Abstract
Background
An early repolarization pattern (ERP) has been hypothesized to be arrhythmogenic in experimental studies, but the prognostic significance of the ERP in the general population is controversial. We performed a meta‐analysis to examine the link between ERP and the risk of sudden cardiac arrest (SCA), cardiac death, and death from any cause.
Methods and Results
We performed a literature search using MEDLINE (January 1, 1966 to July 31, 2015) and EMBASE (January 1, 1980 to July 31, 2015) with no restrictions. Studies that reported relative risk (RR) estimates with 95% confidence intervals (CIs) for the associations of interest were included. Sixteen studies involving 334 524 subjects were identified. Compared with those without ERP, subjects with ERP experienced significantly increased risk for developing SCA (RR 2.18; 95% CI 1.29–3.68), cardiac death (RR 1.48; 95% CI 1.06–2.07), and death from any cause (RR 1.21; 95% CI 1.02–1.42), respectively. The increased risk was present predominantly in Asians and whites but not in African Americans. ERP with J‐point elevation in inferior leads, notching configuration, and horizontal or descending ST segment connote higher risk. ERP was associated with an absolute risk increase of 139.6 (95% CI 130.3–149.3) additional SCAs per 100 000 person‐years and responsible for 7.3% (95% CI 1.9–15.2) of SCA in the general population.
Conclusions
ERP is associated with significant increased risk for SCA, cardiac death, and death from any cause. Future studies should focus on understanding the exact mechanisms for the arrhythmia risk and developing reliable tools for risk stratification.
Keywords: cardiac death, early repolarization pattern, meta‐analysis, risk, sudden cardiac arrest
Subject Categories: Arrhythmias, Sudden Cardiac Death, Ventricular Fibrillation
Introduction
An early repolarization pattern (ERP)—elevation of the QRS‐ST junction (J‐point) and QRS notching or slurring in multiple leads—is a common electrocardiographic finding that affects 1% to 13% of persons.1 Although traditionally viewed as benign, ERP has recently been reported to be a marker of increased transmural heterogeneity of ventricular repolarization in experimental studies, which might increase the vulnerability to ventricular fibrillation.2
The past few years have seen a rapidly growing interest in testing this hypothesis. Many epidemiologic studies have investigated the link between ERP and long‐term cardiovascular risk, but the results are surprisingly conflicting.3, 4, 5 Our group has previously reported a meta‐analysis and revealed that ERP was associated with increased risk for arrhythmia death in the general population.6 However, evidence was limited at that time, and it may be underpowered to detect positive effects for particular outcomes or subgroups. Therefore, we conducted a new meta‐analysis to summarize all published cohort studies and case‐control studies to update the cardiovascular outcomes associated with ERP in the general population.
Methods
Research Objectives
The primary study endpoint was sudden cardiac arrest (SCA), including sudden cardiac arrest (International Classification of Disease, 10th Revision [ICD‐10] codes I46.901, I46.902, I46.051), ventricular fibrillation (I49.002), and sudden cardiac death (I46.101). The secondary study endpoint was cardiac death with the underlying cause of death consistent with a cardiovascular disease (I10‐I79), because we hypothesized that the incidence of cardiac death should be increased if ERP was proarrhythmic. In addition, we included an analysis of death from any cause, which is a hard, verifiable endpoint and not subject to classification errors.
Search Strategy
We searched the publications listed in the electronic databases MEDLINE (source PubMed, January 1, 1966 to July 31, 2015) and EMBASE (January 1, 1980 to July 31, 2015) using the following text and key words in combination both as MeSH terms and text words: “early repolarization,” “J‐wave,” “cardiac,” “cardiovascular,” “death,” “mortality,” “ventricular fibrillation,” “sudden cardiac death,” “cardiac arrest.” We searched articles published in any language and scrutinized references from these studies to identify other relevant studies.
Study Selection
To minimize differences among studies, we imposed the following methodological restrictions for the inclusion criteria: (1) Studies were used that contained the minimum information necessary to estimate the relative risk (RR) associated with ERP and a corresponding measure of uncertainty (ie, 95% confidence interval [CI], standard error, variance, or P value of the significance of the estimate). (2) Cohort studies were used, ie, case‐control studies published as original articles (case reports and prevalence studies were excluded). (3) Studies used had to be independent. In case of multiple reports on the same population or subpopulation, we considered the estimates from the most recent or most informative reports. In instances of multiple publications, the most up‐to‐date or comprehensive information was used. The study was determined to be exempt by the Institutional Review Board of the First Affiliated Hospital of Sun Yat‐Sen University.
Data Abstraction and Quality Assessment
Three authors (Y.J.C., X.X.L., C.C.J.) independently extracted the data. The following data were extracted from each study: first author's name, publication year, geographical location, sex category, mean age, study size, study design, sampling framework, study population, number of cardiovascular events, covariates adjusted for in the multivariable analysis, and RRs and the associated measure of variance. When available, we used the most comprehensively adjusted risk estimates. The Newcastle‐Ottawa quality assessment scale (NOS) was used to evaluate the quality of observational studies. We developed the evaluation criteria. The score ranged from 0 to 9 points for cohort and case‐control studies, with a higher score indicating higher study quality.
Statistical Analysis
The RR was used as a measure of the association between ERP and cardiovascular risk. For case‐control studies, the odds ratios (ORs) were transformed into RRs using the formula RR=OR/[(1−P0)+(P0×OR)], in which P0 is the incidence of the outcome of interest in the reference group.7
When available, we used the most comprehensively adjusted risk estimates reported in the original manuscript for the meta‐analysis. When actual RR was not available, we calculated RRs and 95% CIs using Stata (College Station, TX) version 11.0. Summary RRs (95% CI) were calculated by pooling the study‐specific estimates using a random‐effects model that included between‐study heterogeneity (parallel analyses used fixed‐effects models) because significant heterogeneity was anticipated among studies. Pooled RRs were expressed with 95% CIs. We calculated the I2 (95% CI) statistic to assess heterogeneity across studies, applying the following interpretation for I2: <50%=low heterogeneity; 50% to 75%=moderate heterogeneity; >75%=high heterogeneity.8, 9 Subgroup analyses and meta‐regression models were carried out to investigate potential sources of between‐study heterogeneity.
We calculated absolute difference in risk per 100 000 population year with ERP as ([RR−1]×I0), where RR indicates pooled RRs and I0 was the cumulative incidence of events among the population without ERP. On the basis of population‐based cohort studies, I0 was estimated by weighting each study by its sample size.10, 11 We calculated the population‐attributable fraction (PAF) as {prevalence of ERP×(RR−1)/(prevalence of ERP×[RR−1]+1)}, where RR indicates pooled RRs.12 On the basis of population‐based cohort studies, the average prevalence of ERP was estimated by weighting the result of each study by its sample size. Small study bias, consistent with publication bias, was assessed with a funnel plot, by the Begg adjusted rank correlation test, and by the Egger regression asymmetry test. We used STATA, version 11.0 (Stata Corp, College Station, TX) for all analyses. Statistical tests were 2 sided and used a significance level of P<0.05.
Results
Study Selection
With the search strategy, 1259 unique citations were initially retrieved. Of these, 259 articles were considered of interest, and full text was retrieved for detailed evaluation. Two hundred thirteen articles with no relevant outcomes were excluded, and another 30 articles were excluded because they did not provide enough data to estimate RR, leaving 16 studies for final inclusion in the meta‐analysis. The study by Klatsky et al was excluded because it only examined ECG in a small proportion of patients undergoing health examination, and the prevalence of ERP might be underestimated13 (Figure S1).
Study Characteristics
A total of 334 524 subjects were included in the 16 eligible studies, of whom 60.6% were men. Five studies were based in Europe, 7 in North America, 3 in East Asia, and 1 was multinational. There were 12 population‐based cohort studies and 4 case‐control studies.1, 3, 5, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Four studies investigated only Asians, 5 studies included only whites, and 7 studies involved multiethnic populations. Of the primary studies, 100% had described independent, consecutive sampling of their cohort. Average follow‐up duration ranged from 6.4 to 30.0 years. Patients were followed up for an average of over 10 years in a majority of studies (75.0%). The sizes of the cohorts ranged from 852 to 211 920, with the 5 largest studies recruiting over 10 000 participants. The endpoint of SCA was reported in 8 studies, cardiac death was reported in 8 studies, and death from any cause was reported in 9 studies, respectively (Tables S1 and S2). Adjusted RRs could be determined for all cohort studies and 3 of the case‐control studies. Most risk estimates were adjusted for age (14 studies) and sex (14 studies). Eleven studies (68.8%) reported an adjusted estimate for at least 1 of the cardiovascular risk factors: BMI (9 studies), cholesterol (4 studies), diabetes (4 studies), hypertension (9 studies), smoking (8 studies), heart rate (8 studies), and history of coronary heart disease (5 studies) (Tables S1 and S2).
Association With SCA, Cardiac Death, and Death From Any Cause
Figures showed the results from the random‐effects model (parallel analysis with fixed‐effects model) combining the RRs for the 3 outcomes. Overall, subjects with ERP, compared with the reference group, experienced a significantly increased risk for developing SCA (RR 2.18; 95% CI 1.29–3.68; P=0.003) (Figure 1), cardiac death (RR 1.48; 95% CI 1.06–2.07; P=0.02) (Figure 2), and death from any cause (RR 1.21; 95% CI 1.02–1.42; P=0.03) (Figure 3), respectively. Four cohort studies used sudden cardiac death as the endpoint of interest, involving 39 587 patients and 1118 events. ERP was significantly associated with increased risk of sudden cardiac death (RR 1.33; 95% CI 1.13–1.57; P<0.001]), with no evidence of significant between‐study heterogeneity (I2=0%; 95% CI 0–84.69; P=0.51) (Figure S2).
Figure 1.
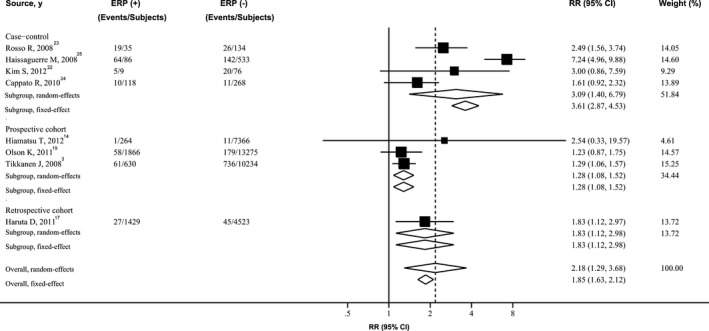
Forest plot showing relative risk for SCA associated with ERP. The size of each square is proportional to the study's weight (inverse of variance). ERP indicates early repolarization pattern; RR, relative risk; SCA, sudden cardiac arrest.
Figure 2.
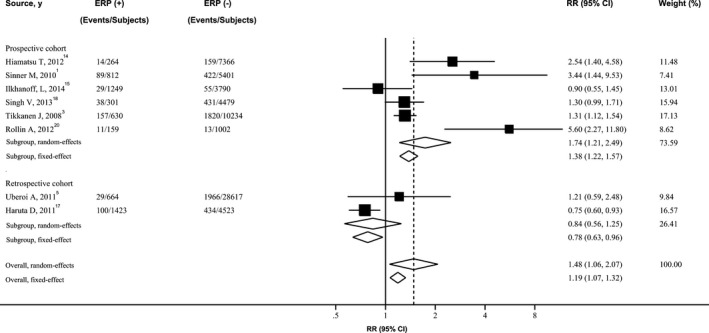
Forest plot showing relative risk for cardiac death associated with ERP. The size of each square is proportional to the study's weight (inverse of variance). ERP indicates early repolarization pattern; RR, relative risk.
Figure 3.
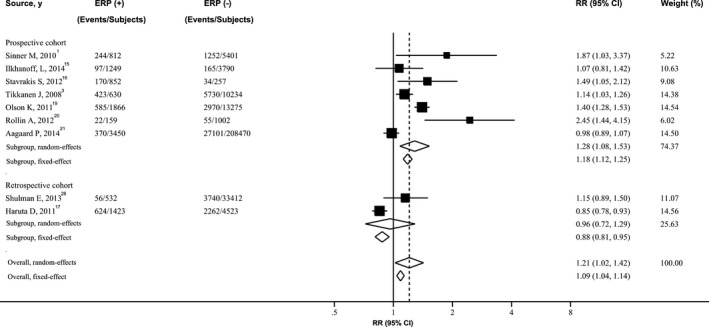
Forest plot showing relative risk for death from any cause associated with ERP. The size of each square is proportional to the study's weight (inverse of variance). ERP indicates early repolarization pattern; RR, relative risk.
There was evidence of considerable heterogeneity of RRs across these studies. Risk estimates did not materially change after analyses with fixed‐effect models, inclusion of the studies with adjusted RRs, or exclusion of the 1 largest and the outlier studies, yet substantial heterogeneity was still present. When the analysis was confined to those large prospective cohort studies, the overall combined RR for SCA still reached statistical significance (RR 1.28; 95% CI 1.08–1.52), but heterogeneity was decreased to 0%. However, moderate to high heterogeneity was still present for risk of cardiac death and death from any cause (Table 1). For the endpoint of SCA, the pooled OR from case‐control studies (for which the OR‐to‐RR conversion was not used) was 4.25 (95% CI 1.84–9.81), significantly higher than the pooled RR from cohort studies (RR 1.33; 95% CI 1.13–1.57; P=0.005).
Table 1.
Sensitivity and Heterogeneity Analysis of Pooled Relative Risks of SCA, Cardiac Death, and Death From Any Cause Associated With ERP
| SCA | Cardiac Death | Death From Any Cause | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n Studies | RR (95% CI) | I2 (95% CI) | P Valuea | n Studies | RR (95% CI) | I2 (95% CI) | P Valuea | n Studies | RR (95% CI) | I2 (95% CI) | P Valuea | |
| Statistical model | ||||||||||||
| Random effects | 8 | 2.18 (1.29‐3.68) | 91.40 (85.46‐94.91) | <0.001 | 8 | 1.48 (1.06‐2.07) | 84.39 (70.95‐91.61) | <0.001 | 9 | 1.21 (1.02‐1.42) | 90.27 (83.80‐94.16) | <0.001 |
| Fixed effects | 8 | 1.85 (1.63‐2.12) | 8 | 1.19 (1.07‐1.32) | 9 | 1.09 (1.04‐1.14) | ||||||
| Analysis of all studies with | ||||||||||||
| Adjusted risk estimate | 3 | 1.80 (1.54‐2.10) | 97.45 (95.02‐98.69) | <0.001 | 6 | 1.88 (1.31‐2.70) | 75.50 (44.74‐89.14) | 0.001 | 7 | 1.17 (1.02‐1.34) | 73.18 (42.37‐87.52) | 0.001 |
| Large cohortb | 3 | 1.28 (1.08‐1.52) | 0.00 (0.00‐89.60) | 0.78 | 5 | 1.75 (1.11‐2.75) | 63.33 (0.00‐87.63) | 0.04 | 5 | 1.20 (1.003‐1.43) | 87.67 (73.69‐94.23) | <0.001 |
| Analysis of all studies except | ||||||||||||
| One largest study | 7 | 2.41 (1.31‐4.43) | 92.02 (86.12‐95.41) | <0.001 | 7 | 1.52 (1.06‐2.19) | 86.62 (74.60‐92.95) | <0.001 | 8 | 1.26 (1.03‐1.54) | 90.78 (84.24‐94.60) | <0.001 |
| One outlier studyc | 7 | 1.61 (1.27‐2.04) | 46.09 (0.00‐77.28) | 0.09 | 7 | 1.16 (1.04‐1.29) | 80.64 (60.76‐90.45) | <0.001 | 8 | 1.15 (0.97‐1.35) | 90.43 (83.54‐94.43) | <0.001 |
ERP indicates early repolarization pattern; RR indicates relative risk; SCA, sudden cardiac arrest.
P‐value for I2.
Large‐cohort studies with sample size over 6000.
Neither funnel plots nor Egger and Begg tests showed evidence of publication bias for aborted SCA (Egger, P=0.56; Begg, P=0.54), cardiac death (Egger, P=0.67; Begg, P=0.27), and death from any cause (Egger, P=0.78; Begg, P=0.18) (Figure S3).
Stratified Analyses
To further explore study heterogeneity, we performed stratified analyses across a number of key study characteristics and clinical factors. Level of adjustment, follow‐up duration, age, publication year, or sex category were not significant sources of heterogeneity. There was some evidence that type of studies and race seemed to be associated with the risk for SCA. Case‐control studies showed higher risk estimates than prospective cohort studies and retrospective cohort studies (P=0.04). In addition, SCA associated with ERP appeared to be more evident in Asians (RR 2.01; 95% CI 1.30–3.10) compared with whites (RR 1.50; 95% CI 1.08–2.07) and African Americans (RR 0.82; 95% CI 0.52–1.30; P=0.01). However, increased risks of cardiac death and death from any cause were observed only in whites (RR 2.19; 95% CI 1.29–3.73 for cardiac death; RR 1.18; 95% CI 1.03–1.35 for death from any cause), not in Asians (RR 1.34; 95% CI 0.41–4.41 for cardiac death; RR 0.85; 95% CI 0.78–0.93 for death from any cause) or African Americans (RR 0.75; 95% CI 0.32–1.79 for cardiac death; RR 0.96; 95% CI 0.83–1.10 for death from any cause). Notably, the finding of increased risk for SCA, cardiac death, and death from any cause in subjects with ERP was consistently found in studies with prospective cohorts or adjustments for multiple cardiovascular risk factors. In addition, risk estimates for all 3 outcomes were consistently higher in women than in men, but the difference was not statistically significant (Table 2).
Table 2.
Stratified Analysis and Heterogeneity Analysis of Relative Risks of Aborted SCA, Cardiac Death and Death From Any Cause Associated With ERP
| Factors Stratified | SCA | Cardiac Death | Death From Any Cause | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERP (+) (Events/Subjects) | ERP (−) (Events/Subjects) | RR (95% CI) | P Valuea | ERP (+) (Events/Subjects) | ERP (−) (Events/Subjects) | RR (95% CI) | P Valuea | ERP (+) (Events/Subjects) | ERP (−) (Events/Subjects) | RR (95% CI) | P Valuea | |
| All studies | 245/4436 | 1170/36 409 | 2.18 (1.29‐3.68) | 467/5502 | 5300/65 412 | 1.48 (1.06‐2.07) | 2591/10 973 | 43 309/280 364 | 1.21 (1.02‐1.42) | |||
| Type of studies | ||||||||||||
| Case‐control | 98/247 | 199/1011 | 3.09 (1.40‐6.79) | 0.04 | — | — | — | — | — | — | ||
| Retrospective cohort | 120/2760 | 926/30 875 | 1.83 (1.12‐2.98) | 129/2087 | 2400/33 140 | 0.84 (0.56‐1.25) | 0.14 | 680/1955 | 6002/37 935 | 0.96 (0.72‐1.29) | 0.12 | |
| Prospective cohort | 27/1429 | 45/4523 | 1.28 (1.08‐1.52) | 338/3415 | 2900/32 272 | 1.74 (1.21‐2.49) | 1911/9018 | 37 307/242 429 | 1.28 (1.08‐1.53) | |||
| Patients, n | ||||||||||||
| ≦6000 | 125/1676 | 244/5534 | 2.77 (1.43‐5.36) | 0.10 | 178/3132 | 933/13 794 | 1.32 (0.76‐2.29) | 0.51 | 913/3683 | 2516/9572 | 1.27 (0.86‐1.88) | 0.96 |
| >6000 | 120/2760 | 926/30 875 | 1.28 (1.08‐1.52) | 289/2370 | 4367/51 618 | 1.75 (1.11‐2.75) | 1678/7290 | 40 793/270 792 | 1.20 (1.003‐1.43) | |||
| Levels of adjustmentsb | ||||||||||||
| − | 162/4310 | 1002/35 742 | 1.79 (1.53‐2.08) | 0.79 | 429/5201 | 4869/60 933 | 1.40 (0.98‐1.99) | 0.32 | 2365/9589 | 39 535/246 695 | 1.26 (1.04‐1.54) | 0.87 |
| + | 165/3954 | 986/28 166 | 1.47 (1.26‐1.72) | 418/5042 | 4856/59 931 | 1.30 (0.88‐1.92) | 2343/9430 | 39 480/245 693 | 1.03 (0.91‐1.16) | |||
| ++ | 183/2581 | 1057/24 042 | 1.80 (1.54‐2.10) | 338/2830 | 4811/57 099 | 1.88 (1.31‐2.70) | 1870/8301 | 40 882/272 051 | 1.17 (1.02‐1.34) | |||
| Follow up, y | ||||||||||||
| ≦15 | 1/264 | 11/7366 | 2.54 (0.33‐19.56) | 0.53 | 92/1388 | 2569/41 464 | 2.05 (1.11‐3.78) | 0.20 | 618/4993 | 30 930/243 141 | 1.32 (0.97‐1.80) | 0.53 |
| >15 | 146/3925 | 960/28 032 | 1.33 (1.13‐1.56) | 375/4114 | 2731/23 948 | 1.14 (0.74‐1.76) | 1793/5980 | 12 379/37 223 | 1.16 (0.91‐1.48) | |||
| Race | ||||||||||||
| Asian | 33/1696 | 76/11 965 | 2.01 (1.30‐3.10) | 0.01 | 114/1687 | 593/11 889 | 1.34 (0.41‐4.41) | 0.91 | 624/1423 | 2262/4523 | 0.85 (0.78‐0.93) | 0.81 |
| White | 32/995 | 68/3078 | 1.50 (1.08‐2.07) | 304/2024 | 3998/41 610 | 2.19 (1.29‐3.73) | 687/2192 | 11 510/54 845 | 1.18 (1.03‐1.35) | |||
| African American | 95/1619 | 860/20 699 | 0.82 (0.52‐1.30) | 10/241 | 195/3644 | 0.75 (0.32‐1.79) | 327/995 | 1056/3078 | 0.96 (0.83‐1.10) | |||
| Age, y | ||||||||||||
| ≦45 | 154/868 | 915/11 169 | 2.47 (1.06‐5.75) | 0.55 | 186/1879 | 1875/14 024 | 1.17 (0.83‐1.64) | 0.35 | 520/1879 | 5895/14 024 | 1.13 (1.03‐1.24) | 0.54 |
| >45 | 91/3568 | 255/25 240 | 1.56 (1.11‐2.20) | 281/3623 | 3425/51 388 | 1.79 (1.04‐3.08) | 2015/8562 | 33 674/232 928 | 1.29 (1.004‐1.65) | |||
| Sex | ||||||||||||
| Men | 62/1822 | 142/7497 | 1.59 (1.15‐2.20) | 0.73 | 80/818 | 365/5924 | 2.92 (1.84‐4.61) | 0.40 | 553/2374 | 3 719/20 269 | 1.14 (0.87‐1.50) | 0.34 |
| Women | 21/823 | 84/10 975 | 2.75 (1.88‐4.04) | 33/459 | 230/7841 | 4.77 (1.66‐13.68) | 212/1037 | 4439/32 817 | 1.85 (0.96‐3.58) | |||
| Publication year | ||||||||||||
| ≦2010 | 154/868 | 915/11 169 | 1.38 (1.14‐1.66) | 0.12 | 246/1442 | 2242/15 635 | 1.45 (0.91‐2.31) | 0.65 | 667/1442 | 6982/15 635 | 1.34 (0.85‐2.10) | 0.50 |
| >2010 | 91/3568 | 255/25 240 | 3.10 (1.23‐7.83) | 221/4060 | 3058/49 777 | 1.89 (0.75‐4.73) | 1924/9531 | 36 327/264 729 | 1.19 (0.97‐1.47) | |||
ERP indicates early repolarization pattern; RR indicates relative risk; SCA, sudden cardiac arrest.
P‐values test homogeneity between strata.
Levels of adjustment in multivariate models: −, not adjusted for any confounding factors; +, adjusted for conventional confounding factors (ie, age, sex); ++, further adjusted by potential cardiovascular risk factors (ie, body mass index, cholesterol, diabetes, hypertension; smoking, heart rate, and history of coronary heart disease).
Assessment of Outcomes According to Early Repolarization Pattern
Subjects with J‐point elevation of at least 0.1 mV in the inferior leads had a higher risk of SCA (RR 2.06; 95% CI 1.31–3.22; P=0.002), cardiac death (RR 1.76; 95% CI 1.14–2.74; P=0.01), and death from any cause (RR 1.41; 95% CI 1.05–1.89; P=0.02) than did those without this abnormality. Subjects with J‐point elevation of more than 0.2 mV on inferior leads had an increased risk of death from any cause (RR 1.54; 95% CI 1.06–2.24; P=0.02) and markedly elevated risks of SCA (RR 2.92; 95% CI 1.45–5.89; P=0.003) and cardiac death (RR 2.98; 95% CI 1.83–4.86; P<0.001). J‐point elevation in the lateral leads was of significance in predicting death from any cause (RR 1.21; 95% CI 1.07–1.36; P=0.002), but it did not predict SCA (RR 1.05; 95% CI 0.64–1.71; P=0.86) or cardiac death (RR 1.35; 95% CI 0.95–1.94; P=0.10). J‐point elevation in both inferior and lateral leads was associated with increased risk for SCA (RR 2.74; 95% CI 1.69–4.44; P<0.001) but not for cardiac death (RR 1.36; 95% CI 0.74–2.48; P=0.32) or death from any cause (RR 1.20; 95% CI 0.85–1.69; P=0.30) (Table 3).
Table 3.
Relative Risks of Various ERP Patterns for SCA, Cardiac Death, and Death From Any Cause
| SCA | Cardiac Death | Death From Any Cause | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERP (+) (Events/Subjects) | ERP (−) (Events/Subjects) | RR (95% CI) | P Value | ERP (+) (Events/Subjects) | ERP (−) (Events/Subjects) | RR (95% CI) | P Value | ERP (+) (Events/Subjects) | ERP (−) (Events/Subjects) | RR (95% CI) | P Value | |
| J‐point elevation | ||||||||||||
| ≧0.1 mV | 245/4436 | 1170/36 409 | 2.18 (1.29‐3.68) | 0.003 | 467/5502 | 5300/65 412 | 1.48 (1.06‐2.07) | 0.02 | 2591/10 973 | 43 309/280 364 | 1.21 (1.02‐1.42) | 0.03 |
| ≧0.1 mV in inferior leads | 72/859 | 838/15 280 | 2.06 (1.31‐3.22) | 0.002 | 199/1557 | 4679/54 727 | 1.76 (1.14‐2.74) | 0.01 | 412/975 | 7038/16 637 | 1.41 (1.05‐1.89) | 0.02 |
| ≧0.1 mV in lateral leads | 30/906 | 838/15 280 | 1.05 (0.64‐1.71) | 0.86 | 123/1181 | 4679/54 727 | 1.35 (0.95‐1.94) | 0.10 | 255/617 | 7038/16 637 | 1.21 (1.07‐1.36)) | 0.002 |
| ≧0.1 mV in both inferior and lateral leads | 19/509 | 792/15 070 | 2.74 (1.69‐4.44) | <0.001 | 10/79 | 2242/15 635 | 1.36 (0.74‐2.48) | 0.32 | 27/79 | 6982/15 635 | 1.20 (0.85‐1.69) | 0.30 |
| ≧0.2 mV | 14/72 | 756/10 310 | 2.68 (1.49‐4.84) | 0.001 | 31/141 | 1833/11 236 | 2.99 (1.41‐6.34) | 0.004 | 57/141 | 5786/11 236 | 1.46 (1.12‐1.89) | 0.005 |
| ≧0.2 mV in inferior leads | 8/36 | 736/10 234 | 2.92 (1.45‐5.89) | 0.003 | 17/36 | 1820/10 234 | 2.98 (1.83‐4.86) | <0.001 | 28/36 | 5730/10 234 | 1.54 (1.06‐2.24) | 0.02 |
| ≧0.2 mV in lateral leads | 2/31 | 736/10 234 | 0.87 (0.22‐3.48) | 0.84 | 9/31 | 1820/10 234 | 1.61 (0.83‐3.11) | 0.16 | 22/31 | 5730/10 234 | 1.26 (0.82‐1.93) | 0.29 |
| Configuration | ||||||||||||
| Notching | 57/1180 | 814/15 227 | 1.46 (1.18‐1.80) | 0.001 | 52/599 | 2401/35 020 | 1.77 (1.33‐2.37) | <0.001 | 98/307 | 1308/6403 | 2.12 (1.15‐3.91) | 0.02 |
| Slurring | 51/520 | 814/15 227 | 1.60 (0.99‐2.58) | 0.06 | 81/991 | 2401/35 020 | 1.21 (0.96‐1.52) | 0.11 | 169/672 | 1308/6403 | 1.19 (1.03‐1.37) | 0.02 |
| ST segment | ||||||||||||
| Rapidly ascending | 35/299 | 808/10 793 | 1.01 (0.72‐1.41) | 0.94 | 51/720 | 1961/29 619 | 1.07 (0.77‐1.48) | 0.70 | 110/701 | 90/1259 | 1.25 (0.95‐1.64) | 0.11 |
| Horizontal or descending | 56/485 | 772/10 684 | 2.03 (1.10‐3.74) | 0.02 | 9/103 | 13/1002 | 6.93 (2.76‐17.43) | <0.001 | 71/310 | 90/1259 | 2.29 (1.52‐3.45) | <0.001 |
ERP indicates early repolarization pattern; RR indicates relative risk; SCA, sudden cardiac arrest.
Notching configuration was associated with increased risks of SCA (RR 1.46; 95% CI 1.18–1.80; P=0.001), cardiac death (RR 1.77; 95% CI 1.33–2.37; P<0.001]), and death from any cause (RR 2.12; 95% CI 1.15–3.91; P=0.02). Slurring configuration conferred slightly increased risk for death from any cause (RR 1.19; 95% CI 1.03–1.37; P=0.02) but not for SCA (RR 1.60; 95% CI 0.99–2.58; P=0.06) or cardiac death (RR 1.21; 95% CI 0.96–1.52; P=0.11). Compared to those without ERP, the population with a horizontal or descending ST segment had increased risks for SCA (RR 2.03; 95% CI 1.10–3.74; P=0.02), cardiac death (RR 6.93; 95% CI 2.76–17.43; P<0.001), and death from any cause (RR 2.29; 95% CI 1.52–3.45; P<0.001). However, a rapidly ascending ST segment did not seem to be associated with all 3 outcomes (RR 1.01; 95% CI 0.72–1.41 for SCA; RR 1.07; 95% CI 0.77–1.48 for cardiac death; RR 1.25; 95% CI 0.95–1.64 for death from any cause) (Table 3). Examples of ERP with benign and malignant patterns are shown in Figure S4.
Absolute Risk Estimates and PAF Calculation
The population without ERP experienced an average of 118.3 (95% CI 110.4–126.5) cases of SCA, 474.2 (95% CI 459.6–489.0) cases of cardiac death, and 1520.9 (95% CI 1505.5–1536.4) cases of death from any cause per 100 000 subjects per year. As compared with no ERP, ERP was associated with an estimated 139.6 (95% CI 130.3–149.3) additional SCAs, 227.6 (95% CI 220.6–234.7) additional cardiac deaths, and 319.4 (95% CI 316.2–322.6) additional deaths from any cause per 100 000 person‐years.
From 8 population‐based studies that reported information on prevalence of ERP, we could calculate that the average prevalence of ERP in the general population was 6.7% (95% CI 2.5–17.7). From the combined summary estimates obtained from all studies, PAF of the 3 outcomes due to ERP could be estimated to be 7.3% (95% CI 1.9–15.2) for SCA, 3.1% (95% CI 0.4–6.7) for cardiac death, and 1.4% (95% CI 0.1–2.7) for death from any cause.
Discussion
The present meta‐analysis, involving 334 524 individuals from 16 studies has found increased risk of SCA, cardiac death, and death from any cause in subjects with ERP. The increased risk was present predominantly in Asians and whites but not in African Americans. J‐point elevation in inferior leads, notching configuration, and horizontal or descending ST segment appeared to connote poor cardiovascular outcomes. In absolute risk, ERP would account for an estimated 139.6 additional SCAs per 100 000 subjects per year and 7.3% of SCAs in the general population.
Early repolarization is very commonly observed in the pediatric population, men, athletes, and African Americans.27 After puberty, the prevalence of an ERP pattern increases in men and decreases in women, suggesting a possible influence of reproductive hormones.28 J‐wave amplitude increases during slow heart rates as well as in a short‐long‐short sequence.29 Circadian variation of the J‐wave amplitude is also known to occur in concordance with vagal tone. Sympathetic stimulation by isoproterenol and sodium channel blockers could attenuate the J‐wave amplitude.30
The underlying mechanisms involved in the association between ERP and mortality remain uncertain, but several plausible explanations have been suggested. The J wave on electrocardiography is inscribed as a consequence of the presence of a prominent Ito‐mediated action potential notch in epicardium, but not endocardium giving rise to a transmural voltage gradient. Further accentuation of Ito eventually leads to disproportionate shortening of the epicardial action potential and phase 2 reentry and thus to ventricular fibrillation or SCA.31 However, it is also possible that an ERP pattern might serve as an underlying electrophysiological substrate that may require a trigger to increase sudden death risk. Under certain conditions known to predispose to additional repolarization heterogeneity, such as ST‐segment elevation myocardial infarction, hypokalemia, and heart failure, patients with ERP may be at increased arrhythmic risk.32
There are several findings regarding ERP pattern and stratified analyses that need to be addressed. First, our results indicated that J‐point elevation in inferior leads rather than lateral leads was associated with an increased risk of SCA. One explanation was that J‐point elevation in inferior leads might represent a moderately arrhythmogenic substrate that facilitates polymorphic ventricular arrhythmias, supported by evidence that arrhythmias in idiopathic VF are usually triggered by ventricular extrasystoles with an inferior origin.33 Nevertheless, presence of a J‐point in lateral leads often involves vagal‐mediated bradycardia and subsequent increase of Ito current in the lateral left ventricle, which might not be arrhythmogenic even in the presence of ventricular extrasystoles.34 Second, notching configuration rather than slurring configuration confers increased arrhythmic risk. The possibility exists that some instances of slurring configuration may be due to delayed depolarization, which may be completely benign while producing ECG findings similar to ERP. However, the study by Haissaguerre et al found no significant association between presence of late ventricular potentials and QRS slurring.25 Third, ERP with a horizontal or descending ST segment is associated with poor cardiovascular outcomes, whereas this is not the case for ERP with a rapidly ascending ST segment. ERP with a rapidly ascending ST segment is generally believed to result from transmural heterogeneity mediated by Ito current in the plateau phase of the myocardial action potential. It usually occurs during vagal stimulation, which generally protects from lethal ventricular arrhythmias.35 Fourth, why does ERP confer increased risk of SCA and cardiac death in whites and Asians but not in African Americans? One explanation is that ERP could be a single phenotypic manifestation of a diverse array of genotypes, which might be differentially distributed among various racial groups. Some genotypes may inherently increase risk of malignant arrhythmia, whereas others might be relatively benign while producing similar ECG findings.19 It is also possible that ERP with a rapidly ascending ST segment, which is a relatively benign phenotype, is more prevalent in African Americans than in other races.36 In addition, African Americans with ERP were younger and less likely to have pathologic Q waves or coronary heart disease than other populations, which may account partly for the racial disparity of cardiovascular outcomes.36
Compared with our previous meta‐analysis, the present study included an addition of 8 new primary studies and ~200 000 subjects, which might provide more robust findings.14, 15, 16, 18, 21, 22, 24, 26 Therefore, the higher risk estimates observed for all 3 outcomes in this study might be attributable to the larger sample size. In absolute terms an addition of 139.6 SCAs and 474.2 cases of cardiac death occurred per 100 000 subjects per year associated with ERP in this study. Given that the absolute risk is not small and that ERP is a common electrocardiographic finding around the world, the total number of excess SCAs and cardiac deaths may not be negligible. Therefore, developing a reliable way is of great importance to risk‐stratify the patients at high risk. On the other side, the recognition of the benign forms is equally important to reassure the patients. Currently, however, there is neither a reliable provocative drug being tested to augment ERP, nor any value in performing electrophysiological studies and familial genetic screening.37 Therefore, further studies, including well‐designed clinical and experiment studies, are warranted to develop a reliable prognostic tool for risk stratification of patients with ERP pattern.
Strengths of this meta‐analysis include the strict inclusion criteria, the large number of patients analyzed, the robustness of the findings in sensitivity analyses, and the fact that all subgroup analyses were prespecified a priori. The absence of important publication bias supports the robustness of the study findings. Still, there were some study limitations. First, a large amount of heterogeneity was observed in the results of the various studies. Inclusion of different types of studies into 1 meta‐analysis may also introduce heterogeneity into the results. Although study type and race adjustment seem, at least in part, to explain this finding, heterogeneity still exists in the outcomes of cardiac death and death from any cause. Despite this, the consistency of the finding of an increased risk of SCA among individuals with ERP in both case‐control and cohort studies suggests that the association is valid. Second, although our study showed a significant association between ERP and risk of death, we could not establish a causal link between ERP and poor cardiovascular outcomes based on the data from observational studies. Third, as the prevalence of ERP differed among the selected studies, we could not exclude the possibility of misdiagnosis of ERP. Fourth, lack of individual participant data may preclude determining the independent associations of individual variables with study outcomes. Instead, we used between‐study meta‐regressions when possible.
In conclusion, the results from this meta‐analysis suggest that ERP was associated with increased risk of SCA, cardiac death, and death from any cause, independent of conventional cardiovascular risk factors. ERP with J‐point elevation in inferior leads, in both inferior and lateral leads, notching configuration, and horizontal or descending ST segment appears to connote a higher risk for SCA. The increased risk was present predominantly in Asians and whites but not in African Americans. J‐point elevation in lateral leads, slurring configuration, and rapidly ascending ST segment appear to be relatively benign ERP phenotypes. Future clinical and experiment studies should focus on populations with ERP at high risk, on understanding the exact mechanisms for cardiovascular risk, on developing reliable tools for risk stratification, and ultimately on devising strategies to prevent SCA in subjects with this pattern.
Sources of Funding
This work was supported by National Natural Science Foundation of China (No. 81370285), Guangdong Province Science and Technology Program (No. 2012B031800091), and Guangzhou City Science and Technology Program (No. 201508020057) to Dr Wu; and National Natural Science Foundation of China for Young Scholar (No. 81600260) and Natural Science Foundation of Guangdong Province (No. 2016A030313210) to Dr Cheng.
Disclosures
None.
Supporting information
Table S1. Cohort Studies Reporting Incidence Risk Estimates
Table S2. Case‐Control Studies Reporting Incidence Risk Estimates
Figure S1. Flowchart of the selection of studies included in meta‐analysis.
Figure S2. Forest plot showing relative risk for sudden cardiac death associated with ERP. The size of each square is proportional to the study's weight (inverse of variance).
Figure S3. Funnel plots showing risks for SCA, cardiac death, and death from any cause associated with ERP. The horizontal line in the funnel plot indicates the random‐effects summary estimate (using inverse‐variance weighting), and the sloping lines indicate the expected 95% confidence intervals for a given standard error, assuming no heterogeneity between studies. Symmetrical distribution of circles below and above the horizontal line indicates no evidence of publication bias.
Figure S4. Representative examples of ERP with benign and malignant patterns. A, Example of benign ERP with slurring pattern and ascending ST segment in a healthy subject. B, Example of malignant ERP with notching pattern and horizontal/descending ST segment in a subject.
(J Am Heart Assoc. 2016;5:e003375 doi: 10.1161/JAHA.116.003375)
References
- 1. Sinner MF, Reinhard W, Muller M, Beckmann BM, Martens E, Perz S, Pfeufer A, Winogradow J, Stark K, Meisinger C, Wichmann HE, Peters A, Riegger GA, Steinbeck G, Hengstenberg C, Kaab S. Association of early repolarization pattern on ECG with risk of cardiac and all‐cause mortality: a population‐based prospective cohort study (MONICA/KORA). PLoS Med. 2010;7:e1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. [DOI] [PubMed] [Google Scholar]
- 3. Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long‐term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. [DOI] [PubMed] [Google Scholar]
- 4. Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, Sager SJ, Rissanen HA, Myerburg RJ, Reunanen A, Huikuri HV. Early repolarization: electrocardiographic phenotypes associated with favorable long‐term outcome. Circulation. 2011;123:2666–2673. [DOI] [PubMed] [Google Scholar]
- 5. Uberoi A, Jain NA, Perez M, Weinkopff A, Ashley E, Hadley D, Turakhia MP, Froelicher V. Early repolarization in an ambulatory clinical population. Circulation. 2011;124:2208–2214. [DOI] [PubMed] [Google Scholar]
- 6. Wu SH, Lin XX, Cheng YJ, Qiang CC, Zhang J. Early repolarization pattern and risk for arrhythmia death: a meta‐analysis. J Am Coll Cardiol. 2013;61:645–650. [DOI] [PubMed] [Google Scholar]
- 7. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 8. Cheng YJ, Zhang J, Li WJ, Lin XX, Zeng WT, Tang K, Tang AL, He JG, Xu Q, Mei MY, Zheng DD, Dong YG, Ma H, Wu SH. More favorable response to cardiac resynchronization therapy in women than in men. Circ Arrhythm Electrophysiol. 2014;7:807–815. [DOI] [PubMed] [Google Scholar]
- 9. Zeng WT, Sun XT, Tang K, Mei WY, Liu LJ, Xu Q, Cheng YJ. Risk of thromboembolic events in atrial fibrillation with chronic kidney disease. Stroke. 2015;46:157–163. [DOI] [PubMed] [Google Scholar]
- 10. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng YJ, Nie XY, Chen XM, Lin XX, Tang K, Zeng WT, Mei WY, Liu LJ, Long M, Yao FJ, Liu J, Liao XX, Du ZM, Dong YG, Ma H, Xiao HP, Wu SH. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol. 2015;66:2173–2184. [DOI] [PubMed] [Google Scholar]
- 12. Cheng YJ, Liu ZH, Yao FJ, Zeng WT, Zheng DD, Dong YG, Wu SH. Current and former smoking and risk for venous thromboembolism: a systematic review and meta‐analysis. PLoS Med. 2013;10:e1001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klatsky AL, Oehm R, Cooper RA, Udaltsova N, Armstrong MA. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. 2003;115:171–177. [DOI] [PubMed] [Google Scholar]
- 14. Hisamatsu T, Ohkubo T, Miura K, Yamamoto T, Fujiyoshi A, Miyagawa N, Kadota A, Takashima N, Nagasawa SY, Kita Y, Murakami Y, Okayama A, Horie M, Okamura T, Ueshima H. Association between J‐point elevation and death from coronary artery disease—15‐year follow up of the NIPPON DATA90. Circ J. 2013;77:1260–1266. [DOI] [PubMed] [Google Scholar]
- 15. Ilkhanoff L, Soliman EZ, Prineas RJ, Walsh JR, Ning H, Liu K, Carr JJ, Jacobs DJ, Lloyd‐Jones DM. Clinical characteristics and outcomes associated with the natural history of early repolarization in a young, biracial cohort followed to middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Circ Arrhythm Electrophysiol. 2014;7:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stavrakis S, Patel N, Te C, Golwala H, George A, Lozano P, Lazzara R. Development and validation of a prognostic index for risk stratification of patients with early repolarization. Ann Noninvasive Electrocardiol. 2012;17:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haruta D, Matsuo K, Tsuneto A, Ichimaru S, Hida A, Sera N, Imaizumi M, Nakashima E, Maemura K, Akahoshi M. Incidence and prognostic value of early repolarization pattern in the 12‐lead electrocardiogram. Circulation. 2011;123:2931–2937. [DOI] [PubMed] [Google Scholar]
- 18. Singh V, Patel NJ, Badheka AO, Rathod A, Deshmukh A, Marzouka G, Tuliani T, Moscucci M, Cohen M. J point elevation and cardiovascular mortality: insights from National Health and Nutrition Examination Survey‐III. Circulation. 2012;126:A17513. [Google Scholar]
- 19. Olson KA, Viera AJ, Soliman EZ, Crow RS, Rosamond WD. Long‐term prognosis associated with J‐point elevation in a large middle‐aged biracial cohort: the ARIC study. Eur Heart J. 2011;32:3098–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rollin A, Maury P, Bongard V, Sacher F, Delay M, Duparc A, Mondoly P, Carrie D, Ferrieres J, Ruidavets JB. Prevalence, prognosis, and identification of the malignant form of early repolarization pattern in a population‐based study. Am J Cardiol. 2012;110:1302–1308. [DOI] [PubMed] [Google Scholar]
- 21. Aagaard P, Shulman E, Di Biase L, Fisher JD, Gross JN, Kargoli F, Kim SG, Palma EC, Ferrick KJ, Krumerman A. Prognostic value of automatically detected early repolarization. Am J Cardiol. 2014;114:1431–1436. [DOI] [PubMed] [Google Scholar]
- 22. Kim SH, Kim DY, Kim HJ, Jung SM, Han SW, Suh SY, Ryu KH. Early repolarization with horizontal ST segment may be associated with aborted sudden cardiac arrest: a retrospective case control study. BMC Cardiovasc Disord. 2012;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M, Katz A, Viskin S. J‐point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. [DOI] [PubMed] [Google Scholar]
- 24. Cappato R, Furlanello F, Giovinazzo V, Infusino T, Lupo P, Pittalis M, Foresti S, De Ambroggi G, Ali H, Bianco E, Riccamboni R, Butera G, Ricci C, Ranucci M, Pelliccia A, De Ambroggi L. J wave, QRS slurring, and ST elevation in athletes with cardiac arrest in the absence of heart disease: marker of risk or innocent bystander? Circ Arrhythm Electrophysiol. 2010;3:305–311. [DOI] [PubMed] [Google Scholar]
- 25. Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquie JL, Nogami A, Babuty D, Yli‐Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O'Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clementy J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. [DOI] [PubMed] [Google Scholar]
- 26. Shulman E, Aagaard P, Kargoli F, Lener S, Fisher J, Gross J, Kim S, Palma E, DiBiase L, Ferrick K, Krumerman A. Early repolarization with J‐point elevation predicts mortality in the Hispanic female population. Circulation. 2014;130:A12714. [Google Scholar]
- 27. Junttila MJ, Sager SJ, Freiser M, McGonagle S, Castellanos A, Myerburg RJ. Inferolateral early repolarization in athletes. J Interv Card Electrophysiol. 2011;31:33–38. [DOI] [PubMed] [Google Scholar]
- 28. Sager SJ, Hoosien M, Junttila MJ, Tanawuttiwat T, Perry AC, Myerburg RJ. Comparison of inferolateral early repolarization and its electrocardiographic phenotypes in pre‐ and postadolescent populations. Am J Cardiol. 2013;112:444–448. [DOI] [PubMed] [Google Scholar]
- 29. Nam GB, Ko KH, Kim J, Park KM, Rhee KS, Choi KJ, Kim YH, Antzelevitch C. Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs. Brugada syndrome. Eur Heart J. 2010;31:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haissaguerre M, Sacher F, Nogami A, Komiya N, Bernard A, Probst V, Yli‐Mayry S, Defaye P, Aizawa Y, Frank R, Mantovan R, Cappato R, Wolpert C, Leenhardt A, de Roy L, Heidbuchel H, Deisenhofer I, Arentz T, Pasquie JL, Weerasooriya R, Hocini M, Jais P, Derval N, Bordachar P, Clementy J. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. 2009;53:612–619. [DOI] [PubMed] [Google Scholar]
- 31. Macfarlane PW, Antzelevitch C, Haissaguerre M, Huikuri HV, Potse M, Rosso R, Sacher F, Tikkanen JT, Wellens H, Yan GX. The early repolarization pattern: a consensus paper. J Am Coll Cardiol. 2015;66:470–477. [DOI] [PubMed] [Google Scholar]
- 32. Naruse Y, Tada H, Harimura Y, Hayashi M, Noguchi Y, Sato A, Yoshida K, Sekiguchi Y, Aonuma K. Early repolarization is an independent predictor of occurrences of ventricular fibrillation in the very early phase of acute myocardial infarction. Circ Arrhythm Electrophysiol. 2012;5:506–513. [DOI] [PubMed] [Google Scholar]
- 33. Haissaguerre M, Shah DC, Jais P, Shoda M, Kautzner J, Arentz T, Kalushe D, Kadish A, Griffith M, Gaita F, Yamane T, Garrigue S, Hocini M, Clementy J. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet. 2002;359:677–678. [DOI] [PubMed] [Google Scholar]
- 34. Gussak I, Bjerregaard P, Egan TM, Chaitman BR. ECG phenomenon called the J wave. History, pathophysiology, and clinical significance. J Electrocardiol. 1995;28:49–58. [DOI] [PubMed] [Google Scholar]
- 35. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST‐segment elevation. Circulation. 1999;100:1660–1666. [DOI] [PubMed] [Google Scholar]
- 36. Perez MV, Uberoi A, Jain NA, Ashley E, Turakhia MP, Froelicher V. The prognostic value of early repolarization with ST‐segment elevation in African Americans. Heart Rhythm. 2012;9:558–565. [DOI] [PubMed] [Google Scholar]
- 37. Mahida S, Derval N, Sacher F, Leenhardt A, Deisenhofer I, Babuty D, Schlapfer J, de Roy L, Frank R, Yli‐Mayry S, Mabo P, Rostock T, Nogami A, Pasquie JL, de Chillou C, Kautzner J, Jesel L, Maury P, Berte B, Yamashita S, Roten L, Lim HS, Denis A, Bordachar P, Ritter P, Probst V, Hocini M, Jais P, Haissaguerre M. Role of electrophysiological studies in predicting risk of ventricular arrhythmia in early repolarization syndrome. J Am Coll Cardiol. 2015;65:151–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cohort Studies Reporting Incidence Risk Estimates
Table S2. Case‐Control Studies Reporting Incidence Risk Estimates
Figure S1. Flowchart of the selection of studies included in meta‐analysis.
Figure S2. Forest plot showing relative risk for sudden cardiac death associated with ERP. The size of each square is proportional to the study's weight (inverse of variance).
Figure S3. Funnel plots showing risks for SCA, cardiac death, and death from any cause associated with ERP. The horizontal line in the funnel plot indicates the random‐effects summary estimate (using inverse‐variance weighting), and the sloping lines indicate the expected 95% confidence intervals for a given standard error, assuming no heterogeneity between studies. Symmetrical distribution of circles below and above the horizontal line indicates no evidence of publication bias.
Figure S4. Representative examples of ERP with benign and malignant patterns. A, Example of benign ERP with slurring pattern and ascending ST segment in a healthy subject. B, Example of malignant ERP with notching pattern and horizontal/descending ST segment in a subject.


