
| Age at fever onset, y | ||
| <1 | 167 (33%) | 143 (33%) |
| 1 to 4 | 235 (47%) | 207 (48%) |
| ≥5 | 98 (20%) | 81 (19%) |
| Male sex | 360 (72%) | 312 (72%) |
| Asian race | 112 (22%) | 104 (24%) |
| Location of aneurysm | ||
| Left anterior descending | 221 (44%) | 202 (47%) |
| Right coronary | 117 (23%) | 95 (22%) |
| Both | 162 (33%) | 134 (31%) |
| z‐Score of largest CAA at diagnosis, median (IQR)a | 4.3 (3.4, 7.0) | 4.6 (3.6–8.9) |
| z‐Score of largest CAA at diagnosisa | ||
| z‐Score <5.0 | 313 (63%) | 289 (67%) |
| z‐Score ≥5, <10 | 97 (19%) | 78 (18%) |
| z‐Score ≥10 | 90 (18%) | 64 (15%) |
| IVIG treatment | ||
| Yes | 456 (91%) | 431 (100%) |
| No | 31 (6%) | 0 |
| Unknown | 13 (3%) | 0 |
| If IVIG Yes, IVIG re‐treatment | ||
| Yes | 164/456 (36%) | 148/431 (34%) |
| No | 290/456 (64%) | 282/431 (65%) |
| Unknown | 2/456 (<1%) | 1/431 (<1%) |
| Adjunctive anti‐inflammatory medication | ||
| Yes | 133 (27%) | 123 (29%) |
| No | 356 (71%) | 308 (71%) |
| Unknown | 11 (2%) | 0 (0%) |
IVIG indicates intravenous immunoglobulin; KD, Kawasaki disease; MACE, major adverse cardiac event.
Larger coronary artery between left anterior descending and right coronary artery used.
Patient outcomes for all 500 CAA patients are shown in Figure 2. Of the 361 patients with adequate follow‐up imaging data, 269 (75%) had CAA regression within 2 years of KD episode. MACE occurred in 24 patients (Table 2), 8 of whom had known persistent CAA and 16 who did not have serial follow‐up imaging (12 followed at outside institutions and 4 lost to follow‐up). No patients with CAA regression had MACE. MACE occurred at a median age of 3.5 years (range 0.2‐29.7 years) and median time from KD of 1.5 years (range 14 days to 27 years). Median follow‐up time for the entire cohort was 11.7 years (IQR 6.9–16.6 years).
Figure 2.
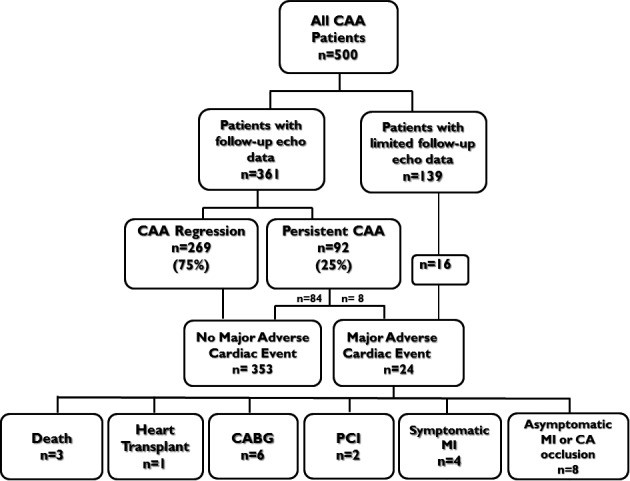
Patient outcomes. CA indicates coronary artery; CAA, coronary artery aneurysm; CABG, coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary artery intervention.
Table 2.
Description of MACE
| MACE | Age at KD, years | KD Year | IVIG | Age at MACE, y | MACE Description | |
|---|---|---|---|---|---|---|
| 1 | MI, OHT | 2.5 | 1981 | No | 29.3 | Acute KD with 6‐mm bilateral CAA, represented at 29 years with cardiomyopathy (EF ≈15%), severe LAD stenosis |
| 2 | MI, PCI | 0.8 | 1982 | No | 21.6 | Giant CAA, lost to follow‐up until he presented with acute chest pain and MI due to high‐grade stenosis of proximal LAD |
| 3 | CA occlusion | 1.3 | 1983 | No | 22.4 | Asymptomatic LAD occlusion on surveillance catheterization |
| 4 | CA Occlusion | 12.2 | 1984 | No | 33.0 | Asymptomatic, incidentally found to have calcified chest mass at 33 years old, giant/thrombosed RCA CAA |
| 5 | CA occlusion | 3.8 | 1985 | No | 6.9 | Asymptomatic, cath 3 years post‐KD with RCA occlusion |
| 6 | MI, Death | 1.1 | 1988 | Late | 1.2 | Acute thrombosis and fatal MI CAA 3 weeks post‐acute KD |
| 7 | MI, CABG, Death | 0.6 | 1988 | Late | 0.7 | Thrombosis of giant LAD CAA 8 weeks post‐acute KD, failed thrombolysis and attempted CABG |
| 8 | CA occlusion | 0.5 | 1989 | No | 20.0 | Asymptomatic, lost to follow‐up, surveillance catheterization with occluded RCA |
| 9 | MI, CA occlusion | 0.9 | 1990 | Yes | 5.2 | Asymptomatic, LAD occlusion by cath with rwma on resting echocardiogram |
| 10 | MI, VT, CABG, PCI | 4.1 | 1993 | Late | 6.0 | Giant bilateral CAA, complete LAD occlusion, high‐grade RCA stenosis with inducible perfusion defect on dobutamine MRI |
| 11 | CABG, MI | 1.3 | 1994 | Late | 4.1 | Thrombotic LAD occlusion in acute phase with rwma and inducible ischemia on dobumatine MRI |
| 12 | MI, CA occlusion | 9.1 | 1994 | No | 21.2 | Asymptomatic, RCA occlusion, delayed enhancement and rwma on dobutamine MRI |
| 13 | MI | 0.6 | 1994 | Late | 0.6 | Acute phase LAD thrombosis with apical MI |
| 14 | MI, PCI | 4.7 | 1995 | Late | 14.4 | Severe stenosis of proximal LAD CAA with exertional chest pain and reversible perfusion defect on stress MIBI |
| 15 | CABG | 0.6 | 1997 | No | 11.9 | RCA occlusion, severe LAD stenosis, ST elevation on exercise stress test |
| 16 | MI, CABG | 1.6 | 1999 | Late | 4.5 | Acute chest pain and MI due to thrombotic occlusion of giant RCA CAA |
| 17 | MI, CA occlusion | 0.4 | 1999 | Late | 1.3 | Asymptomatic, thrombotic LAD occlusion with apical/anterior rwma, fixed perfusion defect and +MDE on MRI |
| 18 | PCI | 0.3 | 2002 | Late | 5.4 | Exertional angina at age 5 years leading to catheterization showing severe RCA stenosis, failed attempt at RCA angioplasty |
| 19 | MI, CA occlusion | 2.5 | 2002 | Late | 2.6 | Thrombotic occlusion of giant circumflex CAD 1 month post‐acute KD, residual +MDE and resting rwma |
| 20 | MI, Death | 0.1 | 2003 | Yes | 0.3 | Giant bilateral CAA. Sudden cardiac arrest at home 10 weeks post‐KD. |
| 21 | MI | 0.3 | 2003 | Late | 4.7 | Asymptomatic, severe RCA stenosis with rwma on rest echo and +MDE on MRI |
| 22 | CABG | 9.4 | 2007 | Yes | 11.6 | RCA occlusion, reversible perfusion defect on stress MIBI and inducible rwma on stress echo |
| 23 | MI, CA occlusion | 0.6 | 2007 | Yes | 0.7 | Occlusive LAD thrombus and MI in acute stage treated with heparin |
| 24 | CA Occlusion | 1.7 | 2010 | Yes | 0.7 | LAD thrombosis in acute phase, successfully treated with thrombolytic |
CAA indicates coronary artery aneurysm; CABG, coronary artery bypass graft; KD, Kawasaki disease; LAD, left anterior descending; MACE, major adverse cardiac event; MDE, myocardial delayed enhancement; MI, myocardial infarction, MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention; RCA, right coronary artery; rwma, regional wall motion abnormality; VT, ventricular tachycardia.
MACE included 3 deaths, 1 orthotopic heart transplant (OHT), 6 CABG, and 2 PCI (Table 2). The deaths all occurred in infants who had thrombosis of the LAD in the acute or subacute period. First, a 13‐month‐old male 3 weeks after illness onset suffered acute thrombosis of giant proximal LAD CAA and fatal MI. Second, a 7‐month‐old male 8 weeks after illness onset had thrombosis of a giant LAD aneurysm and acute MI. After thrombolytic therapy failed, he was taken to the operating room for a CABG, which was unsuccessful, and the patient died in the early postoperative period. Third, a 6‐week‐old female with giant bilateral CAA who died suddenly at home 2.5 months into illness from presumed MI; her family refused autopsy. One patient underwent OHT. He developed KD at 2.5 years of age with large bilateral CAA. He presented again at age 29 years with congestive heart failure symptoms, high‐grade LAD stenosis, and cardiomyopathy (ejection fraction ≈15%). Six patients underwent CABG, and 2 underwent PCI at a median age of 6 years (range 4.1‐12.4 years) and median interval of 3 years post–acute KD (range 1.7‐11.3 years). Of the 12 patients with MI or CA occlusion but no other MACE, 4 had clinical symptoms of MI, and 8 had no recognized clinical symptoms and were diagnosed on surveillance imaging (eg, radionuclear imaging, cardiac MRI with myocardial delayed enhancement, or PET scan).
Factors associated with MACE in univariate and multivariable analysis are shown in Table S2 and Table 3, respectively. Larger CAA size at diagnosis had the highest discrimination for prediction of MACE, with a c‐statistic of 0.92. MACE occurred in 21 of 90 patients (23%) with z‐score ≥10 at diagnosis, 3 of 97 (3%) with z‐score of 5 to 10 at diagnosis, and in none of 313 patients with CA z‐score <5 at diagnosis. All patients with MACE had a history of giant CAA at some time point. The 3 patients with z‐score 5 to 10 at diagnosis who experienced MACE all had progression in size of CAA to z‐score >10 during follow‐up. Lack of IVIG treatment was also associated with MACE in multivariable analysis. MACE occurred in 11/44 (25%) patients who did not receive IVIG, 9/171 (5%) patients who received IVIG ≥10 days after fever onset, and in only 4/285 (1.4%) patients who were treated within 10 days. When follow‐up duration is included in the multivariate model, both size of CAA at diagnosis (OR 1.1, P<0.001) and lack of IVIG treatment (OR 4.4, P=0.03) as well as follow‐up duration (OR=1.1, P=0.02) are associated with MACE. Demographic factors, decade of treatment, site of treatment, use of additional IVIG doses and/or adjunctive anti‐inflammatory medications were not independent risk factors for MACE. In a subgroup analysis restricted to patients who received IVIG within 10 days of treatment, CAA size at diagnosis was the only factor associated with MACE.
Table 3.
Multivariable Analysis of Factors Associated With Major Adverse Cardiac Events
| Odds Ratio | P Value | c‐Statistic | |
|---|---|---|---|
| Size of CAA at diagnosis (for each 1 unit increase in z‐score)a | 1.1 | <0.001 | 0.93 |
| No IVIG treatment | 9.0 | <0.001 |
CAA indicates coronary artery aneurysm; IVIG, intravenous immunoglobulin.
Larger coronary artery between left anterior descending and right coronary artery used.
In the 431 patients with follow‐up CA imaging, the highest zMax occurred on the baseline echocardiogram, ie, at diagnosis, in 273 patients (63%) and at follow‐up in 158 patients (37%). In the latter group the median time to highest zMax was 12 days (interquartile range 0‐27 days). Median highest zMax was 4.5 (range 3.1‐29.9). Univariate and multivariable analyses of factors associated with zMax are shown in Table S3 and Table 4, respectively. In multivariable analysis only larger CAA z‐score at diagnosis and bilateral CAA remained associated with higher zMax. Although IVIG re‐treatment and treatment with adjunctive anti‐inflammatory medications were both associated with higher zMax in univariate analysis, additional KD treatments were not associated with larger zMax after adjustment for baseline CA z‐score.
Table 4.
Multivariable Analysis of Factors Associated With Higher Maximal CA z‐Score
| Coefficient | P Value | |
|---|---|---|
| Largest CAA size at diagnosisa | ||
| z<5.0 | 1.00 | — |
| z≥5, <10 | 2.65 | <0.001 |
| z≥10 | 18.2 | <0.001 |
| CAA location | ||
| LAD or RCA alone | 1.00 | — |
| Both LAD and RCA | 1.10 | 0.01 |
CA indicates coronary arteries; CAA, coronary artery aneurysm; LAD, left anterior descending coronary artery; RCA, right coronary artery.
Larger coronary artery between left anterior descending and right coronary artery used.
CAA regression to normal internal lumen diameter within 2 years occurred in 269 of 361 patients (75%) (Figure 3). Kaplan‐Meier curves (Figure 4) and Table 5 show factors associated with CAA regression. In multivariable analysis, lack of CAA regression was associated with earlier era, larger CAA z‐score at diagnosis, and bilateral CAA. The CAA regression rate in the most recent 5‐year period was 91% compared with 77% from 2000 to 2009 and 49% prior to 2000. Only 19% of patients with CAA z‐score ≥10 at diagnosis had CAA regression, whereas 55% with z‐score between 5 and 9.99 and 87% with z<5 had CAA regression. Patients with bilateral CAA had a lower incidence of regression (45%) than those with only LAD (86%) or RCA (84%) CAA. IVIG re‐treatment and/or use of adjunctive anti‐inflammatory medications were not associated with time to CAA regression. Evaluation of CAA regression of individual CA segments showed 458 total aneurysmal CA segments (LAD and RCA) in the 361 patients. The regression rate was modestly higher in LCA (211/280, 75%) compared to RCA (119/178, 65%). When the analysis is limited to patients who received IVIG within 10 days of fever onset (n=250), the same risk factors remain significant for lack of CAA regression in multivariable analysis: earlier time period of KD (1984‐1999 HR=1.00, 2000‐2009 HR=2.08, 2010‐2014 HR=3.40, P<0.001); larger CAA (CAA z‐score ≥10 HR=1.00, z≥5, <10 HR=4.83 [P=0.01], z<5 HR=11.70 [P<0.001]); and bilateral CAA (HR=0.38, P<0.001).
Figure 3.
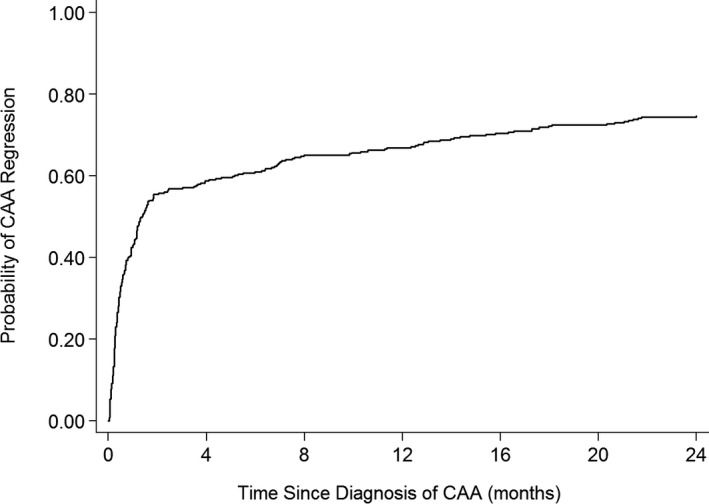
Kaplan‐Meier curve for coronary artery aneurysm regression: entire cohort. CAA indicates coronary artery aneurysm.
Figure 4.
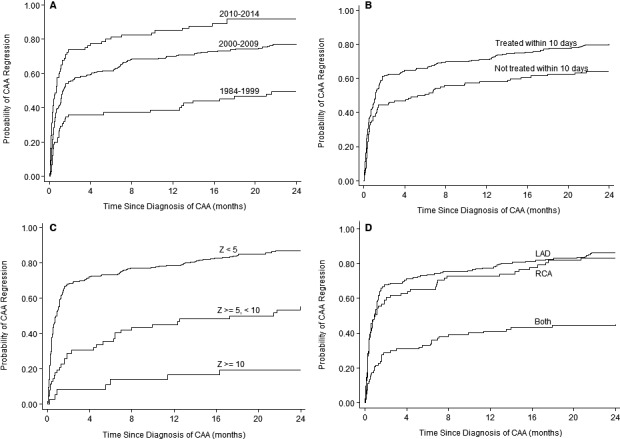
Kaplan‐Meier curves for coronary artery aneurysm regression. A, Decade of Kawasaki disease episode. B, IVIG treatment within 10 days of fever onset. C, Maximum coronary artery z‐score at diagnosis. D, Location of coronary artery aneurysm. CAA indicates coronary artery aneurysm; IVIG, intravenous immunoglobulin; LAD, left anterior descending coronary artery; RCA, right coronary artery.
Table 5.
Factors Associated With CAA Regression
| n | Number (%) With CAA Regression | Hazard Ratio | P Value | |
|---|---|---|---|---|
| Univariate analysis | 361 | 269 (75) | ||
| Time period of KD | ||||
| 1979–1999 | 75 | 37 (49) | 1.00 | — |
| 2000–2009 | 206 | 159 (77) | 2.13 | <0.001 |
| 2000–2014 | 80 | 73 (91) | 3.58 | <0.001 |
| Site | ||||
| Boston | 208 | 143 (69) | 1.00 | — |
| San Diego | 153 | 126 (82) | 1.37 | 0.01 |
| Sex | ||||
| Female | 99 | 68 (69) | 1.00 | — |
| Male | 262 | 201 (77) | 1.15 | 0.33 |
| Age at fever onset | ||||
| ≥5 y | 69 | 52 (75) | 1.00 | — |
| 1 to 4 y | 178 | 146 (82) | 1.17 | 0.33 |
| <1 y | 114 | 71 (62) | 0.72 | 0.07 |
| Asian race | ||||
| No/unknown | 269 | 189 (70) | 1.00 | — |
| Yes | 92 | 80 (87) | 1.47 | 0.004 |
| Largest CAA size at diagnosisa | ||||
| z<5.0 | 263 | 228 (87) | 9.48 | <0.001 |
| z≥5, <10 | 62 | 34 (55) | 3.53 | 0.002 |
| z≥10 | 36 | 7 (19) | 1.00 | — |
| Size of CAA at diagnosis (for each 1 unit increase in z‐score)a | — | — | 0.87 | <0.001 |
| CAA location | ||||
| Left anterior descending coronary artery | 183 | 158 (86) | 3.16 | <0.001 |
| Right coronary artery | 81 | 67 (83) | 2.88 | <0.001 |
| Both | 97 | 44 (45) | 1.00 | — |
| Timing of IVIG treatment | ||||
| <10 days of fever | 239 | 191 (80) | 2.06 | <0.001 |
| >10 days of fever | 55 | 29 (53) | 1.00 | — |
| Unknown timing | 67 | 49 (73) | 1.69 | 0.03 |
| IVIG re‐treatment | ||||
| No | 234 | 193 (82) | 1.00 | — |
| Yes | 111 | 71 (64) | 0.65 | 0.002 |
| Use of adjunctive anti‐inflammatory meds | ||||
| No | 259 | 195 (75) | 1.00 | — |
| Yes | 102 | 74 (73) | 1.00 | 0.98 |
| Multivariable analysis | ||||
| Time period of KD | ||||
| 1977–1999 | 1.00 | — | ||
| 2000–2009 | 2.17 | <0.001 | ||
| 2000–2014 | 3.49 | <0.001 | ||
| Largest CAA size at diagnosis | ||||
| z<5.0 | 6.73 | <0.001 | ||
| z≥5, <10 | 3.00 | 0.009 | ||
| z≥10 | 1.00 | — | ||
| CAA location—both RCA and LAD | 0.61 | 0.007 | ||
CAA indicates coronary artery aneurysm; KD, Kawaski disease; IVIG, intravenous immunoglobulin.
Larger coronary artery between left anterior descending and right coronary artery use.
Finally, we analyzed the effects of treatment strategy on likelihood of CAA regression within 2 years of disease onset using 4 matched groups of 34 patients each, with matching based on age group and CAA z‐score at diagnosis (Table 6). Median age (1.7‐2.0 years) and CAA z‐score at diagnosis (4.2‐4.4) were similar between groups. The CAA regression rate was 91% in patients who received IVIG and adjunctive anti‐inflammatory medication compared to 68% in the other 3 groups (single dose of IVIG, IVIG ≥2 doses, IVIG ≥2 doses and adjunctive anti‐inflammatory medication) (OR=1.95, P=0.02). Treatment strategy was collinear with site and era of treatment and differed between comparison groups (Table 6).
Table 6.
Matched Treatment Effect Analysis
| IVIG Alone (n=34) | IVIG×2 Doses (n=34) | IVIG+Anti‐inflammatory (n=34) | IVIG×2 Doses and Anti‐inflammatory (n=34) | P Value | |
|---|---|---|---|---|---|
| CAA regression rate | 68% | 68% | 91% | 68% | 0.02 |
| Age at fever onset, y | 1.9 (0.8, 4.4) | 2.0 (0.7, 4.3) | 1.7 (0.6, 4.3) | 1.8 (0.7, 4.0) | 0.94 |
| <1 year | 12 (35%) | 12 (35%) | 12 (35%) | 12 (35%) | 1.0 |
| ≥1 year | 22 (65%) | 22 (65%) | 22 (65%) | 22 (65%) | |
| CAA z‐score at diagnosis | 4.0 (3.5, 5.2) | 4.3 (3.4, 5.2) | 4.1 (3.4, 5.4) | 3.9 (3.5, 5.0) | 0.99 |
| z‐score <5.0 | 25 (74%) | 25 (74%) | 25 (74%) | 25 (74%) | 1.0 |
| z‐score ≥5.0, <10.0 | 6 (18%) | 6 (18%) | 6 (18%) | 6 (18%) | |
| z‐score ≥10.0 | 3 (9%) | 3 (9%) | 3 (9%) | 3 (9%) | |
| Male sex | 23 (68%) | 29 (85%) | 24 (71%) | 24 (71%) | 0.34 |
| Asian race | 10 (29%) | 5 (15%) | 10 (29%) | 9 (26%) | 0.44 |
| Time period of KD | <0.001 | ||||
| 1984–1999 | 7 (21%) | 13 (38%) | 1 (3%) | 5 (15%) | |
| 2000–2009 | 18 (53%) | 19 (56%) | 13 (38%) | 17 (50%) | |
| 2010–2014 | 9 (26%) | 2 (6%) | 20 (59%) | 12 (35%) | |
| Treatment ≥10 days of fever | 5 (21%) | 2 (6%) | 5 (16%) | 3 (10%) | 0.41 |
| Site | <0.001 | ||||
| Boston | 17 (50%) | 28 (82%) | 5 (15%) | 20 (59%) | |
| San Diego | 17 (50%) | 6 (18%) | 29 (85%) | 14 (41%) | |
| CAA location | 0.79 | ||||
| LAD | 20 (59%) | 19 (56%) | 20 (59%) | 17 (50%) | |
| RCA | 5 (15%) | 8 (24%) | 7 (21%) | 5 (15%) | |
| Both LAD and RCA | 9 (26%) | 7 (21%) | 7 (21%) | 12 (35%) |
CAA indicates coronary artery aneurysm; KD, Kawasaki disease; LAD, left anterior descending; RCA, right coronary artery.
Discussion
In this 2‐center study, we describe outcomes in the largest cohort of US KD patients with CAA reported to date. We found an overall CAA regression rate of ≈75%. MACE occurred in ≈5% of CAA patients, almost exclusively in those with large/giant CAA at diagnosis. Initial CA z‐score was highly predictive of CA regression, highest CA zMax over time, and clinical events. Because echocardiograms are now routinely obtained at diagnosis, it has become increasingly clear that initial CAA size provides excellent risk stratification.8, 28 This study adds to existing evidence that CAA size at diagnosis is highly predictive of both CAA persistence and cardiac events.28, 29
The CAA regression rate of 75% in our cohort is higher than reported by prior studies.6, 17, 18 A large Japanese study from the pre‐IVIG era performed serial angiograms in KD patients with CAA and showed that ≈55% to 60% had CAA regression, typically within 1 to 2 years of KD.6 Notably, none of the patients with giant aneurysms (n=26) in that study had CAA regression. Similarly, we found that CAA size at diagnosis is highly predictive of CAA regression rate, with a low CAA regression rate (16%) in patients with large/giant CAA and a high regression rate in those with small CAA at diagnosis (85%). More aggressive therapy, particularly increased use of repeat IVIG or additional anti‐inflammatory medications, may decrease CA inflammation and progressive dilation and thereby account for improved CAA regression rate.26, 30, 31 Improved echocardiographic techniques and routine use of CA z‐scores for definition of CAA may lead to identification of more small CAA, and this may also contribute to the higher CAA regression rate in our study compared to prior studies.19
MACE occurred exclusively in patients with giant CAA; 23% of patients in this group had at least 1 MACE. The cardiac event rate in the current study is lower than that in prior Japanese studies, which report cardiac event‐free survival ranging from ≈30% to 50% in KD patients with giant CAA.13, 15, 32 Possible explanations for this difference include the definition of giant CAA; z‐score >10 in this study compared to >8 mm absolute dimension in older literature, which uses Japanese Ministry of Health Criteria. Additionally, more aggressive anticoagulation and medical management may play a role in improved outcomes in this population.12, 17, 33 Duration of follow‐up in this study was shorter than those in Japanese studies of KD patients in the pre‐IVIG era. Conversely, 8 events were clinically silent and were only detected on surveillance imaging showing myocardial scar and/or fixed perfusion defect. Importantly, absence of IVIG administration was an independent risk factor for MACE. In the entire cohort only 4 patients treated with IVIG within 10 days of fever onset developed MACE, whereas 19 patients who had late or no IVIG treatment developed MACE. This provides a strong argument that the most effective intervention for reducing cardiac sequalae from KD involves improved education and awareness in order to prevent missed or delayed diagnosis of KD.34, 35, 36
Our results also suggest that KD recognition and prompt treatment have improved over time with resultant improvement in outcomes. Specifically, in the most recent 5‐year period, the CAA regression rate was ≈90%, and there was only 1 MACE. This CAA regression rate is higher than those of prior eras (50% to 75%) and also higher than those previously reported.6 Improvement in outcomes may be related to greater recognition of KD, leading to fewer patients with late and missed diagnoses. It is also possible that better outcomes are a consequence of more aggressive acute‐phase therapies for patients with CA abnormalities at diagnosis in the more recent era.26, 30, 31 The latter hypothesis is supported by a subgroup analysis of our patients who received IVIG within 10 days of fever onset; those treated in the most recent 5‐year period were more likely to receive additional KD medications and had a higher rate of CAA regression than patients treated in prior eras. Although administration of anti‐inflammatory medications adjunctive to IVIG was associated with improved outcomes, our study design did not permit causal inference and should be viewed as hypothesis generating. Future large randomized controlled trials are needed to determine optimal therapy in KD patients who present with CAA.
Other limitations of this study include its retrospective design, with less optimal data capture in the early era. The precision of our estimates of MACE was limited by losses to follow‐up and inability of young children to report symptoms, such as MI. There is potential ascertainment bias in diagnosis of MACE, particularly clinically silent MI, as follow‐up diagnostic testing and follow‐up duration varied. Both centers in this study are referral centers for management of KD with CA complications, and this may bias the MACE rate. Many covariates were highly correlated, eg, treatment regimen and era, preventing causal inference. The matched analysis of treatment effect is subject to confounding by indication; we attempted to mitigate this by matching based on CAA size at diagnosis. KD therapy varied over time and by site. Indications for additional IVIG and adjunctive anti‐inflammatory medications were not standardized over the study period. We cannot determine if anticoagulation had an effect on CAA regression rate or MACE in patients with giant CAA because all giant CAA patients were on long‐term anticoagulants. Because the sample size in our matched treatment analysis was small, we grouped all adjunctive anti‐inflammatory medications together and had insufficient power to analyze the treatment effect of specific anti‐inflammatory medications or steroid regimens. The matched analysis was further limited by the small number of patients with large/giant CAA who could be matched (n=5 in each group), as well as the inability to evaluate the timing of adjunctive anti‐inflammatory therapies relative to disease onset. Finally, regression of lumen diameter in CAA is not equivalent to normalization of the arterial wall, particularly after remodeling of large and giant CAAs in which layering thrombus and luminal myofibroblastic proliferation occur, leaving these patients at risk for cardiac events.37, 38
Conclusions
In summary, in the largest cohort of US patients with KD and CAA reported to date, 71% had CAA regression over a 2‐year period. Clinical outcomes were associated with CAA z‐score at diagnosis as well as with prompt treatment with IVIG and adjunctive therapies. Once large/giant CAAs develop, the risks of CAA persistence and MACE are high; early recognition of KD and mitigation of CAA progression are thus key.28, 39, 40, 41 Randomized, clinical trials of adjunctive anti‐inflammatory therapies for KD patients who present with CAA are needed to improve outcomes for this vulnerable patient population.
Sources of Funding
This work was supported by The McCance Foundation, Gordon and Marilyn Macklin Foundation, and The Tommy Kaplan Family Fund.
Disclosures
None.
Supporting information
Table S1. Comparison of Study Sites
Table S2. Univariate Analysis of Factors Associated With Major Adverse Cardiac Events
Table S3. Univariate Analysis of Factors Associated With Higher Maximal CA z‐Score
(J Am Heart Assoc. 2016;5:e003289 doi: 10.1161/JAHA.116.003289)
References
- 1. Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA; Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association . Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. [DOI] [PubMed] [Google Scholar]
- 3. Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. [DOI] [PubMed] [Google Scholar]
- 4. McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, Vetter VL, Gersony WM, Mitchell PD, Newburger JW; Pediatric Heart Network I . Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–179. [DOI] [PubMed] [Google Scholar]
- 5. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131:888–893. [DOI] [PubMed] [Google Scholar]
- 6. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long‐term consequences of Kawasaki disease. A 10‐ to 21‐year follow‐up study of 594 patients. Circulation. 1996;94:1379–1385. [DOI] [PubMed] [Google Scholar]
- 7. Newburger JW. Treatment of Kawasaki disease. Lancet. 1996;347:1128. [DOI] [PubMed] [Google Scholar]
- 8. Ogata S, Tremoulet AH, Sato Y, Ueda K, Shimizu C, Sun X, Jain S, Silverstein L, Baker AL, Tanaka N, Ogihara Y, Ikehara S, Takatsuki S, Sakamoto N, Kobayashi T, Fuse S, Matsubara T, Ishii M, Saji T, Newburger JW, Burns JC. Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int J Cardiol. 2013;168:3825–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP, Shulman ST, Wiggins JW, Hicks RV, Fulton DR, Lewis AB, Leung DY, Colton T, Rosen FS, Melish ME. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. [DOI] [PubMed] [Google Scholar]
- 10. Kusakawa S. [Kawasaki disease: results of study by a research group of the Ministry of Health and Welfare]. Nihon Rinsho. 1983;41:1970–1977. [PubMed] [Google Scholar]
- 11. Ronai C, Hamaoka‐Okamoto A, Baker AL, de Ferranti SD, Colan SD, Newburger JW, Friedman KG. Coronary artery aneurysm measurement and Z score variability in Kawasaki disease. J Am Soc Echocardiogr. 2015;29:150–157. [DOI] [PubMed] [Google Scholar]
- 12. Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z‐scores after Kawasaki disease. Pediatr Cardiol. 2010;31:242–249. [DOI] [PubMed] [Google Scholar]
- 13. Tsuda E, Hamaoka K, Suzuki H, Sakazaki H, Murakami Y, Nakagawa M, Takasugi H, Yoshibayashi M. A survey of the 3‐decade outcome for patients with giant aneurysms caused by Kawasaki disease. Am Heart J. 2014;167:249–258. [DOI] [PubMed] [Google Scholar]
- 14. Holve TJ, Patel A, Chau Q, Marks AR, Meadows A, Zaroff JG. Long‐term cardiovascular outcomes in survivors of Kawasaki disease. Pediatrics. 2014;133:e305–e311. [DOI] [PubMed] [Google Scholar]
- 15. Suda K, Iemura M, Nishiono H, Teramachi Y, Koteda Y, Kishimoto S, Kudo Y, Itoh S, Ishii H, Ueno T, Tashiro T, Nobuyoshi M, Kato H, Matsuishi T. Long‐term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single‐institution experience. Circulation. 2011;123:1836–1842. [DOI] [PubMed] [Google Scholar]
- 16. Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther. 2010;8:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levy DM, Silverman ED, Massicotte MP, McCrindle BW, Yeung RS. Longterm outcomes in patients with giant aneurysms secondary to Kawasaki disease. J Rheumatol. 2005;32:928–934. [PubMed] [Google Scholar]
- 18. Takahashi M, Mason W, Lewis AB. Regression of coronary aneurysms in patients with Kawasaki syndrome. Circulation. 1987;75:387–394. [DOI] [PubMed] [Google Scholar]
- 19. de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–258. [DOI] [PubMed] [Google Scholar]
- 20. Beiser AS, Takahashi M, Baker AL, Sundel RP, Newburger JW. A predictive instrument for coronary artery aneurysms in Kawasaki disease. US Multicenter Kawasaki Disease Study Group. Am J Cardiol. 1998;81:1116. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, Kobayashi T, Morikawa A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. [DOI] [PubMed] [Google Scholar]
- 22. Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, Atz AM, Li JS, Takahashi M, Baker AL, Colan SD, Mitchell PD, Klein GL, Sundel RP; Pediatric Heart Network I . Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356:663–675. [DOI] [PubMed] [Google Scholar]
- 23. Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, Baker A, Fulton DR, Sundel RP, Newburger JW. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. J Pediatr. 2011;158:644.e1–649.e1. [DOI] [PubMed] [Google Scholar]
- 24. Son MB, Gauvreau K, Ma L, Baker AL, Sundel RP, Fulton DR, Newburger JW. Treatment of Kawasaki disease: analysis of 27 US pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8. [DOI] [PubMed] [Google Scholar]
- 25. Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, Martin DD, Newburger JW, Burns JC. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tremoulet AH, Jain S, Jaggi P, Jimenez‐Fernandez S, Pancheri JM, Sun X, Kanegaye JT, Kovalchin JP, Printz BF, Ramilo O, Burns JC. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double‐blind, placebo‐controlled trial. Lancet. 2014;383:1731–1738. [DOI] [PubMed] [Google Scholar]
- 27. Tremoulet AH, Pancoast P, Franco A, Bujold M, Shimizu C, Onouchi Y, Tamamoto A, Erdem G, Dodd D, Burns JC. Calcineurin inhibitor treatment of intravenous immunoglobulin‐resistant Kawasaki disease. J Pediatr. 2012;161:506–512.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsuda E, Kamiya T, Ono Y, Kimura K, Kurosaki K, Echigo S. Incidence of stenotic lesions predicted by acute phase changes in coronary arterial diameter during Kawasaki disease. Pediatr Cardiol. 2005;26:73–79. [DOI] [PubMed] [Google Scholar]
- 29. Mueller F, Knirsch W, Harpes P, Pretre R, Valsangiacomo Buechel E, Kretschmar O. Long‐term follow‐up of acute changes in coronary artery diameter caused by Kawasaki disease: risk factors for development of stenotic lesions. Clin Res Cardiol. 2009;98:501–507. [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, Kato T, Hara T, Hamaoka K, Ogawa S, Miura M, Nomura Y, Fuse S, Ichida F, Seki M, Fukazawa R, Ogawa C, Furuno K, Tokunaga H, Takatsuki S, Hara S, Morikawa A; RAISE Study Group Investigators . Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open‐label, blinded‐endpoints trial. Lancet. 2012;379:1613–1620. [DOI] [PubMed] [Google Scholar]
- 31. Shafferman A, Birmingham JD, Cron RQ. High dose Anakinra for treatment of severe neonatal Kawasaki disease: a case report. Pediatr Rheumatol Online J. 2014;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuda E, Hirata T, Matsuo O, Abe T, Sugiyama H, Yamada O. The 30‐year outcome for patients after myocardial infarction due to coronary artery lesions caused by Kawasaki disease. Pediatr Cardiol. 2011;32:176–182. [DOI] [PubMed] [Google Scholar]
- 33. Manlhiot C, Brandao LR, Somji Z, Chesney AL, MacDonald C, Gurofsky RC, Sabharwal T, Chahal N, McCrindle BW. Long‐term anticoagulation in Kawasaki disease: initial use of low molecular weight heparin is a viable option for patients with severe coronary artery abnormalities. Pediatr Cardiol. 2010;31:834–842. [DOI] [PubMed] [Google Scholar]
- 34. Dominguez SR, Anderson MS, El‐Adawy M, Glode MP. Preventing coronary artery abnormalities: a need for earlier diagnosis and treatment of Kawasaki disease. Pediatr Infect Dis J. 2012;31:1217–1220. [DOI] [PubMed] [Google Scholar]
- 35. Sudo D, Monobe Y, Yashiro M, Mieno MN, Uehara R, Tsuchiya K, Sonobe T, Nakamura Y. Coronary artery lesions of incomplete Kawasaki disease: a nationwide survey in Japan. Eur J Pediatr. 2012;171:651–656. [DOI] [PubMed] [Google Scholar]
- 36. Wilder MS, Palinkas LA, Kao AS, Bastian JF, Turner CL, Burns JC. Delayed diagnosis by physicians contributes to the development of coronary artery aneurysms in children with Kawasaki syndrome. Pediatr Infect Dis J. 2007;26:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sugimura T, Kato H, Inoue O, Fukuda T, Sato N, Ishii M, Takagi J, Akagi T, Maeno Y, Kawano T. Intravascular ultrasound of coronary arteries in children. Assessment of the wall morphology and the lumen after Kawasaki disease. Circulation. 1994;89:258–265. [DOI] [PubMed] [Google Scholar]
- 39. Sudo D, Monobe Y, Yashiro M, Sadakane A, Uehara R, Nakamura Y. Case‐control study of giant coronary aneurysms due to Kawasaki disease: the 19th nationwide survey. Pediatr Int. 2010;52:790–794. [DOI] [PubMed] [Google Scholar]
- 40. Tsuda E, Kamiya T, Kimura K, Ono Y, Echigo S. Coronary artery dilatation exceeding 4.0 mm during acute Kawasaki disease predicts a high probability of subsequent late intima‐medial thickening. Pediatr Cardiol. 2002;23:9–14. [DOI] [PubMed] [Google Scholar]
- 41. Broderick L, Tremoulet AH, Burns JC, Hoffman HM. Prolonged urticaria and fever in a toddler. Allergy Asthma Proc. 2012;33:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of Study Sites
Table S2. Univariate Analysis of Factors Associated With Major Adverse Cardiac Events
Table S3. Univariate Analysis of Factors Associated With Higher Maximal CA z‐Score


