Abstract
Background
Physical activity (PA) has an established favorable impact on cardiovascular disease (CVD) outcomes and quality of life. In this study, we aimed to estimate the economic effect of moderate‐vigorous PA on medical expenditures and utilization from a nationally representative cohort with and without CVD.
Methods and Results
The 2012 Medical Expenditure Panel Survey data were analyzed. Our study population was limited to noninstitutionalized US adults ≥18 years of age. Variables of interest included CVD (coronary artery disease, stroke, heart failure, dysrhythmias, or peripheral artery disease) and cardiovascular modifiable risk factors (CRFs; hypertension, diabetes mellitus, hypercholesterolemia, smoking, and/or obesity). Two‐part econometric models were utilized to study cost data; a generalized linear model with gamma distribution and link log was used to assess expenditures per capita. The final study sample included 26 239 surveyed individuals. Overall, 47% engaged in moderate‐vigorous PA ≥30 minutes, ≥5 days/week, translating to 111.5 million adults in the United States stratifying by CVD status; 32% reported moderate‐vigorous PA among those with CVD versus 49% without CVD. Generally, participants reporting moderate‐vigorous PA incurred significantly lower health care expenditures and resource utilization, displaying a step‐wise lower total annual health care expenditure as moving from CVD to non‐CVD (and each CRF category).
Conclusions
Moderate‐vigorous PA ≥30 minutes, ≥5 days/week is associated with significantly lower health care spending and resource utilization among individuals with and without established CVD.
Keywords: cardiovascular disease, cost, exercise, risk factors
Subject Categories: Exercise, Lifestyle, Cardiovascular Disease, Risk Factors
Cardiovascular disease (CVD) continues to be the leading cause of mortality and disability world‐wide.1 One of the most important cardiovascular modifiable risk factors (CRFs) is lack of physical activity (PA), including prominently the lack of intentional moderate‐vigorous PA. It has been documented that 150 minutes per week of moderate‐vigorous physical activity is sufficient to observe a reduction in all‐cause mortality risks,2, 3 aside from also aiding in morbidity,4 and overall decrease in CVD risk.5 In spite of the benefits of PA, and its widely reported positive impact on most CRFs,4, 6 its prevalence is well below desired levels, despite numerous interventions to increase PA.7, 8, 9, 10, 11 In addition, many previous studies have sought to describe the economic impact of PA on health status,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 but these either exclusively studied older populations and/or evaluated health‐related charges (rather than expenditures). In addition, no nation‐wide economic impact of PA has been reported in recent years.
In this study, we aimed to describe the impact of moderate‐vigorous PA on health care expenditure and resource utilization across the spectrum of CVD status and CRF profiles from a sample generalizable to the entire US population, and hypothesized that health care expenditures and utilization were lower among individuals who engaged in moderate‐vigorous PA.
Methods
Study Design and Sampling
This retrospective study used data from the Medical Expenditure Panel Survey (MEPS), 2012. The MEPS, led by the Agency for Healthcare Research and Quality, is a set of large‐scale, national surveys about individuals and families and their medical providers and employers. The Household Component (HC) of the MEPS collects data about health services used, their frequency and cost, charges, source of payment, income, employment, as well as ample data on insurance used by and available to US workers.23 The MEPS respondents are enrolled for 2 years of data collection, with a new panel beginning each year. The sampling frame for the MEPS‐HC is drawn from respondents to the National Health Interview Survey, and the design of the MEPS‐HC survey includes sampling weights, stratification, and clustering. The MEPS sampling weights incorporate adjustment for the complex sample design and reflect survey nonresponse and population totals from the Current Population Survey.23
Of all files in the HC of the survey, we used the full‐year consolidated data files and medical conditions files. The full‐year consolidated data files contain most demographics at a person level, including information on resource utilization and costs, whereas the medical conditions files include each diagnosis a person has, which, after being transcribed verbatim at each survey, are translated into International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) by professional coders. These files were linked together in order to determine accurate results for each individual. Because MEPS is comprised of publicly available, de‐identified data files, this study was exempt of institutional review board approval.24
Participants
The initial sample consisted of 38 974 surveyed individuals. We limited our study population to noninstitutionalized US adults ≥18 years of age (excluding 11 154 individuals <18 years of age). Individuals with body mass index (BMI) <18.5 kg/m2 (532) were further excluded from the sample given that they often represent a sicker patient population. Because of the subject matter of this study, individuals who, for the entire survey period, were either completely unable to walk or unable to walk up 10 steps (130) or pregnant at any point during the survey (919) were also excluded from all analyses.
Study Variables
Physical activity
Individuals in the study sample were classified into PA based on their answers to the question, “Do you currently spend half hour or more in moderate to vigorous physical activity, at least five times a week?” The 2010 MEPS glossary (valid for all subsequent surveys) states, “moderate physical activity causes only light sweating or a slight or moderate increase in breathing or heart rate and would include activities such as fast walking, raking leaves, mowing the lawn, or heavy cleaning. Vigorous physical activity causes heavy sweating or large increases in breathing or heart rate and would include activities such as running, race walking, lap swimming, aerobic classes, or fast bicycling.”25 Participants who answered “yes” to this question were categorized as “optimal PA,” and “nonoptimal PA” otherwise. An important point to be made is that most respondents who engaged in this level of PA closely resemble what most researchers refer to as “exercise.” Consequently, our categorization of nonoptimal PA individuals does not necessarily mean “sedentary,” but that the 2008 Physical Activity Guidelines Advisory Committee Report recommendations for PA were not met.4
Cardiovascular disease and average cardiovascular modifiable risk factor profile
Individuals in the study sample that had a diagnosis of coronary artery disease, stroke, heart failure, dysrhythmias, and/or peripheral artery disease (ascertained by ICD‐9‐CM codes: 410, 413, 414, 433–437, 427, 428, 440, 443, and 447, respectively) were classified as having diagnosed CVD. CRFs were ascertained using self‐reported questionnaires in the MEPS‐HC survey, where individuals with presence of 1 or more of: hypertension, diabetes mellitus, hypercholesterolemia, smoking, and/or obesity (BMI ≥30 kg/m2, a constructed variable using self‐reported weight and height) were included. Based on the presence of these individual risk factors, survey participants were profiled as “poor” (≥3 cardiovascular risk factors), “average” (2 cardiovascular risk factors), or “optimal” (0–1 cardiovascular risk factors).
Expenditures and resource utilization
Total annual direct medical expenditures were calculated for each person. Data for the calculation of this variable included expenditures from all payer groups and out‐of‐pocket spending, including information from hospitalizations, prescribed medications, outpatient visits (hospital outpatient visits and office‐based visits), emergency department (ED) visits, and other expenditures (dental visits, vision aid, home health care, and other medical supplies). In a similar fashion, resource utilization analysis assessed the total number of outpatient and ED visits, number of hospitalizations, and number of prescription medications’ purchases/refills each surveyed individual incurred.
Covariates
Other variables included in the study were age, sex, health status, family income, race/ethnicity, employment, metropolitan statistical area, insurance type, education, geographical region, and modified Charlson Comorbidity Index (without cardiovascular components). Categorical variables were classified as follows: 5 categories were used for age (18–39, 40–54, 55–64, 65–74, and ≥75); 3 categories for family income (poor/near poor [<125% of the 2012 federal poverty level], low/middle income [125% to <400% federal poverty level], and high income [≥400% federal poverty level]); 5 categories for race/ethnicity (non‐Hispanic white, non‐Hispanic black, non‐Hispanic Asian, non‐Hispanic other, and Hispanic); 3 categories for insurance type (private, public only, and uninsured); 5 categories for education (less than high school, high school diploma, some college, college [bachelor's degree] and masters, doctorate, or professional); 4 categories for geographical region (Northeast, Midwest, South, and West); and 3 categories for modified Charlson Comorbidity Index (0, 1, and ≥2).
Statistical Analysis
For comparison of demographic characteristics in our sample, chi‐square tests were performed.26 Because of the right‐skewedness of expenditures data (ie, most expenditures are seen in only a small proportion of the population), two‐part models were utilized to study expenditures.27 Two‐part models are often used to model health care expenditures, and are the product of: (1) the probability that any given individual had any expenditures and (2) their mean expenditures.28 The first part of the model consists on a probabilistic regression model (probit), which estimates the probability of zero versus positive expenditures. Contingent upon having a positive annual health care expenditure, a generalized linear model (glm) with gamma distribution and a logarithmic‐link function estimates the average expenditure per capita28, 29; we determined the distribution of the glm using the modified Park Test.30 For resource utilization, unadjusted and adjusted logistic regression models were utilized. Unadjusted means and proportions were calculated, adjusting for the survey design and sampling weight. For multivariate analyses, variable selection for inclusion into the model was determined using a combination of the Akaike information criterion and their relevance toward cost analysis. Collinearity was assessed using the variance inflated factor. For all statistical analyses, P<0.05 was considered statistically significant. All analyses were carried out using Stata software (version 13.1; StataCorp LP, College Station, TX). Total and marginal expenditures were estimated using the “margins” command after the two‐part models.28 All analyses took into consideration the MEPS complex survey design.
Results
Population Characteristics
The final study population consisted of 26 239 participants ≥18 years of age (47.6±17 years; 51.5% female), which translates to ≈223.7 million US adults; demographic information is presented in Table 1. Overall, 1896 (9%) had a CVD diagnosis, representing 19.4 million of the noninstitutionalized adult population in the United States. Forty‐nine percent of those without CVD and 32% of those with CVD reported engaging in moderate‐vigorous PA. Irrespective of CVD status, participants with optimal PA were less likely to have underlying CVD risk factors, as well as reporting better health status, higher socioeconomic and education strata, and lower prevalence of comorbid conditions (Table 1).
Table 1.
Sample Characteristics from the Medical Expenditure Panel Survey 2012, Stratified by Physical Activity and CVD Status
| CVD | P Value | Non‐CVD | P Value | |||
|---|---|---|---|---|---|---|
| Nonoptimal PA | Optimal PA | Nonoptimal PA | Optimal PA | |||
| Sample, n | 1293 | 603 | 12 510 | 11 833 | ||
| Weighted sample | 12 539 605 | 6 822 007 | 99 586 391 | 104 714 281 | ||
| Age strata, y, n (%) | <0.001 | <0.001 | ||||
| 18–39 | 54 (4.2) | 36 (6.0) | 5016 (40.1) | 5642 (47.7) | ||
| 40–54 | 184 (14.2) | 101 (16.7) | 3720 (29.7) | 3304 (27.9) | ||
| 55–64 | 289 (22.4) | 146 (24.2) | 1993 (15.9) | 1671 (14.1) | ||
| 65–74 | 334 (25.8) | 185 (30.7) | 1094 (8.7) | 844 (7.1) | ||
| ≥75 | 432 (33.4) | 135 (22.4) | 687 (5.5) | 372 (3.1) | ||
| Sex, n (%) | <0.001 | <0.001 | ||||
| Female | 656 (50.7) | 248 (41.1) | 7127 (57.0) | 5479 (46.3) | ||
| Male | 637 (49.3) | 355 (58.9) | 5383 (43.0) | 6354 (53.7) | ||
| Hypertension, n (%) | <0.001 | <0.001 | ||||
| No | 240 (18.6) | 161 (26.7) | 8425 (67.3) | 8952 (75.7) | ||
| Yes | 1053 (81.4) | 442 (73.3) | 4085 (32.7) | 2881 (24.3) | ||
| Diabetes mellitus, n (%) | <0.001 | <0.001 | ||||
| No | 855 (66.1) | 446 (74.0) | 11 243 (89.9) | 11 079 (93.6) | ||
| Yes | 438 (33.9) | 157 (26.0) | 1267 (10.1) | 754 (6.4) | ||
| Hypercholesterolemia, n (%) | 0.02 | <0.001 | ||||
| No | 362 (28.0) | 200 (33.2) | 9039 (72.3) | 9314 (78.7) | ||
| Yes | 931 (72.0) | 403 (66.8) | 3471 (27.7) | 2519 (21.3) | ||
| Current smoker, n (%) | 0.12 | 0.11 | ||||
| No | 1074 (83.1) | 518 (85.9) | 10 611 (84.8) | 9949 (84.1) | ||
| Yes | 219 (16.9) | 85 (14.1) | 1899 (15.2) | 1884 (15.9) | ||
| Obese, n (%) | <0.001 | <0.001 | ||||
| No | 715 (55.3) | 399 (66.2) | 8142 (65.1) | 8951 (75.6) | ||
| Yes | 578 (44.7) | 204 (33.8) | 4368 (34.9) | 2882 (24.4) | ||
| CRF profilea, n (%) | <0.001 | <0.001 | ||||
| Optimal | 263 (20.3) | 182 (30.2) | 8164 (65.3) | 8913 (75.3) | ||
| Average | 366 (28.3) | 181 (30.0) | 2414 (19.3) | 1823 (15.4) | ||
| Poor | 664 (51.4) | 240 (39.8) | 1932 (15.4) | 1097 (9.3) | ||
| Health status, n (%) | <0.001 | <0.001 | ||||
| Excellent | 44 (3.4) | 44 (7.3) | 2059 (16.5) | 3134 (26.5) | ||
| Very good | 198 (15.3) | 190 (31.5) | 4603 (36.8) | 5186 (43.8) | ||
| Good | 470 (36.3) | 239 (39.6) | 4068 (32.5) | 2786 (23.5) | ||
| Fair | 422 (32.6) | 111 (18.4) | 1436 (11.5) | 630 (5.3) | ||
| Poor | 159 (12.3) | 19 (3.2) | 268 (2.1) | 61 (0.5) | ||
| Family income, n (%) | <0.001 | <0.001 | ||||
| Poor/near poor | 417 (32.3) | 133 (22.1) | 3291 (26.3) | 2585 (21.8) | ||
| Low/middle income | 579 (44.8) | 264 (43.8) | 5898 (47.1) | 5525 (46.7) | ||
| High income | 297 (23.0) | 206 (34.2) | 3321 (26.5) | 3723 (31.5) | ||
| Race/ethnicity, n (%) | 0.06 | <0.001 | ||||
| Non‐Hispanic White | 748 (57.8) | 375 (62.2) | 4732 (37.8) | 5060 (42.8) | ||
| Non‐Hispanic Black | 276 (21.3) | 105 (17.4) | 2570 (20.5) | 2357 (19.9) | ||
| Non‐Hispanic Asian | 45 (3.5) | 31 (5.1) | 1031 (8.2) | 857 (7.2) | ||
| Non‐Hispanic Other | 24 (1.9) | 13 (2.2) | 265 (2.1) | 273 (2.3) | ||
| Hispanic | 200 (15.5) | 79 (13.1) | 3912 (31.3) | 3286 (27.8) | ||
| Metropolitan area, n (%) | 0.25 | 0.97 | ||||
| No | 227 (17.6) | 93 (15.4) | 1424 (11.4) | 1345 (11.4) | ||
| Yes | 1066 (82.4) | 510 (84.6) | 11 086 (88.6) | 10 488 (88.6) | ||
| Insurance status, n (%) | <0.001 | <0.001 | ||||
| Private | 582 (45.0) | 359 (59.5) | 6608 (52.8) | 7046 (59.5) | ||
| Public only | 641 (49.6) | 204 (33.8) | 2737 (21.9) | 1908 (16.1) | ||
| Uninsured | 70 (5.4) | 40 (6.6) | 3165 (25.3) | 2879 (24.3) | ||
| Education, n (%) | <0.001 | <0.001 | ||||
| Less than high school | 356 (27.5) | 107 (17.7) | 2958 (23.6) | 2197 (18.6) | ||
| GED or high school diploma | 428 (33.1) | 202 (33.5) | 3645 (29.1) | 3502 (29.6) | ||
| Some college | 281 (21.7) | 124 (20.6) | 3025 (24.2) | 3246 (27.4) | ||
| College (bachelor's degree) | 139 (10.8) | 103 (17.1) | 1734 (13.9) | 1813 (15.3) | ||
| Masters, doctorate or professional | 74 (5.7) | 65 (10.8) | 891 (7.1) | 956 (8.1) | ||
| Region, n (%) | 0.75 | <0.001 | ||||
| Northeast | 233 (18.0) | 118 (19.6) | 2084 (16.7) | 1860 (15.7) | ||
| Midwest | 282 (21.8) | 133 (22.1) | 2200 (17.6) | 2232 (18.9) | ||
| South | 537 (41.5) | 250 (41.5) | 4820 (38.5) | 4295 (36.3) | ||
| West | 241 (18.6) | 102 (16.9) | 3406 (27.2) | 3446 (29.1) | ||
| Modified Charlson Comorbidity Indexb, n (%) | <0.001 | <0.001 | ||||
| 0 | 842 (65.1) | 454 (75.3) | 11 009 (88.0) | 10 791 (91.2) | ||
| 1 | 275 (21.3) | 93 (15.4) | 1053 (8.4) | 744 (6.3) | ||
| ≥2 | 176 (13.6) | 56 (9.3) | 448 (3.6) | 298 (2.5) | ||
CRF indicates cardiovascular risk factor; CVD, cardiovascular disease; GED, general education development; PA, physical activity.
Cardiovascular risk factors: hypertension, diabetes mellitus, hypercholesterolemia, obesity, and smoking.
Charlson Comorbidity Index without cardiovascular components.
Healthcare Expenditures
Univariate and multivariate models estimating average per capita health care expenditures are presented in Table 2. Presence of CVD was independently associated with higher health care expenditures when compared to individuals without CVD. Despite this, presence of optimal PA was associated with lower health care expenditures across CVD status and CRF spectrum. Among those without CVD, those with optimal CRF and PA had a mean annual expenditure of $2328 (95% CI, 1932, 2726), compared to $5475 (95% CI, 4668, 6283) of those with poor CRF and PA (Table 2, model 1). After adjusting for other covariates (demographics, socioeconomic status, insurance type, and comorbidities), these marked differences remained (Table 2, model 3). Moreover, when comparing the marginal expenditures of optimal PA versus nonoptimal PA, the impact of PA on health care expenditures was highest for those with established CVD, followed by participants without CVD but underlying poor CRF profile (Table 2).
Table 2.
Average Expenditures of Physical Activity Per Capita, by CVD Status and CRF Category
| Total | CVD | Non‐CVD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular Risk Factor Profile | ||||||||||
| Poor | Average | Optimal | ||||||||
| Expenditure | 95% CI | Expenditure | 95% CI | Expenditure | 95% CI | Expenditure | 95% CI | Expenditure | 95% CI | |
| Model 1 | ||||||||||
| Nonoptimal PA | $5397.61 | (5064, 5731) | $14 569.82 | (13 209, 15 931) | $7567.77 | (6700, 8436) | $5191.04 | (4568, 5814) | $3053.79 | (2713, 3394) |
| Optimal PA | $3443.45 | (3111, 3776) | $10 586.93 | (8508, 12 666) | $5475.72 | (4668, 6283) | $4476.12 | (3709, 5243) | $2328.69 | (1932, 2726) |
| Model 2 | ||||||||||
| Nonoptimal PA | $5012.54 | (4691, 5334) | $14 564.70 | (11 057, 18 072) | $6636.04 | (5755, 7517) | $4542.76 | (4000, 5086) | $3496.36 | (3048, 3945) |
| Optimal PA | $3787.56 | (3451, 4124) | $10 743.88 | (7347, 14 140) | $5173.92 | (4413, 5935) | $4003.99 | (3380, 4628) | $2847.15 | (2374, 3320) |
| Model 3 | ||||||||||
| Nonoptimal PA | $4867.82 | (4552, 5183) | $12 659.57 | (8992, 16 327) | $6339.80 | (5515, 7164) | $4577.91 | (4044, 5112) | $3733.97 | (3258, 4210) |
| Optimal PA | $4153.53 | (3821, 4486) | $10 092.42 | (6556, 13 629) | $5279.12 | (4477, 6081) | $4147.37 | (3552, 4743) | $3240.39 | (2765, 3716) |
Cardiovascular risk factors: hypertension, diabetes mellitus, hypercholesterolemia, obesity, and smoking. Model 1: unadjusted. Model 2: adjusted for age, sex, family income, and race/ethnicity. Model 3: adjusted for variables in model 2 plus insurance type, geographical region, and modified Charlson Comorbidity Index (Charlson Comorbidity Index without cardiovascular components). CRF indicates cardiovascular risk factor; CVD, cardiovascular disease; PA, physical activity.
Weighted and adjusted estimates by specific expenditure category (health care utilization expenditures: hospitalizations, prescription medications, ED visits, outpatient visits, and other) according to CRF profile and CVD status are presented in Figure 1. Among those with CVD, the highest expenditures pertained to hospitalizations (with expenditures of $5644 and $4233 for nonoptimal PA and optimal PA, respectively), followed by prescription medications and outpatient visits. Among those without CVD, the major part of costs among non‐CVD individuals was attributed to outpatient visits, with those with poor CRF spending an annual average of $2019 and $1918 (nonoptimal PA and optimal PA, respectively), compared to $1319 and $1215 of those with optimal CRF. The highest differences were noted in prescription medications, with those with a poor CRF profile and nonoptimal PA spending $400 more than their optimal PA counterparts. The lowest expenditures were noted among those without CVD, with optimal CRF profile and optimal PA, with an average of $810 on hospitalizations, $587 on prescription medications, and $1215 on outpatient visits (Figure 1).
Figure 1.
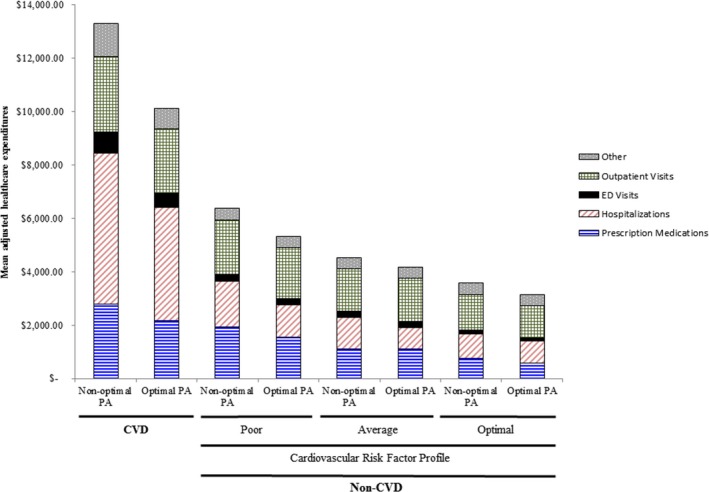
Weighted, adjusted mean health care expenditures by category, among optimal and nonoptimal PA, with and without CVD, further subclassified by CRF profile. CRF indicates cardiovascular risk factor; CVD, cardiovascular disease; ED, emergency department. Other=dental, vision aid, home healthcare, medical devices, others; PA, physical activity. Cardiovascular risk factors: hypertension, diabetes mellitus, hypercholesterolemia, obesity, and smoking. Non‐CVD: adjusted for age, sex, family income, race/ethnicity, insurance type, geographical region, and modified Charlson Comorbidity Index (without cardiovascular components). CVD: adjusted for “non‐CVD” covariates plus cardiovascular risk factors.
Healthcare Resource Utilization
Table 3 summarizes health care utilization rates across PA, CRF, and CVD categories. Overall, optimal PA was associated with significantly less use of health care resources. Among surveyed participants with CVD, those with optimal versus nonoptimal PA were less likely to have an ED visit (24% vs 31%) or any hospitalization (21% vs 27%). The lowest rate of ED visits (9.1%) and hospitalizations (2.6%) was noted among those reporting optimal PA without a CVD diagnosis and presence of optimal CRF profile. After adjusting for key covariates, among individuals without CVD, those with optimal PA had lower odds of being hospitalized (optimal CRF: odds ratio [OR], 0.75; 95% CI [0.60, 0.93]; average CRF: OR, 0.70; 95% CI [0.51, 0.95]; poor CRF: OR, 0.65; 95% CI [0.45, 0.93]), lower odds of having an outpatient visit (optimal CRF: OR, 0.88; 95% CI [0.80, 0.97], average CRF: OR, 0.67; 95% CI [0.55, 0.82], poor CRF: OR, 0.69; 95% CI [0.51, 0.94]), and lower odds of purchasing/refilling a prescription medication (optimal CRF: OR, 0.83; 95% CI [0.75, 0.92], average CRF: OR, 0.73; 95% CI [0.60, 0.89], poor CRF: OR, 0.65; 95% CI [0.48, 0.88]). Similar trends were noted for “high health care utilizers” (at or above the 75th percentile for usage at each particular healthcare resource; Figure 2A and 2B).
Table 3.
Health Care Resource Utilization Physical Activity Categories, by CVD Status and CRF Category
| CVD | Non‐CVD | |||||||
|---|---|---|---|---|---|---|---|---|
| Cardiovascular Risk Factor Profile | ||||||||
| Poor | Average | Optimal | ||||||
| Optimal PA | Nonoptimal PA | Optimal PA | Nonoptimal PA | Optimal PA | Nonoptimal PA | Optimal PA | Nonoptimal PA | |
| Health care utilization | ||||||||
| Hospitalizations | ||||||||
| Proportion with any hospitalizations, % | 21.0 | 27.2 | 7.9 | 11.8% | 5.7 | 8.0 | 2.6 | 3.4 |
| Average hospitalizations among those with ≥1 hospitalization | 1.26 | 1.44 | 1.19 | 1.34 | 1.23 | 1.28 | 1.24 | 1.21 |
| ED visits | ||||||||
| Proportion with any visit | 24.1 | 30.9 | 15.3 | 18.8 | 12.5 | 15.8 | 9.1 | 9.2 |
| Average visits among those with ≥1 visit | 1.47 | 1.61 | 1.31 | 1.44 | 1.38 | 1.45 | 1.31 | 1.31 |
| Outpatient visits | ||||||||
| Proportion with any visit, % | 94.7 | 95.6 | 85.0 | 89.1 | 74.1 | 81.0 | 60.5 | 63.5 |
| Average visits among those with ≥1 visit | 11.55 | 14.94 | 8.22 | 9.99 | 7.96 | 8.55 | 5.88 | 6.81 |
| Prescription medications | ||||||||
| Proportion with ≥1 purchase/refill, % | 97.2 | 97.3 | 87.7 | 91.7 | 76.6 | 81.7 | 48.9 | 53.5 |
| Average purchases/refills among those with ≥1 purchase/refill | 31.93 | 45.96 | 24.92 | 33.45 | 16.53 | 19.44 | 8.71 | 11.95 |
| High (≥75th percentile) health care utilization | ||||||||
| Hospitalizations | ||||||||
| Proportion ≥75th percentile, % | 21.0 | 27.2 | 7.9 | 11.8 | 5.7 | 8.0 | 2.6 | 3.4 |
| Average hospitalizations among those ≥75th percentile | 1.26 | 1.44 | 1.19 | 1.34 | 1.23 | 1.28 | 1.24 | 1.21 |
| ED visits | ||||||||
| Proportion ≥75th percentile, % | 7.0 | 10.9 | 3.3 | 5.1 | 3.0 | 4.4 | 1.9 | 1.9 |
| Average visits among those ≥75th percentile | 2.61 | 2.73 | 2.46 | 2.64 | 2.58 | 2.6 | 2.5 | 2.5 |
| Outpatient visits | ||||||||
| Proportion ≥75th percentile, % | 48.1 | 56.6 | 25.9 | 35.9 | 23.2 | 26.9 | 12.4 | 16.2 |
| Average visits among those ≥75th percentile | 18.82 | 22.59 | 19.66 | 19.53 | 18.36 | 19.34 | 18.57 | 18.77 |
| Prescription medications | ||||||||
| Proportion ≥75th percentile, % | 48.2 | 64.5 | 32.8 | 45.1 | 15.9 | 22.8 | 3.6 | 7.2 |
| Average visits among those ≥75th percentile | 50.67 | 62.34 | 48.09 | 56.25 | 44.46 | 45.12 | 37.61 | 44.11 |
Cardiovascular risk factors: hypertension, diabetes mellitus, hypercholesterolemia, obesity, and smoking. CRF indicates cardiovascular risk factor; CVD, cardiovascular disease; ED, emergency department; PA, physical activity.
Figure 2.
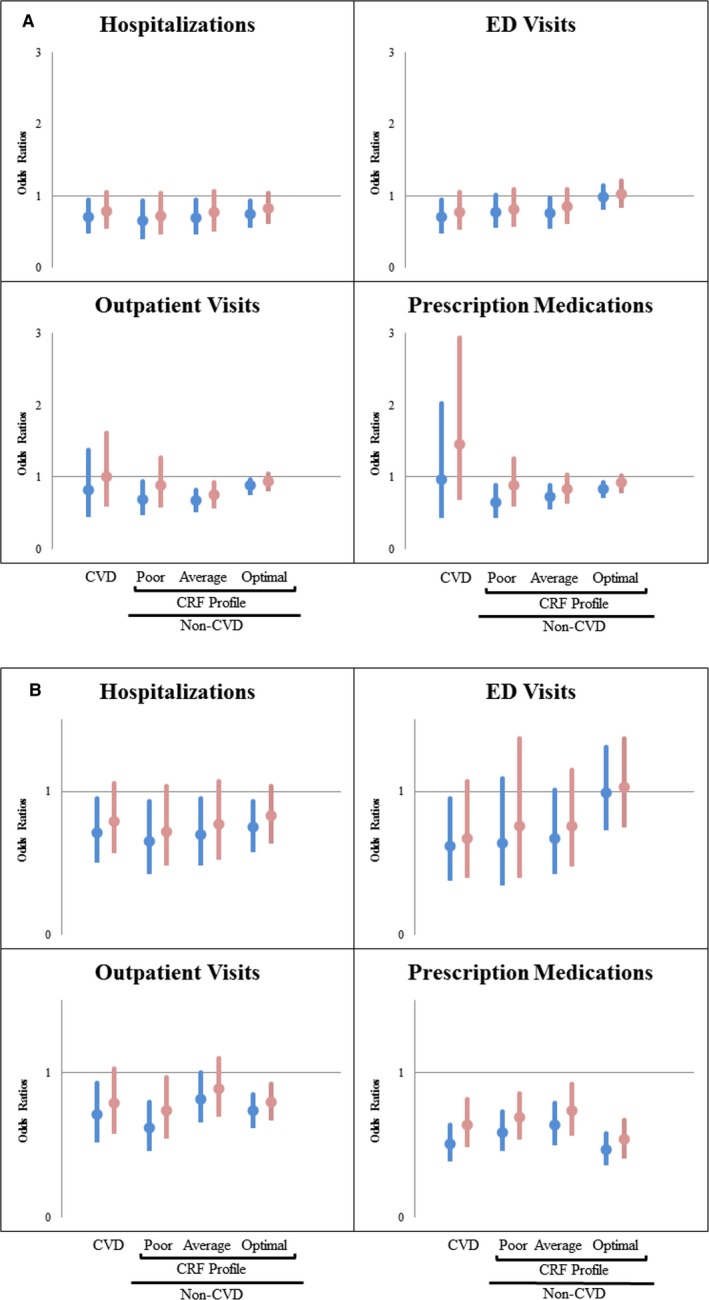
A, Odds ratios for health care utilization of optimal PA versus nonoptimal PA. CRF indicates cardiovascular risk factor profile; ED, emergency department; PA, physical activity. Color scheme: blue bars, univariate model; red bars; multivariate model. Cardiovascular risk factors: hypertension, diabetes mellitus, hypercholesterolemia, obesity, and smoking. Non‐CVD: adjusted for age, sex, family income, race/ethnicity, insurance type, geographical region, and modified Charlson Comorbidity Index (without cardiovascular components). CVD: adjusted for “non‐CVD” covariates plus cardiovascular risk factors. B, Odds ratios for “high” (≥75% percentile) healthcare utilization of optimal PA versus nonoptimal PA. CRF indicates cardiovascular risk factor; ED, emergency department. Color scheme: blue bars, univariate model; red bars, multivariate model. Cardiovascular risk factors: hypertension, diabetes mellitus, hypercholesterolemia, obesity, and smoking. Non‐CVD: adjusted for age, sex, family income, race/ethnicity, insurance type, geographical region, and modified Charlson Comorbidity Index (without cardiovascular components). CVD: adjusted for “non‐CVD” covariates plus cardiovascular risk factors. Note: “High hospitalizations” equaled 1 hospitalization, given the right shift of utilization.
Discussion
Past reports have described the relationship between some level of PA and costs13, 17, 19, 20, 22, 31 however, none have recently shown this on the basis of CVD,16 with a focus on CRFs. This study provides current estimates from a nationally representative sample of the US population. We found lower health care expenditures and resource utilization associated with moderate‐vigorous PA, regardless of CVD status. Additionally, we found significantly lower health care expenditures of PA individuals by each individual CRF profile.
Our results are consistent with most past reports and are an important addition to the understanding of health care expenditures relating to PA. First, this study adds to current literature by providing current national estimates of health care savings among those who engage in moderate‐vigorous PA. Second, this project not only ascertains the differences between CVD and non‐CVD populations, but also brings a special focus on non‐CVD individuals and their CRF profile to demonstrate that efforts concerning prevention are still much needed. Third, to the authors’ knowledge, this is the first attempt to describe both costs and their relating resource utilization estimates regarding PA in a single study.
It has become widely accepted that PA leads to better health outcomes and enhances the prevention of diseases like CVD, diabetes mellitus, cancer, hypertension, obesity, depression, and osteoporosis.32 Consequently, many studies have assessed PA's economic impact. Some have reported no differences in savings related to PA; Chevan et al. found no short‐term savings related to PA,13 whereas Martinson et al. found no differences for those in their forties, but significant lower short‐term charges in those physically active aged 50 and above, compared to those inactive.15 Others have found the opposite to be true. Wang et al. reported savings of more than 50% when comparing CVD versus non‐CVD individuals from a nationally representative sample.16 From our results, we estimate that PA results in a 20% reduction in health care expenditure among those with CVD and in a 50% reduction when comparing those with CVD versus non‐CVD and having poor CRF (the most affected individuals in the non‐CVD categorization; Table 2, model 3). Similarly, Pronk et al. studied modifiable health risks and short‐term health care–related charges, and found that those that engaged in PA had a 4.7% reduction in median charges when compared to those not engaged in PA. Additionally, they reported that in a general population of ≥60 years of age, without diabetes mellitus, or congenital heart disease, high‐risk individuals (BMI ≥25 kg/m2, current smokers, and no PA) had a consistent 49% increase in mean annual charges compared to low‐risk individuals.14 Likewise, even with a slightly different classification of risk factors, our results show health care savings of more than 50% when comparing poor versus optimal CRF, regardless of PA (Table 2, model 3). Furthermore, our results show relative savings of 16.7% and 13% when comparing optimal PA versus nonoptimal PA among those with poor and optimal CRF, respectively. More recently, Carlson et al. reported that inactive adults spent $920 more than their active equivalents.12 Although Carlson et al. did not focus on CVD, our results seem to be in line with their findings, with −$723 as the difference between optimal PA versus nonoptimal PA in the multivariate model, in the general population. Another recent study analyzed costs related to PA using the Cooper Center Longitudinal Study (CCLS).17 They found that expenditures in later life (≥65 years of age) were greatly diminished if cardiorespiratory fitness was achieved in midlife years (around 50 years of age). Our results are not readily comparable with theirs given that they only studied Medicare‐ and Medicaid‐covered individuals. Moreover, individuals from the CCLS tend to be healthier than the general US population.33 Notwithstanding, their conclusions and ours support the same concept: PA needs to be promoted and/or improved, with data to bolster third‐party payers into action for a less‐devastating economic future for the nation, especially given that chronic disease prevalence is on the rise.34, 35 From the health care resource utilization point of view, a Canadian study reported an inverse association with PA and utilization among individuals 65 years and older.36 Our results too show a tendency of diminishing resource utilization when comparing optimal PA versus nonoptimal PA, especially when comparing CVD and non‐CVD individuals.
Overwhelming evidence points toward a world‐wide increase in prevalence of noncommunicable chronic diseases. Dall et al. estimated that by the year 2025, most growth in outpatient visits will be reflected primarily in the field of cardiology, which, along with vascular surgery, will have the highest projected growth in demand.37 Consequently, several initiatives have been set up with a goal to help mitigate the increase in noncommunicable diseases, such as the American Heart Association's (AHA) 2020 Strategic Goals,34 Health 2020,38 or the “25×25” Strategy.39 Unsurprisingly, PA is an integral part of all these efforts, and yet the current prevalence of PA engagement remains below 50% for non‐CVD and below 35% for CVD individuals, according to our results, including the advent of recent reports favoring interventions among middle‐aged individuals,40 or even identifying the benefits of simpler strategies, such as walking, in the prevention of CVD.41
Several limitations of this study should be noted. First, because of the limited assessment of PA degree in the MEPS questionnaires, we were only able to dichotomize PA levels as those engaging versus not in self‐reported moderate‐vigorous activity, ≥30 minutes, ≥5 days/week (optimal vs nonoptimal). This limitation precludes the opportunity to robustly describe the interplay of reported PA levels across a wider spectrum with CVD status as well as burden of modifiable CRF on the overall health care expenditures. Given that the nonoptimal PA category likely included individuals with minimal PA, it likely resulted in more‐conservative estimated differences of medical expenditures between the 2 groups. Furthermore, because of the nature of self‐reported PA, there is a risk for misclassification, though likely random. As a result, this can attenuate the observed findings toward the null. It is important to note that self‐reported PA has been shown to have moderate validity in other national surveys.42 Second, as with any observational study, residual confounding is a possibility. Even though efforts were made to prevent this by controlling for most important variables, there is potential for unmeasured characteristics, which could affect our study outcomes. Third, the prevalence of CVD in this study is lower than past national estimates (9% vs 36%).1 This is because the diagnosis of CVD in our study did not consider hypertension and instead was included in the spectrum of CRF profile assessment. Fourth, because CVD and modifiable CRF were self‐reported, underestimation of the true national prevalence is likely, as has been previously described, especially with chronic conditions.43 As a result, the estimates in our study are likely to be conservative. Because of lack of information on dietary habits, we were not able to account for this important modifiable risk factor. In addition, because of reliance on self‐reported risk factors and lack of information on clinical values (eg, blood pressure), we were unable to estimate the prevalence of the AHA defined “ideal CV health status” in our study. Fifth, other analyses have found that MEPS data tend to underestimate total medical expenditures.44, 45, 46 This limitation would lead to a likely underestimation of the actual cost associated with increasing burden of modifiable CRF as well estimating savings from primordial prevention strategies.
In conclusion, from a national representative population, we provide strong evidence of the association between moderate‐vigorous PA and significantly lower health care expenditures and resource utilization, irrespective of CVD status and/or CRF burden. These robust estimates for potential health care savings strongly support the AHA's strategic goals for optimizing PA levels as a mean to favorably impact the increasing burden of CVD and associated costs.
Disclosures
Dr Nasir is on the advisory board for Quest Diagnostic and is a consultant for Regeneron. No other potential conflicts of interest relevant to this article were reported.
(J Am Heart Assoc. 2016;5: e003614 doi: 10.1161/JAHA.116.003614)
This project is the recipient of the Steven N. Blair Award for Excellence in Physical Activity Research and was presented at the American Heart Association Epi/Lifestyle Scientific Sessions in Phoenix, Arizona, on March 2, 2016.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2015;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Schoenborn CA, Stommel M. Adherence to the 2008 adult physical activity guidelines and mortality risk. Am J Prev Med. 2011;40:514–521. [DOI] [PubMed] [Google Scholar]
- 3. Hamer M, Stamatakis E. Low‐dose physical activity attenuates cardiovascular disease mortality in men and women with clustered metabolic risk factors. Circ Cardiovasc Qual Outcomes. 2012;5:494–499. [DOI] [PubMed] [Google Scholar]
- 4. Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong ME, Green J, Reeves GK, Beral V, Cairns BJ. Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131:721–729. [DOI] [PubMed] [Google Scholar]
- 6. Gomez E, Fernandez‐Alvira JM, Vilanova M, Haro D, Martinez R, Carvajal I, Carral V, Rodriguez C, de Miguel M, Bodega P, Santos‐Beneit G, Penalvo JL, Marina I, Perez‐Farinos N, DalRe M, Villar C, Robledo T, Vedanthan R, Bansilal S, Fuster V. A comprehensive lifestyle peer‐group‐based intervention on cardiovascular risk factors: the randomized controlled Fifty‐Fifty Program. J Am Coll Cardiol. 2016;67:476–485. [DOI] [PubMed] [Google Scholar]
- 7. Roux L, Pratt M, Tengs TO, Yore MM, Yanagawa TL, Van Den Bos J, Rutt C, Brownson RC, Powell KE, Heath G, Kohl HW III, Teutsch S, Cawley J, Lee IM, West L, Buchner DM. Cost effectiveness of community‐based physical activity interventions. Am J Prev Med. 2008;35:578–588. [DOI] [PubMed] [Google Scholar]
- 8. Wu S, Cohen D, Shi Y, Pearson M, Sturm R. Economic analysis of physical activity interventions. Am J Prev Med. 2011;40:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, Stone EJ, Rajab MW, Corso P. The effectiveness of interventions to increase physical activity. A systematic review. Am J Prev Med. 2002;22:73–107. [DOI] [PubMed] [Google Scholar]
- 10. Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;118:576–585. [DOI] [PubMed] [Google Scholar]
- 11. Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo‐Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost‐effectiveness. Lancet. 2010;376:1775–1784. [DOI] [PubMed] [Google Scholar]
- 12. Carlson SA, Fulton JE, Pratt M, Yang Z, Adams EK. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis. 2015;57:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chevan J, Roberts DE. No short‐term savings in health care expenditures for physically active adults. Prev Med. 2014;63:1–5. [DOI] [PubMed] [Google Scholar]
- 14. Pronk NP, Goodman MJ, O'Connor PJ, Martinson BC. Relationship between modifiable health risks and short‐term health care charges. JAMA. 1999;282:2235–2239. [DOI] [PubMed] [Google Scholar]
- 15. Martinson BC, Crain AL, Pronk NP, O'Connor PJ, Maciosek MV. Changes in physical activity and short‐term changes in health care charges: a prospective cohort study of older adults. Prev Med. 2003;37:319–326. [DOI] [PubMed] [Google Scholar]
- 16. Wang G, Pratt M, Macera CA, Zheng ZJ, Heath G. Physical activity, cardiovascular disease, and medical expenditures in U.S. adults. Ann Behav Med. 2004;28:88–94. [DOI] [PubMed] [Google Scholar]
- 17. Bachmann JM, DeFina LF, Franzini L, Gao A, Leonard DS, Cooper KH, Berry JD, Willis BL. Cardiorespiratory fitness in middle age and health care costs in later life. J Am Coll Cardiol. 2015;66:1876–1885. [DOI] [PubMed] [Google Scholar]
- 18. Jones TF, Eaton CB. Cost‐benefit analysis of walking to prevent coronary heart disease. Arch Fam Med. 1994;3:703–710. [DOI] [PubMed] [Google Scholar]
- 19. Garrett NA, Brasure M, Schmitz KH, Schultz MM, Huber MR. Physical inactivity: direct cost to a health plan. Am J Prev Med. 2004;27:304–309. [DOI] [PubMed] [Google Scholar]
- 20. Anderson LH, Martinson BC, Crain AL, Pronk NP, Whitebird RR, O’’Connor PJ, Fine LJ. Health care charges associated with physical inactivity, overweight, and obesity. Prev Chronic Dis. 2005;2:A09. [PMC free article] [PubMed] [Google Scholar]
- 21. Pratt M, Macera CA, Wang G. Higher direct medical costs associated with physical inactivity. Phys Sportsmed. 2000;28:63–70. [DOI] [PubMed] [Google Scholar]
- 22. Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31:S663–S667. [DOI] [PubMed] [Google Scholar]
- 23. Medical Expenditure Panel Survey. Available at: http://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp. Accessed October 1, 2015.
- 24. IRB Exemption. Available at: http://www.hhs.gov/ohrp/regulations-and-policy/decision-trees/#c2. Accessed January 21, 2016.
- 25. MEPS Glossary. Available at: https://meps.ahrq.gov/survey_comp/hc_ques_glossary.shtml. Accessed February 16, 2016.
- 26. Ozieh MN, Dismuke CE, Lynch CP, Egede LE. Medical care expenditures associated with chronic kidney disease in adults with diabetes: United States 2011. Diabetes Res Clin Pract. 2015;109:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mihaylova B, Briggs A, O'Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20:897–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belloti F, Deb P, Manning WG, Norton EC. Twopm: two‐part models. Stata J. 2015;15:3–20. [Google Scholar]
- 29. Hardin J, Hilbe J. Generalized Linear Models and Extensions. 2nd Edition College Station, Texas: StataCorp LP: Stata Press; 2007. [Google Scholar]
- 30. Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. [DOI] [PubMed] [Google Scholar]
- 31. Bell JF, Zimmerman FJ, Arterburn DE, Maciejewski ML. Health‐care expenditures of overweight and obese males and females in the medical expenditures panel survey by age cohort. Obesity (Silver Spring). 2011;19:228–232. [DOI] [PubMed] [Google Scholar]
- 32. Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Willis BL, DeFina LF, Bachmann JM, Franzini L, Shay CM, Gao A, Leonard D, Berry JD. Association of ideal cardiovascular health and long‐term healthcare costs. Am J Prev Med. 2015;49:678–685. [DOI] [PubMed] [Google Scholar]
- 34. Sacco RL. The New American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown). 2011;12:255–257. [DOI] [PubMed] [Google Scholar]
- 35. Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384:45–52. [DOI] [PubMed] [Google Scholar]
- 36. Liu‐Ambrose TY, Ashe MC, Marra C. Independent and inverse association of healthcare utilisation with physical activity in older adults with multiple chronic conditions. Br J Sports Med. 2010;44:1024–1028. [DOI] [PubMed] [Google Scholar]
- 37. Dall TM, Gallo PD, Chakrabarti R, West T, Semilla AP, Storm MV. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff (Millwood). 2013;32:2013–2020. [DOI] [PubMed] [Google Scholar]
- 38. Jakab Z, Tsouros AD. Health 2020—achieving health and development in today's Europe. Cent Eur J Public Health. 2014;22:133–138. [DOI] [PubMed] [Google Scholar]
- 39. Pearce N, Ebrahim S, McKee M, Lamptey P, Barreto ML, Matheson D, Walls H, Foliaki S, Miranda J, Chimeddamba O, Marcos LG, Haines A, Vineis P. The road to 25x25: how can the five‐target strategy reach its goal? Lancet Glob Health. 2014;2:e126–e128. [DOI] [PubMed] [Google Scholar]
- 40. Roux L, Pratt M, Lee IM, Bazzarre T, Buchner D. Does age modify the cost‐effectiveness of community‐based physical activity interventions? J Phys Act Health. 2015;12:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murtagh EM, Nichols L, Mohammed MA, Holder R, Nevill AM, Murphy MH. The effect of walking on risk factors for cardiovascular disease: an updated systematic review and meta‐analysis of randomised control trials. Prev Med. 2015;72:34–43. [DOI] [PubMed] [Google Scholar]
- 42. Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the behavioral risk factor surveillance system (BRFSS). Soz Praventivmed. 2001;46(suppl 1):S3–S42. [PubMed] [Google Scholar]
- 43. Evaluation of National Health Interview Survey diagnostic reporting. Vital Health Stat 2. 1994;120:1–116. [PubMed] [Google Scholar]
- 44. Aizcorbe A, Liebman E, Pack S, Cutler DM, Chernew ME, Rosen AB. Measuring health care costs of individuals with employer‐sponsored health insurance in the U.S.: a comparison of survey and claims data. Stat J IAOS. 2012;28:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sing M, Banthin JS, Selden TM, Cowan CA, Keehan SP. Reconciling medical expenditure estimates from the MEPS and NHEA, 2002. Health Care Financ Rev. 2006;28:25–40. [PMC free article] [PubMed] [Google Scholar]
- 46. Trogdon JG, Murphy LB, Khavjou OA, Li R, Maylahn CM, Tangka FK, Nurmagambetov TA, Ekwueme DU, Nwaise I, Chapman DP, Orenstein D. Costs of chronic diseases at the state level: the chronic disease cost calculator. Prev Chronic Dis. 2015;12:E140. [DOI] [PMC free article] [PubMed] [Google Scholar]


