Abstract
Background
Asthma is a heterogeneous syndrome with different clinical subtypes that is associated with an increased risk for cardiovascular disease (CVD). We hypothesized that the late‐onset subtype of asthma is associated with a higher risk of incident CVD.
Methods and Results
Participants from the Wisconsin Sleep Cohort free of CVD at baseline were followed for a mean (SD) of 13.9 (5.9) years for development of CVD (myocardial infarction, angina, stroke, coronary revascularization, heart failure, or CVD death). Late‐onset asthma was defined as physician‐diagnosed asthma at age ≥18 years. Multivariable Cox regression models adjusted for age, sex, and CVD risk factors were used to assess associations of late‐onset asthma and incident CVD. The 1269 participants were 47.3 (8.0) years old; 166 participants had asthma (111 late‐onset, 55 early‐onset). Participants with late‐onset asthma compared to nonasthmatics were more likely to be female (67% versus 44%) and to have a higher body‐mass index (32.2 versus 29.4 kg/m2) (P<0.05). Mean age of asthma diagnosis in the late‐onset group was 39.5 (9.6) years versus 8.9 (5.7) years in the early‐onset group (P<0.0001). Late‐onset asthmatics had a higher adjusted risk of incident CVD than nonasthmatics (hazard ratio 1.57, 95% CI 1.01–2.45, P=0.045). There was no interaction between body‐mass index and age of asthma diagnosis on incident CVD (P=0.83).
Conclusions
In a large cohort study of adults followed prospectively for over a decade, late‐onset asthmatics had an increased risk of incident CVD events that persisted after adjustment for age, sex, and CVD risk factors.
Keywords: asthma, atherosclerosis, epidemiology, risk factors
Subject Categories: Epidemiology, Cardiovascular Disease
Introduction
Asthma is an inflammatory disorder that poses a significant public health burden.1 In the United States over 25 million individuals have asthma and the prevalence of asthma continues to rise.1 Cardiovascular disease (CVD) also poses a significant public health burden and remains the leading cause of death among adults in the United States.2 Asthma and CVD share an underlying inflammatory pathophysiology.3, 4, 5 An increased risk of CVD events has been found in individuals with other chronic inflammatory diseases, including those with higher levels of subclinical systemic inflammation.3, 6, 7, 8, 9, 10, 11, 12, 13, 14
We and others have demonstrated an increased risk of CVD events in asthmatics.15, 16, 17, 18, 19, 20, 21, 22 However, asthma is a heterogeneous clinical syndrome that can be divided into distinct phenotypes, each possessing a unique pathophysiology.23 One classification of asthma phenotypes is by the age of disease onset. Late‐onset asthma commences at older ages and often is more severe and refractory to standard pharmacotherapeutic regimens than early‐onset asthma.23 In many of these prior investigations, however, CVD risk within a specific asthma subtype was not investigated.15, 16, 17, 18, 22, 24 We hypothesized that the late‐onset phenotype of asthma is associated with a higher CVD risk in the Wisconsin Sleep Cohort (WSC).
Materials and Methods
Participants
The WSC Study is an ongoing population‐based longitudinal cohort study investigating sleep, respiratory, and cardiovascular outcomes in adults followed prospectively since 1988.25 The design of the WSC has been described previously.25 Study protocols and informed consent documents were approved by the University of Wisconsin Health Sciences Institutional Review Board. Participants were selected from a random sample from payroll records of State of Wisconsin employees aged 30 to 60 years. Of the 2940 individuals invited to undergo a baseline overnight in‐laboratory protocol, 1546 (53%) participated. Compared to the entire sampling frame, cohort participants had a slightly healthier profile and lower death rate.25, 26, 27
The participants in the current analysis (n=1267) were those who, at the time of the baseline polysomnography study, had not experienced a CVD event, had complete baseline covariate data, and had at least 1 polysomnography study to assess CVD outcomes and asthma diagnosis (Figure 1). Baseline visits were completed between July 1989 and April 2003; follow‐up was observed through December 2013. Incident CVD information was obtained from self‐reported detailed health history questionnaires regarding specific types of physician‐diagnosed CVD with year of diagnosis as well as medications or other treatments during (1) 1 or more follow‐up in‐laboratory polysomnography studies scheduled at 4‐yearly intervals; and/or (2) mailed health surveys querying all CVD outcomes; and/or death records searches. Health surveys were mailed to all participants in 2008 and during 2010–2013. Deaths were assessed in 2013, as described in the following paragraphs.
Figure 1.
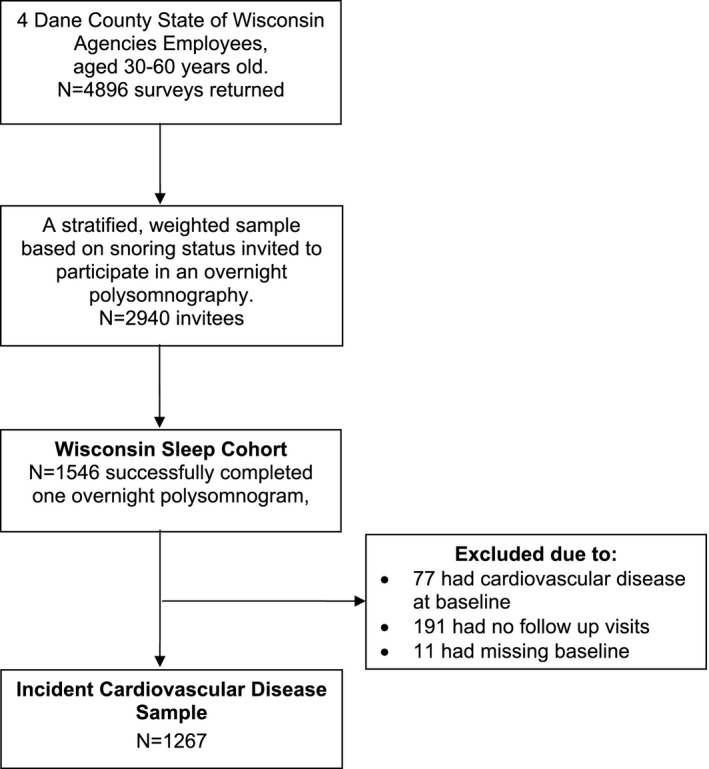
STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) diagram.
Asthma Definitions
To account for the heterogeneity of asthma phenotypes, we stratified asthma into 2 subgroups: those with early‐onset asthma and those with late‐onset asthma at the baseline visit. The definition of asthma in the WSC has been described previously.28 Similar to previous reports, asthma in the current study was defined as a self‐reported history of physician‐diagnosed asthma.28 Late‐onset asthma was defined as asthma diagnosed at age 18 years or older, whereas early‐onset asthma was defined as asthma diagnosed prior to 18 years of age. Further adjudication of the asthma diagnosis was performed by a WSC physician on any participant indicating a history of physician‐diagnosed asthma. Participants had to indicate they had physician‐diagnosed asthma on at least 2 interviews and have provided the year of the asthma diagnosis to be classified as asthmatic in the current study. If a participant indicated a diagnosis of asthma on 1 interview, but was never treated and did not indicate asthma on subsequent interviews, they were not considered to have asthma.28
CVD Event Assessment
The outcome measure was occurrence of a CVD event during follow‐up (through December 2013). CVD events were defined as the occurrence of coronary death, myocardial infarction, angina, stroke, coronary revascularization, heart failure, or CVD death. CVD events were identified through 2 methods: health history questionnaires performed at the time of follow‐up visits and mailed health surveys. Any inconsistencies in CVD events between serial sampling were adjudicated by a WSC study physician. Identification of CVD deaths in the cohort occurring up to May 2013 was performed as reported previously.29 Deaths were identified by matching Social Security numbers with 2 death record sources: the National Death Index and the Wisconsin State Bureau of Health Information and Policy, Vital Records Section. Matches on Social Security numbers were verified with participants' age and sex. All deaths in Wisconsin, reported by the vital records of Wisconsin, also were identified in the National Death Index; in addition, deaths occurring outside of Wisconsin were identified by the National Death Index. Date of death was available for all decedents. Underlying and contributory causes of deaths were available from Wisconsin Vital Statistics. Wisconsin Vital Statistics and the National Death Index supplied files with data on each individual death, including the cause of death and corresponding description as abstracted from individual death certificates. For all deaths, cause of death was further ascertained and adjudicated by examining each death certificate for primary cause, secondary cause, and underlying conditions by a WSC study physician.
CVD Risk Factors
Biologic confounders were assessed at the baseline visit in the WSC through standardized interviews and objective clinical assessments. Hypertension status was defined as baseline systolic blood pressure >140 mm Hg or diastolic pressure >90 mm Hg or the use of antihypertensive medications. Smoking status was defined as never, past, or current smoking. Diabetes mellitus was defined as self‐reported diabetes or use of diabetic medications. Body‐mass index was calculated as weight in kilograms divided by height in meters squared.
Polysomnography
Polysomnograms (Grass Instruments, Quincy, MA) were performed at the University of Wisconsin Hospital. Sleep state was determined by electroencephalography, electrooculography, and electromyography. Arterial oxyhemoglobin saturation, oral and nasal airflow, nasal air pressure, and thoracic cage and abdominal respiratory motion were used to detect obstructive sleep apnea (OSA) events. Sleep state and respiratory event scoring were performed by trained sleep technicians. The polysomnograms used in this analysis were obtained between 1989 and 2004; each 30‐s epoch of the each polysomnographic record was scored for breathing events and for sleep stage using criteria described by Rechtschaffen and Kales.30 Cessation of airflow lasting ≥10 s defined an apnea event. A discernible reduction in the sum of thoracic plus abdomen respiratory inductance plethysmography amplitude that lasted at least 10 s and that was associated with a ≥4% reduction in oxyhemoglobin saturation defined a hypopnea event. The average number of apnea plus hypopnea events/hour of sleep defined the apnea–hypopnea index.
Statistical Methods
Baseline descriptive statistics are reported as means (SD) for continuous variables and percentages for categorical variables. Incident CVD was analyzed as time‐to‐event by asthma categories (early‐onset, late‐onset, no asthma) and by age of asthma diagnosis. Censoring time was defined as the years from the baseline visit date to the date of the first occurrence of the CVD event or CVD death, if more than 1 event was reported. For participants who did not experience a CVD event, lost‐to‐follow‐up time was defined as the years between the baseline visit and the participant's last in‐laboratory polysomnography study. Unadjusted CVD‐free survival rates comparing participants with late‐onset asthma, early‐onset asthma, and those without asthma were calculated using the Kaplan–Meier method. Unadjusted incident CVD rates were calculated for those with late‐onset asthma, early‐onset asthma, and those without asthma. Cox proportional hazard models were utilized to compare the survival distribution of the 3 groups while adjusting for potential confounders. The proportional hazards assumption was evaluated using Schoenfield's test. A series of models were created by adding potential known confounders into each model. Model 0 was unadjusted. Model 1 adjusted for age and sex. Model 2 adjusted additionally for smoking history, lipid medication use, and diabetes mellitus. Model 3 included the confounders in model 2 and hypertensive status. Model 4, the primary prespecified analysis, included the covariates in model 3 and body‐mass index. Effect modification by sex and by body‐mass index (eg, interaction models with age of asthma diagnosis) was tested using Cox proportional hazard models on the final model with all potential confounders included. Secondary analysis of baseline OSA25 interacting with age of asthma diagnosis was also tested using Cox proportional hazard models on the final model with all potential confounders included. Statistical significance was set at a 2‐sided P<0.05 for the main analyses and at 2‐sided P<0.01 for interactions. Analyses were performed in SAS (Version 9.2; SAS Institute Inc, Cary, NC).
Results
Descriptive Characteristics
The 1269 participants were followed for 13.9 (5.9) years. At baseline, participants were 47.3 (8.0) years old; 46% were female and 166 participants had asthma. The 111 participants with late‐onset asthma and the 55 participants with early‐onset asthma were compared to the 1103 participants without asthma. The distribution of CVD risk factors between those with late‐onset and early‐onset asthma differed slightly compared to those without asthma (Table 1). Those with late‐onset asthma were more likely to be female (67% versus 44%), had higher body‐mass index (BMI, 32.2 versus 29.4 kg/m2), and were more likely to be taking antihypertensive medications (22% versus 13%).
Table 1.
Baseline Participant Statistics
| Nonasthmatic (N=1103) | Early‐Onset Asthma (N=55) | Late‐Onset Asthma (N=111) | |
|---|---|---|---|
| Age, y | 47.3 (8.0) | 45.4 (8.3) | 47.2 (8.0) |
| Sex (female), n (%) | 484 (44) | 21 (38) | 74 (67)a |
| BMI, kg/m2 | 29.4 (6.2) | 30.3 (7.2) | 32.2 (8.0)a |
| Systolic blood pressure, mm Hg | 125 (14) | 126 (12) | 125 (17) |
| Antihypertensive medication use, n (%) | 142 (13) | 9 (16) | 24 (22)a |
| Hypertension, n (%) | 316 (29) | 14 (26) | 42 (38)a |
| Diabetes mellitus, n (%) | 32 (3) | 0 | 5 (4.5) |
| Smoking history, n (%) | |||
| Never | 491 (45) | 26 (47) | 53 (48) |
| Past smoker | 413 (37) | 19 (35) | 45 (40) |
| Current smoker | 199 (18) | 10 (18) | 13 (12) |
| Lipid medication use, N (%) | 41 (4) | 1 (2) | 3 (3) |
| Age of asthma diagnosis, y | N/A | 8.9 (5.7) | 39.5 (9.6) |
All values are mean (SD) unless otherwise noted. BMI indicates body‐mass index; N/A, not applicable.
P<0.05, no asthma as the reference group.
Asthma and CVD Events
A total of 223 CVD events (179 in the nonasthma group, 22 in the late‐onset asthma group, and 7 in the early‐onset asthma group) occurred during the observation period. The incidence rate for CVD events was highest in late‐onset asthmatics. The 10‐year CVD‐event rates were 12.7% (95% CI 5.9–19.6) for those with late‐onset asthma, 3.8% (0.1–9.0%) for those with early‐onset asthma, and 8.9% (7.1–10.7%) for nonasthmatics (P=0.16). In multivariate models adjusted for potential confounders, having late‐onset asthma was associated with a significantly higher risk of CVD events (Table 2). In models adjusted for age and sex (Table 2, Model 1), participants with late‐asthma had a higher risk of CVD events (hazard ratio [HR] 1.62 [1.04–2.51], P=0.029). This association persisted in models fully adjusted for potential confounders as participants with late‐onset asthma had a higher risk of CVD events compared to nonasthmatics (HR 1.57 [1.01–2.45], P=0.045) (Table 2, Model 4; Figure 2). Participants with early‐onset asthma had no difference in CVD events compared to nonasthmatics in models adjusted for age and sex (HR 0.81 [0.40–1.65], P=0.56) and fully adjusted models (HR 0.94 [0.46–1.92], P=0.873) (Table 2, Model 4, Figure 2).
Table 2.
Associations of Asthma With CVD Events
| Model | Late‐Onset Asthmaa | Early‐Onset Asthmaa | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Model 0 | 1.47 (0.96–2.27) | 0.079 | 0.79 (0.39–1.50) | 0.51 |
| Model 1 | 1.62 (1.04–2.51) | 0.029 | 0.81 (0.40–1.65) | 0.56 |
| Model 2 | 1.78 (1.14–2.76) | 0.010 | 0.92 (0.45–1.86) | 0.81 |
| Model 3 | 1.64 (1.06–2.56) | 0.027 | 0.94 (0.46–1.91) | 0.86 |
| Model 4 | 1.57 (1.01–2.45) | 0.045 | 0.94 (0.46–1.92) | 0.87 |
CVD indicates cardiovascular disease. Model 0: unadjusted. Model 1: adjusted for age and sex. Model 2: Model 1+smoking status, diabetes mellitus, and lipid medications. Model 3: Model 2+hypertension status. Model 4: Model 3+body‐mass index.
No‐asthma group as reference.
Figure 2.

Cardiovascular disease survival based on asthma status. CVD indicates cardiovascular disease.
Interaction effects of sex×age of asthma diagnosis and BMI×age of asthma diagnosis were not significant (P interaction=0.93 and 0.83, respectively). In separate instances of Model 4, the HR for late‐onset asthma and future CVD events was 1.46 (95% CI 0.78–2.75, P=0.24) in men (N=689) and 1.65 (0.85–3.19, P=0.14) in women (N=578), values that are similar to each other and the overall effect we observed. Considering years postmenopause as an independent variable (in women only) did not appreciably change the effect of late‐onset asthma in Model 4 (HR 1.79 [0.93–3.48], P=0.08). For participants with BMI <30 kg/m2 (N=759), the HR for late‐onset asthma was 1.90 (95% CI 0.98–3.71, P=0.06) without BMI in the model and it was 1.92 (95% CI 0.99–3.75, P=0.055) with BMI in the model. For BMI >30 kg/m2 (N=508), the HR for late‐onset asthma was 1.37 (95% CI 0.75–2.48, P=0.31) without BMI in the model and it was 1.34 (95% CI 0.74–2.45, P=0.34) with BMI in the model. In the latter model, the effect of BMI on future CVD events was statistically significant and in the expected direction (HR 1.06, 95% CI 1.03–1.08, P<0.0001).
Because OSA plausibly could confound or mediate an association between asthma and CVD, we added a measure of OSA severity (the logarithm10 of the apnea–hypopnea index +1, due to the high prevalence of zero values) to the final models for early‐ and late‐onset asthma predicting CVD. In both cases, there was minimal (<4%) change in the magnitude of the asthma coefficient when OSA severity was added to the model. Furthermore, in an exploratory analysis of 731 participants with recorded nocturnal oxygen desaturation, percent of time with oxygen saturation <90% and minimum nocturnal oxygen saturation were not significant predictors of CVD events and had similar beta coefficients for asthma as did the logarithm10 of the apnea–hypopnea index +1. Oxygen desaturation parameters also did not interact with asthma to predict CVD events. Thus, we found little evidence of either substantial confounding or mediation by OSA. In exploratory analyses, we added the 13 positive airway pressure therapy users excluded from the models above to them; however, their inclusion did not substantially alter the reported HRs for asthma predicting later CVD events (data not shown).
In an exploratory analysis, we also looked for an association between a participant report of physician‐diagnosed gastroesophageal reflux disease and CVD events among 997 individuals in our cohort but did not identify one, nor did we identify significant interactions between gastroesophageal reflux disease and asthma or sex. Neither hours/week of physical activity nor year of study entry was associated with future CVD events or substantively altered the HR for the relationship between late‐onset asthma and CVD events.
Discussion
After over a decade of prospective observation, participants with late‐onset asthma but not early‐onset asthma had a 1.6‐fold higher rate of CVD events than nonasthmatics in models adjusted for potential confounders. Prior studies investigating the associations of asthma and CVD yielded varying associations, with some finding no significant association with CVD, others finding limited associations with specific end points, and others finding consistent associations with CVD.15, 16, 17, 18, 19, 20, 21, 22 Many reports have described an association of asthma and CVD; however, few have investigated a possible association between asthma subtype and CVD.19, 20 The heterogeneity of associations between asthma and CVD observed in the prior reports may be due, in part, to the treatment of asthma as a homogeneous condition, when in fact asthma is a heterogeneous condition with unique pathophysiology that describes specific subtypes.23
Two previous reports have investigated the association of age of asthma onset and CVD risk. Onufrak et al investigated adult‐onset asthma and coronary heart disease and stroke in the Atherosclerosis Risk in Communities (ARIC) Study and found that only women with adult‐onset asthma had a higher risk of coronary heart disease or stroke.19 Lee et al performed a cross‐sectional analysis in the 1999–2006 National Health and Nutrition Examination Survey (NHANES) investigating the association of adult‐onset asthma and CVD and found increased risk of CVD with adult‐onset asthma and effect modification by sex.20
Our investigation supports these findings, though it differs in both design and the specificity of certain results. First, as opposed to the cross‐sectional nature of the NHANES investigation, ours is a prospective longitudinal cohort study with over a decade of follow‐up allowing for observation of the natural course of the disease. Second, in both prior investigations there was significant effect modification by sex in the association of late‐onset asthma and CVD. We did not find significant effect modification by sex; however, our study is smaller and may not have adequate power to detect effect modification. Despite the study size, we saw a similar magnitude of association in fully adjusted models, further strengthening the support for the association of late‐onset asthma and CVD. Third, we used the definition of late‐onset asthma as asthma diagnosed at age 18 years or older, but the ARIC study defined late‐onset asthma with an age of diagnosis of 21 years or older. The NHANES investigation used the same age threshold that we used. The age definition of late‐onset asthma varies significantly in the literature, with some reports defining late‐onset asthma with an onset as young as the age of 12 years while others defining onset at age 21 years or even older ages. We performed sensitivity analyses that varied the age threshold for a diagnosis of onset of adult‐onset asthma to age 12 years or greater or age 21 years or greater and found a similar magnitude of association.
Early‐onset and late‐onset asthma are 2 substantially different disease processes and differ in their risk factors, pathophysiology, and responses to treatment, though they often are lumped together. The common risk factors for early‐onset asthma include family history of atopic disease, viral and bacterial infections, and tobacco exposure.31 Early‐onset asthma is generally responsive to treatment with inhaled corticosteroids.32 Late‐onset asthma has myriad different risk factors including environmental irritants, obesity, female sex hormones, respiratory infections, stressful life events, and aspirin use.33 Late‐onset asthma often is more severe and, as opposed to early‐onset asthma, refractory to standard treatments.23 Previous investigations have hypothesized the increased risk of CVD may stem from an effect of estrogen‐modulated inflammation in adult‐onset asthmatic women.19 This remains a plausible explanation for this specific phenotype of late‐onset asthma; however, there is significant phenotypic variation within late‐onset asthma. Different phenotypes of late‐onset asthma that have been identified include the following: late‐onset obese female phenotype, late‐onset nonatopic inflammation predominant phenotype, late‐onset mild asthma, and smoking‐related late‐onset asthma.33 The effect of estrogens on asthma and CVD risk provides a hypothesis for the increased CVD risk in the late‐onset obese female phenotype; however, the other subtypes of late‐onset asthma such as the late‐onset inflammation predominant phenotype with fixed airway obstruction may have unique features that increase CVD risk. Eosinophils, which are increased in the late‐onset nonatopic inflammation phenotype, also may play a role in the increased CVD risk.
Elevated levels of eosinophils have been associated with an increased risk of atherosclerosis and in 1 study, levels of eotaxin, an eosinophilic specific chemoattractant directly correlated with the extent of angiographic epicardial atherosclerotic disease.34, 35, 36 In addition, as opposed to early‐onset asthma, risk factors for late‐onset asthma include many exogenous environmental triggers; the triggers that serve as risk factors for lung disease also serve as risk factors for CVD.33 There are many prior investigations that well document the associations of levels of particulate matter with lung and CVD disease.37, 38, 39 Finally, a feature more commonly observed in the late‐onset asthma phenotype is more severe and refractory lung disease with a more precipitous decline in lung function.40 Previous investigations have well characterized lung function as an independent predictor of CVD events.41, 42, 43
Limitations
Despite the numerous strengths of this study, there are some limitations. Asthma was a self‐reported physician diagnosis and therefore may be prone to misclassification bias. However, previous studies have demonstrated a high sensitivity (91%) and specificity (97%) for self‐reported prevalent asthma in epidemiologic studies.44 The very strict definition of asthma used in the current study, which included participants indicating asthma on 2 separate interviews with the date of diagnosis, considering use of medications, and physician adjudication, further strengthens the specificity of our asthma diagnosis. Furthermore, a misclassification bias would bias the results of our analyses toward the null. Age at asthma diagnosis also was self‐reported and may also be prone to misclassification bias; however, self‐reported age of asthma onset has been used in many prior epidemiological investigations and has been found to be an accurate assessment of age of onset of the disease.45 We do not have objective measures of asthma severity.
The number of CVD events, especially in individuals with early‐onset asthma, was small, so some associations may have been missed. As an observational study, the described associations do not confirm causation. Regression models were adjusted for measured known confounders; however, unmeasured confounders, including exposure to air pollution, stressful life events, or regular aspirin use may affect both asthma and CVD risk and could thus result in residual confounding biasing away (pollution, stress) or toward (aspirin) the null. Finally, the WSC is a predominantly white, Wisconsin‐based cohort so the generalizability of these findings to populations with different characteristics may be limited.
Conclusions
Late‐onset, but not early‐onset asthma was associated with an increased risk of CVD events in this prospective observational study with over a decade of follow‐up. Given the public health burden of asthma, further investigations into the mechanisms of this association in specific asthma phenotypes are needed.
Sources of Funding
Dr Korcarz was supported by NIH grant K23 HL094760. Drs Peppard, Hagen, and Stein and Ms Barnet were supported by R01 HL062252, R01 AG036838, and UL1 RR025011. The funding institutes played no role in the design and conduct of the study; no role in the collection, management, analysis, or interpretation of the data; and no role in the preparation, review, or approval of the manuscript.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003448 doi: 10.1161/JAHA.116.003448)
Presented as an abstract at the American Heart Association's Epidemiology and Prevention/Lifestyle and Cardiometabolic Health 2016 Scientific Sessions in Phoenix, AZ, March 1–4, 2016.
References
- 1. Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 4. Di Gennaro A, Haeggstrom JZ. The leukotrienes: immune‐modulating lipid mediators of disease. Adv Immunol. 2012;116:51–92. [DOI] [PubMed] [Google Scholar]
- 5. Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos MP, Kaiser B, Cohnert TU, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht AJ. Expanding expression of the 5‐lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci USA. 2003;100:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM, Hennekens CH, Buring JE, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 8. Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol. 2007;26:1228–1233. [DOI] [PubMed] [Google Scholar]
- 9. Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. [DOI] [PubMed] [Google Scholar]
- 10. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 11. Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep. 2013;10:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shrestha S, Irvin MR, Grunfeld C, Arnett DK. HIV, inflammation, and calcium in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;34:244–250. [DOI] [PubMed] [Google Scholar]
- 13. Stein JH. Cardiovascular risk and dyslipidemia management in HIV‐infected patients. Top Antivir Med. 2012;20:129–133; quiz 123–124. [PMC free article] [PubMed] [Google Scholar]
- 14. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD; Group ISS . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tattersall MC, Guo M, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Barr RG, Donohue KM, McClelland RL, Delaney JA, Stein JH. Asthma predicts cardiovascular disease events: the Multi‐Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1520–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iribarren C, Tolstykh IV, Miller MK, Sobel E, Eisner MD. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol. 2012;176:1014–1024. [DOI] [PubMed] [Google Scholar]
- 17. Iribarren C, Tolstykh IV, Eisner MD. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol. 2004;33:743–748. [DOI] [PubMed] [Google Scholar]
- 18. Schanen JG, Iribarren C, Shahar E, Punjabi NM, Rich SS, Sorlie PD, Folsom AR. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax. 2005;60:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Onufrak SJ, Abramson JL, Austin HD, Holguin F, McClellan WM, Vaccarino LV. Relation of adult‐onset asthma to coronary heart disease and stroke. Am J Cardiol. 2008;101:1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee HM, Truong ST, Wong ND. Association of adult‐onset asthma with specific cardiovascular conditions. Respir Med. 2012;106:948–953. [DOI] [PubMed] [Google Scholar]
- 21. Enright PL, Ward BJ, Tracy RP, Lasser EC. Asthma and its association with cardiovascular disease in the elderly. The Cardiovascular Health Study Research Group. J Asthma. 1996;33:45–53. [DOI] [PubMed] [Google Scholar]
- 22. Knoflach M, Kiechl S, Mayr A, Willeit J, Poewe W, Wick G. Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY studies. Arch Intern Med. 2005;165:2521–2526. [DOI] [PubMed] [Google Scholar]
- 23. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. [DOI] [PubMed] [Google Scholar]
- 24. Toren K, Lindholm NB. Do patients with severe asthma run an increased risk from ischaemic heart disease? Int J Epidemiol. 1996;25:617–620. [DOI] [PubMed] [Google Scholar]
- 25. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med. 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 26. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 27. Young T, Finn L, Peppard PE, Szklo‐Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen‐year follow‐up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 28. Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 2015;313:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hla KM, Young T, Hagen EW, Stein JH, Finn LA, Nieto FJ, Peppard PE. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep. 2015;38:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rechtscahaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. National Institutes of Health publication no. 204; 1968.
- 31. Bisgaard H, Bonnelykke K. Long‐term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126:187–197; quiz 198‐189. [DOI] [PubMed] [Google Scholar]
- 32. Castro‐Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta‐analysis. Pediatrics. 2009;123:e519–e525. [DOI] [PubMed] [Google Scholar]
- 33. de Nijs SB, Venekamp LN, Bel EH. Adult‐onset asthma: is it really different? Eur Respir Rev. 2013;22:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prentice RL, Szatrowski TP, Fujikura T, Kato H, Mason MW, Hamilton HH. Leukocyte counts and coronary heart disease in a Japanese cohort. Am J Epidemiol. 1982;116:496–509. [DOI] [PubMed] [Google Scholar]
- 35. Niccoli G, Ferrante G, Cosentino N, Conte M, Belloni F, Marino M, Baca M, Montone RA, Sabato V, Schiavino D, Patriarca G, Crea F. Eosinophil cationic protein: a new biomarker of coronary atherosclerosis. Atherosclerosis. 2010;211:606–611. [DOI] [PubMed] [Google Scholar]
- 36. Emanuele E, Falcone C, D'Angelo A, Minoretti P, Buzzi MP, Bertona M, Geroldi D. Association of plasma eotaxin levels with the presence and extent of angiographic coronary artery disease. Atherosclerosis. 2006;186:140–145. [DOI] [PubMed] [Google Scholar]
- 37. Weiden MD, Naveed B, Kwon S, Cho SJ, Comfort AL, Prezant DJ, Rom WN, Nolan A. Cardiovascular biomarkers predict susceptibility to lung injury in World Trade Center dust‐exposed firefighters. Eur Respir J. 2013;41:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. [DOI] [PubMed] [Google Scholar]
- 39. Lucking AJ, Lundback M, Mills NL, Faratian D, Barath SL, Pourazar J, Cassee FR, Donaldson K, Boon NA, Badimon JJ, Sandstrom T, Blomberg A, Newby DE. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008;29:3043–3051. [DOI] [PubMed] [Google Scholar]
- 40. Ulrik CS, Lange P. Decline of lung function in adults with bronchial asthma. Am J Respir Crit Care Med. 1994;150:629–634. [DOI] [PubMed] [Google Scholar]
- 41. Lee HM, Liu MA, Barrett‐Connor E, Wong ND. Association of lung function with coronary heart disease and cardiovascular disease outcomes in elderly: the Rancho Bernardo study. Respir Med. 2014;108:1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population‐based study and a systematic review of the literature. Chest. 2005;127:1952–1959. [DOI] [PubMed] [Google Scholar]
- 43. Engstrom G, Lind P, Hedblad B, Wollmer P, Stavenow L, Janzon L, Lindgarde F. Lung function and cardiovascular risk: relationship with inflammation‐sensitive plasma proteins. Circulation. 2002;106:2555–2560. [DOI] [PubMed] [Google Scholar]
- 44. Oksanen T, Kivimaki M, Pentti J, Virtanen M, Klaukka T, Vahtera J. Self‐report as an indicator of incident disease. Ann Epidemiol. 2010;20:547–554. [DOI] [PubMed] [Google Scholar]
- 45. Toren K, Palmqvist M, Lowhagen O, Balder B, Tunsater A. Self‐reported asthma was biased in relation to disease severity while reported year of asthma onset was accurate. J Clin Epidemiol. 2006;59:90–93. [DOI] [PubMed] [Google Scholar]


