Abstract
Background
The effects of some atherosclerotic cardiovascular disease (ASCVD) risk factors vary according to whether an individual has a family history (FHx) of premature coronary heart disease (CHD). Physical activity (PA) is associated with reduced risk of ASCVD, but whether this association varies by FHx status is not well established.
Methods and Results
We evaluated 9996 participants free of ASCVD at baseline. FHx of premature CHD was defined as CHD occurring in a father before age 55 or mother before age 60. PA, assessed by a Baecke questionnaire, was converted into minutes/week of moderate or vigorous exercise and categorized per American Heart Association guidelines as recommended, intermediate, or poor. Incident ASCVD was defined as incident myocardial infarction, fatal CHD, or stroke. Multivariable‐adjusted Cox hazard models were used. The mean age was 54±6 years, 56% were women, and 21% of black race. Participants with and without a FHx of premature CHD reported similar levels of PA at baseline (423 versus 409 metabolic equivalents of task×min/week, respectively, P=0.852), and ≈40% of both groups met American Heart Association recommended PA levels. Over a mean follow−up of 20.9 years, there were 1723 incident ASCVD events. Compared to those with poor PA adherence to American Heart Association guidelines, participants who reported PA at recommended levels had significantly lower risk of incident ASCVD after adjustment for demographics and lifestyle factors (hazard ratio 0.84, 95% CI 0.74–0.94), but this association was not modified by FHx status (P−interaction=0.680).
Conclusions
PA was associated with a reduced risk of ASCVD among individuals with and without a FHx of premature CHD.
Keywords: cardiovascular disease prevention, cardiovascular disease risk factors, cardiovascular events, family history, physical exercise
Subject Categories: Exercise, Epidemiology, Risk Factors, Primary Prevention, Lifestyle
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in developed countries. A history of premature coronary heart disease (CHD) among first‐degree relatives has been shown in multiple epidemiologic studies to be strongly associated with incident myocardial infarction, coronary death, and stroke.1, 2, 3, 4 Studies have demonstrated a 2‐ to 7‐fold increase in risk of CHD associated with a positive family history (FHx).5
Familial clustering of CHD risk factors such as hypertension, smoking, and dyslipidemia is well established.5, 6 Such clustering may be explained by varying degrees of genetic predisposition as well as shared habits among family members.5 Interestingly, prior data suggest that the risk factors for ASCVD may have differential effects among those with and without a FHx of premature CHD. For example, comparable risk factor profiles have been associated with increased atherosclerotic disease among those with a FHx.6, 7, 8 Similarly, the risk reduction associated with certain interventions such as smoking cessation may differ according to the presence of a FHx of CHD.9, 10
A fundamental component of preventive strategies to reduce cardiovascular risk is to increase physical activity (PA). Higher levels of PA are associated with improvement in all forms of ASCVD, including risk factors, CHD, heart failure, and mortality,11, 12 and as such, achieving and maintaining adequate levels of PA is endorsed by all major guidelines and societies.13, 14
Although clustering of behavioral cardiovascular risk factors has been noted in many studies, data have been conflicting regarding levels of PA performed by those with compared to those without a FHx of CHD.15 Given their higher risk status, it would be important to understand whether individuals with a FHx of premature CHD are more (or less) likely to meet AHA recommended levels of PA than those without a similar FHx. Furthermore, the relative impact of higher levels of PA for ASCVD prevention among those specifically with a FHx of premature CHD compared to those without such a history is currently unknown. Lastly, we pose the question of whether increasing PA levels over time (ie, going from poor levels to recommended levels) could further reduce ASCVD risk compared to those whose PA levels remained poor, and if that differs among those with and without a FHx.
We hypothesized that baseline PA levels would be higher among those with a FHx of CHD because persons with FHx may be more likely to adopt healthy lifestyle because of knowledge of their baseline risk. We also hypothesized that a positive FHx of premature CHD would modify the association between PA and incident ASCVD.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a multicenter, predominantly biracial, prospective cohort of middle‐aged men and women from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. The ARIC Study design has been previously reported.16 Individuals aged 45 to 64 years were enrolled between 1987 and 1989 when they took part in a baseline (visit 1) clinic examination. Participants have taken part in up to 4 additional main visits: 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5).
Of 15 792 participants evaluated at ARIC visit 1, we excluded those with prevalent CHD (n=766), defined by self‐reported prior physician diagnosis of myocardial infarction or coronary revascularization, or prevalent myocardial infarction by 12‐lead ECG at visit 1. We also excluded those with prevalent heart failure at visit 1 (n=599), defined by (1) those with an affirmative response to the question: “Were any of the medications you took during the last 2 weeks for heart failure?” or (2) those with stage 3 or “manifest heart failure” as determined by Gothenburg criteria.17 Participants with prevalent CHD and heart failure were excluded because their underlying health conditions may limit their ability to maximally perform PA, or they may have restrictions placed on their allowed PA intensity/duration as prescribed by their healthcare provider. Additionally, because of small numbers, individuals who were nonblack or nonwhite (n=46), as well as blacks from the Minnesota and Maryland centers (n=49) were not included. We also excluded 2391 participants with missing data on parental history of premature CHD or who answered “don't know” when asked about parental history of premature CHD and 1945 participants with missing data on PA. A total of 9996 individuals were eligible for the present analysis.
The Institutional Review Boards at all ARIC study sites and the Coordinating Center approved study protocols, and all participants provided written informed consent at each study visit.
Exposures
The primary exposure of interest was PA at visit 1, with a focus on its relationship with incident ASCVD. A FHx of premature CHD was considered as a possible effect modifier of this association. FHx of premature CHD was assessed at visit 1 by self‐report via a questionnaire administered to each ARIC participant. Subjects were asked if they had a parent who had a heart attack occurring in a father before the age 55 or in a mother before age 60. Subjects who answered “yes” were counted as yes (“a positive FHx”), whereas those who said “no” were counted as no FHx of premature CHD. Participants who answered “don't know” were excluded from the analyses.
Sports, leisure, and work PA were assessed via a modified Baecke questionnaire at visit 1 and visit 3. For our study, we used data from the sports questionnaire in which individuals reported participation in up to 4 sports within the previous year, as well as the number of hours per week and months per year spent on each sport. As has been done in prior ARIC studies,18, 19 each sport was converted into metabolic equivalents of task (METS) as per the Compendium of Physical Activities. Moderate activities were defined as those involving a workload of 3 to 6 METS and vigorous activities were those involving a workload of ≥6 METS. For both visit 1 and visit 3 separately, information from the questionnaires was subsequently converted into minutes per week of moderate or vigorous PA, and categorized as per AHA recommendations into 3 groups: recommended (≥75 min/week of vigorous intensity or ≥150 min/week of any combination of moderate+vigorous intensity), intermediate (1–74 min/week of vigorous intensity or 1–149 min/week of any combination of moderate+vigorous intensity), or poor (0 min/week of moderate or vigorous exercise). We also modeled PA 3 additional ways: (1) continuously as METS×min/week, (2) categorized into quartiles (in METS×min/week), and (3) by comparing the 90th to the 10th percentile of PA levels. Furthermore, we examined change in PA from visit 1 to visit 3; this was modeled as change in overall METS×min/week (continuous) as well as change in AHA‐defined categories (ie, poor, intermediate, and recommended).
Covariates of Interest
Demographics and information on other covariates of interest were obtained from history, physical examination, and laboratory data at visit 1. Body mass index (BMI) was calculated from measured height and weight. Smoking status was categorized as never, former, and current smoker, and alcohol consumption as current drinkers versus former/never drinkers. Diabetes mellitus was defined as a fasting (≥8 hours) glucose ≥126 mg/dL, a nonfasting glucose ≥200 mg/dL, a self‐reported physician diagnosis of diabetes mellitus or the reported use of hypoglycemic agents. Systolic blood pressure was the mean calculated from the second and third measurements obtained at the visit examination. Hypertension was defined as systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg, or reported use of blood pressure–lowering medications. Total cholesterol, high‐density lipoprotein cholesterol, and triglycerides were measured using standardized enzymatic assays and reported in mg/dL. Estimated glomerular filtration rate was calculated from serum creatinine from blood samples collected at visit 1 using the CKD‐Epi equation.20
Outcomes
The primary outcome was incident ASCVD determined from hospital discharge codes or death certificates. Incident ASCVD was defined as definite or probable myocardial infarction, definite coronary death, and definite or probable stroke, defined as sudden or rapid onset of neurological symptoms that lasted for 24 hours or led to death in the absence of another cause.17, 21, 22 Information on hospitalizations was obtained from participants via yearly telephone calls, and vital records were examined for all deaths. Additionally, ARIC investigators conducted continuous surveillance for all cardiovascular disease–related hospitalizations and deaths. Study participants contributed follow‐up time from the date of the participant's baseline visit until the date of incident ASCVD event, death, loss‐to‐follow‐up, or the end of follow‐up (December 31, 2012), whichever came first.
Statistical Analysis
Baseline characteristics of the study population were described using means, medians, and proportions stratified by status of FHx of premature CHD. We used χ2 and ANOVA tests for categorical and continuous variables, in order to assess for differences in demographic, clinical, and laboratory characteristics among the study population. Kaplan–Meier curves were constructed for ASCVD‐free survival by FHx groups.
We used multivariable‐adjusted multinomial logistic regression and linear regression methods to determine whether baseline PA groups and change in PA levels between visit 1 and visit 3 differed by FHx of premature CHD status. We then used multivariable‐adjusted Cox proportional hazard models to evaluate the associations of PA at visit 1 with incident ASCVD events stratified by FHx of premature CHD status. PA was modeled according to AHA categories, as quartiles, and continuous (90th compared to 10th percentile). All analyses were progressively adjusted as follows: Model 1 included demographics of age, sex, and race/center. Model 2 included the variables from Model 1 as well as possible confounding lifestyle factors of education, BMI, smoking status, and current alcohol intake. Model 2 was considered our primary model. We also examined a third model that included the variables from Model 2 as well as possible mediators of the association of PA and ASCVD including systolic blood pressure, antihypertensive use, diabetes mellitus, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, use of lipid‐lowering medications, and estimated glomerular filtration rate.
We tested for whether there was a multiplicative interaction between PA and FHx of premature CHD for incident ASCVD using Wald Tests, with adjustment for Model 2 covariates.
Additionally, to investigate for a possible dose–response relationship, in analyses stratified by FHx of premature CHD, we modeled the continuous association of PA in METS×min/week with risk of incident ASCVD using restricted cubic splines with knots at 5th, 35th, 65th, and 95th percentiles, adjusted for the factors in Model 2.
Finally, using the same progressively adjusted regression models as above, we assessed the risk of incident ASCVD for change in PA category from visit 1 to visit 3, stratified by FHx of premature CHD status. For this analysis, we excluded 263 participants who developed ASCVD before or on visit 3.
Results
Characteristics of the study population among individuals with and without a FHx of premature CHD are presented in Table 1. Of 9996 ARIC participants included in the study, 990 (9.9%) reported a FHx for premature CHD. The mean age of the study population was 54±6 years, 56% were women and 21% of black race. Compared to those without a FHx of premature CHD, participants with a known FHx of premature CHD were slightly younger, had higher levels of total and low‐density lipoprotein cholesterol and higher triglycerides, and were more likely to be current smokers.
Table 1.
Baseline Characteristicsa of Study Population by Family History of Premature CHD Status: The ARIC Study (1987–1989)
| No FHx of Premature CHD (9006) | FHx of Premature CHD (990) | Overall (9996) | P‐Value | |
|---|---|---|---|---|
| AHA‐defined PA categories, N (%) | 0.913 | |||
| Recommended | 3612 (40.1) | 404 (40.8) | 4016 (40.2) | |
| Intermediate | 2329 (25.9) | 253 (25.6) | 2582 (25.8) | |
| Poor | 3065 (34.0) | 333 (33.6) | 3398 (34.0) | |
| PA levels (METs×min/week [continuous])b | 408.6 (0.0, 1008.9) | 423.4 (0.0, 995.0) | 414.0 (0.0, 1006.8) | 0.852 |
| Age, y | 53.9±5.7 | 53.1±5.5 | 53.8±5.7 | <0.001 |
| BMI, kg/m2 | 27.3±5.0 | 27.4±5.2 | 27.3±5.0 | 0.679 |
| Male, N (%) | 3993 (44.3) | 396 (40.0) | 4389 (43.9) | 0.009 |
| Race/center, N (%) | <0.001 | |||
| Minneapolis, MN whites | 2597 (28.8) | 269 (27.2) | 2866 (28.7) | |
| Washington County, MD Whites | 2372 (26.3) | 327 (33.0) | 2699 (27.0) | |
| Forsyth County, NC Whites | 2158 (24.0) | 289 (29.2) | 2447 (24.5) | |
| Forsyth County, NC blacks | 227 (2.5) | 11 (1.1) | 238 (2.4) | |
| Jackson, MS blacks | 1652 (18.3) | 94 (9.5) | 1746 (17.5) | |
| Education, N (%) | 0.991 | |||
| <High School | 1614 (17.9) | 176 (17.8) | 1790 (17.9) | |
| High School or Vocational School | 3808 (42.3) | 419 (42.3) | 4227 (42.3) | |
| College, Graduate, or Professional School (%) | 3575 (39.7) | 395 (39.9) | 3970 (39.8) | |
| Smoking status, N (%) | 0.060 | |||
| Never | 4107 (45.6) | 418 (42.3) | 4525 (45.3) | |
| Former | 2882 (32.0) | 321 (32.5) | 3203 (32.1) | |
| Current | 2013 (22.4) | 250 (25.3) | 2263 (22.7) | |
| Current alcohol consumption, N (%) | 5364 (59.7) | 602 (61.0) | 5966 (59.9) | 0.446 |
| Total cholesterol, mg/dL | 213.5±41.0 | 219.3±40.8 | 214.1±41.0 | <0.001 |
| HDL, mg/dL | 52.5±17.0 | 51.6±16.0 | 52.4±16.9 | 0.124 |
| LDL, mg/dL | 136.2±38.2 | 141.0±37.8 | 136.6±38.1 | <0.001 |
| Triglycerides, mg/dL | 126.7±84.6 | 135.1±78.9 | 127.5±84.1 | 0.003 |
| Diabetes mellitus N (%) | 773 (8.6) | 92 (9.3) | 865 (8.7) | 0.485 |
| Systolic BP, mm Hg | 119.5±17.4 | 119.9±17.3 | 119.5±17.4 | 0.463 |
| Hypertension, N (%) | 2553 (28.4) | 304 (31.0) | 2857 (28.7) | 0.094 |
| Estimated GFR, mL/min per 1.73 m2, N (%) | 0.028 | |||
| ≥90 | 7543 (84.2) | 860 (87.2) | 8403 (84.5) | |
| 60 to <90 | 1344 (15.0) | 117 (11.9) | 1461 (14.7) | |
| <60 | 68 (0.8) | 9 (0.9) | 77 (0.8) |
AHA indicates American Heart Association; ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; FHx, family history; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; METS, metabolic equivalents of task; PA, physical activity.
Data are means (SDs) or number (%).
Median (interquartile interval).
Participants with and without a FHx of premature CHD reported similar levels of PA at baseline visit 1 (423 versus 409 MET×min/week, respectively, P=0.852), and ≈40% of both groups met AHA recommended PA levels. In multivariable‐adjusted models, individuals with a FHx of premature CHD were not more likely to engage in intermediate (odds ratio 0.94 [95% CI 0.79, 1.12], Model 2) or recommended (odds ratio 0.97 [0.83, 1.14]) levels of PA compared to those without a FHx (Table 2).
Table 2.
Association of Family History of Premature CHD Status With Baseline AHA‐Defined Physical Activity Categories (Odds Ratios, 95% CI) at ARIC Visit 1 (1987–1989) and With Change in Physical Activity Levels (METS×min/week) From ARIC Visit 1 to Visit 3 (1987–1995)
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| Intermediate compared with poord | |||
| No FHx | Reference (1) | Reference (1) | Reference (1) |
| FHx | 0.91 (0.77, 1.09) | 0.94 (0.79, 1.12) | 0.98 (0.82, 1.18) |
| Recommended compared with poord | |||
| No FHx | Reference (1) | Reference (1) | Reference (1) |
| FHx | 0.93 (0.80, 1.09) | 0.97 (0.83, 1.14) | 1.01 (0.86, 1.20) |
| Change of PA from visit 1 to visit 3 | −0.11 (−0.30, 0.08) | −0.06 (−0.24, 0.13) | −0.01 (−0.20, 0.18) |
AHA indicates American Heart Association; ARIC, Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CHD, coronary heart disease; FHx, family history; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; METS, metabolic equivalents of task; PA, physical activity.
Model 1: Age, sex, race/center.
Model 2: Model 1+education, BMI, smoking status, and alcohol intake.
Model 3: Model 2+additional potential mediating variables (of the association between PA and ASCVD risk)−systolic blood pressure, antihypertensive medication use, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, use of lipid‐lowering medications, and estimated GFR.
Multivariable‐adjusted multinomial regression.
Over a mean follow‐up of 20.9 years, there were a total of 1545 ASCVD events within the group without a FHx of CHD (incidence rate: 8.2 per 1000 person‐years), and 178 ASCVD events among individuals with a positive parental history of premature CHD (incidence rate: 8.7 per 1000 person‐years). The time to 90% event‐free survival was 14.7 years for those without a FHx and 13.8 years for those with a FHx (Figure 1).
Figure 1.
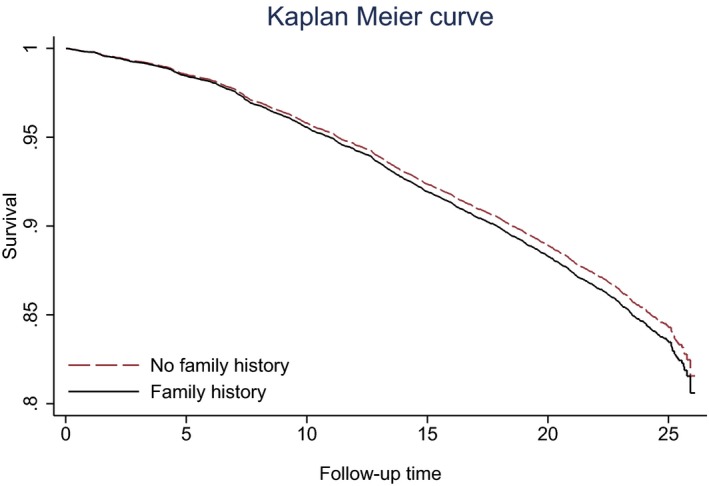
Time (years) to first ASCVD event by FHx status. ASCVD indicates atherosclerotic cardiovascular disease; FHx, family history.
In the overall cohort, after adjustment for demographics and lifestyle factors, meeting AHA‐recommended levels of PA, versus poor levels of PA, was associated with lower risk of incident ASCVD (hazard ratio (HR) 0.84 [0.74, 0.94], Model 2), which remained statistically significant after further adjustment for ASCVD risk factors that may possibly mediate this association (HR 0.83 [0.73, 0.93], Model 3). These associations were similar among individuals with and without a FHx of premature CHD (HR 0.92 [0.63, 1.33] and 0.83 [0.73, 0.94], respectively, Model 2), without significant interaction by FHx status (P‐interaction=0.680) (Table 3).
Table 3.
Hazard Ratios and 95% CI for the Association of AHA‐Defined Categories of Physical Activity With Risk for Incident ASCVD Over 21 Years Follow‐Up by Family History Status: The ARIC Study 1987–2012
| Poor | Intermediate | Recommended | |
|---|---|---|---|
| No family history of premature CHDe (n=9006) | |||
| N events | 618 | 377 | 550 |
| N total | 3065 | 2329 | 3612 |
| Incidence ratea | 9.8 (9.1, 10.6) | 7.7 (6.9, 8.5) | 7.2 (6.6, 7.8) |
| Model 1b | Ref | 0.82 (0.72, 0.93) | 0.70 (0.62, 0.79) |
| Model 2c | Ref | 0.91 (0.80, 1.04) | 0.83 (0.73, 0.94) |
| Model 3d | Ref | 0.89 (0.78, 1.02) | 0.83 (0.73, 0.94) |
| Family history of premature CHDe (n=990) | |||
| N events | 67 | 46 | 65 |
| N total | 333 | 253 | 404 |
| Incidence ratea | 10.1 (7.9, 12.8) | 8.7 (6.5, 11.6) | 7.6 (6.0, 9.7) |
| Model 1b | Ref | 0.92 (0.63, 1.35) | 0.76 (0.54, 1.09) |
| Model 2c | Ref | 1.02 (0.69, 1.51) | 0.92 (0.63, 1.33) |
| Model 3d | Ref | 0.98 (0.66, 1.46) | 0.80 (0.55, 1.17) |
AHA indicates American Heart Association; ARIC, Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CHD, coronary heart disease; FHx, family history; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PA, physical activity.
Incidence rate is per 1000 person‐years.
Model 1: Age, sex, race/center.
Model 2: Model 1+education, BMI, smoking status, and alcohol intake.
Model 3: Model 2+additional potential mediating variables (of the association between PA and ASCVD risk)−systolic blood pressure, antihypertensive medication use, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, use of lipid‐lowering medications, and estimated GFR.
P‐interaction for FHx (Model 2)=0.680.
Consistent results were also found when evaluating quartiles of PA, with those in the highest quartile, compared to the lowest quartile, having lower risk of ASCVD in the overall cohort after adjustment for demographics/lifestyle factors (HR 0.79 [0.69, 0.91], Model 2) and also with further adjustment for ASCVD risk factors (HR 0.80 [0.70, 0.92], Model 3). Results were again similar among those with and without a FHx of premature CHD (0.90 [0.59, 1.39] and 0.78 [0.68, 0.90], Model 2), without significant interaction by FHx status (P‐interaction=0.939) (Table 4). Finally, when comparing the 90th to the 10th percentile of PA levels, the benefit conferred by high PA for ASCVD was modestly stronger among those with a FHx (0.76 [0.51, 1.12], Model 2) compared to those without a FHx (0.83 [0.73, 0.94]) but confidence intervals were wide and overlapping for those with a FHx and no significant interaction by FHx status was found (P‐interaction=0.533).
Table 4.
Hazard Ratios and 95% CI for the Association of Physical Activity by Quartilesa and by the 90th (Compared to 10th) Percentileb With Risk for Incident ASCVD Events Over 21 Years Follow‐Up by Family History Status: The ARIC Study 1987–2012
| No Family History of Prematureg CHD (n=9006) | |||||
|---|---|---|---|---|---|
| Quartile 1 (n=3009) | Quartile 2 (n=1550) | Quartile 3 (n=2192) | Quartile 4 (n=2255) | 90th vs 10th Percentile | |
| Incidence ratec | 9.7 (9.0, 10.6) | 7.4 (6.5, 8.4) | 8.1 (7.3, 9.0) | 6.8 (6.1, 7.6) | |
| Model 1d | Ref | 0.80 (0.68, 0.93) | 0.83 (0.73, 0.94) | 0.65 (0.57, 0.75) | 0.71 (0.63, 0.80) |
| Model 2e | Ref | 0.88 (0.75, 1.02) | 0.93 (0.82, 1.07) | 0.78 (0.68, 0.90) | 0.83 (0.73, 0.94) |
| Model 3f | Ref | 0.85 (0.73, 1.00) | 0.92 (0.81, 1.06) | 0.80 (0.69, 0.92) | 0.85 (0.75, 0.97) |
| Family History of Premature CHDg (n=990) | |||||
|---|---|---|---|---|---|
| Quartile 1 (n=328) | Quartile 2 (n=162) | Quartile 3 (n=256) | Quartile 4 (n=244) | 90th vs 10th Percentile | |
| Incidence ratec | 9.7 (7.6, 12.4) | 9.7 (6.9, 13.8) | 8.1 (6.0, 10.9) | 7.3 (5.3, 10.1) | |
| Model 1d | Ref | 1.01 (0.66, 1.56) | 0.90 (0.61, 1.34) | 0.74 (0.49, 1.13) | 0.65 (0.44, 0.96) |
| Model 2e | Ref | 1.10 (0.71, 1.70) | 1.06 (0.71, 1.58) | 0.90 (0.59, 1.39) | 0.76 (0.51, 1.12) |
| Model 3f | Ref | 1.04 (0.67, 1.61) | 0.88 (0.58, 1.34) | 0.82 (0.53, 1.26) | 0.71 (0.47, 1.07) |
ARIC indicates Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; FHx, family history; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; METS, metabolic equivalents of task; PA, physical activity.
Quartiles of PA were determined for overall population as follows (in METS×min/week): Quartile 1: 0; Quartile 2: >0 to 414; Quartile 3: 415 to 1006; Quartile 4: 1007 to 8032.
90th percentile of PA level (1719 METS×min/week) vs 10th percentile of PA (0 METS×min/week).
Incidence rate is per 1000 person‐years (95% CI).
Model 1: Age, sex, race/center.
Model 2: Model 1+education, BMI, smoking status, and alcohol intake.
Model 3: Model 2+additional potential mediating variables (of the association between PA and ASCVD risk)−systolic blood pressure, antihypertensive medication use, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, use of lipid‐lowering medications, and estimated GFR.
P‐interaction for FHx (Model 2) was 0.939 for quartile analysis and 0.533 for 90th vs 10th percentile analysis.
Similar patterns were observed when considering a FHx of any CHD (regardless of whether premature or not), with no significant interactions by FHx status (Table 5).
Table 5.
Hazard Ratios and 95% CI for Incident ASCVD by AHA‐Defined PA Categories Stratified by Any Family History of CHD (Regardless of Premature or Not)
| No FHx of CHD | FHx of CHD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N Events/n Total | IRa | Model 1b | Model 2c | Model 3d | N Events/n Total | IRa | Model 1b | Model 2c | Model 3d | |
| PA levels | ||||||||||
| Poor (reference) | 399/1991 | 9.8 | Reference (1) | Reference (1) | Reference (1) | 286/1407 | 10.1 | Reference (1) | Reference (1) | Reference (1) |
| Intermediate | 229/1516 | 7.7 | 0.77 (0.65, 0.91) | 0.86 (0.73, 1.02) | 0.83 (0.70, 0.99) | 194/1066 | 8.7 | 0.91 (0.75, 1.09) | 1.01 (0.83, 1.21) | 0.98 (0.81, 1.19) |
| Recommended | 345/2369 | 7.2 | 0.68 (0.58, 0.78) | 0.79 (0.67, 0.92) | 0.77 (0.66, 0.90) | 270/1647 | 7.6 | 0.76 (0.64, 0.90) | 0.91 (0.76, 1.09) | 0.89 (0.75, 1.07) |
| P‐interactione | 0.620 | |||||||||
| PA level categoriesa | ||||||||||
| 1st quartile | 391/1954 | 9.7 | Reference (1) | Reference (1) | Reference (1) | 275/1383 | 9.7 | Reference (1) | Reference (1) | Reference (1) |
| 2nd quartile | 147/1007 | 7.4 | 0.75 (0.62, 0.91) | 0.84 (0.69, 1.01) | 0.80 (0.65, 0.97) | 126/705 | 9.7 | 0.91 (0.73, 1.12) | 0.97 (0.78, 1.20) | 0.96 (0.77, 1.19) |
| 3rd quartile | 236/1435 | 8.1 | 0.80 (0.68, 0.94) | 0.90 (0.76, 1.07) | 0.89 (0.75, 1.05) | 183/1013 | 8.1 | 0.89 (0.74, 1.08) | 1.00 (0.83, 1.22) | 0.97 (0.80, 1.19) |
| 4th quartile | 199/1480 | 6.8 | 0.61 (0.51, 0.73) | 0.72 (0.60, 0.87) | 0.73 (0.60, 0.88) | 166/1019 | 7.3 | 0.73 (0.60, 0.90) | 0.89 (0.72, 1.09) | 0.89 (0.72, 1.09) |
| P‐interactione | 0.793 | |||||||||
AHA indicates American Heart Association; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CHD, coronary heart disease; FHx, family history; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PA, physical activity.
Incidence rate (IR) is per 1000 person‐years.
Model 1: Age, sex, race/center.
Model 2: Model 1+education, BMI, smoking status, and alcohol intake.
Model 3: Model 2+additional potential mediating variables (of the association between PA and ASCVD risk)−systolic blood pressure, antihypertensive medication use, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, use of lipid‐lowering medications, and estimated GFR.
P for interaction by FHx status was assessed using model 2.
We also modeled PA as a continuous variable measured in METS×min/week. Using restricted cubic spline models, we found a graded association of higher levels of PA and lower ASCVD risk in those without a FHx of premature CHD (Figure 2A). Among those with a FHx of premature CHD the confidence intervals are wide, making interpretation difficult (Figure 2B).
Figure 2.
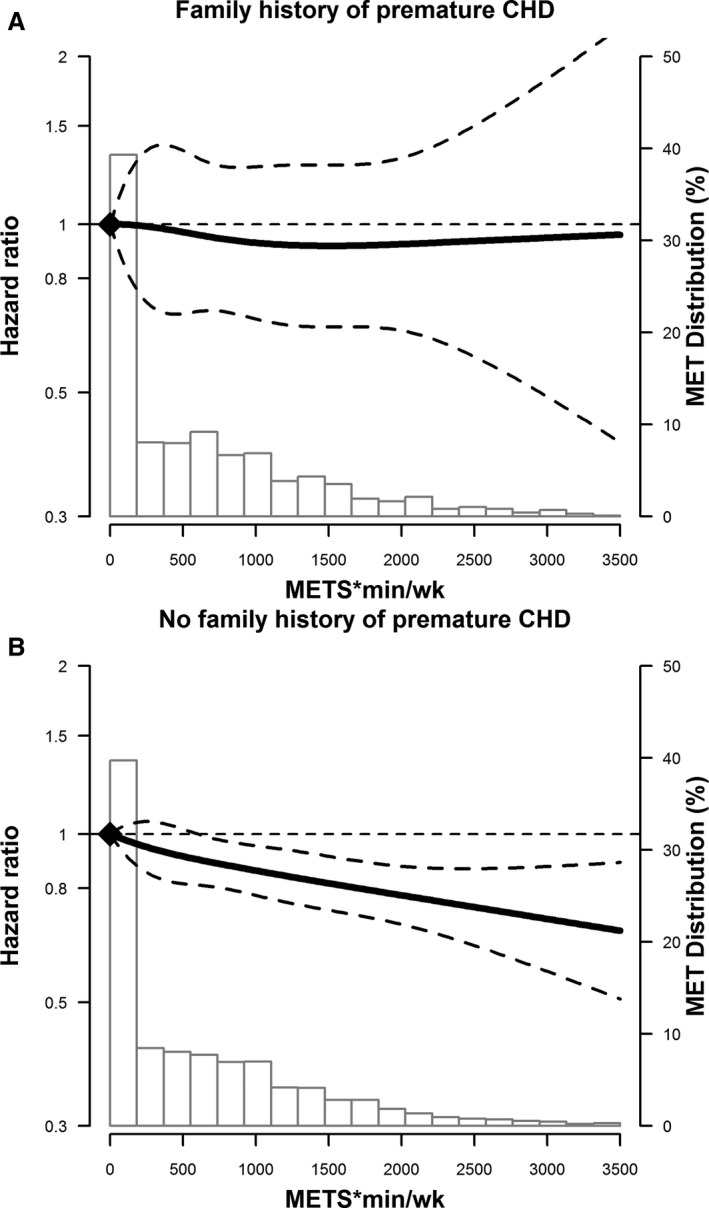
Adjusted* restricted cubic spline model showing the hazard ratios (95% CI) for the association of physical activity levels at visit 1 (METS×min/week) and incident ASCVD events, stratified for those without (A) and with (B) a FHx of premature CHD. The solid line represents the hazard ratios and the dashed lines represent the 95% CIs. Knots at 5th, 35th, 65th, and 95th percentiles. Spline centered at score 0. Histogram shows the distribution of METS score. Restricted cubic spline truncated at 1st and 99th percentile of METS score. *Model is adjusted for age, sex, race/center, education, BMI, smoking status, and alcohol intake. ASCVD indicates atherosclerotic cardiovascular disease; BMI, body mass index; CHD, coronary heart disease; FHx, family history; METS, metabolic equivalents of task.
When assessing changes in AHA categories of PA from visit 1 to visit 3 in the overall cohort, we found that the majority of study participants, 54%, maintained the same levels of PA from visit 1 to visit 3. Over this 6‐year period, 22% of the participants decreased, and 24% increased their levels of PA. In multivariable‐adjusted analyses, there was no significant difference in the change in PA levels from visit 1 to visit 3 among those with a FHx of premature CHD compared to those without such a history (Table 2). Compared to those whose PA levels remained poor, we observed that maintaining recommended levels of PA or increasing levels of PA over time are associated with lower risk of incident ASCVD in both individuals with and without a FHx of premature CHD (P‐interaction=0.678) (Table 6).
Table 6.
Hazard Ratios and 95% CI for Incident ASCVD for Change in AHA‐Defined PA Categories From ARIC Visit 1 (1987–1989) to Visit 3 (1993–1995), Stratified by Family History of Premature CHDf
| No FHx | FHx | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N Events/n Total | IRa | Model 1b | Model 2c | Model 3d | N Events/n Total | IRa | Model 1b | Model 2c | Model 3d | |
| V1 poor V3 poor | 312/1685 | 12.5 | Reference (1) | Reference (1) | Reference (1) | 39/200 | 13.4 | Reference (1) | Reference (1) | Reference (1) |
| V1 poor V3 intermediate | 107/651 | 10.6 | 0.86 (0.69, 1.07) | 0.91 (0.73, 1.13) | 0.86 (0.69, 1.08) | 13/67 | 13.7 | 1.14 (0.61, 2.14) | 1.26 (0.67, 2.39) | 1.12 (0.57, 2.20) |
| V1 poor V3 recommended | 101/639 | 10.2 | 0.69 (0.55, 0.86) | 0.74 (0.59, 0.93) | 0.73 (0.58, 0.93) | 7/60 | 8.1 | 0.54 (0.24, 1.21) | 0.59 (0.26, 1.33) | 0.55 (0.24, 1.26) |
| V1 intermediate V3 poor | 117/731 | 10.5 | 0.85 (0.69, 1.06) | 0.93 (0.75, 1.16) | 0.93 (0.74, 1.16) | 8/72 | 7.1 | 0.58 (0.27, 1.26) | 0.61 (0.28, 1.32) | 0.55 (0.25, 1.20) |
| V1 intermediate V3 intermediate | 89/730 | 7.7 | 0.67 (0.53, 0.85) | 0.79 (0.62, 1.01) | 0.73 (0.57, 0.94) | 17/84 | 13 | 1.01 (0.56, 1.79) | 1.20 (0.67, 2.17) | 1.10 (0.60, 2.04) |
| V1 intermediate V3 recommended | 117/819 | 9.1 | 0.69 (0.55, 0.85) | 0.80 (0.65, 1.00) | 0.80 (0.64, 1.00) | 13/90 | 9.4 | 0.70 (0.37, 1.32) | 0.82 (0.43, 1.56) | 0.75 (0.38, 1.46) |
| V1 recommended V3 poor | 90/584 | 10.2 | 0.79 (0.62, 1.00) | 0.83 (0.65, 1.05) | 0.80 (0.63, 1.02) | 12/69 | 12 | 0.87 (0.45, 1.67) | 0.97 (0.50, 1.87) | 0.73 (0.37, 1.46) |
| V1 recommended V3 intermediate | 72/616 | 7.4 | 0.61 (0.47, 0.79) | 0.71 (0.54, 0.92) | 0.71 (0.54, 0.92) | 10/79 | 7.9 | 0.59 (0.29, 1.18) | 0.67 (0.33, 1.36) | 0.65 (0.32, 1.34) |
| V1 recommended V3 recommended | 314/2348 | 8.5 | 0.61 (0.52, 0.72) | 0.77 (0.65, 0.92) | 0.77 (0.65, 0.92) | 29/242 | 7.5 | 0.54 (0.33, 0.89) | 0.69 (0.41, 1.17) | 0.61 (0.36, 1.03) |
| P‐valuee | 0.678 | |||||||||
AHA indicates American Heart Association; ARIC, Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CHD, coronary heart disease; FHx, family history; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PA, physical activity.
Incidence rate (IR) is per 1000 person years.
Model 1: Age, sex, race/center.
Model 2: Age, education, BMI, smoking status, and alcohol intake.
Model 3: Model 2+additional potential mediating variables (of the association between PA and ASCVD risk)−systolic blood pressure, antihypertensive medication use, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, use of lipid‐lowering medications, and estimated GFR.
P for interaction was assessed using model 2.
We excluded 263 participants who developed ASCVD before or on visit 3.
Discussion
In this analysis of a large cohort of black and white middle‐aged men and women free of ASCVD or heart failure at baseline, we found that less than half of the study participants engaged in AHA‐recommended levels of PA. Individuals with a positive FHx of premature CHD had higher levels of total and low‐density lipoprotein cholesterol and higher triglycerides, but had similarly low levels of PA both at baseline and at a 6‐year follow‐up compared to those without a positive FHx of premature CHD. Our findings confirm prior studies that higher levels of PA are associated with lower incidence ASCVD, and we now suggest that this association could be similar among individuals with and without a FHx of premature CHD, although more definitive conclusions are limited by the small number of individuals in the former group.
Our study findings corroborate recent data that greater than 50% of the American population perform less than the recommended levels of PA23 and that ≈250 000 annual deaths can be attributed to the consequences of physical inactivity.24 The AHA considers meeting recommended levels of PA as one of the metrics with the greatest potential for improvement,14 and our study further highlights the importance of increasing PA for ASCVD risk prevention. In our study, despite having more adverse lipid profiles and being at an increased risk for ASCVD, only 40% of participants with a FHx of premature CHD performed recommended levels of PA. Such findings emphasize the importance of assessing PA levels at every patient encounter and increasing efforts to counsel patients on the benefits of exercise.
To our knowledge, this is the first study to evaluate the impact of higher levels of PA among individuals with a FHx of premature CHD compared to those without. No interaction was found between higher levels of PA and a FHx of premature CHD. However, despite the lack of statistical significance among the group with a FHx of premature CHD, both groups had similar magnitude of decrease in the risk of ASCVD events. The lack of statistical significance within the group with a FHx of premature CHD is likely explained by the small number of individuals in that group leading to reduced power to detect a significant difference.
FHx of premature CHD has been associated with a significantly increased risk of ASCVD events,25 and prior studies have indicated that these individuals are more susceptible to the deleterious effects of certain behaviors such as smoking.9 Screening for FHx of premature CHD is an effective way of identifying a high‐risk group that may benefit from receiving more intensive preventative interventions.26 Tailored counseling of high‐risk individuals identified through family risk assessment has been shown to increase healthy behaviors such as consumption of fruits and vegetables, and performing PA for 30 minutes 5 to 6 times a week.27 Our study demonstrates the high prevalence of modifiable behavior within this high‐risk population and suggests a potential benefit with targeted approaches that would lead to increases in PA levels.
In our study, ≈10% of the population reported a parental history of premature CHD. In contrast to other studies, the crude (unadjusted) incidence rates of ASCVD events was not much higher among this subgroup when compared to individuals without such FHx, with only a small difference in time to first event on average. Although simple definitions of FHx have been shown to be associated with ASCVD risk, not everyone has the same family structure, or knowledge of their family members’ health status. FHx of premature CHD was ascertained at ARIC visit 1 (1987–1989); during this time period, there might have been less emphasis on the importance of FHx compared to the present, and perhaps participants were less knowledgeable about their FHx status, potentially leading to misclassification of study participants.
A FHx of premature CHD in a sibling may be a stronger risk for ASCVD compared to a parental FHx.28 Furthermore, a more comprehensive approach called “family risk scores,” which compare observed and expected number of relatives with ASCVD in a family compared to population estimates of age/sex disease rates, may further improve risk stratification and allow for inclusion of second‐ and even third‐degree relatives into risk estimates.3, 29 These data were not available in the overall ARIC cohort. Finally, the relative risks associated with a FHx of premature CHD tend to be largest for people in middle age; the mean age of our study population at baseline was 54 years, so by the end of follow‐up, the mean age of the cohort is in their mid‐70s. Thus, the relative difference that the presence of a positive FHx confers on incident ASCVD rates would likely be more pronounced in a younger cohort.
Our study has some limitations. As with all observational studies, there is the potential for residual confounding. PA was assessed via a questionnaire with the potential for reporting bias. Directly measured fitness correlates better with cardiometabolic risk factors30 and CHD risk31 than self‐reported PA. Another important limitation is ascertainment of FHx of premature CHD. In ARIC, FHx of premature CHD was self‐reported, leading to possible misclassification. Participants may not have been aware of the medical history of family members, or may have reported premature deaths that were not from CHD. However, self‐reported FHx of premature CHD is what is commonly used in multiple large population‐based cohorts. Furthermore, when evaluating a patient in a clinical setting, ascertainment of FHx is also usually self‐reported and not validated. Prior studies have found that self‐reported FHx of CHD is reasonably accurate compared to validated FHx using medical records obtained from first‐degree relatives of the proband.32, 33
Finally, we were limited by the small number of individuals in the group with a parental history of premature CHD, which decreased the power to detect statistically significant differences. Since the lack of interaction by FHx status was actually contrary to our original a priori hypotheses, we performed a post‐hoc power calculation. We determined that our study had adequate power to detect moderate to large differences in the associations between participants with and without a FHx of CHD, although it had limited power to detect small differences. For instance, in the fully adjusted models, we had ≈92% power to detect hazard ratios twice as large in one FHx group compared to the other, but only 50% power to detect hazard ratios 1.5 times as large.
Despite these limitations, our study has many strengths. ARIC is a well‐characterized bi‐ethnic cohort of middle‐aged individuals, with long‐term (21‐year) follow‐up, and ASCVD events adjudicated by an expert panel. Another strength of the study is the assessment of PA at 2 points in time, over a 6‐year period to evaluate change in PA levels by FHx status.
In summary, our study demonstrated that despite more adverse lipid profiles, individuals with a FHx of premature CHD had similar PA patterns as compared to a lower‐risk group without such FHx, with less than half meeting AHA recommended levels. PA was associated with a lower risk for incident ASCVD in those without a FHx, and a similar trend was observed within the smaller group of participants with a FHx of premature CHD. These findings have significant clinical implications for strategies to prevent ASCVD in the general population, as well as within this high‐risk subgroup. Promotion of PA should be encouraged for all regardless of FHx status. Better strategies for increasing PA levels in the general population as well as in high‐risk subgroups are needed. Better ascertainment of FHx (such as using a validated FHx or Family Risk Scores) and of PA (such as using objectively measured PA and/or measured fitness) will enhance the quality of future studies in this field.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Dr Michos is supported by a grant R01NS072243 from National Institutes of Health/National Institute of Neurological Disorders and Stroke and the Blumenthal Scholars Award in Preventive Cardiology.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc. 2016;5:e003505 doi: 10.1161/JAHA.116.003505)
The abstract for this work was presented at the 2016 American Heart Association Epidemiology and Lifestyle Meeting, March 1–4 in Phoenix, Arizona.
References
- 1. Myers RH, Kiely DK, Cupples LA, Kannel WB. Parental history is an independent risk factor for coronary artery disease: the Framingham Study. Am Heart J. 1990;120:963–969. [DOI] [PubMed] [Google Scholar]
- 2. Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104:393–398. [DOI] [PubMed] [Google Scholar]
- 3. Kardia SL, Modell SM, Peyser PA. Family‐centered approaches to understanding and preventing coronary heart disease. Am J Prev Med. 2003;24:143–151. [DOI] [PubMed] [Google Scholar]
- 4. Patel J, Al Rifai M, Blaha MJ, Budoff MJ, Post WS, Polak JF, Bluemke DA, Scheuner MT, Kronmal RA, Blumenthal RS, Nasir K, McEvoy JW. Coronary artery calcium improves risk assessment in adults with a family history of premature coronary heart disease: results from Multiethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jorde LB, Williams RR. Relation between family history of coronary artery disease and coronary risk variables. Am J Cardiol. 1988;62:708–713. [DOI] [PubMed] [Google Scholar]
- 6. Juonala M, Viikari JS, Rasanen L, Helenius H, Pietikainen M, Raitakari OT. Young adults with family history of coronary heart disease have increased arterial vulnerability to metabolic risk factors: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006;26:1376–1382. [DOI] [PubMed] [Google Scholar]
- 7. Michos ED, Nasir K, Rumberger JA, Vasamreddy C, Braunstein JB, Budoff MJ, Blumenthal RS. Relation of family history of premature coronary heart disease and metabolic risk factors to risk of coronary arterial calcium in asymptomatic subjects. Am J Cardiol. 2005;95:655–657. [DOI] [PubMed] [Google Scholar]
- 8. Cuomo S, Guarini P, Gaeta G, De Michele M, Boeri F, Dorn J, Bond M, Trevisan M. Increased carotid intima‐media thickness in children‐adolescents, and young adults with a parental history of premature myocardial infarction. Eur Heart J. 2002;23:1345–1350. [DOI] [PubMed] [Google Scholar]
- 9. Hopkins PN, Williams RR, Hunt SC. Magnified risks from cigarette smoking for coronary prone families in Utah. West J Med. 1984;141:196–202. [PMC free article] [PubMed] [Google Scholar]
- 10. Khaw KT, Barrett‐Connor E. Family history of heart attack: a modifiable risk factor? Circulation. 1986;74:239–244. [DOI] [PubMed] [Google Scholar]
- 11. Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, Linet MS, Lee IM, Matthews CE. Leisure time physical activity and mortality: a detailed pooled analysis of the dose‐response relationship. JAMA Intern Med. 2015;175:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bertoni AG, Whitt‐Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. [DOI] [PubMed] [Google Scholar]
- 14. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task F and Statistics C . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 15. Slattery ML, Schumacher MC, Hunt SC, Williams RR. The associations between family history of coronary heart disease, physical activity, dietary intake and body size. Int J Sports Med. 1993;14:93–99. [DOI] [PubMed] [Google Scholar]
- 16. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 18. Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in Atherosclerosis Risk in Communities. Med Sci Sports Exerc. 2013;45:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Autenrieth CS, Evenson KR, Yatsuya H, Shahar E, Baggett C, Rosamond WD. Association between physical activity and risk of stroke subtypes: the Atherosclerosis Risk in Communities Study. Neuroepidemiology. 2013;40:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD EPI . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 22. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease C and Prevention . Adult participation in recommended levels of physical activity–United States, 2001 and 2003. MMWR Morb Mortal Wkly Rep. 2005;54:1208–1212. [PubMed] [Google Scholar]
- 24. Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol (1985). 2000;88:774–787. [DOI] [PubMed] [Google Scholar]
- 25. Lloyd‐Jones DM, Nam BH, D'Agostino RB Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O'Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle‐aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. [DOI] [PubMed] [Google Scholar]
- 26. Hunt SC, Gwinn M, Adams TD. Family history assessment: strategies for prevention of cardiovascular disease. Am J Prev Med. 2003;24:136–142. [DOI] [PubMed] [Google Scholar]
- 27. Ruffin MTt, Nease DE Jr, Sen A, Pace WD, Wang C, Acheson LS, Rubinstein WS, O'Neill S, Gramling R; Family History Impact Trial G . Effect of preventive messages tailored to family history on health behaviors: the Family Healthware Impact Trial. Ann Fam Med. 2011;9:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murabito JM, Pencina MJ, Nam BH, D'Agostino RB Sr, Wang TJ, Lloyd‐Jones D, Wilson PW, O'Donnell CJ. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle‐aged adults. JAMA. 2005;294:3117–3123. [DOI] [PubMed] [Google Scholar]
- 29. Li R, Bensen JT, Hutchinson RG, Province MA, Hertz‐Picciotto I, Sprafka JM, Tyroler HA. Family risk score of coronary heart disease (CHD) as a predictor of CHD: the Atherosclerosis Risk in Communities (ARIC) study and the NHLBI family heart study. Genet Epidemiol. 2000;18:236–250. [DOI] [PubMed] [Google Scholar]
- 30. Minder CM, Shaya GE, Michos ED, Keenan TE, Blumenthal RS, Nasir K, Carvalho JA, Conceicao RD, Santos RD, Blaha MJ. Relation between self‐reported physical activity level, fitness, and cardiometabolic risk. Am J Cardiol. 2014;113:637–643. [DOI] [PubMed] [Google Scholar]
- 31. Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta‐analysis. Med Sci Sports Exerc. 2001;33:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pereira MA, Schreiner PJ, Pankow JS, Williams RR, Higgins M, Province MA, Rao DC. The Family Risk Score for coronary heart disease: associations with lipids, lipoproteins, and body habitus in a middle‐aged bi‐racial cohort: the ARIC study. Ann Epidemiol. 2000;10:239–245. [DOI] [PubMed] [Google Scholar]
- 33. Silberberg JS, Wlodarczyk J, Fryer J, Ray CD, Hensley MJ. Correction for biases in a population‐based study of family history and coronary heart disease. The Newcastle Family History Study I. Am J Epidemiol. 1998;147:1123–1132. [DOI] [PubMed] [Google Scholar]


