Abstract
Background
There is limited knowledge on the short‐term influence of radiofrequency ablation (RFA) of atrial fibrillation (AF) on 2 cardiac biomarkers; the N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and the midregional fragment of the N‐terminal of pro‐ANP (MR‐proANP) and 2 extracardiac biomarkers; the c‐terminal provasopressin (copeptin) and the midregional portion of proadrenomedullin (MR‐proADM). There are also limited data concerning cardiac production of the latter two.
Methods and Results
We studied 192 consecutive patients eligible for RFA of AF referred to the University Hospital, Linköping, Sweden. NT‐proBNP, MR‐proANP, copeptin, and MR‐proADM levels were measured in peripheral blood, the coronary sinus (CS), and the left atrium before ablation, and in peripheral blood immediately and the day after RFA. The level of NT‐proBNP decreased the day after RFA in participants in AF at the time of RFA, compared to the participants in sinus rhythm who showed a slight increase (P<0.001). Furthermore, regardless of the actual rhythm, the level of MR‐proANP showed an increase immediately after RFA (P<0.001), followed by a decrease the day after ablation (P<0.001). Copeptin level showed a 6‐fold increase immediately after RFA compared to baseline (P<0.001), whereas MR‐proADM level increased the day after RFA (P<0.001). Levels of copeptin and MR‐proADM were not higher in the CS compared to peripheral blood.
Conclusions
RFA of AF is a strong stimulus with a significant and direct impact on different neurohormonal systems. We found no sign of a cardiac release of MR‐proADM or copeptin.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique Identifier: NCT01553045.
Keywords: atrial fibrillation, biomarkers, catheter ablation
Subject Categories: Arrhythmias
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia with an estimated prevalence of 2.9% in the Swedish population.1 Pulmonary vein isolation, either by radiofrequency ablation (RFA) or by cryoablation, has become a standard therapeutic alternative for patients with paroxysmal and persistent AF.2
Although several hundred thousand catheter ablation procedures for AF were performed during the last decade,3 there is a significant knowledge gap concerning the hemodynamic and neurohormonal changes that affect these patients during, directly after, and on the first day after the procedure. These changes are of short‐term significance, and they can contribute to explaining procedure‐related complications and symptoms. In order to give a more complete picture of these changes, both cardiac and extracardiac biomarkers can be used in the evaluation of patients with AF.
It is well known that the heart has endocrine functions that among others are mediated by A‐ and B‐type natriuretic peptides (ANP and BNP),4 which are synthesized and secreted from atrial and ventricular myocytes.5 Although the most common pathological cause of BNP production is heart failure (HF), elevated concentrations of the N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) have been reported in patients with AF even without overt HF.6 Furthermore, even though there are certain differences between BNP and NT‐proBNP, the information gained by those natriuretic peptides is, in general, similar.
Two cardiac peptides, NT‐proBNP and N‐terminal atrial natriuretic peptide (N‐ANP) have been introduced as biomarkers of AF.7, 8 A stable method for quantifying the midregional fragment of proANP (MR‐proANP) has been introduced.9 MR‐proANP seems to correlate with the duration of AF episodes.10
The extracardiac peptides, that is, vasopressin and adrenomedullin (ADM), play a central role in cardiovascular endocrine metabolism. Vasopressin is produced in the hypothalamus, released from the neurohypophysis in response to hypervolemia and changes in plasma osmolality, and is important for the fluid regulation of the body. The plasma concentration of vasopressin increases in patients with HF attributed to inadequate cardiac output, low blood pressure, or increased vascular resistance.11, 12 Vasopressin is a small unstable molecule that is mainly attached to platelets and rapidly cleared.13 In contrast, c‐terminal pro‐vasopressin (copeptin), which shares the same parental molecule as arginine vasopressin (AVP), is a more‐stable biomarker for clinical evaluation of cardiovascular disease.11 ADM is a peptide with vasoactive and natriuretic properties. It is secreted in response to several hormonal agents, such as corticosteroids, and cytokines, such as tumor necrosis factor, but also attributed to physical stimulants, such as shear stress and stretch.14, 15, 16, 17 MR‐proADM, a product of the parental molecule of ADM, is stable and better suited for evaluation in clinical routines.18 In a recent study, MR‐proADM was proved to be associated with the occurrence of AF in the general population,19 and elevated MR‐proADM levels have been reported to predict the recurrence of AF after RFA.20
These biomarkers (ie, NT‐proBNP, MR‐proANP, copeptin, and MR‐proADM) are important regulators of the hemodynamic status, but their role in patients with AF and, particularly, their immediate response to RFA have not been previously investigated.
The aim of this study was to analyze these biomarker responses in relation to presenting rhythm and their response to RFA, and to investigate whether or not any cardiac production of these peptides could be found.
Methods
Study Design
This is an observational, single‐center cohort study based on data from the SMURF (Symptom burden, metabolic profile, ultrasound findings, rhythm, neurohormonal activation, hemodynamics, and health‐related quality of life in patients with AF) study.21
Settings/Participants
The study was conducted between January 2012 and April 2014. All patients referred for RFA of AF to the University Hospital in Linköping, Sweden, were considered for participation. The inclusion criteria were: (1) patients with paroxysmal or persistent AF; (2) patient age above 17 years; (3) patients referred to the hospital for the first RFA treatment; and (4) sufficient knowledge of the Swedish language to independently fill out the study questionnaires.
Patients were excluded if they: (1) had previously undergone catheter or surgical AF ablation; (2) had or were expected to require heart surgery; (3) suffered from severe HF with ejection fraction (EF) <35%; or (4) had acute coronary syndrome during the past 3 months before ablation.21
In total, 338 patients with AF were referred to the Department of Cardiology, University Hospital in Linköping, Sweden, between January 2012 and April 2014 and selected for first‐time RFA. Of those, 19 patients were excluded because of previous heart surgery, 8 because of EF <35%, 107 because of logistical reasons (we could only include 4 patients per week in the study), and 12 declined participation. Thus, 192 participants, 141 in sinus rhythm (SR) and 51 in AF, were included in the study (Figure 1), 64 of those with lone AF (ie, CHA2DS2 VASc score22 of 0 for male and 1 for female, and no other known cardiac comorbidities). There were no baseline differences between participants included in our study and those excluded because of logistical difficulties or because they declined participation (Table 1).
Figure 1.
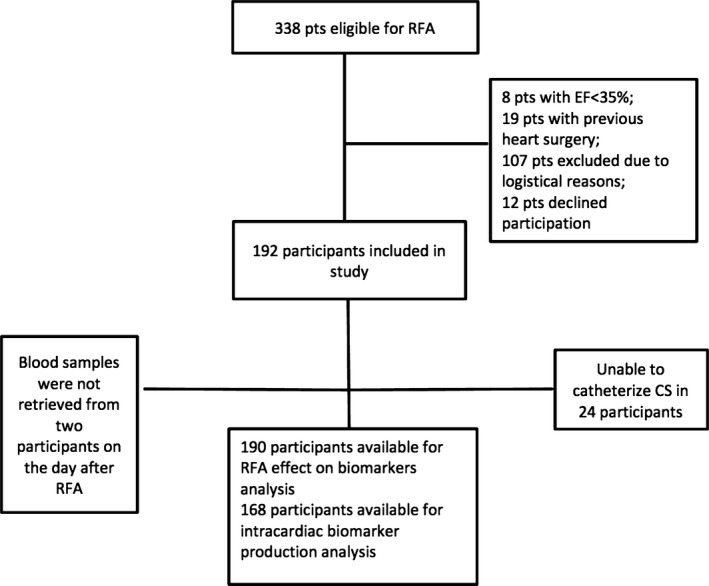
Flow chart of the inclusion of the study population. CS indicates coronary sinus; EF, ejection fraction; pts, patients; RFA, radiofrequency ablation.
Table 1.
Comparison of the Study Population and Patients Excluded Attributed to Logistical Difficulties or Declined Participation
| Variables | Patients Included Into the SMURF Study N=192 | Patients Excluded Attributed to Logistical Difficulties or Declined Participation N=118 | P Value |
|---|---|---|---|
| Age, mean | 60.5±10.5 | 60.3±11.9 | 0.871 |
| Female sex (%) | 56 (29.2) | 42 (35.3) | 0.259 |
| BMI | 27.9 (IQR 4.2) | 27.4 (IQR 6.5) | 0.984 |
| Paroxysmal AF (%) | 71 (37.4) | 42 (35.3) | 0.764 |
| Hypertension (%) | 80 (42.1) | 59 (49.6) | 0.173 |
| Diabetes mellitus (%) | 15 (7.8) | 12 (10.2) | 0.489 |
| Heart diseasea | 26 (13.5) | 11 (9.2) | 0.24 |
| IHD (%) | 15 (7.8) | 6 (5) | 0.516 |
| SVT (%) | 38 (19.8) | 24 (20.2) | 0.936 |
| Stroke/TIA (%) | 19 (10) | 9 (7.6) | 0.485 |
| Beta‐blocker (%) | 139 (73.2) | 81 (68.1%) | 0.332 |
| AAD (%) | 105 (54.7) | 66 (55.5) | 0.894 |
| ACEi or ARB (%) | 77 (48.5) | 51 (42.9) | 0.632 |
| Statins (%) | 56 (29.5) | 26 (21.8) | 0.155 |
| AF at the ablation lab (%) | 51 (27) | 34 (28.8) | 0.666 |
| Procedural time, min | 186±49.6 | 173±49.8 | 0.025 |
| Fluoroscopy time, minutes | 21 (IQR 13) | 21.5 (IQR 14) | 0.215 |
| Primary successful procedure (%) | 172 (90.5) | 100 (84) | 0.151 |
| Complications (%) | 7 (3.7) | 5 (4.2) | 0.805 |
| Normal LV function (%) | 143 (74.5) | 90 (75.6) | 0.854 |
Normal disturbed data are presented as mean values±SD and possible differences examined with t test, nonparametric data are presented as median values with IQR and tested with Mann–Whitney U test, and categorical data are presented as counts with percent values within brackets and tested with chi‐square test. AAD indicates antiarrhythmic drugs; ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index (kg/m2); EF, ejection fraction; IHD, ischemic heart disease; IQR, interquartile range; SVT, supraventricular tachycardia; TIA, transient ischemic attack.
Patients with dilated, hypertrophic, ischemic, restrictive cardiomyopathy, and perimyocarditis.
Informed Consent and Ethical Considerations
The regional ethical review board of the Faculty of Health Sciences, Linköping, Sweden, approved the protocol for this study (Registration No.: 2011/40‐31). All participants gave their written consent, and study participation details were documented in the patient's medical record. The study complies with the Declaration of Helsinki.23
Echocardiography
All participants underwent transthoracic and transoesophageal echocardiographic examinations (TTE and TEE) before RFA. GE Vivid 7 or GE Vivid E9 systems (GE Healthcare, Horten, Norway) were utilized with a 3.5‐MHz transducer for TTE and a 7‐MHz transducer for TEE. Measurements and evaluations were performed according to the guidelines of the European Society of Echocardiography.24 Blood pressure was measured in the left arm with the patient in the supine position, and heart rate was monitored during the examination.
Left ventricular EF was calculated according to Simpson's biplane method. Left atrium (LA) volume was measured using the biplane area‐length method. Left ventricular filling velocities (E and A) were obtained by pulsed‐wave Doppler in the apical 4‐chamber (4CH) view. Tissue Doppler images in the 4CH view were obtained and used offline in order to measure early diastolic velocity (E′) in the mitral septal annulus. The E/E′ ratio was then calculated.
The left atrium appendage was visualized and examined thoroughly with TEE for the possible presence of thrombus.
Ablation Procedure
All procedures were performed under conscious sedation using propofol and remifentanil. Two transseptal sheaths were inserted into the LA through the right femoral vein and perfused with heparinized saline. Heparin was used to maintain an activated clotting time of >350 seconds throughout the procedure. RFA was conducted under the guidance of a computer‐based mapping system (CARTO; Biosense Webster, Diamond Bar, CA). Mapping and ablation were performed with an open‐irrigated catheter (ThermoCool; Biosense Webster). A 7‐F multipolar (20‐pole) circumferential diagnostic catheter was used for the assessment of pulmonary vein activation and isolation (Lasso; Biosense Webster). Radiofrequency energy was delivered in a power‐controlled mode with a maximum energy setting of 35 W, but reduced to 25 W in the posterior wall. The endpoint of the procedure was electrical disconnection of all pulmonary veins by antral ablation, verified by entry and exit block of all pulmonary veins. With participants with persistent AF, additional ablation to create LA lines was at the discretion of the operator and verified by pacing maneuvers. Participants in AF were routinely converted to SR on completion of the RFA procedure.
Biomarkers
Blood samples were collected in plastic vials containing ETDA. Vials were centrifuged at 3100g for 20 minutes and then frozen at −70°C. No sample was thawed more than twice.
Concentrations of proBNP 1‐76 (NT‐proBNP) were analyzed on the Elecsys 2010 platform (Roche Diagnostics, Mannheim, Germany). The total coefficient of variation (CV) was 4.6% at 426.5 pg/mL (n=487) and 3.2% at 2308 pg/mL (n=495). Plasma concentrations of MR‐proADM, copeptin, and MR‐proANP were analyzed on the Kryptor platform (Brahms AG, Hennigsdorf, Germany). Total CV for copeptin was 4% at a concentration of 15 pmol/L (n=18) and 3.5% at concentrations of 100 pmol/L (n=18). For MR‐proADM, the intraassay CV, according to the manufacturer, was ≤10% for concentrations between 0.2 and 0.5 nmol/L, <4% for concentrations between 0.5 and 2 nmol/L, <2% for concentrations between 2 and 6 nmol/L, and <3.5% for concentrations over 6 nmol/L. The intraassay CV for MR‐proANP according to the manufacturer was ≤5% for concentrations between 10 and 20 pmol/L, <3.5% for concentrations between 20 and 1000 pmol/L, and <3.5% for concentrations over 1000 pmol/L.
Subject Measurements
After screening, all potentially eligible patients received written information on the study and RFA procedure.
Before RFA, all participants received further oral information about the procedure and then signed the informed consent. A full baseline evaluation was performed, including medical history, physical examination, and a 12‐lead electrocardiogram. All participants underwent TTE, TEE, and a computerized tomographic scan of the heart according to the clinical routine.
All participants were catheterized, and blood samples for the analysis of biomarkers (NT‐proBNP, MR‐proANP, copeptin, and MR‐proADM) were drawn from the femoral vein, well into the coronary sinus (CS; the position of the catheter was evaluated in the left anterior oblique projection) and the LA (baseline sampling) by use of a multipurpose MR A1 high‐flow catheter (Cordis, Johnson & Johnson Medical, Fremont, CA).
After baseline evaluation, the electrophysiologist in charge proceeded with the planned RFA as described previously. At the end of the procedure, blood samples were drawn from the femoral vein for biomarker analysis.
Blood samples were drawn from a peripheral vein the day after the RFA procedure while the actual rhythm and heart rate were noted down.
Evaluations
Measurement of possible changes in NT‐proBNP, MR‐proANP, copeptin, and MR‐proADM concentrations in relation to EF and the heart rhythm at the time of RFA, immediately after and the day after the RFA procedure.
Measurement of a possible concentration gradient between intracardiac and peripheral venous blood of copeptin and MR‐proADM indicating a possible cardiac production of these 2 biomarkers and the levels of NT‐proBNP and MR‐proANP in LA and CS in relation to the heart rhythm at the time of RFA and EF.
Measurements of a possible correlation between the levels of 4 biomarkers.
Statistical Analysis
For baseline data, continuous variables are expressed as means±SD. Variables, not normally distributed, are presented as median with interquartile range (IQR). Categorical data are presented as percentages. Participants with missing values were excluded from the planned follow‐up analyses.
In order to analyze the first and second outcomes, a repeated‐measurement ANOVA was used. To compare the different levels in the repeated‐measures ANOVA, a simple contrast comparison was used. The actual rhythms at the time of RFA and EF (<50%) were used as within‐subject factors. The analysis was adjusted for covariates: age >65 years, sex, heart rate >100/min, body mass index (BMI) >30, type of AF (paroxysmal or persistent) and glomerular filtration rate (GFR) of <60 mL/min per 1.73 m2 that were calculated by using a previously described cystatin‐C formula.25 The analysis for the primary outcome was additionally adjusted for the echocardiographical septal wall index E/E′ >15 in order to adjust for signs of elevated filling pressure.26
Logarithmic transformations of NT‐proBNP, MR‐proANP, and copeptin levels were used, because they were not normally distributed.
Spearman's correlation was used in order to study possible correlations between biomarker levels.
All reported P values are 2‐sided, and a P<0.05 was considered statistically significant. In order to control the false discovery rate in multiple testing, the Benjamini and Hochberg method was used.27 Analyses were performed using SPSS software (version 22.0; SPSS, Inc., Chicago, IL).
Results
Baseline Characteristics
In total, 192 participants were included in the SMURF study (56 women and 136 men), 190 were evaluated for the effect of RFA on biomarker levels, and 168 were available for the analysis of biomarker levels in different locations (Figure 1). Baseline characteristics are presented in detail in Table 2.
Table 2.
Baseline Characteristics
| Variables | Total N=192 |
|---|---|
| Age, mean | 60.5±10.2 |
| Female sex (%) | 56 (29.2) |
| BMI, kg/m2 | 27.9 (IQR 4.2) |
| Paroxysmal AF | 71 (37.4%) |
| Months in AF | 48 (IQR 84) |
| No. of DC conversions/patient with persistent AF | 3 (IQR 3) |
| Heredity (%) | 62 (32.6) |
| Hypertension (%) | 80 (42.1) |
| Diabetes mellitus (%) | 15 (7.8) |
| Heart failure (%) | 17 (8.9) |
| CKD (GFR <60 mL/min per 1.73 m2) (%) | 40 (20.1) |
| SVT (%) | 38 (19.8) |
| Stroke/TIA (%) | 19 (10) |
| CHA2DS2 VASc | 1.63 (IQR 2) |
| Beta‐blocker (%) | 139 (73.2) |
| AAD (%) | 105 (54.7) |
| Amiodarone (%) | 42 (22) |
| Flecainide (%) | 35 (18) |
| Dronedarone (%) | 23 (12.1) |
| ACEi or ARB (%) | 77 (48.5) |
| Statins (%) | 56 (29.5) |
| AF at the ablation lab (%) | 51 (27) |
| Arrival heart rate, bpm | 60 (IQR 22.5) |
| Procedural time, minute | 186±49.6 |
| Fluoroscopy time, minute | 21 (IQR 13) |
| Primary successful procedure (%) | 172 (90.5) |
| Complications (%) | 7 (3.7) |
| EF, % | 55.9±11.3 |
| LA volume, mL | 56.7±19.7 |
| E/E′ | 11.9 (IQR 6.1) |
Normally distributed continuous data are presented as means with SD; if not, they are presented as median values with IQR. Discrete data are presented as counts with percent values within brackets. AAD indicates antiarrhythmic drugs; ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; CHA2DS2 VASc, congestive heart failure, hypertension, age >75, diabetes, stroke, vascular disease, sex; CKD, chronic kidney failure; DC, direct current; EF, ejection fraction; GFR, glomerular filtration rate; IQR, interquartile range; LA, left atrium; SVT, supraventricular tachycardia; TIA, transient ischemic attack.
At the time of baseline blood sampling, 51 of 192 participants were in AF. Mean procedural time was 186±50 minutes with a median fluoroscopy time of 21 (IQR, 13) minutes. Total radiofrequency time was 40±14 minutes. Additional ablation lines were performed in the LA in 17 participants whereas a right atrium (RA) isthmus line was drawn in 11 participants (Table 3). In 90.5% of cases, the ablation was considered preliminarily successful, that is, all pulmonary veins were isolated with complete entry and exit blocks.
Table 3.
Lines Performed During Radiofrequency Ablation, in Addition to Pulmonary Vein Isolation
| Additional Lines | Total |
|---|---|
| RA isthmus (%) | 11 (5.7) |
| LA isthmus (%) | 1 (0.5) |
| LA roof (%) | 15 (7.8) |
| CS line (%) | 1 (0.5) |
| No additional line | 164 (85.4) |
Data are presented as counts with percent values within brackets. CS indicates coronary sinus; LA, left atrium, RA, right atrium.
Complications occurred in 7 participants (3.6%), where 2 suffered from cardiac tamponade requiring pericardiocentesis and 1 suffered from pericardial effusion not requiring pericardial drainage. Moreover, 3 participants developed pseudoaneurysm, and another patient developed a larger‐than‐normal hematoma of the groin.
RFA Effect on NT‐proBNP and MR‐proANP
RFA caused a significant change in NT‐proBNP level immediately after and the day after the RFA procedure, compared to the baseline measurement, depending on the underlying rhythm (SR or AF) at the time of RFA (F=43.4; P<0.001). Participants in AF at baseline showed a significant decrease in NT‐proBNP level on the day after RFA whereas those in SR before RFA showed a slightly increased level (F=43.2; P<0.001; Figure 2). Furthermore, the NT‐proBNP level in participants with EF <50% was reduced more on the day after the RFA procedure compared to those with EF >50% (F=15.8; P<0.001). The complication of pericardial effusion was entered as a covariate in these analyses without any significant impact on the result.
Figure 2.
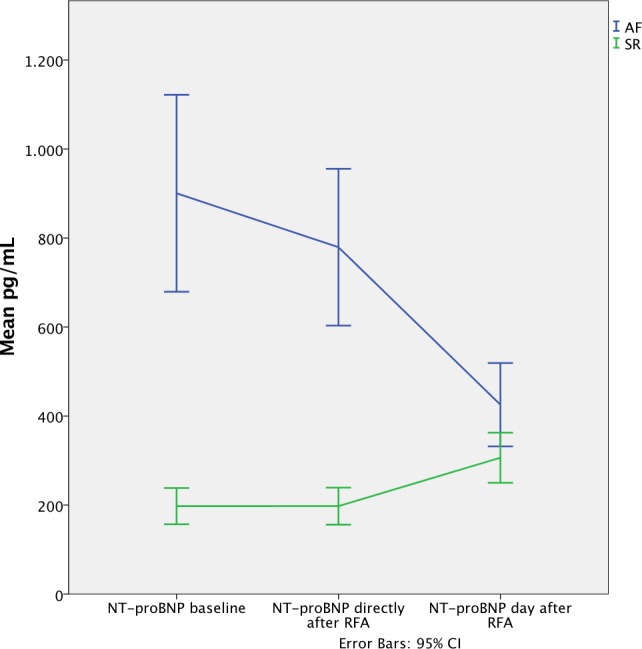
Changes in concentration of NT‐proBNP after radiofrequency ablation depending on rhythm. NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; RFA, radiofrequency ablation.
The level of MR‐proANP differed between the different sampling occasions (F=114.2; P<0.001; Table 4; Figure 3). The MR‐proANP level was higher immediately after RFA compared to the baseline measurement (F=35.9; P<0.001) and decreased the day after RFA compared to baseline (F=116.7; P<0.001). Participants in AF at baseline had a higher increase in MR‐proANP concentration directly after RFA compared to those in SR (F=9.5; P<0.002). The same reaction was also observed in participants with EF >50% whose MR‐proANP concentration increased more, directly after RFA compared with those having an EF >50% (F=12.2; P<0.001).
Table 4.
Radiofrequency Ablation Effect on Biomarkers
| Biomarkers N=190 | Baseline | 95% CI | Post‐RFA | 95% CI | Day After RFA | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| NT‐proBNP, pg/mL | 187.1 | 156.6 to 223.4 | 189.8 | 161.4 to 223.2 | 243.5 | 217.9 to 272.1 | 0.886 |
| MR‐proANP, pmol/L | 132.5 | 122.7 to 143.1 | 196.7 | 182.9 to 211.6 | 100.5 | 93.2 to 108.5 | <0.001 |
| Copeptin, pmol/L | 8.6 | 7.57 to 9.88 | 53.5 | 44.7 to 64.1 | 8.19 | 7.42 to 9.05 | <0.001 |
| MR‐proADM, nmol/L | 0.682 | 0.655 to 0.708 | 0.6211 | 0.598 to 0.644 | 0.811 | 0.778 to 0.844 | <0.001 |
Data are presented as geometric means with 95% CI, but MR‐proADM is presented as mean with 95% CI. Analyses of the course of peptides were performed with repeated‐measurements ANOVA and were adjusted for covariates: BMI >30, GFR <60 mL/min per 1.73 m2, EF <50%, age >65 years, heart rate >100/min, E/E′, and sex. The potential statistical significance of those analyses are presented with P values. BMI indicates body mass index; EF, ejection fraction; GFR, glomerular filtration rate; MR‐proADM, midregional portion of proadrenomedullin; MR‐proANP, midregional fragment of the N‐terminal precursor of atrial natriuretic peptide; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Figure 3.
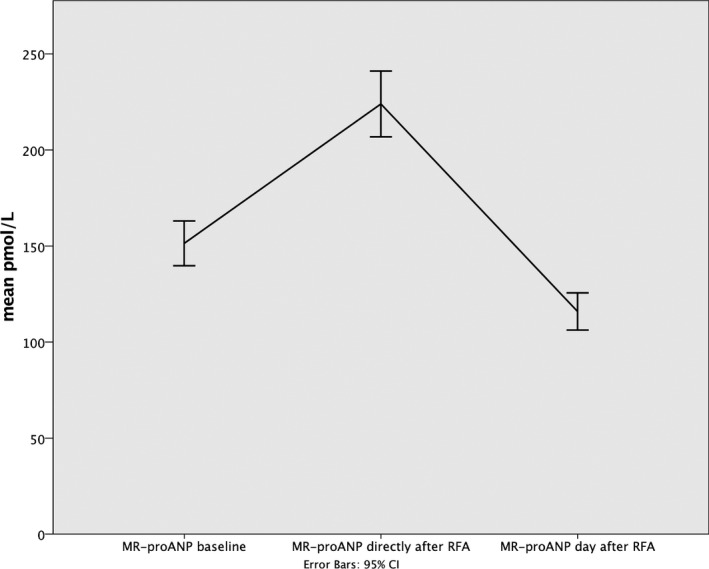
Changes in concentration of MR‐proANP after radiofrequency ablation. MR‐proANP indicates midregional fragment of the N‐terminal precursor of atrial natriuretic peptide; RFA, radiofrequency ablation.
RFA Effect on Copeptin and MR‐proADM
The level of copeptin increased 6‐fold immediately after RFA compared to baseline (F=131.2; P<0.001) and then normalized on the day after the ablation (F=0.753; P=0.387; Table 4; Figure 4). Neither the actual rhythm nor the EF played any significant role in the copeptin reaction on RFA.
Figure 4.
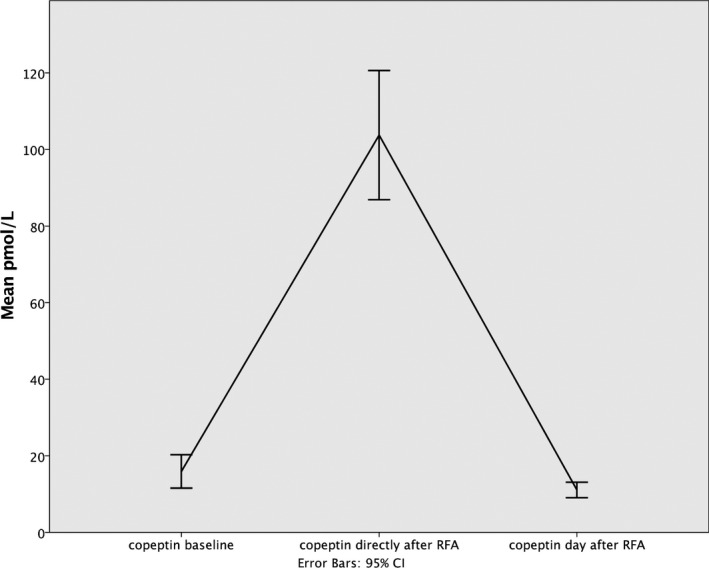
Changes in concentration of copeptin after radiofrequency ablation. RFA indicates radio frequency ablation.
The MR‐proADM level decreased when measured immediately after RFA (F=80.9; P<0.001), but increased significantly on the day after RFA (F=34.9; P<0.001; Table 4; Figure 5). These changes occurred regardless of the actual rhythm. The MR‐proADM level in participants with EF <50% reacted differently compared to those with EF >50% (F=4.6; P=0.02). Participants with EF <50% showed a greater decrease in their MR‐proADM level (F=6.2; P=0.014), immediately after the RFA procedure.
Figure 5.
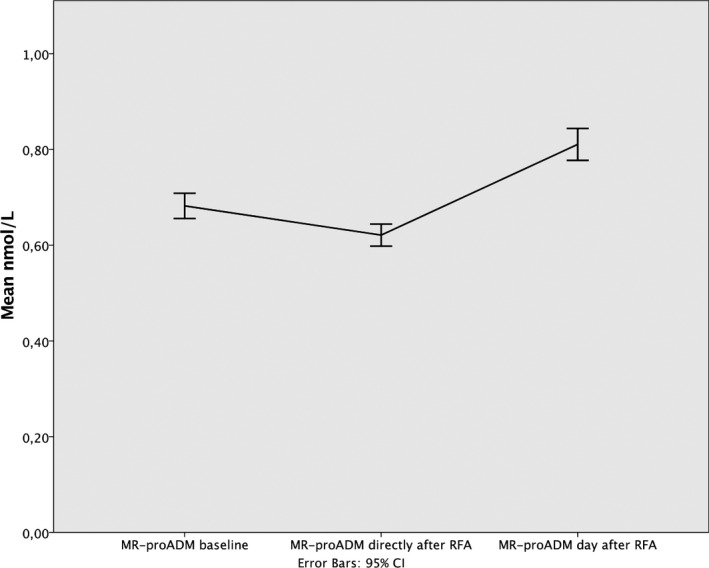
Changes in concentration of MR‐proADM after radiofrequency ablation. CI indicates confidence interval; MR‐proADM, midregional portion of proadrenomedullin; RFA, radiofrequency ablation.
Cardiac Production of Biomarkers
The MR‐proADM level in peripheral venous blood was higher compared to the level both in CS (F=11.8; P<0.001) and LA (F=49.4; P<0.001). No such difference was found in copeptin (F=0.83; P=0.402; Table 5).
Table 5.
Biomarker Levels in Different Sites
| Biomarkers N=168 | Femoral Vein | 95% CI | CS | 95% CI | LA | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| NT‐proBNP, pg/mL | 177.3 | 147.4 to 213.4 | 230.8 | 192.5 to 276.9 | 171.9 | 142.8 to 207 | <0.001 |
| MR‐proANP, pmol/L | 128.7 | 118.6 to 139.6 | 184.7 | 170.3 to 200.4 | 132 | 121.7 to 143.2 | <0.001 |
| Copeptin, pmol/L | 8.59 | 7.46 to 9.88 | 8.56 | 7.39 to 9.91 | 8.39 | 7.24 to 9.73 | 0.402 |
| MR‐proADM, nmol/L | 0.676 | 0.648 to 0.704 | 0.646 | 0.619 to 0.674 | 0.623 | 0.594 to 0.639 | <0.001 |
Data are presented as geometric means with 95% CI, but MR‐proADM is presented as mean with 95% CI. Analyses were performed with repeated‐measurements ANOVA and were adjusted for covariates: BMI >30, GFR <60 mL/min per 1.73 m2, EF <50%, age >65 years, heart rate >100/min, E/E′, and sex. The potential statistical significance of those analyses is presented with P values. BMI indicates body mass index; EF, ejection fraction; GFR, glomerular filtration rate; MR‐proADM, midregional portion of proadrenomedullin; MR‐proANP, midregional fragment of the N‐terminal precursor of atrial natriuretic peptide; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
NT‐proBNP and the MR‐proANP levels differed significantly between the 3 different blood sampling sites (F=35.9; P<0.001 and F=33.2; P<0.001, respectively). Levels of both NT‐proBNP and MR‐proANP were higher in CS compared to peripheral venous blood (F=38; P<0.001/F=48.1; P<0.001), but no such difference was found between peripheral venous blood and LA (F=2.8; P<0.097/F=0.27; P<0.603, respectively; Table 5). Neither the actual rhythm nor the EF level had any influence on this measurement.
Correlations Between Biomarker Levels
The NT‐proBNP level correlated significantly with the MR‐proANP level at each sampling site (peripheral blood: R=0.788; P<0.001; CS: R=0.795; P<0.001; LA: R=0.801; P<0.001).
Furthermore, the copeptin level seemed to have a weak, but significant, correlation with the MR‐proADM level in peripheral venous blood (R=0.2; P<0.001).
Discussion
In this study, we examined the levels of 2 peptides with cardiac origin (the 2 natriuretic peptides) and 2 peptides with extracardiac origin (copeptin, MR‐proADM) at 3 different sites (peripheral vein, CS, and LA) and their reaction to RFA. The main findings were (1) an immediate impact of RFA on all 4 biomarkers and (2) no cardiac release of MR‐proADM or copeptin.
Although some contribution of myocyte injury on the effect on biomarkers cannot be excluded, myocyte injury was not the major cause of this immediate impact. We studied high‐sensitivity troponin T (hsTropT) in a small number of patients in our cohort (32 patients) and found no correlation between hsTropT levels after RFA and the reaction of NT‐proBNP, MR‐proANP, copeptin, or MR‐proADM after RFA. Furthermore, concerning the NT‐proBNP reaction, participants with different rhythms reacted differently, although all were ablated.
RFA Effect on Biomarkers
Cardiac biomarkers
RFA effect on NT‐proBNP
Interestingly, a decrease in NT‐proBNP was observed after the RFA procedure in participants in AF, who cardioverted or were cardioverted to SR during the procedure; in contrast, there was a slight increase in the level in participants in SR. The major cause of production and secretion of BNP is the increase in myocardial wall tension.28 In addition, BNP increases after RFA in patients with supraventricular tachycardias (SVTs), probably attributed to myocyte injury.29 Moreover, a previous study on patients with lone fibrillation reported higher levels of NT‐proBNP, compared to healthy controls,6 whereas restoration of SR by cardioversion led to a decreased BNP level.30 Thus, several stimuli can increase the BNP level: one is myocyte injury29 and another is volume overload during the RFA procedure,31 whereas the restoration of SR can reduce the BNP level, probably attributed to reduced ventricular rate and changes in atrial and ventricular wall tension. In the present study, participants in AF showed a significant decrease in NT‐proBNP on the day after RFA, which was probably secondary to the restoration of SR leading to reduced wall tension. On the other hand, participants in SR showed an increase in NT‐proBNP on the day after RFA, which was probably secondary to myocardial injury and volume overload. Our reported NT‐proBNP reaction concurred with 2 previous studies reporting similar BNP reactions post‐RFA, 1 by Sacher et al.32 on participants with persistent AF, and a recent study by Pillarisetti et al.33 However, Gould et al.34 reported a decrease in BNP level postablation in 20 participants (18 in SR, 2 in AF). This conflicting result might be explained by the fact that all selected participants had paroxysmal AF, and that the blood samples were retrieved immediately after RFA. Moreover, all RFA procedures were performed under general anesthesia in that study.34
RFA effect on MR‐proANP
We also observed an increase in MR‐proANP immediately after RFA, followed by a decrease on the day after ablation. ProANP is known to be stored in secretory granules in atrial myocytes, and later to split into ANP and NT‐proANP fragments upon secretion. This process is secondary to increased wall tension.5, 35 The observed increase in MR‐proANP can be explained by myocardial injury and volume overload during the RFA procedure, leading to an immediate release of the granule and thus secretion of MR‐proANP. The reduction in MR‐proANP the day after the RFA procedure can be explained by depletion of MR‐proANP in the atrial myocyte granule after the initial secretion during RFA, and is also the result of the cardioversion to SR resulting in less wall tension, and thus stimulates the secretion of the biomarker.
Extracardiac biomarkers
RFA effect on copeptin
Copeptin level increased immediately after RFA. RFA is known to cause myocardial damage and necrosis with considerable troponin elevation.36 Copeptin level increases acutely in response to an acute myocardial infarction (AMI)11 and stabilizes on a plateau for 3 to 5 days, and then returns to normal unless HF develops, which then causes the level to rise and remain high.37 It is also known that the copeptin concentration changes in response to changes in plasma osmolality38 and in response to the relatively large fluid volume administered during an RFA procedure performed with open‐tip irrigation, as shown by Seiler et al.31 Furthermore, AVP and copeptin contribute to the complex stimulation of the humoral stress response, resulting in adrenocorticotropic and cortisol release.39 Thus, the fast increase in copeptin concentration can be explained by myocardial injury, volume overload, and endocrine stress response to the RFA procedure.
Our findings are in agreement with those of Jochberger et al., who reported a significant decrease in copeptin in patients undergoing noncardiac surgery without systematic inflammatory response syndrome (SIRS) compared to those with SIRS on the second postoperative day compared to the first postoperative day. This indicated an association between the postoperative copeptin response and the severity of systemic inflammation and cardiovascular failure.40
RFA effect on MR‐proADM
MR‐proADM level in our cohort decreased immediately after RFA and increased on the day after RFA compared to the baseline measurement. Previous studies have shown that ADM synthesis and secretion are stimulated by several hormones and cytokines,41 and the level is elevated in patients with sepsis and is associated with short‐term mortality.42 Ventricular‐derived, and not atrial, ADM production is activated in patients with HF.43 In patients with AMI, ADM levels increased44 and seemed to have cardioprotective actions against the increased excessive vasoconstrictors in AMI. The slight decrease in the MR‐proADM level in our study can possibly be explained by the administration of benzodiazepines and propofol during RFA, which attenuated the production of ADM.45, 46 The consecutive increase in MR‐proADM on the day after RFA was regarded as the main effect of RFA on MR‐proADM. This increase can possibly be attributed to the triggered inflammatory process and the volume overload.
Cardiac production of biomarkers
Another important point is that no cardiac release of copeptin or MR‐proADM was observed in our cohort.
As in previous studies, copeptin and MR‐proADM were not studied extensively in patients with AF. We compared the levels of copeptin and MR‐proADM in participants with lone AF in our cohort to the levels in a healthy population between the ages of 55 and 64, described by Morgenthaler et al.18, 47 We found higher MR‐proADM levels in the lone AF population, compared to the healthy population described by Morgenthaler.
In contrast, Jougasaki et al.,48 in a previous study on patients with a mean EF of 33%, reported that ADM levels were higher in CS compared to the aorta, which we were unable to confirm in our study. However, our tested population consisted of patients with EF between 35% and 50%, thus comprising a population with a lesser degree of myocardial impairment.
Regarding NT‐proBNP and MR‐proANP levels in different locations, previous studies have established that BNP and ANP are produced in the heart, and thus higher levels of these peptides can be measured in CS compared to peripheral blood, even in patients with normal systolic function, which the present study could also confirm.28, 35 We observed that the participants in our cohort had 31% higher levels of NT‐proBNP and 39% higher levels of MR‐proANP in CS compared to the measurement in LA.
Correlations between biomarkers
The strong correlation between MR‐proANP and NT‐proBNP is not surprising given that cardiac ANP and BNP gene expression is always regulated synchronously as the disease progresses. This means that the increased plasma concentration of 1 peptide is followed by increased concentration of the other.5
Clinical and investigational importance
To the best of our knowledge, there is no previous study providing such a comprehensive picture of different neurohormonal systems and the hemodynamical changes demonstrated in the same study population, evaluated at the same time. Furthermore, it is of clinical importance to recognize that RFA causes an immediate response in the different biomarkers evaluated in our study attributed to myocyte injury, volume overload, and stress, so the use of them for clinical reasons directly after an RFA should proceed with caution. On the other hand, these responses seem to be transient, as reflected by the copeptin and MR‐proANP reaction, and of lesser importance compared to the hemodynamic changes of the AF conversion, as shown by NT‐proBNP reaction. In addition, our study also showed the importance of being aware of the patient's actual rhythm when evaluating NT‐proBNP levels. Patients with AF had higher NT‐proBNP levels compared to patients with SR, and restoration of SR led to a rapid restoration of NT‐proBNP levels not only in patients with persistent or longstanding persistent AF, but also for the whole AF population eligible for RFA.
Limitations
Our study is a single‐center observational cohort study with a moderate sample size and no control group. Our sample consisted of a relatively heterogenic group of participants, including those with both paroxysmal and persistent AF, participants with normal or reduced EF, presenting in either SR or AF. Furthermore, we observed the same sex imbalance, as observed in previous studies, concerning RFA of AF.32, 49 We only examined the levels of these biomarkers in intracardiac blood samples at the end of the RFA procedure and the day after the procedure. Additional analyses on the second day and after 1 week might have provided us with further insights into their production; however, this was not possible in the clinical setting we had.
Conclusion
RFA is a strong stimulus with a significant and direct impact on different neurohormonal systems. The level of NT‐proBNP after RFA depended on the patient's actual rhythm, that is, the level decreased in patients with AF whereas it increased in patients with SR. On the other hand, the levels of MR‐proANP, copeptin, and MR‐proADM increased regardless of the current rhythm. No sign of a cardiac release of copeptin or MR‐proADM was found. This study provides new data that needs to be evaluated through further research regarding the mechanisms of the biomarker balance and its effects on the body and should also be assessed for potential clinical use in patients with AF.
Sources of Funding
The SMURF study is supported by grants from ALF grants (County Council of Östergötland), the Carldavid Jönsson Research Foundation, the Heart Foundation, Linköping University, and by unrestricted grants from Biosense Webster, Johnson & Johnson.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003557 doi: 10.1161/JAHA.116.003557)
References
- 1. Friberg L, Bergfeldt L. Atrial fibrillation prevalence revisited. J Intern Med. 2013;274:461–468. [DOI] [PubMed] [Google Scholar]
- 2. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 3. Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, Grover P, Singh V, Vallurupalli S, Savani GT, Badheka A, Tuliani T, Dabhadkar K, Dibu G, Reddy YM, Sewani A, Kowalski M, Mitrani R, Paydak H, Viles‐Gonzalez JF. In‐hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128:2104–2112. [DOI] [PubMed] [Google Scholar]
- 4. Flynn TG, de Bold ML, de Bold AJ. The amino acid sequence of an atrial peptide with potent diuretic and natriuretic properties. Biochem Biophys Res Commun. 1983;117:859–865. [DOI] [PubMed] [Google Scholar]
- 5. Goetze JP, Friis‐Hansen L, Rehfeld JF, Nilsson B, Svendsen JH. Atrial secretion of B‐type natriuretic peptide. Eur Heart J. 2006;27:1648–1650. [DOI] [PubMed] [Google Scholar]
- 6. Ellinor PT, Low AF, Patton KK, Shea MA, Macrae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol. 2005;45:82–86. [DOI] [PubMed] [Google Scholar]
- 7. Rossi A, Enriquez‐Sarano M, Burnett JC Jr, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler‐echocardiographic study. J Am Coll Cardiol. 2000;35:1256–1262. [DOI] [PubMed] [Google Scholar]
- 8. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro‐atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. [DOI] [PubMed] [Google Scholar]
- 10. Meune C, Vermillet A, Wahbi K, Guerin S, Aelion H, Weber S, Chenevier‐Gobeaux C. Mid‐regional pro atrial natriuretic peptide allows the accurate identification of patients with atrial fibrillation of short time of onset: a pilot study. Clin Biochem. 2011;44:1315–1319. [DOI] [PubMed] [Google Scholar]
- 11. Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O, Bergmann A, Squire I, van Veldhuisen DJ, Dickstein K. C‐terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J. 2009;30:1187–1194. [DOI] [PubMed] [Google Scholar]
- 12. Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol. 2005;95:8B–13B. [DOI] [PubMed] [Google Scholar]
- 13. Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension. 1983;5:I129–I138. [DOI] [PubMed] [Google Scholar]
- 14. Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160–1166. [DOI] [PubMed] [Google Scholar]
- 15. Isumi Y, Shoji H, Sugo S, Tochimoto T, Yoshioka M, Kangawa K, Matsuo H, Minamino N. Regulation of adrenomedullin production in rat endothelial cells. Endocrinology. 1998;139:838–846. [DOI] [PubMed] [Google Scholar]
- 16. Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor‐alpha. Biochem Biophys Res Commun. 1994;203:719–726. [DOI] [PubMed] [Google Scholar]
- 17. Chun TH, Itoh H, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Doi K, Inoue M, Masatsugu K, Korenaga R, Ando J, Nakao K. Shear stress augments expression of C‐type natriuretic peptide and adrenomedullin. Hypertension. 1997;29:1296–1302. [DOI] [PubMed] [Google Scholar]
- 18. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. [DOI] [PubMed] [Google Scholar]
- 19. Schnabel RB, Wild PS, Wilde S, Ojeda FM, Schulz A, Zeller T, Sinning CR, Kunde J, Lackner KJ, Munzel T, Blankenberg S. Multiple biomarkers and atrial fibrillation in the general population. PLoS ONE. 2014;9:e112486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parwani AS, von Haehling S, Kolodziejski AI, Huemer M, Wutzler A, Attanasio P, Stojakovic T, Scharnagl H, Haverkamp W, Boldt LH. Mid‐regional proadrenomedullin levels predict recurrence of atrial fibrillation after catheter ablation. Int J Cardiol. 2014;180C:129–133. [DOI] [PubMed] [Google Scholar]
- 21. Charitakis E, Walfridsson U, Nystrom F, Nylander E, Stromberg A, Alehagen U, Walfridsson H. Symptom burden, Metabolic profile, Ultrasound findings, Rhythm, neurohormonal activation, haemodynamics and health‐related quality of life in patients with atrial Fibrillation (SMURF): a protocol for an observational study with a randomised interventional component. BMJ Open. 2015;5:e008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 23. Rickham PP. Human experimentation. Code of ethics of the World medical association. Declaration of Helsinki. Br Med J 1964;2:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. [DOI] [PubMed] [Google Scholar]
- 25. Grubb A, Nyman U, Bjork J, Lindstrom V, Rippe B, Sterner G, Christensson A. Simple cystatin C‐based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan‐Barratt prediction equations for children. Clin Chem. 2005;51:1420–1431. [DOI] [PubMed] [Google Scholar]
- 26. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate ‐ a practical and powerful approach to multiple testing. J Roy Stat Soc B Methodol. 1995;57:289–300. [Google Scholar]
- 28. Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, Nakao K. Localization and mechanism of secretion of B‐type natriuretic peptide in comparison with those of A‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. [DOI] [PubMed] [Google Scholar]
- 29. Chen L, Wei T, Zeng C, Chen Q, Shi Z, Wang L. Effect of radiofrequency catheter ablation on plasma B‐type natriuretic peptide. Pacing Clin Electrophysiol. 2005;28:200–204. [DOI] [PubMed] [Google Scholar]
- 30. Wozakowska‐Kaplon B. Effect of sinus rhythm restoration on plasma brain natriuretic peptide in patients with atrial fibrillation. Am J Cardiol. 2004;93:1555–1558. [DOI] [PubMed] [Google Scholar]
- 31. Seiler J, Steven D, Roberts‐Thomson KC, Inada K, Tedrow UB, Michaud GF, Stevenson WG. The effect of open‐irrigated radiofrequency catheter ablation of atrial fibrillation on left atrial pressure and B‐type natriuretic peptide. Pacing Clin Electrophysiol. 2014;37:616–623. [DOI] [PubMed] [Google Scholar]
- 32. Sacher F, Corcuff JB, Schraub P, Le Bouffos V, Georges A, Jones SO, Lafitte S, Bordachar P, Hocini M, Clementy J, Haissaguerre M, Bordenave L, Roudaut R, Jais P. Chronic atrial fibrillation ablation impact on endocrine and mechanical cardiac functions. Eur Heart J. 2008;29:1290–1295. [DOI] [PubMed] [Google Scholar]
- 33. Pillarisetti J, Reddy N, Biria M, Ryschon K, Nagarajan D, Murray C, Atkins D, Bommana S, Reddy MY, DiBiase L, Pimentel R, Berenbom L, Dawn B, Natale A, Lakkireddy D. Elevated brain natriuretic peptide level in patients undergoing atrial fibrillation ablation: is it a predictor of failed ablation or a mere function of atrial rhythm and rate at a point in time? J Interv Card Electrophysiol. 2014;40:161–168. [DOI] [PubMed] [Google Scholar]
- 34. Gould PA, Gula LJ, Bhayana V, Subbiah RN, Bentley C, Yee R, Klein GJ, Krahn AD, Skanes AC. Characterization of cardiac brain natriuretic peptide release in patients with paroxysmal atrial fibrillation undergoing left atrial ablation. Circ Arrhythm Electrophysiol. 2010;3:18–23. [DOI] [PubMed] [Google Scholar]
- 35. Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, Kambayashi Y, Inouye K, Imura H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haegeli LM, Kotschet E, Byrne J, Adam DC, Lockwood EE, Leather RA, Sterns LD, Novak PG. Cardiac injury after percutaneous catheter ablation for atrial fibrillation. Europace. 2008;10:273–275. [DOI] [PubMed] [Google Scholar]
- 37. Khan SQ, Dhillon OS, O'Brien RJ, Struck J, Quinn PA, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL. C‐terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation. 2007;115:2103–2110. [DOI] [PubMed] [Google Scholar]
- 38. Szinnai G, Morgenthaler NG, Berneis K, Struck J, Muller B, Keller U, Christ‐Crain M. Changes in plasma copeptin, the c‐terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metabol. 2007;92:3973–3978. [DOI] [PubMed] [Google Scholar]
- 39. Rivier C, Vale W. Modulation of stress‐induced ACTH release by corticotropin‐releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–327. [DOI] [PubMed] [Google Scholar]
- 40. Jochberger S, Dorler J, Luckner G, Mayr VD, Wenzel V, Ulmer H, Morgenthaler NG, Hasibeder WR, Dunser MW. The vasopressin and copeptin response to infection, severe sepsis, and septic shock. Crit Care Med. 2009;37:476–482. [DOI] [PubMed] [Google Scholar]
- 41. Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. [DOI] [PubMed] [Google Scholar]
- 42. Marino R, Struck J, Maisel AS, Magrini L, Bergmann A, Somma S. Plasma adrenomedullin is associated with short‐term mortality and vasopressor requirement in patients admitted with sepsis. Crit Care. 2014;18:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jougasaki M, Grantham JA, Redfield MM, Burnett JC Jr. Regulation of cardiac adrenomedullin in heart failure. Peptides. 2001;22:1841–1850. [DOI] [PubMed] [Google Scholar]
- 44. Kobayashi K, Kitamura K, Hirayama N, Date H, Kashiwagi T, Ikushima I, Hanada Y, Nagatomo Y, Takenaga M, Ishikawa T, Imamura T, Koiwaya Y, Eto T. Increased plasma adrenomedullin in acute myocardial infarction. Am Heart J. 1996;131:676–680. [DOI] [PubMed] [Google Scholar]
- 45. Korbonits M, Trainer PJ, Edwards R, Besser GM, Grossman AB. Benzodiazepines attenuate the pituitary‐adrenal responses to corticotrophin‐releasing hormone in healthy volunteers, but not in patients with Cushing's syndrome. Clin Endocrinol (Oxf). 1995;43:29–35. [DOI] [PubMed] [Google Scholar]
- 46. Hayashi Y, Minamino N, Isumi Y, Kangawa K, Kuro M, Matsuo H. Effects of thiopental, ketamine, etomidate, propofol and midazolam on the production of adrenomedullin and endothelin‐1 in vascular smooth muscle cells. Res Commun Mol Pathol Pharmacol. 1999;103:325–331. [PubMed] [Google Scholar]
- 47. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. [DOI] [PubMed] [Google Scholar]
- 48. Jougasaki M, Rodeheffer RJ, Redfield MM, Yamamoto K, Wei CM, McKinley LJ, Burnett JC Jr. Cardiac secretion of adrenomedullin in human heart failure. J Clin Invest. 1996;97:2370–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, Vardas P, Laroche C, Anselme F, Inama G, Jais P, Kalarus Z, Kautzner J, Lewalter T, Mairesse GH, Perez‐Villacastin J, Riahi S, Taborsky M, Theodorakis G, Trines SA. Atrial Fibrillation Ablation Pilot Study I. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J. 2014;35:1466–1478. [DOI] [PubMed] [Google Scholar]


